Abstract
Purpose
This is a case- control study to determine whether G1733A polymorphism of androgen receptor gene is associated with an increased risk for recurrent spontaneous abortion (RSA).
Method
A total of 85 women with at least two recurrent spontaneous abortion before 20th week of gestation composed the study group. Subjects were genotyped by the polymerase chain reaction restriction fragment length polymorphism method.
Results
The observed frequencies of GG, GA and AA genotypes of the G1733A polymorphism were 5.89 %, 82.35 % and 11.76 %, respectively, for the patient group and 71.76 %, 23.51 % and 4.71 %, respectively, for the control group. Allele frequencies of the G1733A polymorphism among patients and controls were 0.47 and 0.84, respectively, for the dominant allele (G) (wild type) and 0.53 and 0.16, respectively, for the A allele (mutant type).
Conclusions
These results indicated that the androgen receptor G1733A polymorphism is strongly associated with increased risk for RSA.
Keywords: Recurrent spontaneous abortion, Androgen receptor, Polymorphism, RFLP PCR
Recurrent spontaneous abortion (RSA) is a reproductive problem that occurs in women in reproductive age with a frequency of 1 %–3 % [20]. It is defined as two or more repeated pregnancy losses before the 20th week of gestation [14]. The risk of miscarriage is enhanced by a variety of factors including chromosomal abnormalities, uterine abnormalities, hereditary thrombophilia, endocrinologic disorders,immunologic factors, infections, and nutritional and environmental factors [18, 19]. In women with a history of recurrent miscarriage, the risk of miscarriage in a subsequent pregnancy is about 40 % to 50 %. It has a major influence on the wellbeing and psychological status of patients, therefore improved diagnosis and development of treatment strategies is essential [6, 16, 23]. In the past years, research interest has been focused on the association studies between different genetic polymorphisms and recurrent spontaneous abortion.
Androgens are lipophilic hormones with several physiological effects in both sexes [17]. Androgen signaling in female is very important for the differentiation of human endometrial stromal cells into decidual cell; a process is called decidualization that critically controls embryo implantation and placentation [11]. The effect of androgens such as testosterone and dihydrotestosterone mediated by androgen receptor (AR) that is a nuclear receptor [3]. Androgen receptor is widely expressed in female reproductive tissue such as endometrium [1] and binds to a steroid ligand and then is transferred into the nucleus, where it regulates the transcription of androgen-responsive genes [5]. The AR gene is located on the X chromosome between q11 and q12 loci and is composed of eight exons [2] that encode a 110-kd protein that contains an N-terminal transactivation domain, a central DNA-binding domain, and a C-terminal ligand-binding domain [8].
Exon 1 of the gene contains two polymorphic trinucleotide repeats (CAG and GGC) that code for polyglutamine and polyglycine tracts. In vitro studies have shown that there is a relationship between the length of both repeats and AR activity [7, 9]. Between these two microsatellites, a single nucleotide polymorphism determined as a G to A substitution (G1733A) in the third nucleotide position of the 211 codon has been previously described [15]. This codon resides in the N-terminal domain of the AR that harbors the major transcription activation functions [10]. Increased androgen bioactivity may result from higher circulating androgen concentrations or from increased AR transactivational activity, which is influenced by AR gene polymorphisms and can cause increased endometrial proliferative activity [12, 13]. The aim of our study was to determine whether the AR G1733A polymorphism is associated with an increased risk for RSA.
Methods
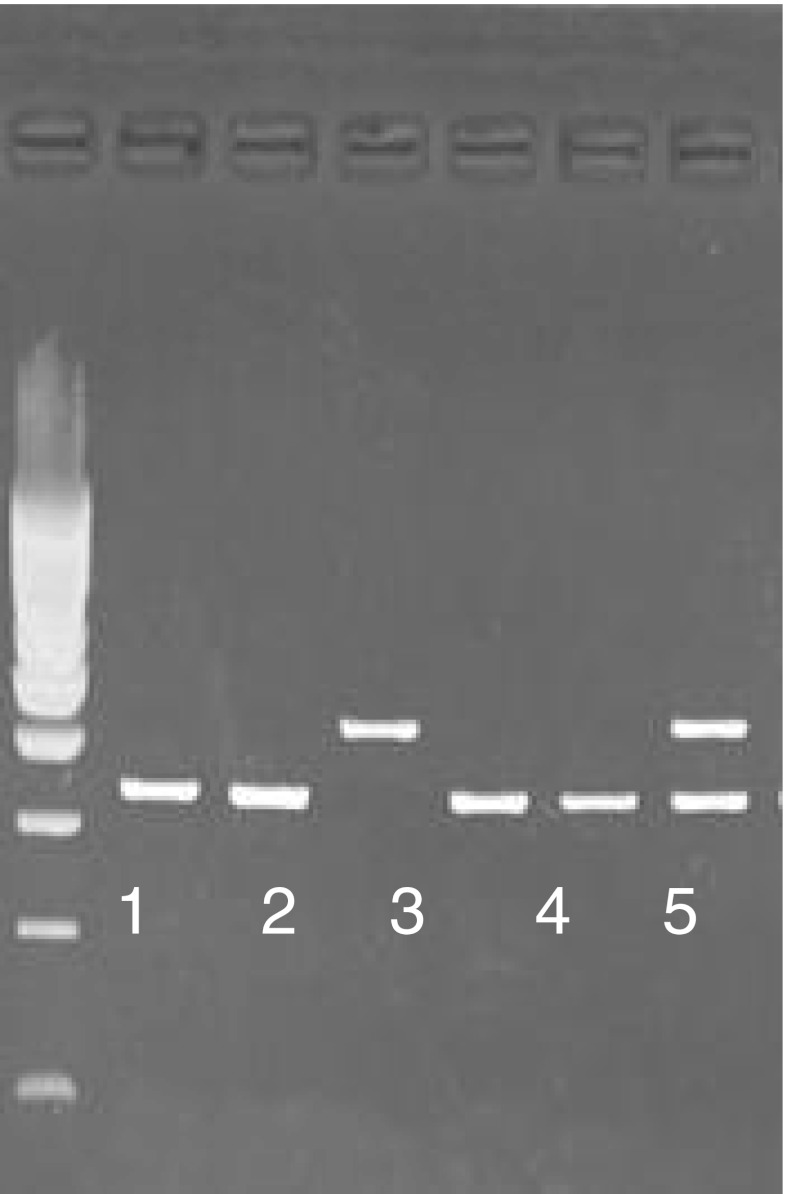
This study was done from April 2011 till February 2012 on 85 patients aged 20 to 42 years (mean 29.63 ± 4.7 years). Control group was 85 healthy women with at least one normal child. Cases and controls were matched according to their age. The study was approved by the ethics committee of recurrent abortion clinic of Yazd Reproductive Sciences Institute, which is a referral center for reproductive disorder, and our patients came from all over the Iran and also informed consent was signed by all participants. The patient group consisted of subjects presenting at least two recurrent spontaneous abortions before 20 weeks of gestation. The patients were investigated to exclude established causes of RSA, such as chromosomal abnormalities, uterine abnormalities, hereditary thrombophilias, endocrinologic disorders, immunologic factors and infections. The control group consisted of women with at least two live births and without history of abortions. Genomic DNA was extracted from peripheral blood leukocytes of the patients and controls, by salting-out method. The genotyping of the G1733Apolymorphism was carried out using the polymerase chainreaction (PCR)–restriction fragment length polymorphism method. The PCR amplification primers were as follows: forward primer: 5′–CTG GAT GAG GAA CAG CAA CC-3′; reverse primer: 5′-CGT TGT CAG AAA TGG TCG AA-3′.Deoxyribonucleic acid was amplified for 35 cycles, each cycle comprising denaturation at 94 °C for 2 min, annealing at 64 °C for 45 s, and extension at 72 °C for 45 s, with a final extension time of 1 min at 72 °C. The 379-base pair (bp) PCR product was digested with the restriction enzyme Stu1 at 37 °C for 5 min and inactivated 80 °C for 10 min. The restricted fragments were separated on 2 % agarose gels with ethidium bromide staining. The presence of the A variant (379-bp fragment) or the G variant (313-bp fragment) indicated by two alleles (Fig. 1). The results of the study and control groups were analyzed for statistical significance at the 95 % confidence interval using chi square test. A P value of < .05 was considered statistically significant. The odds ratio was used as measure ofthe strength of the association between allele frequencies and RSA. Statistical analysis was performed with the Statistical Package for the Social Sciences for Windows, version 16.
Fig. 1.
Photograph of 2 % ethidium bromide stained agarose gel used to resolve the restriction fragment length polymorphism G1733A in androgen receptor of 6 randomly selected case and control. The wild type G allele is represented by a 313 bp band. The mutant A allele is represented by a 379 bp band. Column 1,2,4,5 show homozygous wild type, column 3 shows homozygous mutant and column 6 shows heterozygous pattern
Results
Demographic data of the patient group and control group are shown in Table 1. The mean age between two groups was similar. All 85 patients and 85 controls were examined for the G1733A polymorphism of the AR gene. The results are shown in Table 2. The frequencies of the GG, GA, and AA genotypes of the G1733A polymorphism were 5.89 %, 82.35%and 11.76 %, respectively, for the patient group and 71.76 %, 23.51%and 4.71 %, respectively, for the control group. Allele frequencies of the G1733A polymorphism among patients and controls were 0.47 and 0.84, respectively, for the dominant allele (G) (wild type) and 0.53 and 0.16, respectively, for the A allele (mutant type). Statistical analysis of the genotype frequencies showed that there are significant differences between the two groups (P-Value < 0.0001). Furthermore, as shown in Table 2, analysis of allele frequencies indicated significant differences (P-Value < 0.0001, odds ratio 5.92) between women with RSA and controls.
Table 1.
Characteristics of women with recurrent spontaneous abortions
| Characteristic | Patients (n = 85) | Controls (n = 85) |
|---|---|---|
| Age | 30.84 ± 5.2 (22–42) | 29 ± 4.4 (20–41) |
| Abortions | 3 ± 1.3 (2–7) | 0 |
| Live births | 0 | 2.3 (2–5) |
Table 2.
Genotype and allele frequencies of G1733A polymorphism of AR gene among patients and controls followed by Hardy-Weinberg equilibrium (P + Q = 1)
| Genotype/Allele | Controls (n = 85) | Patients (n = 85) | P-Value | Odd ratio (CI 95 %) |
|---|---|---|---|---|
| Genotype | ||||
| GG | 61 (71.78 %) | 5 (5.89 %) | <0.0001 | |
| GA | 20 (23.51 %) | 70 (82.35 %) | ||
| AA | 4 (4.71 %) | 10 (11.76 %) | ||
| Allele | ||||
| G | 0.84 | 0.47 | <0.0001 | 5.92 (3.050–11.493) |
| A | 0.16 | 0.53 | ||
Conclusion
The outcome of pregnancy relies on the success rate of various early events, such as implantation, establishment of feto-maternal circulation and maintenance of increased blood flow to the implantation site [12, 13]. The development of RSA is complex and is regulated by multiple genetic pathways. Different genes encoding for proteins involved in various biologic pathways have been reported to be associated with recurrent spontaneous abortion [4, 22].
There is evidence that androgens and their receptor are involved in uterine cell proliferation in rats [26]. Recent studies have further explained the important role of androgen receptor in normal female reproduction as described above [21, 24]. So, we hypothesized that it is possible that polymorphisms in this gene may affect normal gene function and it could be associated with RSA.
In the present study we investigated the potential effect of the G1733A polymorphism of the AR gene in the pathogenesis of RSA.
Our result shows that the frequency of A allele in Iranian population is 0.53. The observed frequency of homozygous AA is 0.04 and 0.11in the control and patient groups respectively, whereas the frequency of heterozygous GA is 0.23 for the control group and 0.82 for the patient group. Statistical analysis of the results indicates significant difference between the two groups for studied polymorphism. Although heterozygote females initially display similar fertility to wild-type females, they exhibit recurrent spontaneous miscarriages and this indicates that quantitative variations in AR activity via gene dosage may play a role in determining female fertility [25]. Our findings are comparable with study performed by Karvela et al. They managed a case–control study with 131women with RSA and found that there are significant differences between the two groups in terms of both genotype distribution and allele frequencies (2008).
We concluded that the presence of the A allele of the G1733A polymorphism seems to be associated with an increased risk for recurrent miscarriage and it could be a useful genetic marker for the assessment of a woman’s risk for RSA.
Footnotes
Capsule Our results indicated that the androgen receptor G1733A polymorphism is strongly associated with increased risk for RSA.
References
- 1.Apparao KB, Lovely LP, Gui Y, Lininger RA, Lessey BA. Elevated endometrial androgen receptor expression in women with polycystic ovarian syndrome. Biol Reprod. 2002;66:297–304. doi: 10.1095/biolreprod66.2.297. [DOI] [PubMed] [Google Scholar]
- 2.Brosens IA, De Sutter P, Hamerlynck T, Imeraj L, Yao Z, Cloke B, Brosens JJ, Dhont M. Endometriosis is associated with a decreased risk of preeclampsia. Hum Reprod. 2007;22:1725–1729. doi: 10.1093/humrep/dem072. [DOI] [PubMed] [Google Scholar]
- 3.Brown CJ, Goss SJ, Lubahn DB, Joseph DR, Wilson EM, French FS, et al. Androgen receptor locus on the human X chromosome: regional localization to Xq11-12 and description of a DNA polymorphism. Am J Hum Genet. 1989;44:264–9. [PMC free article] [PubMed] [Google Scholar]
- 4.Buchholz T, Lohse P, Kosian E, Thaler CJ. Vasoconstrictively acting AT1R A1166C and NOS3 4/5 polymorphisms in recurrent spontaneous abortions (RSA) Am J Reprod Immunol. 2004;51(5):323–8. doi: 10.1111/j.1600-0897.2004.00163.x. [DOI] [PubMed] [Google Scholar]
- 5.Burger HG. Androgen production in women. Fertil Stril. 2002;77:S3–S5. doi: 10.1016/S0015-0282(02)02985-0. [DOI] [PubMed] [Google Scholar]
- 6.Carrington B, Sacks G, Regan L. Recurrent miscarriage: pathophysiology and outcome. Curr Opin Obstet Gynecol. 2005;17(6):591–7. doi: 10.1097/01.gco.0000194112.86051.26. [DOI] [PubMed] [Google Scholar]
- 7.Chamberlain NL, Driver ED, Miesfeld RL. The length and location of CAG trinucleotide repeats in the androgen receptor N-terminal domain affect transactivation function. Nucleic Acids Res. 1994;22:3181–6. doi: 10.1093/nar/22.15.3181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chang C, Saltzman A, Yeh S, Young W, Keller E, Lee HJ, Wang C, Mizokami A. Androgen receptor: an overview. Crit Rev Eukaryot Gene Expr. 1995;5:97–125. doi: 10.1615/CritRevEukarGeneExpr.v5.i2.10. [DOI] [PubMed] [Google Scholar]
- 9.Ding D, Xu L, Menon M, Reddy GP, Barrack ER. Effect of GGC (glycine) repeat length polymorphism in the human androgen receptor on androgen action. Prostate. 2005;62:133–9. doi: 10.1002/pros.20128. [DOI] [PubMed] [Google Scholar]
- 10.Grad JM, Lyons LS, Robins DM, Burnstein KL. The androgen receptor (AR) amino-terminus imposes androgen-specific regulation of AR gene expression via an exonic enhancer. Endocrinology. 2001;142:1107–16. doi: 10.1210/en.142.3.1107. [DOI] [PubMed] [Google Scholar]
- 11.Guay A, Munarriz R, Jacobson J, Talakoub L, Quirk F, Goldstein I, Spark R. Serum androgen levels healthy premenopausal women with and without sexual dysfunction: part A. Serum androgen levels in women aged 20–49 years with no complaints of sexual dysfunction. Int J Import. 2004;16:112–120. doi: 10.1038/sj.ijir.3901178. [DOI] [PubMed] [Google Scholar]
- 12.Karvela M, Papadopoulou S, Tsaliki E, Konstantakou E, Hatzaki A, Florentin-Arar L, Lamnissou K. Endothelial nitric oxide synthase gene polymorphisms in recurrent spontaneous abortions. Arch Gynecol Obstet. 2008;278(4):349–52. doi: 10.1007/s00404-008-0577-8. [DOI] [PubMed] [Google Scholar]
- 13.Karvela M, Stefanakis N, Papadopoulou S, Tsitilou SG, Tsilivakos V and Lamnissou K. Evidence for association of the G1733A polymorphism of the androgen receptor gene with recurrent spontaneous abortions. Fertil Stril. 2008;90. [DOI] [PubMed]
- 14.Li TC, Makris M, Tomsu M, Tuckerman E, Laird S. Recurrent miscarriage: aetiology, management and prognosis. Hum Reprod Update. 2002;8:463–81. doi: 10.1093/humupd/8.5.463. [DOI] [PubMed] [Google Scholar]
- 15.Lu J, Danielsen M. A Stu I polymorphism in the human androgen receptor gene (AR) Clin Genet. 1996;49:323–4. doi: 10.1111/j.1399-0004.1996.tb03800.x. [DOI] [PubMed] [Google Scholar]
- 16.Monien S, Kadecki O, Baumgarten S, Salama A, Dörner T, Kiesewetter H. Use of heparin in women with early and late miscarriages with and without thrombophilia. Clin Appl Thromb Hemost. 2009;15(6):636–44. doi: 10.1177/1076029609335501. [DOI] [PubMed] [Google Scholar]
- 17.Quigley CA, De Bellis A, Marschke KB, Marschke KB, Marschke KB, el-Awady MK, Wilson EM, French FS. Androgen receptor defects: historical, clinical, and molecular perspectives. Endocr Rev. 1995;16:271–321. doi: 10.1210/edrv-16-3-271. [DOI] [PubMed] [Google Scholar]
- 18.Rai R, Regan L. Recurrent miscarriage. Lancet. 2006;368(9535):601–11. doi: 10.1016/S0140-6736(06)69204-0. [DOI] [PubMed] [Google Scholar]
- 19.Redline RW. Thrombophilia and placental pathology. Clin Obstet Gynecol. 2006;49(4):885–94. doi: 10.1097/01.grf.0000211957.68745.6b. [DOI] [PubMed] [Google Scholar]
- 20.Regan L. Overview of recurrent miscarriage. Gynaecol Forum. 1998;3:3–7. [Google Scholar]
- 21.Shiina H, Matsumoto T, Sato T, Igarashi K, Miyamoto J, Takemasa S, Sakari M, Takada I, Nakamura T, Metzger D, Chambon P, Kanno J, Yoshikawa H, Kato S. Premature ovarian failure in androgen receptor-deficient mice. Proc Natl Acad Sci USA. 2006;103(1):224–9. doi: 10.1073/pnas.0506736102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tempfer C, Unfried G, Zeillinger R, Hefler L, Nagele F, Huber JC. Endothelial nitric oxide synthase gene polymorphism in women with idiopathic recurrent miscarriage. Hum Reprod. 2001;16(8):1644–7. doi: 10.1093/humrep/16.8.1644. [DOI] [PubMed] [Google Scholar]
- 23.Toth B, Jeschke U, Rogenhofer N, Scholz C, Würfel W, Thaler CJ, Makrigiannakis A. Recurrent miscarriage: current concepts in diagnosis and treatment. J Reprod Immunol. 2010;85(1):25–32. doi: 10.1016/j.jri.2009.12.006. [DOI] [PubMed] [Google Scholar]
- 24.Walters KA, Allan CM, Jimenez M, Lim PR, Davey RA, Zajac JD, Illingworth P, Handelsman DJ. Female mice haploin sufficient for an inactivated androgen receptor (AR) exhibit age-dependent defects that resemble the AR null phenotype of dysfunctional late follicle development, ovulation, and fertility. Endocrinology. 2007;148(8):3674–84. doi: 10.1210/en.2007-0248. [DOI] [PubMed] [Google Scholar]
- 25.Walter KA, Simanainen U, Handelsman DJ. Molecular insights into androgen actions in male and female reproductive function from androgen receptor knockout models. Hum Reprod Update. 2010;16(5):543–558. doi: 10.1093/humupd/dmq003. [DOI] [PubMed] [Google Scholar]
- 26.Weihua Z, Ekman J, Almkvist A, Saji S, Wang L, Warner M, Gustafsson JA. Involvement of androgen receptor in 17beta-estradiol-induced cell proliferation in rat uterus. Biol Reprod. 2002;67(2):616–23. doi: 10.1095/biolreprod67.2.616. [DOI] [PubMed] [Google Scholar]