Abstract
Purpose
To compare two vitrification methods and two warming methods for human oocyte vitrification using a high security closed device in terms of survival, fertilization and embryo development.
Methods
For vitrification, oocytes were (1) immediately placed in equilibration solution or (2) they were gradually exposed to the cryoprotectants. For warming, oocytes were placed (1) in a 25 μl preheated (37 °C) thawing solution droplet that was put at room temperature for 1 min once the oocytes were inside or (2) in a 150 μl droplet for 1 minute at 37 °C.
Results
Survival and preimplantation development were significantly lower when warming was performed in a small preheated droplet. There was no significant difference in survival and embryo development between the gradual or direct exposure to cryoprotectants.
Conclusions
Using this high security closed vitrification device a 90 % survival rate can be achieved when the oocytes are immediately warmed in a large volume at 37 °C.
Keywords: Oocyte, Vitrification, Human, Closed straw, Survival, In vitro development
Introduction
Oocyte cryopreservation for women in their reproductive age opens new opportunities in IVF because: (i) it permits women to cryopreserve oocytes prior to gonadotoxic radio-or chemotherapy and ovariectomy [3, 60], (ii) it allows women to delay childbearing [58], (iii) it eliminates donor-recipient endometrium synchronization problems and (iv) it avoids ethical and legal concerns regarding supernumerary cryopreserved embryos and embryo ownership [53].
Since the first report of a pregnancy from a frozen-thawed human oocyte in 1986 [11], oocyte cryopreservation has gained increasing interest. Although slow-freezing protocols for oocytes have been modified and improved over time, the outcomes are variable and difficult to reproduce [8, 13, 22, 39, 42, 44, 45, 57, 67]. A consensus has slowly emerged stating that vitrification procedures result in a better embryological and clinical outcome than the slow-freezing procedures [9, 12, 18, 24, 34, 35, 42, 55, 56]. The applied vitrification procedure combines ultra rapid cooling/warming with high cryoprotectant concentrations and minimal volume methods [29–31]. Hence, the surrounding solution is solidified in an amorphous glass-like structure avoiding ice crystal formation [19]. Today, vitrification of oocytes can result in >90 % survival rate, with fertilization (>75 %) and pregnancy rates (32–65 % per embryo transfer) similar to fresh oocytes [3, 15, 40]. Open storage devices -generating a higher cooling rate than closed devices- have been used preferentially, however cross-contamination during vitrification and liquid nitrogen storage cannot be excluded [4, 5, 23, 61]. Bielanski et al. [4] reported that the CBS High Security (HS) Straws (CryoBioSystem, Paris, France) are optimal devices for cryopreservation because they are impermeable to pathogenic agents and resistant to extremely low temperature.
Since the implementation of the EU Cell and Tissue Directive in Belgium, national guidelines encourage to use the CBS-VIT HS system for oocyte and embryo vitrification in order to prevent cross-contamination [58, 63].
Special attention should be paid to oocyte cryopreservation procedures since oocytes are particularly susceptible to cryodamage due to their large surface/volume ratio, plasma membrane permeability properties [47] and intracellular functions (calcium, ribosomes, Golgi apparatus, mitochondria, intracellular trafficking of molecules and organelles) [56]. Moreover, the meiotic spindle, necessary for the correct completion of meiosis and fertilization, is very sensitive to chemical and physical changes. The microtubular structure of the meiotic spindle is affected when oocytes are cooled to room temperature, with a potential deleterious consequences on chromosomal organization [48]. The Polscope, a non-invasive computer-assisted polarized light microscopy system, can be used to study spindles in oocytes [64–66]. The presence of the spindle is suggested to be linked to higher fertilization rates [65, 66] and a higher proportion of good quality embryos [37]. On the other hand, the inability to detect meiotic spindles in oocytes is associated with a higher incidence of fertilization and cleavage abnormalities [49].
The present study aimed to compare two vitrification methods and two warming methods for human oocyte vitrification in a high security closed system in terms of survival, fertilization and blastocyst formation. In two experiments, two vitrification methods and two warming methods were compared on mature sibling oocytes that were donated for research.
Materials and methods
Oocyte source
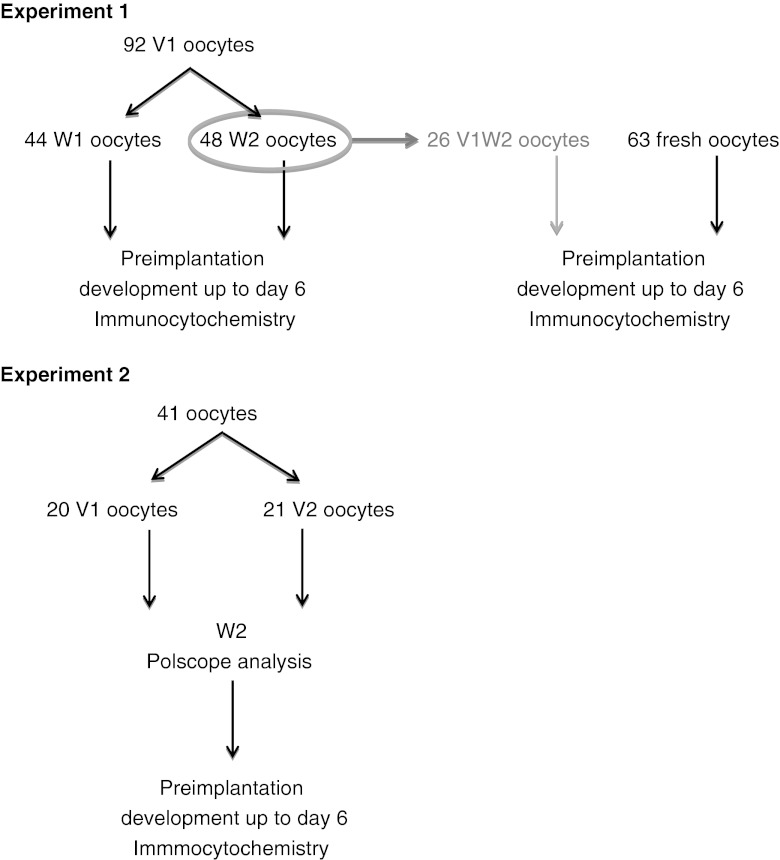
Human oocytes were used to create embryos for research in our centre. For this study, approval of the Local Ethical Committee of UZ Brussel and the Federal Committee for medical and scientific research on human embryos in vitro was obtained. Oocytes were obtained from couples suffering from severe male factor infertility who were unable to undergo ICSI due to the lack of sperm in their testicular biopsy. Oocyte cryopreservation or the use of donor sperm was not an option for them and they donated their oocytes to create embryos for research after written informed consent. Before vitrification, oocytes were denuded with Cumulase® (80 USP Units/ml, Halozyme Therapeutics Inc., San Diego, CA, USA) from surrounding cumulus and corona cells [17]. For experiment 1, a total of 92 oocytes from 16 patients (mean age of 31.8 ± 4.2 year) were vitrified within 7:30 ± 1 h after OPU (Oocyte Pick-Up) between September 2008 and January 2010. For experiment 2, 41 oocytes from 7 patients (mean age 33.6 ± 5.4 year) were vitrified within 7:50 ± 1 h after OPU between April 2010 and June 2011. In a subanalysis of experiment 1, pre-implantation development of 26 vitrified oocytes from 11 patients was compared to the development of 63 fresh oocytes for research from the same patients. In experiment 2, Polscope analysis was performed to evaluate the re-appearance of the spindle after warming.
Oocyte vitrification and warming
For both vitrification methods, the Irvine Scientific® Vitrification Freeze Kit was used containing 7.5 % (v/v) ethylene glycol (EG) +7.5 % (v/v) dimethylsulfoxide (DMSO) in an M-199 HEPES Buffered Medium supplemented with 20 % dextran serum supplement (DSS), referred to as equilibration solution (ES); and vitrification solution (VS) containing 15 % (v/v) EG + 15 % (v/v) DMSO + 0.5 M sucrose. For both warming methods, the Irvine Scientific® Vitrification Thaw Kit was used containing a thawing solution (TS) with 1 M sucrose in an HEPES Buffered Medium supplemented with 20 % DSS, a dilution solution (DS) containing 0.5 M sucrose in an HEPES Buffered Medium supplemented with 20 % DSS and a washing solution (WS) containing HEPES Buffered Medium supplemented with 20 % DSS.
Depending on the number of oocytes available per patient, oocytes were vitrified individually or per two.
Vitrification method 1 (V1)
Denuded oocytes were placed in a pre-equilibrated (37 °C) 25 μl droplet HTF-hepes supplemented with Human Serum Albumin (HSA, Vitrolife, Sweden) at 37 °C for 1 min. Subsequently, they were equilibrated in a 25 μl ES droplet for 10 min at room temperature (RT). Then they were placed in four consecutive 25 μl VS droplets at RT and loaded on the CBS vit straw within 60 s. Straws were thermosealed before plunging into liquid nitrogen.
Vitrification method 2 (V2)
Oocytes were placed in a pre-equilibrated (37 °C) droplet of 25 μl HTF-hepes supplemented with HSA and put at RT for 1 min once the oocytes were inside. After 1 min, this droplet was merged with 25 μl ES for 2 min at RT followed by a second merging with 25 μl ES for 2 min. Then oocytes were transferred to a new 25 μl ES droplet for 10 min, followed by four consecutive 25 μl VS droplets and they were loaded on the CBS vit straw within 60 s. Straws were thermosealed before plunging into liquid nitrogen.
The most important difference between the two vitrification methods is the way the oocytes are exposed to the cryoprotectants. In V1, oocytes are directly exposed to cryoprotectants by putting the oocytes immediately in ES. In V2, oocytes undergo a gradual exposure by merging the HTF-hepes droplet twice with the same volume of ES.
Warming method 1 (W1)
A 25 μl TS droplet was preheated at 37 °C. The dish was put at RT to put the oocytes into the droplet; they were kept at RT for 1 min. This was followed by a second incubation in TS at RT for 1 min. Oocytes were then placed twice in 25 μl DS at RT for 2 min. Finally, three washes of 3 min each were performed in 25 μl WS at RT.
Warming method 2 (W2)
Oocytes were immediately placed in 150 μl preheated TS at 37 °C for 1 min, followed by 3 min in 25 μl DS and two washes for 5 min each in 25 μl WS, both at RT.
The major difference between the two warming methods is the warming rate achieved during the first warming step. In W1, oocytes were places in a small (25 μl) pre-heated (37 °C) TS droplet that was put at RT to put the oocytes inside. In W2, oocytes were places in a large (150 μl) pre-heated (37 °C) TS droplet that was kept at 37 °C.
After washing, oocytes were transferred to individual 25 μl droplets of fertilization medium (Sage, Cooper Surgical) under oil and scored for survival. Subsequently, they were cultured for 2 h in an incubator with 5 % O2 and 6 % CO2.
In experiment 1, V1 was combined with W1 and W2. In experiment 2, V1 and V2 were combined with W2.
Evaluation of survival
Warmed oocytes were considered ‘morphologically surviving’ if there was no dark/degenerated or contracted ooplasm and no cracked zona pellucida.
Polscope analysis
In experiment 2, the presence of the spindle was analysed at 0, 1 and 2 h post-warming using the Polscope imaging system (Research Instruments). Oocytes were placed in a 5 μl drop of HTF-hepes under oil in a glass-bottom culture dish, pre-heated at 37 °C (WillCo-dish, Amsterdam, The Netherlands). An inverted microscope with Polscope equipment and liquid crystal compensator optics (SpindleView, CRI, Cambridge, USA) combined with a computerized image-analysis system (SpindleView Software; CRI) was used at 200x magnification to examine the re-appearance of the spindle on a heating stage at 37 °C.
Fertilization and embryo quality
After 2 h incubation in fertilization medium, ICSI was performed on all surviving oocytes [62] with sperm of a consenting donor. The injected oocytes were cultured in individual 25 μl droplets of cleavage medium (Sage, Cooper Surgical) under oil (Vitrolife) until day 3 and further cultured in blastocyst medium (Sage, Cooper Surgical) until day 5–6.
Fertilization was checked 17–19 h after injection. Preimplantation embryo development was evaluated daily. Good-quality day 3 embryos were defined as having at least six blastomeres and <20 % fragmentation. Blastocyst evaluation relied on the scoring system described by Gardner and Schoolcraft [20]: good-quality blastocysts were defined as being a full blastocyst with an ICM and a TE of type A or B on day 5 or day 6.
Blastocyst fixation, immunostaining and confocal microscopy
All good-quality blastocysts were fixed for further immunofluorescent analysis as described by Cauffman et al. [10]. Immunofluorescent staining with NANOG and KRT-18 was performed to check the quality of the blastocysts. NANOG-positive nuclei should be restricted to the ICM cells. KRT-18 should abundantly be present in the TE and reflect the integrity of the epithelium. Fixation was performed on day 5 or 6 depending on the blastocyst developmental rate and quality. Blastocysts were individually stained in 50 μl droplets in a 96-well plate (650185, Cellstar, Greiner Bio-one, Frickenhausen, Germany). Fixation was performed with 3.7 % formaldehyde (Merck, Darmstadt, Germany) at RT for 10 min and was followed by permeabilization with 0.1 % Triton X-100 (Sigma-Aldrich, St Louis, MO, USA) at RT for 20 min. Both solutions were made in phosphate-buffered saline (PBS). Samples were incubated overnight at 4 °C with a rabbit polyclonal IgG antibody against NANOG (3 μg/ml; ab21624, Abcam, Cambridge, UK) and KRT18 (1 μg/ml; ab668, Abcam). Control reactions for the non-specific binding of the primary antibodies were included in each experiment and carried out by replacing the rabbit antibodies with rabbit serum (R9133, Sigma-Aldrich, St Louis, MO, USA) and the mouse antibodies with mouse IgG1s (349040, Becton Dickinson, Franklin Lakes, NJ, USA) under the same conditions as the primary antibodies. Alexa Fluor® 488 F(ab′)2 fragment of goat anti-rabbit IgG (H + L) (A-11070, Molecular Probes, Invitrogen, Stockholm, Sweden) and Alexa Fluor® 647 F(ab′)2 fragment of goat anti-mouse IgG (H + L) (A-21237, Molecular Probes) were used as secondary antibodies. Samples and controls were incubated at a concentration of 10 μg/ml at RT in the dark for 2 h. All antibody solutions were prepared in PBS supplemented with 2 % bovine serum albumin (BSA) (Sigma-Aldrich). Extensive washing with PBS + 2 % BSA was performed between all steps. After staining, blastocysts were put in 0.5 μl SlowFade® Gold antifade reagent with DAPI (S36939, Invitrogen) and were put between two glass cover slips (24 × 50 mm). To prevent squeezing, round glass cover slips (Ø 10 mm) were put between the cover slips using acrytol mountant (01721, Surgipath Europe LTD, Bretton). Before examination, samples were put at 4 °C in the dark for at least 30 min.
Confocal scanning microscopy was performed with a LD-Ar–HeNe laser (405/488/633) (IX71 Fluoview 300; Olympus, Aartselaar, Belgium) to record the fluorescent images. Control and test images were captured using identical settings. Scans were made every 2–2.5 μm for the samples and every 5 μm for the controls throughout the whole blastocyst.
Statistical analysis
Differences between sibling oocytes were assessed by the Wilcoxon Signed-Rank test. A difference was considered statistically significant when the P-value was <0.05.
Results
We started oocyte vitrification in our lab in 2008. Due to the lack of a standardized protocol for aseptic oocyte vitrification at that time, we applied the technique for blastocyst vitrification (V1W1) since this methodology was well-known and successful for blastocysts in our hands [63]. This technique was applied as the starting point for oocyte vitrification from which further improvements were made in two experiments (Fig. 1).
Fig. 1.
Schematic representation of the two experiments. In experiment 1, all the oocytes were vitrified with V1; half were warmed with W1 and the other half with W2. For some V1W2 oocytes, development was compared with fresh sibling oocytes. In experiment 2, half of the oocytes were vitrified with V1 and the other half with V2. All the oocytes were warmed with W2. After warming Polscope analysis was performed. Preimplantation development was followed in both experiments until day 6 followed by immunocytochemical analysis
Experiment 1
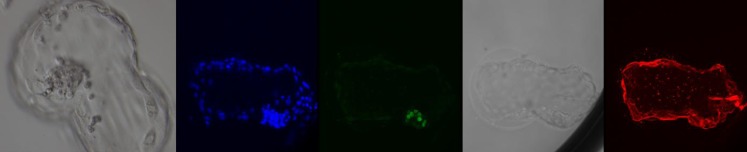
In experiment 1, 92 oocytes were vitrified using V1. Forty-four oocytes were warmed with W1 and 48 oocytes with W2 (Table 1). A significant difference was observed comparing W1 with W2 for survival, fertilization rates, the proportion of good-quality day 3 embryos and good-quality blastocysts on day 5 + 6. The quality of the blastocysts was confirmed by confocal scanning microscopy; good-quality blastocysts showed a well-formed KRT-18 positive TE and an ICM with cells displaying NANOG (1–31 cells) (Fig. 2).
Table 1.
Experiment 1: Survival, fertilization and in vitro development to blastocyst of V1 vitrified human oocytes from 16 patients according to the two warming methods (W1 and W2)
| Variable | Experiment 1 | P-value | |
|---|---|---|---|
| Vitrification method | V1 | V1 | |
| Warming method | W1 | W2 | |
| No. of vitrified-warmed oocytes | 44 | 48 | |
| No. of survived oocytes (%) | 29/44 (65.9) | 43/48 (89.6) | 0.007 |
| No. of fertilized oocytes (%) | 18/29 (62.1) | 35/43 (81.4) | 0.01 |
| No. of good-quality day 3 embryos (%) | 7/18 (38.9) | 16/35 (45.7) | 0.034 |
| No. of good-quality blastocysts on day 5 (%) | 1/18 (5.6) | 5/35 (14.3) | NS |
| No. of good-quality blastocysts on day 5 + 6 (%) | 1/18 (5.6) | 9/35 (25.7) | 0.028 |
| Efficiency (No. Of GQ d5 Bl/warmed oocyte) | 1/44 (2.3) | 5/48 (10.4) | NS |
| Efficiency (No. Of GQ d5 + 6 Bl/warmed oocyte) | 1/44 (2.3) | 9/48 (18.8) | 0.020 |
NS Not Significant; GQ Good Quality
Fig. 2.
Day 6 blastocyst from a V1W2 vitrified oocyte before fixation and the images after fluorescent scanning microscopy: DAPI (blue), NANOG (green), transmission and KRT-18 (red)
Fertilization and embryo development were compared for 26 V1W2 oocytes and 63 fresh sibling oocytes that had been used for other experimental purposes (Table 2). In this subanalysis, no statistical differences were found.
Table 2.
Experiment 1: Survival, fertilization and in vitro development to blastocyst of V1W2 oocytes compared with fresh sibling oocytes from 11 patients
| Variable | Experiment 1 | P-value | |
|---|---|---|---|
| Vitrification method | V1 | fresh | |
| Warming method | W2 | fresh | |
| No. of vitrified-warmed oocytes | 26 | NA | |
| No. of survived oocytes (%) | 25/26 (96.2) | NA | |
| No. of microinjected oocytes (%) | 25/25 (100) | 63/63 (100) | |
| No. of fertilized oocytes (%) | 19/25 (76.0) | 49/63 (77.8) | NS |
| No. of GQ day 3 embryos (%) | 10/19 (52.6) | 32/49 (65.3) | NS |
| No. of GQ blastocysts on day 5 (%) | 3/19 (15.8) | 12/49 (24.5) | NS |
| No. of GQ blastocysts on day 5 + 6 (%) | 6/19 (31.6) | 15/49 (30.6) | NS |
NS Not Significant; NA Not Applicable; GQ Good Quality
Experiment 2
To further increase the survival and developmental rate of the vitrified oocytes, the effect of merging the droplets during vitrification was investigated by gradually exposing the oocytes to the cryoprotectants. Forty-one sibling oocytes were vitrified with V1 (n = 20) or V2 (n = 21) and all oocytes were warmed with W2 (Table 3). Survival and fertilization rates and the proportion of good-quality day 3 embryos, good-quality blastocysts on day 5 and good-quality blastocysts on day 5 + 6 were not different between the two vitrification methods. The presence of NANOG and KRT-18 was confirmed in the blastocysts (data not shown).
Table 3.
Survival, fertilization and in vitro development to blastocyst of V1W2 versus V2W2 sibling oocytes from 7 patients
| Variable | Experiment 2 | P-value | |
|---|---|---|---|
| Vitrification method | V1 | V2 | |
| Warming method | W2 | W2 | |
| No. of vitrified-warmed oocytes | 20 | 21 | |
| No. of survived oocytes (%) | 18/20 (90.0) | 18/21 (85.7) | NS |
| No. of fertilized oocytes (%) | 14/18 (77.8) | 13/18 (72.2) | NS |
| No. of good-quality day 3 embryos (%) | 10/14 (71.4) | 10/13 (76.9) | NS |
| No. of good-quality blastocysts on day 5 (%) | 5/14 (35.7) | 3/13 (23.1) | NS |
| No. of good-quality blastocysts on day 5 + 6 (%) | 7/14 (50.0) | 8/13 (61.5) | NS |
| Efficiency (No. Of GQ d5/warmed oocyte) | 5/20 (25.0) | 3/21 (14.3) | NS |
| Efficiency (No. Of GQ d5 + 6/warmed oocyte) | 7/20 (35.0) | 8/21 (38.1) | NS |
NS Not Significant; GQ Good Quality
Polscope analysis was performed on all surviving oocytes at 0, 1 and 2 h after warming. The results are shown in Table 4. There was no statistical significant difference between V1 and V2 in spindle re-appearance of the surviving oocytes for the different time points: in both V1 and V2, normal fertilization seemed to be higher when the spindle was present (85 % and 81 % respectively); when the spindle was absent fertilization was 60 % and 0 % respectively. The percentage of good-quality blastocysts per surviving oocyte was 46 % and 50 % respectively when the spindle was present; and 20 % and 0 % respectively when the spindle was absent. Abnormal fertilization was only observed for one oocyte; in this case the spindle was not present before injection.
Table 4.
Experiment 2: Spindle re-appearance in surviving oocytes (V1W2: n = 18 and V2W2: n = 18) per time point for the two vitrification methods and subsequent fertilization and blastocyst formation, given as: number (percentage)
| Spindle re-appearance | No | Yes | ||||
|---|---|---|---|---|---|---|
| 0–2 h | 0 h | 1 h | 2 h | |||
| V1W2 | Survival | 5(28) | 13(72) | 6(33) | 5(28) | 2(11) |
| Fertilization | ||||||
| - 2PN | 3(60) | 11(84) | 6(46) | 3(23) | 2(15) | |
| - 1PN | 1(20) | / | / | / | / | |
| - 3PN | / | / | / | / | / | |
| - no PN | 1(20) | 2(15) | / | 2(15) | / | |
| GQ blastocysts | 1(33) | 6(54) | 3(27) | 2(18) | 1(9) | |
| V2W2 | Survival | 2(11) | 16(89) | 7(39) | 5(28) | 4(22) |
| Fertilization | ||||||
| - 2PN | 0(0) | 13(82) | 6(38) | 4(25) | 3(19) | |
| - 1PN | / | / | / | / | / | |
| - 3PN | 1(50) | / | / | / | / | |
| - no PN | 1(50) | 3(18) | 1(6) | 1(6) | 1(6) | |
| GQ blastocysts | 0(0) | 8(61) | 3(23) | 2(15) | 3(23) | |
GQ good quality
Discussion
The aim of this study was to compare two vitrification and two warming procedures for mature human oocyte cryopreservation in a high-security closed system in terms of survival and in vitro developmental competence. Although the numbers are small, we conclude that gradual exposure to cryoprotectants by consecutive merging of the ES droplets during vitrification most likely does not provide any added value to the survival and in vitro competence as compared to direct exposure. However, survival of oocytes was higher (90 %) when put immediately in a large droplet of TS at 37 °C during warming.
During the last decade, the results obtained with oocyte vitrification have improved to a level almost equal to fresh oocytes. Survival rates over 90 % and fertilization rates ranging between 70 and 95 % led to a tremendous increase in clinical outcome. Most of these results are obtained using open devices for cryostorage [1, 2, 14, 15, 26, 27, 30, 33, 50, 51, 53, 68].
Currently, only few reports are available with the use of closed systems for oocyte vitrification [7, 22, 41, 43, 46, 55, 58]. Although acceptable survival (57.9 % to 100 %) and fertilization (57.6 % to 77.5 %) rates have been achieved with closed devices, the overall clinical pregnancy rates per warmed oocyte only ranges from 1.5 % to 10 %, while with open devices the live birth rate per warmed oocyte ranges between 1 % and 22 % [15]. This has been attributed to the higher cooling rate in open devices due to the direct contact of the samples with liquid nitrogen [7, 35, 52], thereby preventing chilling injury [19, 32, 61]. A few studies comparing open and closed devices for oocyte vitrification obtained similar results with both devices ([7, 61], indicating that the minor differences in cooling rate might not be so important and that the warming rate could be more important than the cooling rate. Seki and Mazur [54] and Mazur and Seki [36] emphasized this in their experiments: the highest survival rates were obtained when the highest warming rate was used, independent of the initial cooling rate. During slow warming, there is more time for recrystallization which may lead to irreversible damage to the oocyte. Boldt [6] suggested that devitrification and subsequent ice nucleation during warming can be prevented by increasing the warming rate through (i) the use of very small volumes and (ii) plunging directly from LN2 in 37 °C. The latter may explain the differences in oocyte survival in experiment 1 when comparing two different warming methods. The use of a droplet of 150 μl pre-heated TS at 37 °C that is kept at 37 °C generated a warming rate sufficiently high to obtain a high survival rate (90 %). Apparently, the warming rate was too slow in the 25 μl droplet that was put at RT. Moreover, fertilization rate and embryo developmental rate were also impaired after thawing in a small droplet at RT, indicating that the quality of the survived oocytes is affected by the warming procedure. To further improve the outcome, the effect of gradually exposing the oocytes to the cryoprotectant during vitrification, in order to reduce the osmotic shock, was investigated (experiment 2). Although investigated on a small number of oocytes, merging droplets during vitrification did not further improve the survival, fertilization and in vitro developmental rates.
When looking at the timing of spindle re-appearance after warming, no difference between the two vitrification methods was observed. Because of technical problems with the Polscope, the spindle appearance was not analysed prior to vitrification. However, the results of spindle re-appearance of the warmed oocytes in experiment 2 were higher (72.2–88.9 %) than the range reported for the presence of a spindle in fresh oocytes (53.8–83.5 %), [37, 49, 65, 66]), indicating that the high security closed vitrification system is safe at this level. As reported by Moon et al. [37], the embryo developmental rate was higher when the spindle was present.
The results of the present study might have been compromised by the large interval between oocyte retrieval and vitrification. In clinical practice, oocytes are vitrified within 1 h after retrieval. However, this strict timing was not possible with the present oocytes due to the time required to search for sperm in the testicular biopsies. The oocytes only became available for research when no sperm was found in the treatment cycle for the couple. This interval was on average 7 h after retrieval and accordingly, injection post-warming was performed on aged oocytes. Taking into account that aged oocytes show a decreased fertilization and preimplantation development [21, 28], the use of less in vitro aged oocytes for cryopreservation might even improve the results.
In most literature reports, transfers of embryos derived from vitrified/warmed oocytes are performed on day 2 or 3 and extended culture up to day 5 is rarely performed. Good-quality day 3 embryo formation rate per fertilized oocyte ranges between 39.9 % and 96.7 % [1, 2, 14, 33, 51, 53] for open devices and between 57.1 % and 66.7 % [39, 41, 59] for closed devices. In our experiments with W2, good-quality day 3 embryo formation rate up to 76.9 % was achieved, comparable to the results obtained with the fresh oocytes (65.3 %) for research in this study. The implantation potential of the blastocysts could not be evaluated because they were created for research. However they showed a nice ICM with NANOG-positive nuclei and a cohesive TE epithelium expressing KRT18, confirming their viability and potential to develop beyond day 3. Besides this, the fertilization and embryo development rate obtained in this study were comparable with fresh sibling oocytes.
Notwithstanding the lower cooling rate as compared to open devices, closed devices have the major advantage of totally eliminating the risk of cross-contamination [4, 5], and hereby avoiding the need for special equipment to sterilize the LN2, to use vapour phase nitrogen [16] or hermetical cryostorage [46].
Even after the delivery of many healthy babies after oocyte vitrification, it remains important to further test the safety of the oocyte vitrification procedures [25, 38]. Regardless of the vitrification system used, it is important to determine the effect of shrinkage and re-expansion on cellular structures and organelles; and its impact on intracellular trafficking and epigenetic modifications [56].
Based upon the results of this study and using the vitrification solutions and devices described herein, we have found that vitrification of mature human oocytes in a high security closed system is a promising approach. An important step in order to obtain high survival, fertilization and subsequent embryo development in vitro is the first warming step which should be performed in a large droplet at 37 °C; the outcome is most likely independent of the direct or gradual exposure to cryoprotectants during vitrification.
Acknowledgments
The authors thank the co-workers at the laboratory of the Centre for Reproductive Medicine for their dedicated work.
Grants, funding
Our research is supported by grants from the Scientific Research Foundation—Flanders (FWO-Vlaanderen) and the Research Council (OZR) of the VUB.
Footnotes
Capsule
Using a high security closed vitrification device a 90 % survival rate can be achieved when the oocytes are warmed in a large volume at 37 °C.
References
- 1.Almodin CG, Minguetti-Camara VC, Paixao CL, Pereira PC. Embryo development and gestation using fresh and vitrified oocytes. Hum Reprod. 2010;25(5):1192–1198. doi: 10.1093/humrep/deq042. [DOI] [PubMed] [Google Scholar]
- 2.Antinori M, Licata E, Dani G, Cerusico F, Versaci C, Antinori S. Cryotop vitrification of human oocytes results in high survival rate and healthy deliveries. Reprod Biomed Online. 2007;14(1):72–79. doi: 10.1016/S1472-6483(10)60766-3. [DOI] [PubMed] [Google Scholar]
- 3.Ata B, Chian RC, Tan SL. Cryopreservation of oocytes and embryos for fertility preservation for female cancer patients. Best Pract Res Clin Obstet Gynaecol. 2010;24(1):101–112. doi: 10.1016/j.bpobgyn.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 4.Bielanski A, Nadin-Davis S, Sapp T, Lutze-Wallace C. Viral contamination of embryos cryopreserved in liquid nitrogen. Cryobiology. 2000;40(2):110–116. doi: 10.1006/cryo.1999.2227. [DOI] [PubMed] [Google Scholar]
- 5.Bielanski A, Vajta G. Risk of contamination of germplasm during cryopreservation and cryobanking in IVF units. Hum Reprod. 2009;24(10):2457–2467. doi: 10.1093/humrep/dep117. [DOI] [PubMed] [Google Scholar]
- 6.Boldt J. Current results with slow freezing and vitrification of the human oocyte. Reprod Biomed Online. 2011;23(3):314–322. doi: 10.1016/j.rbmo.2010.11.019. [DOI] [PubMed] [Google Scholar]
- 7.Bonetti A, Cervi M, Tomei F, Marchini M, Ortolani F, Manno M. Ultrastructural evaluation of human metaphase II oocytes after vitrification: closed versus open devices. Fertil Steril. 2010;95(3):928–935. doi: 10.1016/j.fertnstert.2010.08.027. [DOI] [PubMed] [Google Scholar]
- 8.Borini A, Bianchi V, Bonu MA, Sciajno R, Seren E, Cattoli M, et al. Evidence-based clinical outcome of oocyte slow cooling. Reprod Biomed Online. 2007;15(2):175–181. doi: 10.1016/S1472-6483(10)60706-7. [DOI] [PubMed] [Google Scholar]
- 9.Cao YX, Xing Q, Li L, Cong L, Zhang ZG, Wei ZL, et al. Comparison of survival and embryonic development in human oocytes cryopreserved by slow-freezing and vitrification. Fertil Steril. 2009;92(4):1306–1311. doi: 10.1016/j.fertnstert.2008.08.069. [DOI] [PubMed] [Google Scholar]
- 10.Cauffman G, De Rycke M, Sermon K, Liebaers I, Van de Velde H. Markers that define stemness in ESC are unable to identify the totipotent cells in human preimplantation embryos. Hum Reprod. 2009;24(1):63–70. doi: 10.1093/humrep/den351. [DOI] [PubMed] [Google Scholar]
- 11.Chen C. Pregnancy after human oocyte cryopreservation. Lancet. 1986;1(8486):884–886. doi: 10.1016/S0140-6736(86)90989-X. [DOI] [PubMed] [Google Scholar]
- 12.Chen SU, Yang YS. Slow freezing or vitrification of oocytes: their effects on survival and meiotic spindles, and the time schedule for clinical practice. Taiwan J Obstet Gynecol. 2009;48(1):15–22. doi: 10.1016/S1028-4559(09)60030-9. [DOI] [PubMed] [Google Scholar]
- 13.Cobo A, Rubio C, Gerli S, Ruiz A, Pellicer A, Remohí J. Use of fluorescence in situ hybridization to assess the chromosomal status of embryos obtained from cryopreserved oocytes. Fertil Steril. 2001;75(2):354–360. doi: 10.1016/S0015-0282(00)01725-8. [DOI] [PubMed] [Google Scholar]
- 14.Cobo A, Kuwayama M, Pérez S, Ruiz A, Pellicer A, Remohí J. Comparison of concomitant outcome achieved with fresh and cryopreserved donor oocytes vitrified by the Cryotop method. Fertil Steril. 2008;89(6):1657–1664. doi: 10.1016/j.fertnstert.2007.05.050. [DOI] [PubMed] [Google Scholar]
- 15.Cobo A, Vajta G, Remohí J. Vitrification of human mature oocytes in clinical practice. Reprod Biomed Online. 2009;19(Suppl 4):4385. [PubMed] [Google Scholar]
- 16.Cobo A, Pérez S, de los Santos MJ, Meseguer M, Remohí J. Storage of human oocytes in the vapor phase of nitrogen. Fertil Steril. 2010;94(5):1903–1907. doi: 10.1016/j.fertnstert.2009.10.042. [DOI] [PubMed] [Google Scholar]
- 17.De Vos A, Van Landuyt L, Van Ranst H, Vandermonde A, D’Haese V, Sterckx J, et al. Randomized sibling-oocyte study using recombinant human hyaluronidase versus bovine-derived Sigma hyaluronidase in ICSI patients. Hum Reprod. 2008;23(8):1815–1819. doi: 10.1093/humrep/den212. [DOI] [PubMed] [Google Scholar]
- 18.Fadini R, Brambillasc F, Renzini MM, Merola M, Comi R, De Ponti E, et al. Human oocyte cryopreservation: comparison between slow and ultrarapid methods. Reprod Biomed Online. 2009;19(2):171–180. doi: 10.1016/S1472-6483(10)60069-7. [DOI] [PubMed] [Google Scholar]
- 19.Fuller B, Paynter S. Fundamentals of cryobiology in reproductive medicine. Reprod Biomed Online. 2004;9(6):680–691. doi: 10.1016/S1472-6483(10)61780-4. [DOI] [PubMed] [Google Scholar]
- 20.Gardner DK, Schoolcraft WB. In-vitro culture of human blastocysts. In: Jansen R, Mortimer D, editors. Towards reproductive certainty: fertility and genetics beyond 1999. Carnforth: Parthenon Press; 1999. pp. 378–388. [Google Scholar]
- 21.Gook DA, Osborn SM, Bourne H, Johnston WI. Fertilization of human oocytes following cryopreservation; normal karyotypes and absence of stray chromosomes. Hum Reprod. 1994;9(4):684–691. doi: 10.1093/oxfordjournals.humrep.a138572. [DOI] [PubMed] [Google Scholar]
- 22.Grifo JA, Noyes N. Delivery rate using cryopreserved oocytes is comparable to conventional in vitro fertilization using fresh oocytes: potential fertility preservation for female cancer patients. Fertil Steril. 2010;93(2):391–396. doi: 10.1016/j.fertnstert.2009.02.067. [DOI] [PubMed] [Google Scholar]
- 23.Grout BW, Morris GJ. Contaminated liquid nitrogen vapour as a risk factor in pathogen transfer. Theriogenology. 2009;71(7):1079–1082. doi: 10.1016/j.theriogenology.2008.12.011. [DOI] [PubMed] [Google Scholar]
- 24.Herrero L, Martínez M, Garcia-Velasco JA. Current status of human oocyte and embryo cryopreservation. Curr Opin Obstet Gynecol. 2011;23(4):245–250. doi: 10.1097/GCO.0b013e32834874e2. [DOI] [PubMed] [Google Scholar]
- 25.Homburg R, van der Veen F, Silber SJ. Oocyte vitrification–women’s emancipation set in stone. Fertil Steril. 2009;91(4 Suppl):1319–1320. doi: 10.1016/j.fertnstert.2008.02.127. [DOI] [PubMed] [Google Scholar]
- 26.Katayama KP, Stehlik J, Kuwayama M, Kato O, Stehlik E. High survival rate of vitrified human oocytes results in clinical pregnancy. Fertil Steril. 2003;80(1):223–224. doi: 10.1016/S0015-0282(03)00551-X. [DOI] [PubMed] [Google Scholar]
- 27.Kim TJ, Laufer LR, Hong SW. Vitrification of oocytes produces high pregnancy rates when carried out in fertile women. Fertil Steril. 2010;93(2):467–474. doi: 10.1016/j.fertnstert.2008.12.094. [DOI] [PubMed] [Google Scholar]
- 28.Koutlaki N, Schoepper B, Maroulis G, Diedrich K, Al-Hasani S. Human oocyte cryopreservation: past, present and future. Reprod Biomed Online. 2006;13(3):427–436. doi: 10.1016/S1472-6483(10)61449-6. [DOI] [PubMed] [Google Scholar]
- 29.Kuwayama M, Vajta G, Ieda S, Kato O. Comparison of open and closed methods for vitrification of human embryos and the elimination of potential contamination. Reprod Biomed Online. 2005;11(5):608–614. doi: 10.1016/S1472-6483(10)61169-8. [DOI] [PubMed] [Google Scholar]
- 30.Kuwayama M, Vajta G, Kato O, Leibo SP. Highly efficient vitrification method for cryopreservation of human oocytes. Reprod Biomed Online. 2005;11(3):300–308. doi: 10.1016/S1472-6483(10)60837-1. [DOI] [PubMed] [Google Scholar]
- 31.Lane M, Schoolcraft WB, Gardner DK. Vitrification of mouse and human blastocysts using a novel cryoloop container-less technique. Fertil Steril. 1999;72(6):1073–1078. doi: 10.1016/S0015-0282(99)00418-5. [DOI] [PubMed] [Google Scholar]
- 32.Liebermann J, Dietl J, Vanderzwalmen P, Tucker MJ. Recent developments in human oocyte, embryo and blastocyst vitrification: where are we now? Reprod Biomed Online. 2003;7(6):623–633. doi: 10.1016/S1472-6483(10)62084-6. [DOI] [PubMed] [Google Scholar]
- 33.Lucena E, Bernal DP, Lucena C, Rojas A, Moran A, Lucena A. Successful ongoing pregnancies after vitrification of oocytes. Fertil Steril. 2006;85(1):108–111. doi: 10.1016/j.fertnstert.2005.09.013. [DOI] [PubMed] [Google Scholar]
- 34.Magli MC, Lappi M, Ferraretti AP, Capoti A, Ruberti A, Gianaroli L. Impact of oocyte cryopreservation on embryo development. Fertil Steril. 2010;93(2):510–516. doi: 10.1016/j.fertnstert.2009.01.148. [DOI] [PubMed] [Google Scholar]
- 35.Martínez-Burgos M, Herrero L, Megías D, Salvanes R, Montoya MC, Cobo AC, et al. Vitrification versus slow freezing of oocytes: effects on morphologic appearance, meiotic spindle configuration, and DNA damage. Fertil Steril. 2010;95(1):374–377. doi: 10.1016/j.fertnstert.2010.07.1089. [DOI] [PubMed] [Google Scholar]
- 36.Mazur P, Seki S. Survival of mouse oocytes after being cooled in a vitrification solution to −196°C at 95° to 70,000°C/min and warmed at 610° to 118,000°C/min: A new paradigm for cryopreservation by vitrification. Cryobiology. 2011;62(1):1–7. doi: 10.1016/j.cryobiol.2010.10.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moon JH, Hyun CS, Lee SW, Son WY, Yoon QH, Lim JH. Visualization of the metaphase II meiotic spindle in living human oocytes using the Polscope enables the predication of embryonic developmental competence after ICSI. Hum Reprod. 2003;18(4):817–820. doi: 10.1093/humrep/deg165. [DOI] [PubMed] [Google Scholar]
- 38.Nottola SA, Coticchio G, Sciajno R, Gambardella A, Maione M, Scaravelli G, et al. Ultrastructural markers of quality in human mature oocytes vitrified using cryoleaf and cryoloop. Reprod Biomed Online. 2009;19(Suppl 3):17–27. doi: 10.1016/S1472-6483(10)60280-5. [DOI] [PubMed] [Google Scholar]
- 39.Noyes N, Knopman J, Labella P, McCaffrey C, Clark-Williams M, Grifo J. Oocyte cryopreservation outcomes including pre-cryopreservation and post-thaw meiotic spindle evaluation following slow cooling and vitrification of human oocytes. Fertil Steril. 2010;94(6):2078–2082. doi: 10.1016/j.fertnstert.2010.01.019. [DOI] [PubMed] [Google Scholar]
- 40.Noyes N, Boldt J, Nagy ZP. Oocyte cryopreservation: is it time to remove its experimental label? J Assist Reprod Genet. 2010;27(2–3):69–74. doi: 10.1007/s10815-009-9382-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Oakes MB, Gomes CM, Fioravanti J, Serafini P, Motta EL, Smith GD. A case of oocyte and embryo vitrification resulting in clinical pregnancy. Fertil Steril. 2008;90(5):2013–2018. doi: 10.1016/j.fertnstert.2008.03.046. [DOI] [PubMed] [Google Scholar]
- 42.Oktay K, Cil AP, Bang H. Efficiency of oocyte cryopreservation: a meta-analysis. Fertil Steril. 2006;86(1):70–80. doi: 10.1016/j.fertnstert.2006.03.017. [DOI] [PubMed] [Google Scholar]
- 43.Paffoni A, Guarneri C, Ferrari S, Restelli L, Nicolosi AE, Scarduelli C, et al. Effects of two vitrification protocols on the developmental potential of human mature oocytes. Reprod Biomed Online. 2011;22(3):292–298. doi: 10.1016/j.rbmo.2010.11.004. [DOI] [PubMed] [Google Scholar]
- 44.Parmegiani L, Cognigni GE, Bernardi S, Ciampaglia W, Infante F, Pocognoli P, et al. Freezing within 2 h from oocyte retrieval increases the efficiency of human oocyte cryopreservation when using a slow freezing/rapid thawing protocol with high sucrose concentration. Hum Reprod. 2008;23(8):1771–1777. doi: 10.1093/humrep/den119. [DOI] [PubMed] [Google Scholar]
- 45.Parmegiani L, Bertocci F, Garello C, Salvarani MC, Tambuscio G, Fabbri R. Efficiency of human oocyte slow freezing: results from five assisted reproduction centres. Reprod Biomed Online. 2009;18(3):352–359. doi: 10.1016/S1472-6483(10)60093-4. [DOI] [PubMed] [Google Scholar]
- 46.Parmegiani L, Cognigni GE, Bernardi S, Cuomo S, Ciampaglia W, Infante FE, et al. Efficiency of aseptic open vitrification and hermetical cryostorage of human oocytes. Reprod Biomed Online. 2011;23(4):505–512. doi: 10.1016/j.rbmo.2011.07.003. [DOI] [PubMed] [Google Scholar]
- 47.Paynter SJ. A rational approach to oocyte cryopreservation. Reprod Biomed Online. 2005;10(5):578–586. doi: 10.1016/S1472-6483(10)61664-1. [DOI] [PubMed] [Google Scholar]
- 48.Pickering SJ, Braude PR, Johnson MH, Cant A, Currie J. Transient cooling to room temperature can cause irreversible disruption of the meiotic spindle in the human oocyte. Fertil Steril. 1990;54(1):102–108. doi: 10.1016/s0015-0282(16)53644-9. [DOI] [PubMed] [Google Scholar]
- 49.Rienzi L, Ubaldi F, Martinez F, Iacobelli M, Minasi MG, Ferrero S, et al. Relationship between meiotic spindle location with regard to the polar body position and oocyte developmental potential after ICSI. Hum Reprod. 2003;18(6):1289–1293. doi: 10.1093/humrep/deg274. [DOI] [PubMed] [Google Scholar]
- 50.Rienzi L, Romano S, Albricci L, Maggiulli R, Capalbo A, Baroni E, et al. Embryo development of fresh ‘versus’ vitrified metaphase II oocytes after ICSI: a prospective randomized sibling-oocyte study. Hum Reprod. 2010;25(1):66–73. doi: 10.1093/humrep/dep346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rienzi L, Cobo A, Paffoni A, Scarduelli C, Capalbo A, Vajta G, Remohí J, Ragni G, Ubaldi FM. Consistent and predictable delivery rates after oocyte vitrification: an observational longitudinal cohort multicentric study. Hum Reprod. 2012;27(6):1606–12. [DOI] [PubMed]
- 52.Saragusty J, Arav A. Current progress in oocyte and embryo cryopreservation by slow freezing and vitrification. Reproduction. 2011;141(1):1–19. doi: 10.1530/REP-10-0236. [DOI] [PubMed] [Google Scholar]
- 53.Schoolcraft WB, Keller JL, Schlenker T. Excellent embryo quality obtained from vitrified oocytes. Reprod Biomed Online. 2009;19(6):820–823. doi: 10.1016/j.rbmo.2009.09.017. [DOI] [PubMed] [Google Scholar]
- 54.Seki S, Mazur P. The dominance of warming rate over cooling rate in the survival of mouse oocytes subjected to a vitrification procedure. Cryobiology. 2009;59(1):75–82. doi: 10.1016/j.cryobiol.2009.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Smith GD, Serafini PC, Fioravanti J, Yadid I, Coslovsky M, Hassun P, et al. Prospective randomized comparison of human oocyte cryopreservation with slow-rate freezing or vitrification. Fertil Steril. 2010;94(6):2088–2095. doi: 10.1016/j.fertnstert.2009.12.065. [DOI] [PubMed] [Google Scholar]
- 56.Smith GD, Motta EE, Serafini P. Theoretical and experimental basis of oocyte vitrification. Reprod Biomed Online. 2011;23(3):298–306. doi: 10.1016/j.rbmo.2011.05.003. [DOI] [PubMed] [Google Scholar]
- 57.Stachecki JJ, Cohen J. An overview of oocyte cryopreservation. Reprod Biomed Online. 2004;9(2):152–163. doi: 10.1016/S1472-6483(10)62124-4. [DOI] [PubMed] [Google Scholar]
- 58.Stoop D, Nekkebroek J, Devroey P. A survey on the intentions and attitudes towards oocyte cryopreservation for non-medical reasons among women of reproductive age. Human Reprod. 2011;26(3):655–661. doi: 10.1093/humrep/deq367. [DOI] [PubMed] [Google Scholar]
- 59.Stoop D, De Munck N, Jansen E, Platteau P, Van den Abbeel E, Verheyen G, et al. Clinical validation of a closed vitrification system in an oocyte donation programme. Reprod Biomed Online. 2012;24(2):180–185. doi: 10.1016/j.rbmo.2011.10.015. [DOI] [PubMed] [Google Scholar]
- 60.Tao T, Del Valle A. Human oocyte and ovarian tissue cryopreservation and its application. J Assist Reprod Genet. 2008;25(7):287–296. doi: 10.1007/s10815-008-9236-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Vajta G, Kuwayama M. Improving cryopreservation systems. Theriogenology. 2006;65(1):236–244. doi: 10.1016/j.theriogenology.2005.09.026. [DOI] [PubMed] [Google Scholar]
- 62.Van Landuyt L, De Vos A, Joris H, Verheyen G, Devroey P, Van Steirteghem A. Blastocyst formation in in vitro fertilization versus intracytoplasmic sperm injection cycles: influence of the fertilization procedure. Fertil Steril. 2005;83(5):1397–1403. doi: 10.1016/j.fertnstert.2004.10.054. [DOI] [PubMed] [Google Scholar]
- 63.Van Landuyt L, Stoop D, Verheyen G, Verpoest W, Camus M, Van de Velde H, et al. Outcome of closed blastocyst vitrification in relation to blastocyst quality: evaluation of 759 warming cycles in a single-embryo transfer policy. Hum Reprod. 2011;26(3):527–534. doi: 10.1093/humrep/deq374. [DOI] [PubMed] [Google Scholar]
- 64.Wang WH, Meng L, Hackett RJ, Odenbourg R, Keefe DL. Limited recovery of meiotic spindles in living human oocytes after cooling-rewarming observed using polarized light microscopy. Hum Reprod. 2001;16:2374–2378. doi: 10.1093/humrep/16.11.2374. [DOI] [PubMed] [Google Scholar]
- 65.Wang WH, Meng L, Hackett RJ, Keefe DL. Developmental ability of human oocytes with or without birefringent spindles imaged by Polscope before insemination. Hum Reprod. 2001;16:1464–1468. doi: 10.1093/humrep/16.7.1464. [DOI] [PubMed] [Google Scholar]
- 66.Wang WH, Meng L, Hackett RJ, Odenbourg R, Keefe DL. The spindle observation and its relationship with fertilization after intracytoplasmic sperm injection in living human oocytes. Fertil Steril. 2001;75:348–353. doi: 10.1016/S0015-0282(00)01692-7. [DOI] [PubMed] [Google Scholar]
- 67.Wennerholm UB, Söderström-Anttila V, Bergh C, Aittomäki K, Hazekamp J, Nygren KG, et al. Children born after cryopreservation of embryos or oocytes: a systematic review of outcome data. Hum Reprod. 2009;24(9):2158–2172. doi: 10.1093/humrep/dep125. [DOI] [PubMed] [Google Scholar]
- 68.Yoon TK, Kim TJ, Park SE, Hong SW, Ko JJ, Chung HM, et al. Live births after vitrification of oocytes in a stimulated in vitro fertilization-embryo transfer program. Fertil Steril. 2003;79(6):1323–1326. doi: 10.1016/S0015-0282(03)00258-9. [DOI] [PubMed] [Google Scholar]