Abstract
The differential expression and secretion of the neuropeptide kisspeptin from neurons in the arcuate (Arc) and anteroventral periventricular (AVPV) nuclei of the hypothalamus coordinate the temporal release of pituitary gonadotropins that control the female reproductive cycle. However, the molecular basis for this differential regulation is incompletely understood. Here, we report that liver receptor homolog-1 (LRH-1), a member of the nuclear receptor superfamily, is expressed in kisspeptin neurons in the Arc but not in the AVPV in female mice. LRH-1 binds directly to the kisspeptin (Kiss1) promoter and stimulates Kiss1 transcription. Deletion of LRH-1 from kisspeptin neurons in mice decreased Kiss1 expression in the Arc, leading to reduced plasma FSH levels, dysregulated follicle maturation, and prolongation of the estrous cycle. Conversely, overexpression of LRH-1 in kisspeptin neurons increased Arc Kiss1 expression and plasma FSH concentrations. These studies provide a molecular basis for the differential regulation of basal kisspeptin expression in Arc and AVPV neurons and reveal a prominent role for LRH-1 in hypothalamus in regulating the female reproductive axis.
The hypothalamic neuropeptide kisspeptin is a master regulator of the hypothalamic-pituitary-gonadal axis (1, 2). Mutations in the Kiss1 gene result in infertility in mice and humans (3–7). Kisspeptin is synthesized in neurons in two regions of the hypothalamus: the arcuate nucleus (Arc) and the anteroventral periventricular nucleus (AVPV) (8, 9). Secretion of kisspeptin activates its cognate G protein-coupled receptor, GPR54, on GnRH neurons, which direct the pituitary to release FSH and LH (10–16). During diestrus, kisspeptin secreted from the Arc stimulates the release of FSH required for ovarian follicles to mature. During proestrus, follicle maturation concludes and follicular secretion of estradiol (E2) peaks. E2, in turn, has two important downstream effects. First, it induces Kiss1 expression in the AVPV by activating the estrogen receptor α (ERα). This causes a surge in pituitary LH secretion, which triggers ovulation of the mature follicles. Second, E2 represses Kiss1 expression in the Arc, also through an ERα-dependent mechanism. The importance of ERα in coordinating this reproductive cycle is demonstrated by the infertility and polycystic ovarian phenotype that occur in mice selectively lacking ERα in kisspeptin neurons (17). Although it is well established that the AVPV pool of kisspeptin neurons dictates the timing of the LH surge needed for ovulation, it is unknown how Kiss1 expression is maintained in the Arc during diestrus.
Liver receptor homolog-1 (LRH-1; NR5A2) is a nuclear receptor that regulates lipid and sterol metabolism in enterohepatic tissues, steroid synthesis in the ovary, and digestive enzyme expression in the exocrine pancreas (18, 19). LRH-1 is involved in coordinating the actions of endocrine signaling axes across multiple tissues. For example, in the intestine and liver, LRH-1 governs a transcriptional network of genes involved in bile acid signaling, including the hormone fibroblast growth factor 15/19 (20–24). Likewise, in the reproductive axis, LRH-1 acts in multiple tissues including the pituitary and gonads to coordinately regulate genes involved in gonadotropin and steroid hormone synthesis (25, 26).
Although LRH-1 expression has been observed in the human and mouse Arc (27, 28), its role there has not been established. Here, we report that LRH-1 is expressed in kisspeptin neurons of the Arc but not in the AVPV. We further show that LRH-1 maintains Kiss1 expression in the Arc at the level required for normal FSH secretion and follicle maturation.
Materials and Methods
Mouse studies
All animal experiments were approved by the Institutional Animal Care and Research Advisory Committee of the University of Texas Southwestern Medical Center. Lrh1Kiss1−/− mice were generated using previously described mouse lines (24, 29). Npy-Egfp, Pomc-Egfp, and Kiss1-Egfp/Kiss1-βgal mice were as described elsewhere (24, 29). To generate the kisspeptin neuron-specific Lrh1-transgenic line, a lox-stop-lox-Lrh1-Tg mouse was constructed by standard transgenic techniques and crossed with the Kiss1-Cre line. All mice were maintained on a mixed C57 BL/6J × 129/Sv background and littermates were used in all experiments.
To determine the effect of E2 on hypothalamic Kiss1 expression, mice underwent ovariectomy followed by E2 replacement. After recovering from surgery, mice were injected sc at 3:00 pm with either vehicle (100% ethanol), or 6 μg 17β-E2 (Sigma Chemical Co, St Louis, Missouri) as previously described (30, 31). Mice were humanely destroyed 24 hours after injection.
The stage of the estrous cycle was determined daily at 3:00 pm via vaginal lavage and microscopic examination of vaginal epithelial cell morphology (32, 33). For tissue collection, mice verified to be in diestrus or estrus, were humanely destroyed via isoflurane overdose at 3:00 pm. To assess fertility, Lrh1fl/fl and Lrh1Kiss1−/− mice were housed with proven stud males and monitored for litter frequency for at least 4 months. Tissues were snap frozen in liquid nitrogen and stored at −80°C or processed for histologic analysis. To induce superovulation, adult mice in diestrus were injected ip with 5 IU gonadotropin from pregnant mare serum (Sigma) followed 48 hours later by ip injection of 5 IU human chorionic gonadotropin (Sigma). Ovaries were harvested 24 hours later.
Plasma analysis
Plasma levels of FSH and LH were measured at the University of Virginia Ligand Core Laboratory (Charlottesville, Virginia).
Histology and in situ hybridization
For ovarian histology, hematoxylin staining was performed on 5-μm sections that were cut in series at 100-μm intervals throughout the entire ovary. In situ hybridization with a 500-bp riboprobe specific to the 3′-untranslated region of LRH-1 was performed as described previously (34).
Fluorescence-activated cell sorting
Fluorescence-activated cell sorting collection and analysis of neurons were performed as described elsewhere (29). RNA extraction was performed using the PicoPure RNA Isolation kit (Arcturis, St Louis, Missouri). Before quantitative PCR (QPCR), cDNA was preamplified using 2× TaqMan PreAmp Master Mix (Applied Biosystems, Foster City, California).
QPCR analysis
QPCR was conducted as described previously (35, 36). Primer sequences are available upon request.
Chromatin immunoprecipitation (ChIP)
ChIP analysis of the Kiss1 promoter from Arc of female mice in diestrus collected at 3:00 pm was performed using a Magna Chip-G Tissue kit (Millipore Corp, Bedford, Massachusetts) according to manufacturer's directions. Antihuman LRH-1 mouse monoclonal antibody (Perseus Proteomics, Tokyo, Japan; PP-H2325–00, lot A2) was used for ChIP of LRH-1 on the Kiss1 promoter. Primer sequences for ChIP were:
−4325: forward, 5′-AGCCTGTTTCTGCCCTTCA-3′; reverse, 5′-GGCTTTGAGACAGCAGATGTG-3′
−4142: forward, 5′-AAAGCTCCGCCTGCCTTA-3′; reverse, 5′-GGTGGCACATTCCTCCAAT-3′
−3797: forward, 5′-CCGGTCTCAGCTCACAGTACA-3′; reverse, 5′-AAGAAAGCCAGGTCAGATTGG-3′
−2400: forward, 5′-GGAGTTGGTTTTTCCCCTTCT-3′; reverse, 5′-CGCTTGACAATCTGAATTTAATCC-3′
−1110: forward, 5′-CCTAGGCTCCACCTGTTGTG-3′; reverse, 5′-CCCAACAAATGGCATTAAGTG-3′
+196: forward, 5′-GGCTGGTCTAGGCCCTTCT-3′; reverse, 5′-ACAGGACCCCCAACTCTAGCT-3′
+427: forward, 5′-CCATCCCAGCACTTGGAA-3′; reverse, 5′-TGTCCTGGACCAGGCTGAT-3′
+766: forward, 5′-CCCCCCAAGAGCATCATG-3′; reverse, 5′-CGAACAGCCAAGAAGAATTCA-3′
+1034: forward, 5′-GGCCACACTCACCTCCAA-3′; reverse, 5′-GCGGAGGATGGCTTTGA-3′
Plasmids and reporter constructs
Various lengths of the Kiss1 promoter isolated from purified C57 BL/6J mouse genomic DNA were cloned into a pGL4-Luc2 vector (Promega Corp, Madison, Wisconsin) using standard cloning techniques. Mutational insertions were generated with a QuikChange II LX site-directed mutagenesis kit (Stratagene, La Jolla, California). The Lrh1 cDNA sequence was cloned into the multiple cloning site in the CAG-Z-multiple cloning site-IRES-Egfp vector using standard techniques.
Cell-based reporter assays
Cell culture and cotransfection assays were performed in phenol-red free DMEM as described elsewhere (37).
Statistical analysis
Microsoft Excel 2007 was used for statistical analysis of data. Student's test of P < .05 was considered significant when comparing two groups.
Results
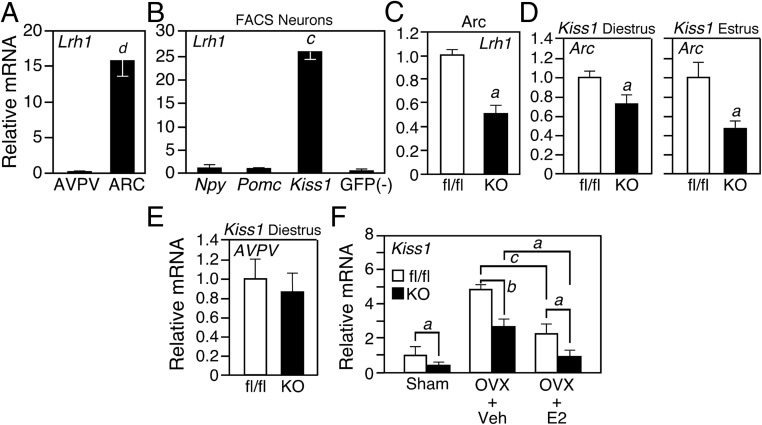
Lrh1 mRNA expression in the central nervous system was previously shown to be highest in the Arc (27, 28). Consistent with these findings, we detected Lrh1 mRNA in the Arc but not the AVPV of dissected female mouse hypothalami by quantitative (Q) PCR (Figure 1A). To determine whether Lrh1 is expressed in kisspeptin, neuropeptide Y, or proopiomelanocortin neurons in the Arc, we used three different reporter mouse lines in which enhanced green fluorescent protein (EGFP) is selectively expressed in these different neuron populations (29). EGFP+ neurons were collected from Arc of female mice using fluorescence-activated cell sorting. Notably, Lrh1 expression was highly enriched in kisspeptin neurons (Figure 1B).
Figure 1.
LRH-1 Regulates Kiss1 in Arc Neurons. A, Lrh1 mRNA levels in dissected samples of AVPV and Arc (n = 10 per group). B, Lrh1 mRNA levels in EGFP+ Arc neurons from Npy-Egfp, Pomc-Egfp, or Kiss1-Egfp mouse lines (n = 6 per group). EGFP-negative neurons were collected as a control. C, Lrh1 mRNA levels in Arc of Lrh1Kiss1−/− (knockout [KO]) mice and control Lrh1fl/fl (fl/fl) littermates (n = 8 per group). D and E, Kiss1 mRNA in Arc (D) or AVPV (E) of female fl/fl or KO mice in diestrus and estrus as indicated (n = 8, diestrus; n = 5, estrus). F, Ovariectomy (OVX) or sham surgery followed by either vehicle (Veh) or E2 replacement in fl/fl and KO mice (n = 6 per group). Error bars represent SEM. Letters above bars denote statistical significance values by Student's t test: a = P < .05; b = P < .01; c = P < .005; d = P < .001. FACS, fluorescence-activated cell sorting; GFP, green fluorescent protein.
To examine the role of LRH-1 in kisspeptin neurons in the Arc, we crossed mice carrying a floxed Lrh1 allele (24) with Kiss1-Cre mice (29) to generate kisspeptin neuron-specific Lrh1 knockout mice (Lrh1Kiss1−/−). Although Kiss1-Cre is active as early as day 14.5 during embryonic development (C.F.E, unpublished data, 2012), we did not detect histologic changes in the Arc or any other region of the hypothalamus in Lrh1Kiss1−/− mice (Supplemental Figure 1A published on The Endocrine Society's Journals Online web site at http://endo.endojournals.org and data not shown). QPCR analysis showed that Lrh1 expression was reduced but not eliminated in the Arc of Lrh1Kiss1−/− mice (Figure 1C). The remaining Lrh1 expression likely occurs in either kisspeptin neurons that do not express Cre at levels sufficient to drive recombination or in an unidentified Arc neuronal population that expresses Lrh1 but not Kiss1. Lrh1 mRNA levels were unchanged in the pituitary, ovary, and other peripheral tissues in Lrh1Kiss1−/− mice (Supplemental Figure 1, B–D). Lrh1Kiss1−/− mice did not have changes in body weight or food intake (Supplemental Figure 1E).
We next measured Kiss1 mRNA levels in Arc samples dissected from female Lrh1Kiss1−/− mice. Kiss1 mRNA levels were significantly reduced during diestrus and estrus (Figure 1D). In contrast, Kiss1 levels were unchanged in the AVPV in the Lrh1Kiss1−/− mice (Figure 1E).
To test the contribution of LRH-1 to E2-mediated repression of Kiss1 expression in Arc neurons, Lrh1Kiss1−/− and control mice homozygous for the floxed Lrh1 allele (Lrh1fl/fl) were ovariectomized and 2 weeks later given an sc injection of either E2 or vehicle alone. As expected, Kiss1 expression was increased in Lrh1fl/fl mice after ovariectomy and repressed approximately 2-fold in response to E2 (Figure 1F). Likewise, Kiss1 mRNA levels were increased in ovariectomized Lrh1Kiss1−/− mice, although to a lower absolute level than in Lrh1fl/fl mice. Importantly, Kiss1 was still repressed 2- to 3-fold by E2 in Lrh1Kiss1−/− mice (Figure 1F). Thus, whereas LRH-1 is required for basal Kiss1 expression in the Arc, it is not required for E2-mediated repression of this gene.
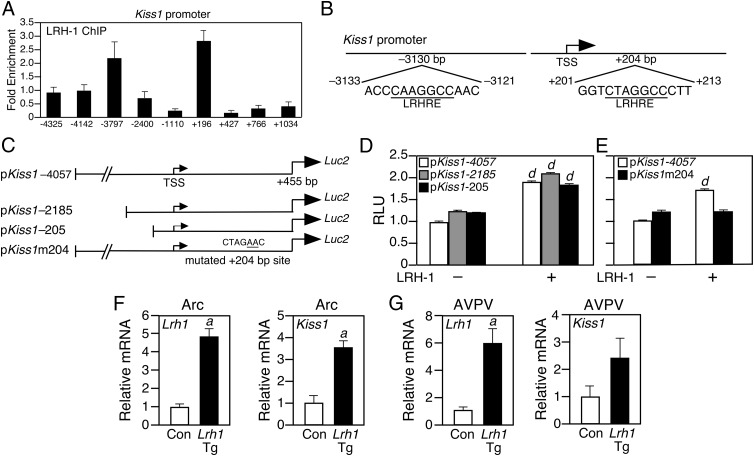
To test whether Kiss1 is a direct LRH-1 target gene, we performed scanning ChIP experiments using Arc tissue from female mice in diestrus. LRH-1 bound to the Kiss1 promoter at two regions: one approximately 3700 bp upstream of the transcriptional start site (TSS) and another about 200 bp downstream of the TSS in the first intron of the Kiss1 gene (Figure 2A). Computational inspection of these regions revealed conserved LRH-1 response elements centered at −3130 bp and +204 bp (Figure 2B).
Figure 2.
Kiss1 Is a Direct LRH-1 Target Gene. A, ChIP for LRH-1 on Arc samples from female mice in diestrus (n = 5). x-Axis labels indicate the binding site of the forward QPCR primer. B, Schematic of the murine Kiss1 promoter with putative LRH-1 response elements (LRHRE) indicated. C, Schematic of Kiss1-luciferase reporter vectors. For pKiss1m204, the mutations in the LRHRE at +204 bp are underlined. D, Transient transfection assays performed with the indicated Kiss1 promoter-luciferase reporter plasmids in either the presence or absence of an LRH-1 expression vector. E, Transient transfection assays performed with the pKiss1-4057 and pKiss1m204 reporter plasmids in either in the presence or absence of an LRH-1 expression vector. F and G, Lrh1 and Kiss1 mRNA levels in Arc (F) or AVPV (G) of the kisspeptin neuron-specific Lrh1-transgenic (Lrh1-Tg) or control (Con) lox-stop-lox-Lrh1 mice (n = 4 mice per group). Error bars represent SEM. Student's t test: a = P < .05; d = P < .001.
To determine the functional significance of the putative LRH-1 binding sites, three luciferase reporter constructs containing differing lengths of the 5′-Kiss1 promoter were generated (Figure 2C) and cotransfected in the absence or presence of an LRH-1 expression vector into HEK293 cells. LRH-1 stimulated reporter activity from the −4057/+455Kiss1 construct containing both the −3130-bp and +204-bp sites by approximately 2-fold (Figure 2D). Reporter plasmids containing the Kiss1 promoter truncated to −2185 bp or −205 bp upstream of the TSS showed reporter activity identical to that of the −4057Kiss1 construct (Figure 2D). To test whether the +204-bp site was responsible for the induction of Kiss1 promoter activity, a reporter plasmid was constructed in which this site was mutated (pKiss1m204; Figure 2C). LRH-1 had no activity on pKiss1m204 (Figure 2E). These data demonstrate that LRH-1 directly activates the Kiss1 promoter by binding to the +204-bp site.
To test whether LRH-1 induces Kiss1 expression in vivo, we generated a Kiss1-specific Lrh1 transgenic mouse line (Kiss1-Lrh1-Tg). The transgenic cassette included a CAG viral promoter and a triple-polyA transcriptional stop signal flanked by LoxP sites upstream of the Lrh1 transgene (Supplemental Figure 2A) (38). Upon Cre induction, LoxP recombination removes the transcriptional stop signal, allowing the CAG promoter to drive Lrh1 expression. Lox-stop-lox-Lrh1-Tg mice were crossed with the Kiss1-Cre line to generate the Kiss1-Lrh1-Tg line with 4-fold overexpression of Lrh1 in the Arc (Figure 2F). Lrh-1 expression was also increased in the AVPV (Figure 2G) but unchanged in the pituitary and ovary of Lrh1-Tg mice (Supplemental Figure 2, B and C). Importantly, Kiss1 levels were increased by nearly 4-fold in Arc samples from Lrh1-Tg mice in diestrus (Figure 2F) and also trended higher in the AVPV (Figure 2G). These gain-of-function data complement the knockout data in demonstrating that LRH-1 regulates Kiss1 in vivo.
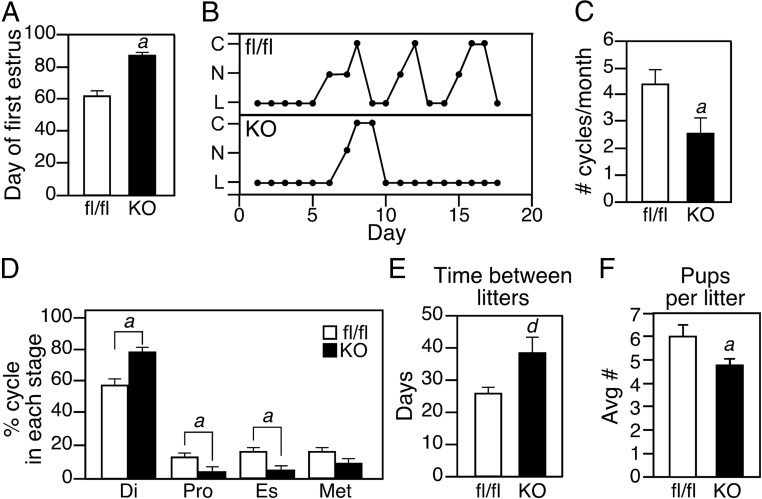
We next assessed female reproductive function in Lrh1Kiss1−/− mice. The age of sexual maturity, as defined by the day of first estrus, was significantly delayed in Lrh1Kiss1−/− mice (Figure 3A). In addition, adult Lrh1Kiss1−/− mice cycled less frequently (Figure 3, B and C) and spent more time in diestrus than control mice (Figure 3D). Although female Lrh1Kiss1−/− mice were fertile, the time between litters was significantly longer than that of control mice (Figure 3E) and they had fewer pups per litter (Figure 3F). Thus, female Lrh1Kiss1−/− mice have reproductive defects.
Figure 3.
LRH-1 in Kisspeptin Neurons Is Required for Optimal Reproductive Fitness. A, Analysis of the first day of estrus during puberty in Lrh1fl/fl (fl/fl) and Lrh1Kiss1−/− knockout (KO) mice (n = 8 per group). B, Representative estrous cycle stage profiles for a fl/fl and KO mouse caged together; C, cornified vaginal epithelia (estrus [Es]); N, nucleated vaginal epithelia (proestrus [Pro]); L, predominately lymphocytic epithelia (diestrus [Di]). C, Average number of estrous cycles per month in fl/fl and KO mice (n = 14 per group). D, Time spent in each stage of the estrous cycle in fl/fl and KO mice for 8 weeks (n = 14 per group). E, Number of days between litters delivered by fl/fl and KO mice (n = 6 per group). F, Number of pups delivered per litter by fl/fl and KO mice (n = 6–7 dams per group, with 2–9 litters per dam). Error bars represent SEM. Student's t test: a = P < .05; d = P < .001.
The delayed sexual maturation and extended estrous cycle in Lrh1Kiss1−/− mice suggested a defect in gonadotopin secretion. Indeed, whereas plasma LH levels were normal in Lrh1Kiss1−/−mice (Figure 4A), plasma FSH levels were reduced significantly during both diestrus and estrus (Figure 4B). Conversely, plasma FSH levels were significantly elevated in Kiss1-Lrh1-Tg mice as were plasma LH concentrations (Figure 4C). Thus, regulation of Kiss1 by LRH-1 in the Arc controls pituitary FSH secretion in vivo.
Figure 4.
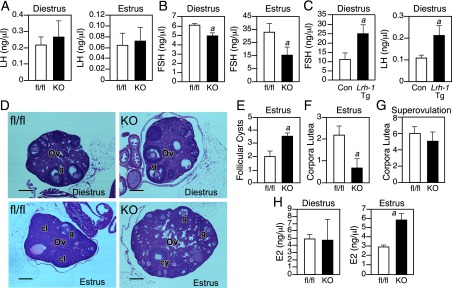
Kiss1Lrh1−/− Mice Have Abnormal Follicles. A and B, Serum LH (A) and FSH (B) levels in female Lrh1fl/fl (fl/fl) and Lrh1Kiss1−/− knockout (KO) mice in diestrus or estrus (n = 5–8 per group). C, Serum FSH and LH levels in female control and kisspeptin neuron-specific Lrh1-Tg mice in diestrus (n = 4 per group). D, Hematoxylin and eosin staining of ovaries (Ov) from fl/fl and KO mice in diestrus or estrus as indicated. 100× magnification, 5-μm sections, scale bars = 100 μm. cl, corpora lutea; g, graafian follicle; cy, follicular cyst. Quantification of follicular cysts (panel E) and corpora lutea (panel F) in ovaries from fl/fl and KO mice (n = sections from 5–8 mice per group). G, Quantification of corpora lutea in superovulated fl/fl and KO mice (n = sections from 3–7 mice per group). H, Plasma E2 concentrations in fl/fl and KO mice in diestrus or estrus (n = 5–8 mice per group). Error bars represent SEM. Student's t test: a = P < .05.
Ovarian histology confirmed defects consistent with FSH deficiency in Lrh1Kiss1−/− mice. During diestrus, when early follicular development occurs in a largely FSH-independent manner (39), there were no histologic differences observed between ovaries from Lrh1fl/fl and Lrh1Kiss1−/− mice (Figure 4D). However, in the postovulation estrus phase, Lrh1Kiss1−/− ovaries contained an abnormal number of unruptured mature follicles and fluid-filled antral follicular cysts (Figure 4, D and E) and fewer corpora lutea (Figure 4F). Notably, there was no difference between Lrh1fl/fl and Lrh1Kiss1−/− mice in the number of corpora lutea after superovulation (Figure 4G), demonstrating that this phenotype could be reversed by administration of exogenous gonadotropins. Consistent with the presence of follicular cysts and impaired ovulation, Lrh1Kiss1−/− mice had elevated plasma E2 levels during estrus but not diestrus (Figure 4H). These data demonstrate that deletion of LRH-1 from kisspeptin neurons decreases plasma FSH levels, resulting in aberrant follicular maturation and an impaired LH response.
Discussion
In this report, we demonstrate that LRH-1 regulates Kiss1 transcription selectively in the Arc of the hypothalamus. Targeted deletion of LRH-1 from Arc kisspeptin neurons decreased Kiss1 expression, leading to decreased serum FSH levels and impaired follicle maturation and ovulation. Conversely, overexpression of LRH-1 in kisspeptin neurons in the Kiss1-Lrh1-Tg line increased Kiss1 expression and serum FSH levels. Using a combination of ChIP and cell-based reporter assays, we further show that LRH-1 directly regulates Kiss1 transcription by binding to a consensus response element approximately 200 bp downstream of the TSS. These data demonstrate that LRH-1 in the Arc plays an important role in regulating follicular biology in the ovary. We note that the LRH-1 paralog, steroidogenic factor-1, which binds to the same consensus DNA response element and also regulates female reproduction, is exclusively expressed in the brain in the ventral medial hypothalamic nucleus and thus does not regulate Kiss1 expression (40).
Female mice that completely lack FSH signaling are infertile due to arrest of follicular maturation at the preantral stage (39). In the present study, elimination of LRH-1 from a subpopulation of kisspeptin neurons reduced plasma FSH concentrations 10%–40% depending on cycle phase. Although this level of FSH was sufficient to allow progression to the graafian stage, the follicles display a perturbed LH response that resulted in the formation of follicular cysts and delayed progression of the next estrous cycle. Comparable models of diminished (rather than absent) FSH signaling are limited and in vivo data regarding the precise timing and intensity of FSH signal required for normal follicular maturation are not available. However, evidence from in vitro cultures of primary follicles supports the notion that in order to achieve normal maturation, the intensity of FSH signaling must be precisely coordinated with the developmental stage of the follicle (41, 42). As such, LRH-1 in kisspeptin neurons plays an important role in maintaining plasma FSH at a level that is required for the formation of mature follicles that are fully capable of responding to the midcycle LH surge.
The presence of LRH-1 in kisspeptin neurons in Arc, but not in the AVPV, provides a mechanistic basis for the differential contributions of these kisspeptin neuron populations to pituitary gonadotropin output. The effect of LRH-1 on basal Kiss1 expression is required for sustaining the levels of FSH needed for normal follicle maturation. Once follicles are mature and plasma E2 levels peak, ERα induces Kiss1 expression in the AVPV (where Kiss1 is not regulated by LRH-1) to drive the LH surge needed for ovulation. At the same time, Arc Kiss1 expression is repressed by ERα, which causes the drop in serum FSH observed at the end of the estrous cycle. Importantly, we show that LRH-1 is not required for E2-mediated repression of Kiss1. Thus, the presence of LRH-1 in the Arc maintains FSH output during diestrus, whereas its absence in the AVPV results in low basal Kiss1 expression and is permissive for the kisspeptin spike that culminates in the LH surge during estrus. Therefore, LRH-1 in kisspeptin neurons plays an important evolutionary role in maintaining optimal reproductive success and thus conferring species fitness.
An important theme in LRH-1 biology is its role in coordinating metabolic processes among different tissues. For example, in the enterohepatic axis, LRH-1 coordinates bile acid homeostasis by regulating programs of genes in both the intestine and liver (23, 24). Here, we extend this paradigm to include the hypothalamic-pituitary-gonadal axis. In the pituitary, LRH-1 is important for gonadotropin subunit expression (26), and in the ovary, LRH-1 cooperates with ERα to regulate Cyp19a1 and progesterone synthesis enzymes in response to gonadotropin hormones (25). We now show that LRH-1 is also required for regulating kisspeptin in the hypothalamus and downstream gonadotropin levels.
Supplementary Material
Acknowledgments
We thank Dr. Jeffery Zigman for the Kiss1-Cre mouse line, Dr Robert Hammer and the University of Texas Southwestern Medical Center (UTSW) Transgenic Core Facility for assistance in generating the Lrh1-Tg mouse, Angela Mobley and the UTSW Flow Cytometry Core Facility, the UTSW Histology Core Facility, and the University of Virginia Center for Research in Reproduction Ligand Assay and Analysis Core.
This research was supported by the Howard Hughes Medical Institute (to D.J.M), National Institutes of Health Grants U19DK62434 (to D.J.M.), GM007062 (to A.L.B.), HD69702 (to C.F.E.), and U54-HD28934 (UVA Ligand Core), and the Robert A. Welch Foundation (I-1275 to D.J.M. and I-1558 to S.A.K.).
Disclosure Summary: The authors have nothing to disclose.
NURSA Molecule Pages†:
Nuclear Receptors: LRH-1.
Annotations provided by Nuclear Receptor Signaling Atlas (NURSA) Bioinformatics Resource. Molecule Pages can be accessed on the NURSA website at www.nursa.org.
- Arc
- arcuate nucleus
- AVPV
- anteroventral periventricular nucleus
- ChIP
- chromatin immunoprecipitation
- E2
- estradiol
- EGFP
- enhanced green fluorescent protein
- ER
- estrogen receptor
- LRH-1
- liver receptor homolog-1
- QPCR
- quantitative PCR
- TSS
- transcriptional start site.
References
- 1. Navarro VM. New insights into the control of pulsatile GnRH release: the role of Kiss1/neurokinin B neurons. Front Endocrinol (Lausanne). 2012;3:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Oakley AE, Clifton DK, Steiner RA. Kisspeptin signaling in the brain. Endocr Rev. 2009;30:713–743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Semple RK, Achermann JC, Ellery J, et al. Two novel missense mutations in g protein-coupled receptor 54 in a patient with hypogonadotropic hypogonadism. J Clin Endocrinol Metab. 2005;90:1849–1855 [DOI] [PubMed] [Google Scholar]
- 4. Cerrato F, Seminara SB. Human genetics of GPR54. Rev Endocr Metab Disord. 2007;8:47–55 [DOI] [PubMed] [Google Scholar]
- 5. d'Anglemont de Tassigny X, Fagg LA, Dixon JP, et al. Hypogonadotropic hypogonadism in mice lacking a functional Kiss1 gene. Proc Natl Acad Sci USA. 2007;104:10714–10719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Madeira da Silva L, Vandepas L, Bianco SD. 2011. Mutagenesis and analysis of genetic mutations in the GC-rich KISS1 receptor sequence identified in humans with reproductive disorders. J Vis Exp.:e2897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Topaloglu AK, Tello JA, Kotan LD, et al. Inactivating KISS1 mutation and hypogonadotropic hypogonadism. N Engl J Med. 2012;366:629–635 [DOI] [PubMed] [Google Scholar]
- 8. Mikkelsen JD, Simonneaux V. The neuroanatomy of the kisspeptin system in the mammalian brain. Peptides. 2009;30:26–33 [DOI] [PubMed] [Google Scholar]
- 9. Yeo SH, Herbison AE. Projections of arcuate nucleus and rostral periventricular kisspeptin neurons in the adult female mouse brain. Endocrinology. 2011;152:2387–2399 [DOI] [PubMed] [Google Scholar]
- 10. Kotani M, Detheux M, Vandenbogaerde A, et al. The metastasis suppressor gene KiSS-1 encodes kisspeptins, the natural ligands of the orphan G protein-coupled receptor GPR54. J Biol Chem. 2001;276:34631–34636 [DOI] [PubMed] [Google Scholar]
- 11. Gottsch ML, Cunningham MJ, Smith JT, et al. A role for kisspeptins in the regulation of gonadotropin secretion in the mouse. Endocrinology. 2004;145:4073–4077 [DOI] [PubMed] [Google Scholar]
- 12. Messager S, Chatzidaki EE, Ma D, et al. Kisspeptin directly stimulates gonadotropin-releasing hormone release via G protein-coupled receptor 54. Proc Natl Acad Sci USA. 2005;102:1761–1766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Roa J, Vigo E, Castellano JM, et al. Follicle-stimulating hormone responses to kisspeptin in the female rat at the preovulatory period: modulation by estrogen and progesterone receptors. Endocrinology. 2008;149:5783–5790 [DOI] [PubMed] [Google Scholar]
- 14. Navarro VM, Gottsch ML, Chavkin C, Okamura H, Clifton DK, Steiner RA. Regulation of gonadotropin-releasing hormone secretion by kisspeptin/dynorphin/neurokinin B neurons in the arcuate nucleus of the mouse. J Neurosci. 2009;29:11859–11866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wakabayashi Y, Nakada T, Murata K, et al. Neurokinin B and dynorphin A in kisspeptin neurons of the arcuate nucleus participate in generation of periodic oscillation of neural activity driving pulsatile gonadotropin-releasing hormone secretion in the goat. J Neurosci. 2010;30:3124–3132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Jayasena CN, Nijher GM, Comninos AN, et al. The effects of kisspeptin-10 on reproductive hormone release show sexual dimorphism in humans. J Clin Endocrinol Metab. 2011;96:E1963–E1972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mayer C, Acosta-Martinez M, Dubois SL, et al. Timing and completion of puberty in female mice depend on estrogen receptor α-signaling in kisspeptin neurons. Proc Natl Acad Sci USA. 2010;107:22693–22698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lee YK, Moore DD. Liver receptor homolog-1, an emerging metabolic modulator. Front Biosci. 2008;13:5950–5958 [DOI] [PubMed] [Google Scholar]
- 19. Sablin EP, Krylova IN, Fletterick RJ, Ingraham HA. Structural basis for ligand-independent activation of the orphan nuclear receptor LRH-1. Mol Cell. 2003;11:1575–1585 [DOI] [PubMed] [Google Scholar]
- 20. Freeman LA, Kennedy A, Wu J, et al. The orphan nuclear receptor LRH-1 activates the ABCG5/ABCG8 intergenic promoter. J Lipid Res. 2004;45:1197–1206 [DOI] [PubMed] [Google Scholar]
- 21. Malerod L, Sporstol M, Juvet LK, et al. Bile acids reduce SR-BI expression in hepatocytes by a pathway involving FXR/RXR, SHP, and LRH-1. Biochem Biophys Res Commun. 2005;336:1096–1105 [DOI] [PubMed] [Google Scholar]
- 22. Out C, Hageman J, Bloks VW, et al. Liver receptor homolog-1 is critical for adequate up-regulation of Cyp7a1 gene transcription and bile salt synthesis during bile salt sequestration. Hepatology. 2011;53:2075–2085 [DOI] [PubMed] [Google Scholar]
- 23. Mataki C, Magnier BC, Houten SM, et al. Compromised intestinal lipid absorption in mice with a liver-specific deficiency of liver receptor homolog 1. Mol Cell Biol. 2007;27:8330–8339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lee YK, Schmidt DR, Cummins CL, et al. Liver receptor homolog-1 regulates bile acid homeostasis but is not essential for feedback regulation of bile acid synthesis. Mol Endocrinol. 2008;22:1345–1356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Duggavathi R, Volle DH, Mataki C, et al. Liver receptor homolog 1 is essential for ovulation. Genes Dev. 2008;22:1871–1876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zheng W, Yang J, Jiang Q, He Z, Halvorson LM. Liver receptor homologue-1 regulates gonadotrope function. J Mol Endocrinol. 2007;38:207–219 [DOI] [PubMed] [Google Scholar]
- 27. Gofflot F, Chartoire N, Vasseur L, et al. Systematic gene expression mapping clusters nuclear receptors according to their function in the brain. Cell. 2007;131:405–418 [DOI] [PubMed] [Google Scholar]
- 28. Higashiyama H, Kinoshita M, Asano S. Expression profiling of liver receptor homologue 1 (LRH-1) in mouse tissues using tissue microarray. J Mol Histol. 2007;38:45–52 [DOI] [PubMed] [Google Scholar]
- 29. Cravo RM, Margatho LO, Osborne-Lawrence S, et al. Characterization of Kiss1 neurons using transgenic mouse models. Neuroscience. 2011;173:37–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Umetani M, Domoto H, Gormley AK, et al. 27-Hydroxycholesterol is an endogenous SERM that inhibits the cardiovascular effects of estrogen. Nat Med. 2007;13:1185–1192 [DOI] [PubMed] [Google Scholar]
- 31. DuSell CD, Nelson ER, Wang X, et al. The endogenous selective estrogen receptor modulator 27-hydroxycholesterol is a negative regulator of bone homeostasis. Endocrinology. 2010;151:3675–3685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Nelson JF, Felicio LS, Randall PK, Sims C, Finch CE. A longitudinal study of estrous cyclicity in aging C57BL/6J mice. I. Cycle frequency, length and vaginal cytology. Biol Reprod. 1982;27:327–339 [DOI] [PubMed] [Google Scholar]
- 33. Thrasher JD, Clark FI, Clarke DR. Changes in the vaginal epithelial cell cyle in relation to events of the estrous cycle. Exp Cell Res. 1967;45:232–236 [DOI] [PubMed] [Google Scholar]
- 34. Zigman JM, Jones JE, Lee CE, Saper CB, Elmquist JK. Expression of ghrelin receptor mRNA in the rat and the mouse brain. J Comp Neurol. 2006;494:528–548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Bookout AL, Cummins CL, Mangelsdorf DJ, Pesola JM, Kramer MF. High-throughput real-time quantitative reverse transcription PCR. Curr Protocol Mol Biol. 2006;73:15.8.1–15.8.28 [DOI] [PubMed] [Google Scholar]
- 36. Dutchak PA, Katafuchi T, Bookout AL, et al. Fibroblast growth factor-21 regulates PPARγ activity and the antidiabetic actions of thiazolidinediones. Cell. 2012;148:556–567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Makishima M, Okamoto AY, Repa JJ, et al. Identification of a nuclear receptor for bile acids. Science. 1999;284:1362–1365 [DOI] [PubMed] [Google Scholar]
- 38. Fukuda T, Scott G, Komatsu Y, et al. Generation of a mouse with conditionally activated signaling through the BMP receptor, ALK2. Genesis. 2006;44:159–167 [DOI] [PubMed] [Google Scholar]
- 39. Kumar TR, Wang Y, Lu N, Matzuk MM. Follicle stimulating hormone is required for ovarian follicle maturation but not male fertility. Nat Genet. 1997;15:201–204 [DOI] [PubMed] [Google Scholar]
- 40. Parker KL, Rice DA, Lala DS, et al. Steroidogenic factor 1: an essential mediator of endocrine development. Recent Prog Horm Res. 2002;57:19–36 [DOI] [PubMed] [Google Scholar]
- 41. Kreeger PK, Fernandes NN, Woodruff TK, Shea LD. Regulation of mouse follicle development by follicle-stimulating hormone in a three-dimensional in vitro culture system is dependent on follicle stage and dose. Biol Reprod. 2005;73:942–950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Adriaens I, Cortvrindt R, Smitz J. Differential FSH exposure in preantral follicle culture has marked effects on folliculogenesis and oocyte developmental competence. Hum Reprod. 2004;19:398–408 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.