Abstract
Expression of pituitary FSH and LH, under the control of pulsatile GnRH, is essential for fertility. cAMP response element-binding protein (CREB) has been implicated in the regulation of FSHβ gene expression, but the molecular mechanisms by which pulsatile GnRH regulates CREB activation remain poorly understood. We hypothesized that CREB is activated by a distinct signaling pathway in response to pulsatile GnRH in a frequency-dependent manner to dictate the FSHβ transcriptional response. GnRH stimulation of CREB phosphorylation (pCREB) in the gonadotrope-derived LβT2 cell line was attenuated by a protein kinase A (PKA) inhibitor, H89. A dominant negative PKA (DNPKA) reduced GnRH-stimulated pCREB and markedly decreased GnRH stimulation of FSHβ mRNA and FSHβLUC activity, but had little effect on LHβLUC activity, indicating relative specificity of this pathway. In perifusion studies, FSHβ mRNA levels and FSHβLUC activities were increased by pulsatile GnRH, with significantly greater increases at low compared with high pulse frequencies. DNPKA markedly reduced these GnRH-stimulated FSHβ responses at both low and high pulse frequencies. Correlating with FSHβ activation, both PKA activity and levels of pCREB were increased to a greater extent by low compared with high GnRH pulse frequencies, and the induction of pCREB was also attenuated by overexpression of DNPKA at both low and high pulse frequencies. Taken together, these data indicate that a PKA-mediated signaling pathway mediates GnRH activation of CREB at low-pulse frequencies, playing a significant role in the decoding of the hypothalamic GnRH signal to result in frequency-dependent FSHβ activation.
Secreted from anterior pituitary gonadotrope cells, both FSH and LH are modulated by the pulsatile action of the hypothalamic neuropeptide, GnRH. GnRH binds to its native high-affinity seven-transmembrane receptor (GnRHR) on the cell surface of the gonadotrope, stimulating signaling cascades that confer the production of these gonadotropins. FSH and LH, in turn, act on the ovaries and testes, resulting in steroidogenesis and gametogenesis, demonstrating their importance for reproductive function (1). Several diseases result from the disruption of the GnRH-, FSH-, and LH-signaling cascades, including idiopathic hypogonadotropic hypogonadism and Kallmann's syndrome, whereby patients present with low levels of GnRH, FSH, and LH, resulting in infertility (2). Conversely, polycystic ovarian syndrome, affecting 5%–15% of the female population within reproductive age, is associated with an accelerated GnRH pulse frequency, which favors LH production over FSH (3). Polycystic ovarian syndrome is associated with infertility and is also linked to obesity, insulin resistance, and metabolic and cardiovascular abnormalities (4–6). These examples highlight the importance of GnRH and the subsequent regulation of FSH and LH for reproductive function.
FSH and LH contain a common α-glycoprotein subunit (αGSU), but distinct β-subunits (FSHβ and LHβ, respectively) that result in the specific actions of these gonadotropins (7). Transcriptional regulation of FSHβ and LHβ is mediated through signaling mechanisms that are not yet fully understood; therefore, the controlled release of FSH and LH by pulsatile GnRH requires further investigation. Differential GnRH pulse frequencies and amplitudes alter the ratio of FSH vs LH secretion (8, 9), with increasing frequencies resulting in preferential secretion of LH, whereas decreasing frequencies result in greater FSH release. The levels of gonadotropin subunit (αGSU, FSHβ, and LHβ) mRNAs are also modulated by varying GnRH pulse frequency, a reflection of changes in gene transcription (10, 11). In the rodent model, FSHβ gene expression is optimally stimulated by GnRH pulses every 2 hours, whereas LHβ gene expression is higher at faster frequencies, every 30 minutes. Although αGSU gene expression also responds to GnRH pulse frequencies, this regulation is less important for overall FSH and LH production because αGSU is produced in excess at both high and low pulse frequencies (12, 13).
A partial cAMP response element (CRE) is present in the rat FSHβ promoter and is bound by the CRE-binding protein (CREB). GnRH stimulates CREB phosphorylation, which results in recruitment of CREB-binding protein (CBP) and increased FSHβ transcription (14–16). This site is fully conserved (100%) in humans (16), highlighting the relevance of understanding the regulation of FSHβ transcription via this response element. Our prior studies showed that mutation of the FSHβ CRE site abolished preferential FSHβ gene expression in response to pulsatile GnRH in perifusion at low-pulse frequency (17), suggesting that modulation of CREB activity could be responsible for the GnRH pulse frequency-dependent regulation of FSH. We hypothesized that distinct signaling mechanisms are stimulated dependent on the pulse frequency of GnRH to determine the relative production of FSH vs LH. In the present study, we demonstrate that FSHβ gene transcription is preferentially stimulated at a low GnRH pulse frequency by a protein kinase A (PKA)-dependent mechanism that is mediated by CREB phosphorylation. Furthermore, we demonstrate that LHβ gene expression is unaffected by this pathway, providing support to the notion that differential signaling pathways are stimulated by pulsatile GnRH to independently regulate FSH and LH production.
Materials and Methods
Materials
Materials were purchased either from Fisher Scientific (Pittsburgh, Pennsylvania) or Sigma Chemical Co (St Louis, Missouri). The PKA (H-89), calmodulin-dependent protein kinase (CamK)II (KN-62) and MAPK kinase (MEK)I/II (U0126) inhibitors were purchased from Sigma. Protein kinase C (PKC) (GF109203X) and CamKII (KN-93) inhibitors were obtained from Tocris Bioscience (Ellisville, Missouri). CREB phosphorylation (pCREB) (06-519; 1:2000 dilution) and CREB (06–863; 1:2000 dilution) antibodies were purchased from Millipore Corp. (Billerica, Massachusetts). Oligonucleotides were synthesized by Invitrogen (Carlsbad, California) (see Supplemental Table 1 published on The Endocrine Society's Journals Online web site at http://mend.endojournals.org). The murine LβT2 cell line was a kind gift from Professor Pamela L. Mellon (University of California, San Diego, California).
Reporter plasmids and expression vectors
The −140/+15 rFSHβLUC and −797/+5 rLHβLUC reporters were generated in pXP2 as previously described (18, 19). The dominant negative PKA MT-REVAB and corresponding backbone (ZEM3EV) vectors were a kind gift from Professor G. Stanley McKnight (University of Washington, Seattle, Washington). As an internal control, a β-galactosidase expression vector (simian virus driven, SV40βGal; Promega Corp., Madison, Wisconsin) was cotransfected with all luciferase vectors. Transfection efficiency was determined using a green fluorescent protein (GFP) expression vector (cytomegalovirus [CMV]-GFP).
Cell culture and transient transfections
LβT2 cells were maintained in high-glucose DMEM (Mediatech, Inc., Manassas, Virginia), supplemented with 10% (v/v) fetal bovine serum (FBS; Omega, Tarzana, California), 100 U of penicillin/mL, and 100 μg of streptomycin sulfate/mL (Invitrogen) in 5% CO2 humidified 37°C air. The cells were transiently transfected using the electroporation method as previously described (14). Unless otherwise stated, 2 μg of total plasmid DNA was transiently transfected across all experimental paradigms. Transfection with a similar amount (2 μg) of the CMV-GFP vector indicated a transfection efficiency of approximately 95% (data not shown). After 8 minutes recovery in PBS supplemented with 5 mM glucose and 20% FBS, transfected LβT2 cells were cultured in 10% (v/v) FBS-supplemented DMEM for 16 hours (all treatment paradigms), before 24-hour serum starvation with FBS-free DMEM (static cultures only), and treatment with 10 nM GnRH (Sigma) with or without pharmacologic pathway inhibitors. This concentration of GnRH was selected for consistency with the perifusion studies presented in this manuscript, as well as other studies from our group (20–22) and others (15, 23–25).
Perifusion studies
Using a previously described perifusion system (17, 22), LβT2 cells were treated with 10 nM GnRH pulses (5 min/pulse) in serum-free DMEM for 20 hours at either high (every 30 min) or low (every 120 min) pulse frequencies. This amplitude of GnRH pulses was selected based on previous optimization studies demonstrating its ability to result in sustained gonadotropin responses without desensitization from dispersed rodent primary pituitary cell cultures (26). After the final pulse, total protein or RNA was isolated at the indicated times with either radioimmune precipitation assay buffer (Santa Cruz Biotechnology, Inc, Santa Cruz, California), or TRI Reagent (Sigma), respectively.
mRNA and protein quantification
LβT2 cells harvested for total RNA with TRI Reagent were processed using the chloroform/isopropanol method. One microgram of total RNA was reverse transcribed using the Superscript III cDNA synthesis kit (Invitrogen), followed by quantitative real-time PCR analysis performed on an ABI PRISM 7000 sequence detection system (Applied Biosystems, Foster City, California) using iQ SYBR Green (Bio-Rad Laboratories, Hercules, California) according to manufacturer's instructions. Results were analyzed using ABI PRISM 7000 SDS software (Applied Biosystems), and products were subsequently electrophoresed on an agarose gel to verify that a single product was amplified. Levels of mRNA were normalized to ribosomal protein L19 as an internal control.
Total protein isolated from LβT2 cells using radioimmune precipitation assay buffer was quantified and 30 μg was separated by SDS-PAGE and transferred onto a nitrocellulose membrane (Whatman, Waltham, Massachsetts) in preparation for Western blot analysis. The membranes were blocked in 3% nonfat milk in TBST (Tris-buffered saline plus Tween 20) for 1 hour at room temperature with shaking, followed by incubation for 16 hours at 4°C with pCREB or CREB primary antibodies (both after 1:2000 dilutions) with gentle shaking. After washes with TBST, the membranes were exposed to donkey antirabbit IgG-horseradish peroxidase (IgG-HRP, Santa Cruz Biotechnology, Santa Cruz, California; sc-2313; 1:5000 dilution) secondary antibody for 1 hour at room temperature with shaking. After further washes in TBST, complexes were detected using HyGLO Quick Spray Chemiluminescent HRP antibody detection reagent (Denville Scientific Inc, Metuchen, New Jersey). pCREB signal intensity was measured by ImageJ software (National Institutes of Health, Bethesda, Maryland), normalized to total CREB detected on separate SDS-PAGE gels run concurrently, and expressed as fold change over control samples.
PKA activity assay
LβT2 cells were lysed with protein extraction buffer (25 mM Tris-HCl [pH 7.4], 0.5 mM EDTA, 0.5 mM EGTA, 10 mM β-mercaptoethanol, 0.5 mM phenylmethanesulfonylfluoride, 1 μg/mL leupeptin/aprotinin) and prepared according to the manufacturer's instructions provided with the PKA kinase activity kit (Promega). PKA activity was represented by absorbance of phosphorylated substrate at 570 nm.
Statistical analysis
All graphs were generated and statistical analysis was performed using Prism software (Prism for Mac OS X, GraphPad Software Inc, San Diego, California). Unless otherwise stated, one-way ANOVA followed by post hoc Tukey multiple-comparison tests were carried out to determine statistical significance. P < .05 was considered to be statistically significantly different. Numerical data presented in this manuscript represent the mean ± SEM from at least three independent experiments, each performed in triplicate.
Results
A PKA inhibitor attenuates GnRH stimulation of CREB phosphorylation
Because CREB was identified as a key transcription factor involved in mediating GnRH stimulation of FSHβ transcription (14), we hypothesized that pathways that stimulate CREB phosphorylation in response to GnRH would likewise be involved in the induction of FSHβ by GnRH. To identify the signaling pathways that mediate GnRH-stimulated CREB phosphorylation, we treated the murine LβT2 gonadotrope cell line with a series of pharmacologic inhibitors of pathways that have been previously associated with gonadotrope signaling in response to GnRH, including calcium/calmodulin-dependent protein kinases (CamK) (27, 28), PKA (29, 30), as well as PKC and MAPK (31–39).
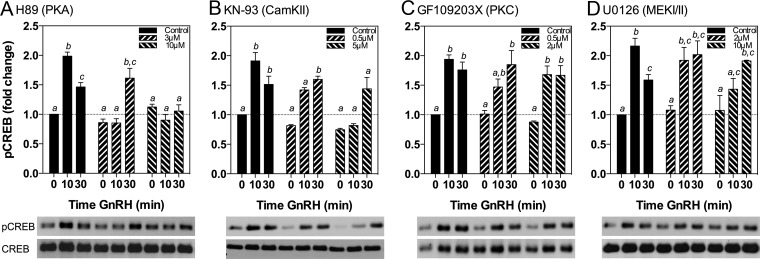
LβT2 cells in static culture were pretreated with serum-free DMEM alone, or with the indicated concentrations of inhibitors for 30 min, followed by stimulation with 10 nM GnRH for 0, 10, or 30 minutes (Figure 1). The ranges of inhibitor concentrations selected were chosen to include the predicted half-maximal IC50, as provided by the respective manufacturer. As expected (14, 40), GnRH significantly increased pCREB levels at 10 minutes (2.0 ± 0.06-fold, P < .05) and 30 minutes (1.58 ± 0.07-fold, P < .05) compared with controls. The PKA inhibitor, H89, significantly reduced pCREB levels at 10 minutes at both 3 and 10 μM concentrations, extending to 30 minutes with 10 μM (Figure 1A), suggesting the phosphorylation of CREB in response to GnRH is mediated by PKA. The effect of H89 was far more striking than those of the other kinase inhibitors. Neither the PKC inhibitor (GF109203X) nor the MEKI/II inhibitor (U0126) resulted in significant inhibition of GnRH-stimulated CREB phosphorylation (Figure 1, C and D). The CamKII inhibitor KN-93 did significantly reduce pCREB levels at 10 minutes, but the effect was not sustained to 30 minutes (Figure 1B). We extended and confirmed these findings with an alternate CamKII inhibitor, KN-62, which similarly had minimal effects on GnRH-induced pCREB levels except at the highest concentration tested (data not shown).
Figure 1.
GnRH stimulation of pCREB Levels Is Attenuated by a PKA Inhibitor. LβT2 cells in static culture were pretreated for 30 minutes with the indicated concentrations of inhibitors of: A, PKA (H89); B, CamKII (KN-93); C, PKC (GF109203X); and D, MEKI/II (U0126), followed by treatment with 10 nM GnRH for 0, 10, or 30 minutes. Bar graphs show relative pCREB levels (mean ± SEM from three independent experiments, each performed in triplicate, normalized to CREB). Western immunoblots shown below graphs are from a representative experiment. Significant differences (P < .05), measured by one-way ANOVA with a post hoc Tukey multiple-comparison test, are indicated by different letters.
Dominant negative inhibition of PKA activity attenuates GnRH-stimulated CREB phosphorylation
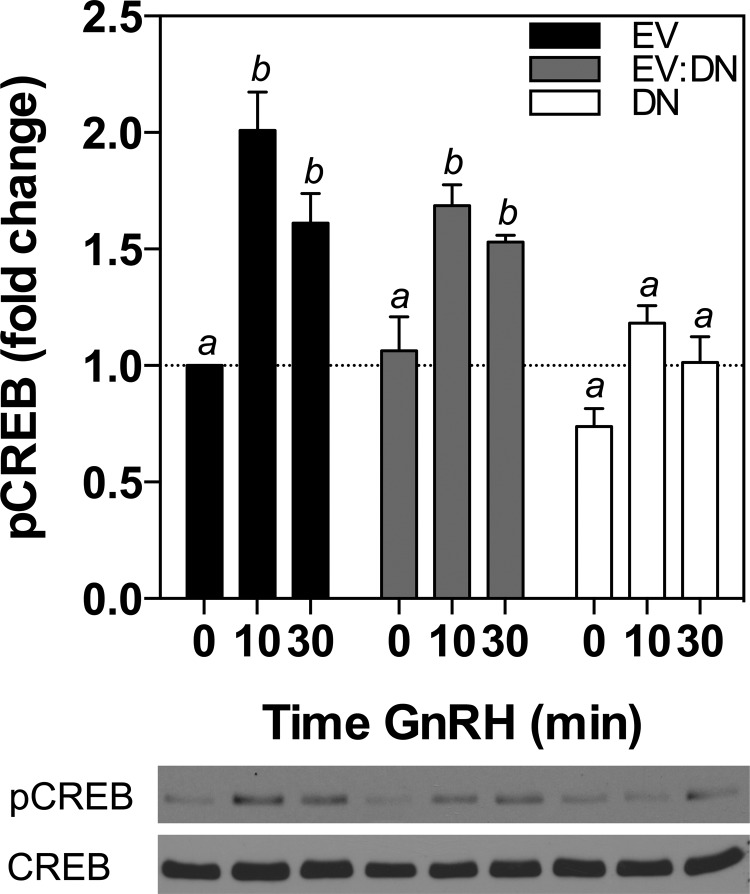
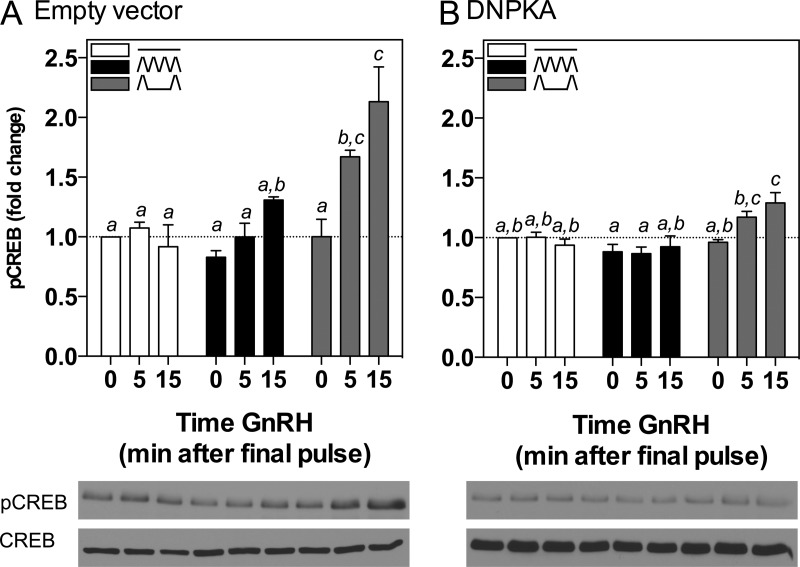
To further investigate the role of PKA in regulating CREB phosphorylation, we performed transient transfections of LβT2 cells with either empty vector or increasing amounts of a regulatory subunit dominant negative PKA (DNPKA). The DNPKA vector contains two-point mutations in cAMP-binding sites A and B within the regulatory subunit of PKA that lead to inhibition of PKA activity (41). Cells were cultured in serum-free media for 16 hours, during which they were stimulated with 10 nM GnRH for the final 0, 10, or 30 min. The control cells transiently transfected with empty vector demonstrated a significant increase in pCREB levels after both 10 minutes (2.01 ± 0.16-fold, P < .05) and 30 minutes (1.61 ± 0.13-fold, P < .05) of GnRH stimulation compared with basal controls (Figure 2, black solid bars). Similarly to cells treated with 10 μM of the PKA inhibitor, H89 (Figure 1A), this effect was attenuated by DNPKA overexpression in a dose-dependent manner, with complete abrogation of GnRH-stimulated CREB phosphorylation at the highest amounts of DNPKA transfected (Figure 2, white solid bars). These results indicate that the inhibition of PKA activity, either pharmacologically or catalytically, reduces the GnRH-stimulated phosphorylation of CREB in LβT2 cells.
Figure 2.
GnRH Stimulation of pCREB Levels Is Attenuated by a DNPKA Inhibitor. LβT2 cells in static culture were transiently transfected with 2 μg total DNA: 2 μg empty vector (EV, black solid bars); 1 μg empty vector in combination with 1 μg DNPKA (EV:DN, gray shaded bars); or 2 μg DNPKA alone (DN, white solid bars). Cells were treated 40 hours after transfection with 10 nM GnRH for 0, 10, or 30 minutes before harvest. Bar graphs show relative pCREB levels (mean ± SEM from three independent experiments, each performed in triplicate, normalized to CREB). Western immunoblot shown below bar graph is from one representative experiment. Significant differences (P < .05), measured by one-way ANOVA with a post hoc Tukey multiple-comparison test, are indicated by different letters.
Inhibition of PKA activity reduces GnRH stimulation of FSHβ transcription
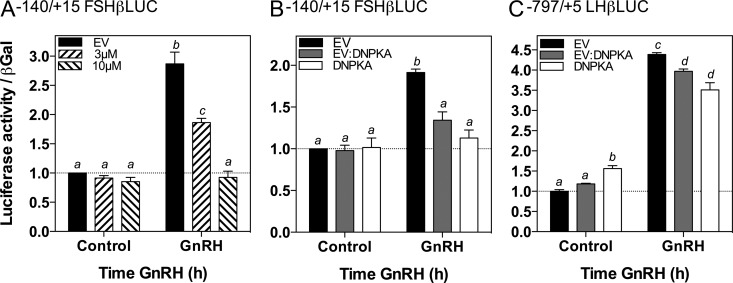
Because inhibition of PKA activity reduced GnRH stimulation of CREB phosphorylation, and GnRH-stimulated CREB phosphorylation results in the recruitment of CBP to the FSHβ promoter to mediate transcriptional activation (14), we hypothesized that inhibition of PKA activity would attenuate the induction of FSHβ by GnRH. Previously, GnRH has been shown to activate Gs and elevate cAMP levels in LβT2 cells (42, 43) and modulate the expression of PKA-catalytic and -regulatory subunits in αT3–1 cells (44). An earlier study also detected elevated cAMP levels within male rat pituitary cells treated with GnRH (45), suggesting potential roles for the cAMP- and PKA-signaling pathway in modulating the gonadotrope response to GnRH. To investigate the role of PKA in GnRH-mediated stimulation of FSHβ transcription, we examined the effects of PKA inhibition on the activity of a luciferase reporter driven by the FSHβ promoter, −140/+15 FSHβLUC, after GnRH stimulation. Stimulation of LβT2 cells transfected with −140/+15 FSHβLUC with 10 nM GnRH for 4 hours resulted in a significant increase in luciferase activity (Figure 3A, black solid bars; 2.87 ± 0.20-fold; P < .05). This response was significantly attenuated by 3 μM H89 (Figure 3A; 1.86 ± 0.07-fold increase over control; P < .05) and completely abrogated at 10 μM H89 (Figure 3A; 0.93 ± 0.11-fold increase over control; P > .05). To confirm these observations, cells were transiently transfected with the DNPKA expression vector or the empty vector control together with −140/+15 FSHβLUC. Increasing amounts of DNPKA significantly reduced GnRH stimulation of −140/+15 FSHβLUC activity (Figure 3B, empty vector alone, 1.91 ± 0.04-fold, P < .05, 1 μg DNPKA, 1.34 ± 0.10-fold, P > .05; 2 μg DNPKA, 1.13 ± 0.10-fold, P > .05, compared with cells treated with vehicle). Similar studies were performed in LβT2 cells transiently transfected with −797/+5 LHβLUC for comparison. Stimulation with 10 nM GnRH for 4 h significantly increased −797/+5 LHβLUC activity (Figure 3C, 4.39 ± 0.04-fold, P < .05); GnRH responsiveness persisted in the presence of DNPKA compared with control values (Figure 3C, 1 μg DNPKA, 3.97 ± 0.06-fold, P < .05; 2 μg DNPKA, 3.65 ± 0.20-fold, P < .05). These results indicate that the effects of PKA inhibition are relatively specific for FSHβ, with complete abrogation of GnRH stimulation of FSHβ transcription but only a modest reduction in LHβ transcription.
Figure 3.
Inhibition of PKA Activity Markedly Attenuated GnRH Stimulation of FSHβ Promoter Activity with Only Modest Effects on LHβ Promoter Activity. A, LβT2 cells in static culture were transiently transfected with 2 μg −140/+15 FSHβLUC and pretreated 40 hours after transfection for 30 min with the indicated concentrations of H89, followed by cotreatment with vehicle or 10 nM GnRH for 4 hours. B, LβT2 cells in static culture were transiently transfected with 2 μg of −140/+15 FSHβLUC, as well as 2 μg empty vector (EV, black solid bars); 1 μg empty vector in combination with 1 μg DNPKA (EV:DN, gray shaded bars); or 2 μg DNPKA alone (DN, white solid bars). C, LβT2 cells were transiently transfected with 2 μg of −797/+5 LHβLUC as well as the EV, EV:DN, and DN vectors as described in panel B. Panels B and C were treated with vehicle or 10 nM GnRH for 4 hours. Bar graphs show relative luciferase activity (mean ± SEM from three independent experiments each performed in triplicate, normalized to β-galactosidase activity). Significant differences (P < .05), measured by one-way ANOVA with a post hoc Tukey multiple-comparison test, are indicated by different letters.
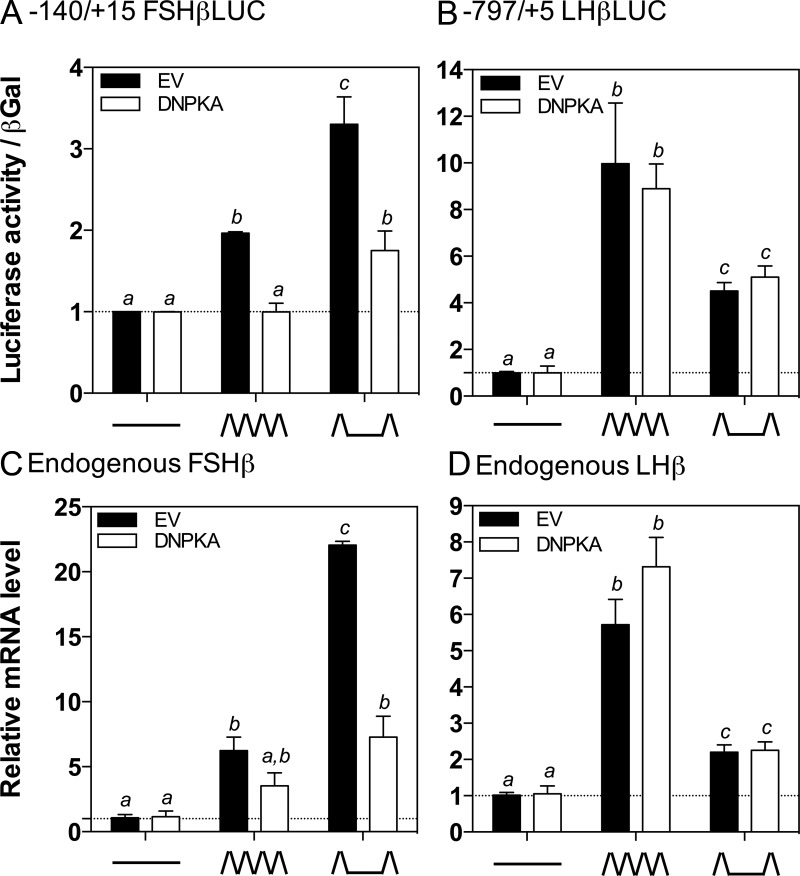
Stimulation of FSHβ by pulsatile GnRH is attenuated by PKA inhibition at both high and low GnRH pulse frequencies
Our ultimate goal is to identify the signaling pathways by which pulsatile GnRH stimulates FSHβ in a pulse frequency-dependent manner, with preferential activation at low-pulse frequencies, to better understand the physiologic responses to pulsatile GnRH at varying GnRH pulse frequencies. Because our prior studies suggested that modulation of CREB activity could be responsible for the GnRH pulse frequency-dependent regulation of FSH (17), we next sought to determine the role of the PKA pathway in mediating the regulation of FSHβ by pulsatile GnRH. In these studies, LβT2 cells were perifused for 20 hours and exposed to pulsatile GnRH at either high (every 30 min) or low (every 120 min) pulse frequencies or with medium alone, as previously described (Figure 4) (17, 22). These pulse frequencies were chosen based on previous studies in both LβT2 cells and in cultured rat pituitary cells, indicating that these frequencies were optimal for LHβ gene expression and LH secretion and FSHβ gene expression and FSH secretion, respectively (10, 11, 17, 22, 46–48). LβT2 cells were transiently transfected with −140/+15 FSHβLUC together with the DNPKA expression vector, or the empty vector as a control (Figure 4A). Stimulation with pulsatile GnRH significantly increased −140/+15 FSHβLUC activity at both high and low pulse frequencies, compared to control samples (high frequency, 1.97 ± 0.01-fold, P < .05; low frequency, 3.30 ± 0.19-fold, P < .05; Figure 4A, solid black bars). Activity was significantly higher at the low compared with the high GnRH pulse frequency, consistent with previous studies (11, 17, 21, 22, 49). Overexpression of the DNPKA attenuated the response to pulsatile GnRH at both high and low pulse frequencies (Figure 4A, open bars). At the high GnRH pulse frequency, the response was completely abrogated, whereas at the low GnRH pulse frequency, the response was markedly reduced, although still significantly higher than the controls (1.75 ± 0.14-fold, P < .05).
Figure 4.
GnRH Induction of FSHβ mRNA and FSHβLUC Activity Is Attenuated at Both High and Low GnRH Pulse Frequencies by PKA Inhibition. LβT2 cells were transiently transfected with (A) 2 μg −140/+15 FSHβLUC or (B) 2 μg −797/+5 LHβLUC, as well as 2 μg of empty vector (EV, black solid bars) or DNPKA (DN, white solid bars). After 20 hours of perifusion with high (ΛΛΛΛ; every 30 min) or low (Λ__Λ; every 120 min) frequencies of GnRH or medium alone (____), luciferase activity was measured. Endogenous (C) FSHβ mRNA levels or (D) LHβ mRNA levels were quantified by real-time RT-PCR in LβT2 cells transfected with 2 μg of empty vector (EV, black solid bars) or 2 ug of DNPKA (DN, white solid bars) followed by 20 hours of perifusion with high (ΛΛΛΛ; every 30 min) or low (Λ__Λ; every 120 min) frequencies of GnRH or medium alone (____). Bar graphs show relative change compared with basal control samples (mean ± SEM from three independent experiments each performed in triplicate, normalized to β-galactosidase activity (A and B) or endogenous ribosomal protein L19 (RPL19) mRNA levels (C and D)). Significant differences (P < .05), measured by one-way ANOVA with a post hoc Tukey multiple-comparison test, are indicated by different letters.
To further investigate whether the signaling mechanisms by which pulsatile GnRH regulates FSHβ and LHβ are distinct, the effects of PKA inhibition on the response of LHβ to pulsatile GnRH was similarly measured after high and low pulse frequency paradigms (Figure 4B). LβT2 cells were transiently transfected with the −797/+5 LHβLUC reporter together with the DNPKA expression vector or empty vector control as above. Similarly to −140/+15 FSHβLUC, luciferase activity was significantly increased in response to pulsatile GnRH at both high and low pulse frequencies compared with control (high frequency, 9.97 ± 1.50-fold, P < .05; low frequency, 4.51 ± 0.21, P < .05-fold). The response was significantly greater at high compared with low GnRH pulse frequency, as expected and in contrast to the FSHβ response (11, 22, 49). In contrast to the effects on FSHβ, overexpression of the DNPKA inhibitor had no effect on the response to pulsatile GnRH at either high or low GnRH pulse frequencies, and the frequency-dependent response persisted (Figure 4B).
To confirm the role of the PKA pathway in the stimulation of FSHβ transcription by pulsatile GnRH, our studies of the rat FSHβ and LHβ promoters using luciferase reporters were extended to measure the effects on the endogenous murine FSHβ and LHβ genes in LβT2 cells. Cells were transfected with DNPKA or empty vector control followed by perifusion for 20 hours and stimulation with pulsatile GnRH at either high (every 30 min) or low (every 120 min) pulse frequencies or with medium alone. FSHβ and LHβ mRNA levels were then measured by quantitative real-time quantitative PCR. FSHβ mRNA levels were stimulated by pulsatile GnRH, with the highest response at the low GnRH pulse frequency, as expected (Figure 4C, solid black bars; high frequency, 6.24 ± 1.04-fold, P < .5; low frequency, 22.06 ± 0.28-fold, P < .05) (17). Conversely, LHβ mRNA, although also stimulated by pulsatile GnRH at both frequencies, was increased to the greatest extent at the high GnRH pulse frequency (Figure 4D, solid black bars; high frequency, 5.72 ± 0.70-fold, P < .05; low frequency, 2.40 ± 0.08-fold, P < .05). Overexpression of DNPKA reduced stimulation of FSHβ mRNA levels by pulsatile GnRH at both high and low pulse frequencies (Figure 4C, open bars). At the low GnRH pulse frequency, FSHβ mRNA levels were greater than control (7.28 ± 1.61-fold, P < .05), but significantly lower than in cells transfected with the empty vector, and there was no difference compared with levels in cells exposed to the high GnRH pulse frequency. In contrast, stimulation of LHβ mRNA levels by pulsatile GnRH was unaffected by DNPKA, consistent with the −797/+5 LHβLUC response (Figure 4D, open bars). Taken together, these data suggest a role for the PKA-signaling pathway in regulating the frequency-dependent stimulation of FSHβ, but not LHβ, by pulsatile GnRH.
CREB phosphorylation is stimulated by pulsatile GnRH in a frequency-dependent manner that is attenuated by PKA inhibition
Because PKA inhibition attenuated stimulation of FSHβ by pulsatile GnRH at both low and high frequencies, and it has been demonstrated that CREB is a key transcription factor involved in mediating GnRH stimulation of FSHβ transcription (14), we hypothesized that the PKA pathway would similarly be involved in the stimulation of CREB phosphorylation by pulsatile GnRH. To test this hypothesis and measure CREB phosphorylation by pulsatile GnRH, LβT2 cells were stimulated in perifusion by pulsatile GnRH at low and high GnRH pulse frequencies followed by measurement of pCREB levels at varying time intervals after the final GnRH pulse (Figure 5A). Cells were lysed and protein collected at 0, 5, or 15 minutes after the final pulse of GnRH. In this experimental paradigm, baseline interpulse pCREB levels were not different in cells treated with pulsatile GnRH at low or high pulse frequency, compared with cells perifused with medium alone. There was a small increase in pCREB levels in cells harvested 15 min after the final GnRH pulse at high frequency, although this did not reach statistical significance compared with the basal control (Figure 5A, solid black bars). However, pCREB levels were significantly increased 5 and 15 minutes after the final GnRH pulse in cells exposed to pulsatile GnRH at the low pulse frequency, compared with control samples (Figure 5A, gray bars; 5 min, 1.67 ± 0.06-fold; 15 min, 2.13 ± 0.29-fold, P < .05). The greater induction of pCREB by pulsatile GnRH at low frequency correlated with the greater induction of FSHβ in response to this GnRH paradigm.
Figure 5.
CREB Phosporylation Is Stimulated by Pulsatile GnRH in a Frequency-Dependent Manner That Is Attenuated by PKA Inhibition. LβT2 cells were transiently transfected with 2 μg of (A) empty vector or (B) DNPKA. Cells were perifused for 20 h and stimulated with high (black solid bars; ΛΛΛΛ; every 30 min) or low (gray shaded bars; Λ__Λ; every 120 min) frequencies of GnRH or medium alone (white solid bars; ____). Bar graphs show relative pCREB levels (mean ± SEM from three independent experiments, each performed in triplicate, normalized to CREB). Western immunoblot shown is representative of one experiment. Significant differences (P < .05), measured by one-way ANOVA with a post hoc Tukey multiple-comparison test, are indicated by different letters.
Having demonstrated that CREB is phosphorylated by pulsatile GnRH preferentially by the low pulse frequency paradigm, the effect of PKA inhibition was determined (Figure 5B). In cells transfected with DNPKA, there was no change in pCREB levels at any time point in response to pulsatile GnRH at high GnRH pulse frequency (Figure 5B, solid black bars). A modest, but significant, increase in pCREB levels in response to the low GnRH pulse frequency was detected (Figure 5B, gray bars; 15 min, 1.29 ± 0.08-fold, P < .05) compared with control samples, suggesting some, albeit markedly reduced, stimulation of this pathway even in the presence of DNPKA.
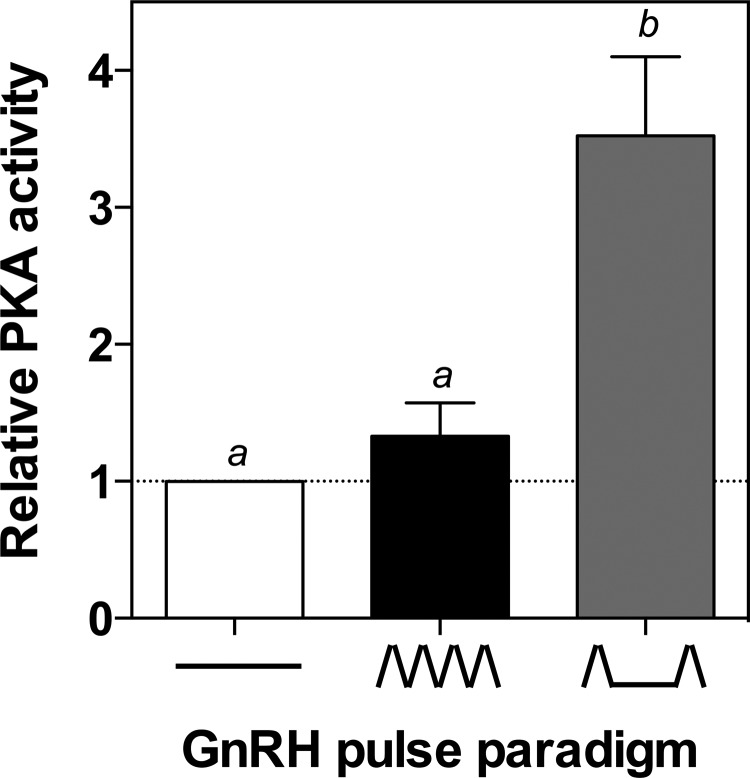
PKA activity is stimulated by pulsatile GnRH at low GnRH pulse frequencies
Because PKA pathway inhibition attenuated GnRH pulse frequency-dependent CREB phosphorylation and FSHβ transcription, both of which are activated to a greater extent at low GnRH pulse frequencies, we hypothesized that PKA activity might similarly be induced by pulsatile GnRH in a frequency-dependent manner. To test this hypothesis, LβT2 cells were perifused and exposed to pulsatile GnRH in the high and low pulse frequency paradigms. Cells were lysed and protein collected for measurement of PKA activity 10 min after the final pulse of GnRH (Figure 6). In cells perifused with GnRH at the high pulse frequency, there was no significant increase in PKA activity compared with basal control samples. However, after stimulation by the low GnRH pulse frequency, PKA activity was significantly higher than the controls (2.69 ± 0.61-fold, P < .05). These findings support the hypothesis that PKA activity is preferentially stimulated by pulsatile GnRH at low pulse frequencies, leading to preferential CREB phosphorylation and a correspondingly greater increase in FSHβ transcription.
Figure 6.
GnRH Induction of PKA Activity Is Stimulated at Low GnRH Pulse Frequencies. LβT2 cells were perifused for 20 h with high (ΛΛΛΛ; every 30 min) or low (Λ__Λ; every 120 min) frequencies of GnRH or medium alone (____), after which PKA activity was measured. The bar graph shows relative change compared with basal control samples (mean ± SEM from three independent experiments, each performed in triplicate). Significant differences (P < .05), measured by one-way ANOVA with a post hoc Tukey multiple-comparison test, are indicated by different letters.
Discussion
The expression and secretion of both FSH and LH are regulated by the pulsatile release of the same hypothalamic neuropeptide, GnRH, suggesting that distinct signaling mechanisms are responsible for the differential production of these two gonadotropins. Input from other neuroendocrine factors, locally produced autocrine/paracrine factors, as well as feedback from downstream endocrine organs, including the testes and ovaries, also influence the transcriptional regulation of the gonadotropin subunits αGSU, FSHβ, and LHβ. The importance of GnRH in FSH and LH regulation is highlighted by the hpg hypogonadal mouse, which harbors a deletion in the GnRH gene (50, 51). With no functional GnRH, these mice have low FSH and LH levels. This GnRH dependence is also demonstrated in patients with Kallmann's syndrome, who are GnRH deficient and have low FSH and LH levels (2).
It is now accepted that FSH and LH production is regulated by the pulse frequency of GnRH (8–11). The murine gonadotrope-derived LβT2 cell line (52) has provided a model for investigating gonadotropin regulation by GnRH. In combination with the αT3–1 cell line (53), many experiments have been performed that have identified signaling pathways involved in FSH and LH regulation by GnRH in static culture (23, 39, 42, 54–58). These are complemented by a smaller number of studies carried out with more physiologically relevant pulsatile GnRH treatment paradigms in LβT2 cells (17, 22, 31, 59, 60) and primary pituitary cultures (20, 21, 46, 49, 61). In this study, our goal was to elucidate the signaling pathways that contribute to GnRH pulse frequency-dependent differential control of FSHβ gene expression.
The rat FSHβ gene is bound by CREB at a CRE/activator protein 1 half-site in the proximal promoter (14). Phosphorylation of CREB, which can be mediated by a number of kinases, leads to the recruitment of the histone acetyltransferase, CBP, resulting in transcriptional activation (62). It has been shown that GnRH stimulates CREB phosphorylation (40), and we have previously demonstrated that an intact CRE site within the FSHβ promoter is essential to maintain the preferential stimulation of FSHβ transcription at low GnRH pulse frequencies (17). Therefore, this CRE has an important role in decoding GnRH pulse frequency, supported by data showing that a mutation within this site prevents CREB binding and reverses preferential stimulation such that FSHβ transcription is greater at high compared with low GnRH pulse frequencies (17). Therefore, the elucidation of the signaling pathways that mediate CREB phosphorylation and subsequent increases in FSHβ transcription in response to pulsatile GnRH would be expected to provide insight into the mechanisms by which the gonadotrope differentially responds to varying GnRH pulse frequencies. We have also previously demonstrated that high frequency pulsatile GnRH increases the expression of inducible cAMP early repressor. Inducible cAMP early repressor competes with CREB for binding to the CRE reducing FSHβ transcription, suggesting that the CRE plays a significant role in regulating FSHβ transcription at both high and low GnRH pulse frequencies. Conversely, regulation of the LHβ promoter is mediated by the transcription factor, Egr-1, which binds to two highly conserved binding sites within the proximal GnRH-responsive region (37, 63–67). Female mice with targeted disruption of Egr-1 had markedly reduced pituitary LHβ mRNA levels and were infertile (68, 69); however, FSHβ mRNA levels were unchanged in these mice (68). These findings suggest that differential mechanisms regulate FSHβ and LHβ expression and led us to focus our study on the signaling pathways that mediate CREB phosphorylation and control FSHβ mRNA levels at high and low GnRH pulse frequencies.
The data described in this study indicate that CREB phosphorylation in response to GnRH stimulation is mediated primarily by the PKA pathway in LβT2 cells. The use of pharmacologic inhibitors demonstrated that a PKA inhibitor, H89, had the greatest relative impact on GnRH-induced CREB phosphorylation, with lesser effects of CamKII, PKC, and MEK inhibitors (Figure 1). GnRH has been shown to induce CamKII activity (27, 28), and the CamKII inhibitor, KN-93, did have modest effects on GnRH stimulation of CREB phosphorylation, but only at one time point and at the highest inhibitor dose tested. To clarify the role of the CamKII pathway, an alternative CamKII inhibitor, KN-62, was tested, which again showed only modest effects on GnRH-stimulated CREB phosphorylation (data not shown) when compared with the effects of the PKA inhibitor, H89 (Figure 1A).
Because pharmacologic inhibitors can at times have nonspecific effects on other kinases, depending on the concentration used, the cell model studied, and cross talk between pathways, such studies are best used as a screen to identify potential pathways for further focus. We confirmed our findings with the pharmacologic PKA inhibitor, H89, by demonstrating that a dominant negative PKA RIAB (DNPKA), which inhibits PKA activity (41), replicated the inhibitory effect of H89 on GnRH-stimulated CREB phosphorylation (Figure 2). In addition, DNPKA was selected as the method of PKA inhibition in perifusion, due to the large amounts of inhibitor that would be required for this system. Testing of our transfection efficiency using a CMV-GFP reporter indicated levels as high as 95% by electroporation (data not shown). This suggests that sufficient levels of transfection efficiency could be achieved for DNPKA to effectively inhibit PKA activity. To confirm that inhibition of CREB phosphorylation indeed resulted in impairment of FSHβ transcriptional activation, we extended our study and determined that PKA inhibition by both H89 and the DNPKA reduced GnRH stimulation of FSHβ promoter activity. Although an effect of the DNPKA on GnRH stimulation of LHβ promoter activity was also observed, the effect was modest and a substantial GnRH induction of LHβ remained, in contrast to the complete abrogation of the induction of FSHβ by GnRH. Thus, the role of the PKA pathway appears to be quite highly selective for the induction of FSHβ rather than LHβ by GnRH.
GnRH has been shown to stimulate cAMP accumulation in LβT2 cells (42, 43, 70), as well as increase PKA activity in both primary rat pituitary (71) and LβT2 cells (70). The cAMP/PKA signaling pathway has also been implicated in the GnRH regulation of follistatin, a suppressor of FSHβ transcription, in LβT2 cells treated with GnRH in static culture (72). Increased follistatin expression at high GnRH pulse frequencies has been correlated with reduced FSHβ expression in male rats (49). However, the former study noted that a dominant negative CREB construct reduced GnRH-stimulated follistatin expression in static cultures only, but not in perifused LβT2 cells stimulated with hourly pulses of GnRH, suggesting that the PKA-signaling network may mediate effects of continuous but not pulsatile GnRH on follistatin (72). Therefore the role of the PKA-signaling system in response to GnRH in the gonadotrope may vary not only with the downstream target but also with the pattern of GnRH input.
Oscillatory regulation of biological systems provides a mechanism by which cells can respond differentially to the same ligand, as exemplified by the GnRH pulse frequency-dependent differential effects on FSH and LH production. FSHβ transcription is preferentially stimulated at low rather than high GnRH pulse frequencies, but the signaling pathways leading to these differential effects remain to be elucidated. As already noted, our group has shown that mutations of the CRE site in the FSHβ promoter disrupt the preferential activation of FSHβ by slow GnRH pulse frequencies (17). Given that CREB binds to this site and is phosphorylated to mediate GnRH-stimulated FSHβ transcription through recruitment of CBP, combined with our evidence that CREB phosphorylation by GnRH is mediated by PKA pathways, we hypothesized that the GnRH/GnRHR/PKA/CREB-signaling pathway would be a major contributor to the GnRH pulse frequency-dependent regulation of FSHβ transcription. It appears that CREB phosphorylation itself is regulated by GnRH pulse frequency, with greater phosphorylation at low GnRH pulse frequencies as a result of increased PKA activity, suggesting that CREB phosphorylation is a mediator of the GnRH pulse frequency code. Furthermore, in our established perifusion system (17, 20–22, 31), the preferential stimulation of FSHβ promoter activity and FSHβ mRNA levels at low pulse frequencies was selectively attenuated by PKA inhibition, with no effect on LHβ (Figure 4). These findings demonstrate: 1) the relative specificity of the PKA pathway to the GnRH stimulation of FSHβ transcription; and 2) the role of the PKA pathway in mediating GnRH-stimulated FSHβ transcription at both high and low pulse frequencies.
Given that the PKA pathway appears to play a role in mediating FSHβ stimulation in response to pulsatile GnRH at both low and high frequencies, the question remained as to whether this pathway contributes to the differential GnRH pulse frequency-dependent responses. As noted, we have shown previously that GnRH stimulates CREB phosphorylation in static culture (14). In this report, we have extended this observation by probing the response to pulsatile GnRH after high and low pulse frequency paradigms (Figure 5). Indeed, a greater induction of CREB phosphorylation was observed after stimulation with pulsatile GnRH at the low pulse frequency. Levels of pCREB after stimulation with pulsatile GnRH at the high pulse frequency were increased only modestly compared with controls and did not reach statistical significance, perhaps reflecting limitations in the sensitivity of this assay in the perifusion system. In addition, the inhibition of PKA did not completely abrogate CREB phosphorylation in response to low GnRH pulse frequency. This may reflect incomplete inhibition of PKA by the DNPKA, or alternatively may reflect a contribution of other additional pathways to mediate CREB phosphorylation upon GnRH stimulation at low GnRH pulse frequencies. For example, the CamKII inhibitor, KN-93, did modestly reduce CREB phosphorylation in response to GnRH (Figure 1B), and it has been demonstrated that CamKII has a role in regulating gonadotropin subunit synthesis in response to pulsatile GnRH (27, 28). Nonetheless, these data provide important insights into a potential mechanism by which the gonadotrope can respond differentially to variable GnRH pulse inputs to result in preferential stimulation of FSHβ at low GnRH pulse frequencies.
The preferential phosphorylation of CREB at the low GnRH pulse frequency, combined with evidence that PKA inhibition blocks this preferential induction (Figure 5), led to the hypothesis that stimulation of PKA activity may occur in a GnRH pulse frequency-dependent pattern. Measurement of PKA activity after stimulation with pulsatile GnRH showed that, much like CREB phosphorylation, PKA activity was induced after stimulation by the low GnRH pulse frequency (Figure 6), whereas there was no measurable significant increase in PKA activity after stimulation by high GnRH pulse frequency. In a recent study (70), both cAMP and PKA signaling have been reported after continuous and pulsatile GnRH treatments. Our data confirm that a low GnRH pulse frequency increases PKA activity, although we did not detect a significant increase in PKA activity at a high GnRH pulse frequency compared with control groups, in contrast to Tsutsumi et al. (70) who saw similar induction of PKA at both high and low GnRH pulse frequencies. Although the frequencies of GnRH pulses are comparable between the two studies, the duration of pulsatile GnRH stimulation was 20 hours for our study compared with 4 hours in their study, which may contribute to the differences. Secondary effects may occur that limit the response at high GnRH pulse frequencies in our longer duration experimental paradigm.
Several other kinase pathways have been implicated in the regulation of gonadotropin subunit gene transcription and LH and FSH secretion by GnRH. PKC isoforms activated in response to GnRH mediate the activation of several MAPK-signaling pathways, including ERK, jun N-terminal kinase, and p38 (42, 73). CamKI and CamKII activation by GnRH and subsequent Ca2+ mobilization has also been shown to stimulate gonadotropin subunit expression (27, 28). Two recent studies examined the role of ERK (35) and calcium (74) signaling in decoding GnRH pulse frequency. Notably, these studies demonstrate that the ERK and Ca2+/calmodulin/calcineurin-signaling pathways contribute to the control of gonadotropin synthesis by pulsatile GnRH and their relative contributions to regulation of FSHβ and LHβ transcription are distinct, yet neither alone is responsible for decoding the pulsatile signal (35, 74). However, mathematical modeling analyses carried out by the same group suggest that these pathways could converge upon stimulation by pulsatile GnRH to provide a potential mechanism by which the gonadotrope may decode the pulse signal (75, 76). Our data implicate PKA in the pathway by which GnRH regulates FSHβ transcription in the LβT2 cell model, as demonstrated by effects on both a rat FSHβLUC reporter and endogenous mouse FSHβ mRNA levels. In contrast, the PKA pathway did not play a major role in the regulation of rat LHβLUC activity and endogenous mouse LHβ mRNA levels, suggesting that this pathway is relatively specific for the regulation of FSHβ by both continuous and pulsatile GnRH.
These studies were carried out using the murine gonadotrope-derived LβT2 cell line, an in vitro model used extensively to investigate GnRH signaling and gonadotropin gene transcription. Nonetheless, all studies using immortalized cell lines ultimately need to be validated in a more physiologic context, including primary mouse gonadotrope cells and murine animal models, in an effort to further correlate these data with the regulation of reproductive function in the human hypothalamic-pituitary-gonadal axis.
Taken together, the data presented in this study implicate PKA as an important mediator of GnRH stimulation of FSHβ, but not LHβ, transcription in LβT2 cells. PKA activity is preferentially stimulated by pulsatile GnRH at low pulse frequencies, leading to preferential CREB phosphorylation and a correspondingly greater increase in FSHβ transcription. These findings suggest that PKA-signaling pathways play a key role in decoding pulsatile GnRH inputs.
Acknowledgments
We thank Professor Pamela L. Mellon (University of California, San Diego, California) for the kind gift of the LβT2 cell line, as well as Professor G. Stanley McKnight (University of Washington, Seattle, Washington) for the generous donation of the DNPKA and corresponding empty vector constructs. We also thank Dr. Yujiang Shi (Brigham and Women's Hospital, Boston, Massachusetts) for invaluable contributions throughout laboratory meeting discussions and Mr. Ahmad Khogeer for maintaining cell cultures and assisting with the assays described.
This work was supported by NIH grants R01 HD33001 and HD19938 (to U.B.K.) and British Endocrine Society Early Career Grant (to I.R.T.).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- CamK
- calmodulin-dependent protein kinase
- CMV
- cytomegalovirus
- CRE
- cAMP response element
- CREB
- CRE-binding protein
- DNPKA
- dominant negative PKA
- FBS
- fetal bovine serum
- GFP
- green fluorescent protein
- αGSU
- α-glycoprotein subunit
- MEK
- MAPK kinase
- pCREB
- CREB phosphorylation
- PKA
- protein kinase A
- PKC
- protein kinase C
- TBST
- Tris-buffered saline plus Tween 20.
References
- 1. Burger LL, Haisenleder DJ, Dalkin AC, Marshall JC. Regulation of gonadotropin subunit gene transcription. J Mol Endocrinol. 2004;33:559–584 [DOI] [PubMed] [Google Scholar]
- 2. Seminara SB, Hayes FJ, Crowley WF., Jr Gonadotropin-releasing hormone deficiency in the human (idiopathic hypogonadotropic hypogonadism and Kallmann's syndrome): pathophysiological and genetic considerations. Endocr Rev. 1998;19:521–539 [DOI] [PubMed] [Google Scholar]
- 3. Ehrmann DA. Polycystic ovary syndrome. N Engl J Med. 2005;352:1223–1236 [DOI] [PubMed] [Google Scholar]
- 4. Blank SK, McCartney CR, Helm KD, Marshall JC. Neuroendocrine effects of androgens in adult polycystic ovary syndrome and female puberty. Semin Reprod Med. 2007;25:352–359 [DOI] [PubMed] [Google Scholar]
- 5. Dunaif A. Insulin resistance and the polycystic ovary syndrome: mechanism and implications for pathogenesis. Endocr Rev. 1997;18:774–800 [DOI] [PubMed] [Google Scholar]
- 6. Hoffman LK, Ehrmann DA. Cardiometabolic features of polycystic ovary syndrome. Nat Clin Pract Endocrinol Metab. 2008;4:215–222 [DOI] [PubMed] [Google Scholar]
- 7. Gharib SD, Wierman ME, Shupnik MA, Chin WW. Molecular biology of the pituitary gonadotropins. Endocr Rev. 1990;11:177–199 [DOI] [PubMed] [Google Scholar]
- 8. Wildt L, Hausler A, Marshall G, et al. Frequency and amplitude of gonadotropin-releasing hormone stimulation and gonadotropin secretion in the rhesus monkey. Endocrinology. 1981;109:376–385 [DOI] [PubMed] [Google Scholar]
- 9. Savoy-Moore RT, Swartz KH. Several GnRH stimulation frequencies differentially release FSH and LH from isolated, perfused rat anterior pituitary cells. Adv Exp Med Biol. 1987;219:641–645 [DOI] [PubMed] [Google Scholar]
- 10. Dalkin AC, Haisenleder DJ, Ortolano GA, Ellis TR, Marshall JC. The frequency of gonadotropin-releasing-hormone stimulation differentially regulates gonadotropin subunit messenger ribonucleic acid expression. Endocrinology. 1989;125:917–924 [DOI] [PubMed] [Google Scholar]
- 11. Haisenleder DJ, Dalkin AC, Ortolano GA, Marshall JC, Shupnik MA. A pulsatile gonadotropin-releasing hormone stimulus is required to increase transcription of the gonadotropin subunit genes: evidence for differential regulation of transcription by pulse frequency in vivo. Endocrinology. 1991;128:509–517 [DOI] [PubMed] [Google Scholar]
- 12. Weiss J, Duca KA, Crowley WF., Jr Gonadotropin-releasing hormone-induced stimulation and desensitization of free α-subunit secretion mirrors luteinizing hormone and follicle-stimulating hormone in perifused rat pituitary cells. Endocrinology 1990;127:2364–2371 [DOI] [PubMed] [Google Scholar]
- 13. Landy H, Boepple PA, Mansfield MJ, et al. Altered patterns of pituitary secretion and renal excretion of free α-subunit during gonadotropin-releasing hormone agonist-induced pituitary desensitization. J Clin Endocrinol Metab 1991;72:711–717 [DOI] [PubMed] [Google Scholar]
- 14. Ciccone NA, Lacza CT, Hou MY, et al. A composite element that binds basic helix loop helix and basic leucine zipper transcription factors is important for gonadotropin-releasing hormone regulation of the follicle-stimulating hormone β gene. Mol Endocrinol. 2008;22:1908–1923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Coss D, Jacobs SB, Bender CE, Mellon PL. A novel AP-1 site is critical for maximal induction of the follicle-stimulating hormone β gene by gonadotropin-releasing hormone. J Biol Chem. 2004;279:152–162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wang Y, Fortin J, Lamba P, et al. Activator protein-1 and smad proteins synergistically regulate human follicle-stimulating hormone β-promoter activity. Endocrinology. 2008;149:5577–5591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ciccone NA, Xu S, Lacza CT, Carroll RS, Kaiser UB. Frequency-dependent regulation of follicle-stimulating hormone β by pulsatile gonadotropin-releasing hormone is mediated by functional antagonism of bZIP transcription factors. Mol Cell Biol. 2010;30:1028–1040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zakaria MM, Jeong KH, Lacza C, Kaiser UB. Pituitary homeobox 1 activates the rat FSHβ (rFSHβ) gene through both direct and indirect interactions with the rFSHβ gene promoter. Mol Endocrinol. 2002;16:1840–1852 [DOI] [PubMed] [Google Scholar]
- 19. Kaiser UB, Sabbagh E, Katzenellenbogen RA, Conn PM, Chin WW. A mechanism for the differential regulation of gonadotropin subunit gene expression by gonadotropin-releasing hormone. Proc Natl Acad Sci USA. 1995;92:12280–12284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kaiser UB, Jakubowiak A, Steinberger A, Chin WW. Regulation of rat pituitary gonadotropin-releasing hormone receptor mRNA levels in vivo and in vitro. Endocrinology. 1993;133:931–934 [DOI] [PubMed] [Google Scholar]
- 21. Kaiser UB, Jakubowiak A, Steinberger A, Chin WW. Differential effects of gonadotropin-releasing hormone (GnRH) pulse frequency on gonadotropin subunit and GnRH receptor messenger ribonucleic acid levels in vitro. Endocrinology. 1997;138:1224–1231 [DOI] [PubMed] [Google Scholar]
- 22. Bedecarrats GY, Kaiser UB. Differential regulation of gonadotropin subunit gene promoter activity by pulsatile gonadotropin-releasing hormone (GnRH) in perifused L β T2 cells: role of GnRH receptor concentration. Endocrinology. 2003;144:1802–1811 [DOI] [PubMed] [Google Scholar]
- 23. Coss D, Hand CM, Yaphockun KK, Ely HA, Mellon PL. p38 mitogen-activated protein kinase is critical for synergistic induction of the FSH(β) gene by gonadotropin-releasing hormone and activin through augmentation of c-Fos induction and Smad phosphorylation. Mol Endocrinol. 2007;21:3071–3086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Do MH, Santos SJ, Lawson MA. GNRH induces the unfolded protein response in the LβT2 pituitary gonadotrope cell line. Mol Endocrinol. 2009;23:100–112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zhang H, Bailey JS, Coss D, et al. Activin modulates the transcriptional response of LβT2 cells to gonadotropin-releasing hormone and alters cellular proliferation. Mol Endocrinol. 2006;20:2909–2930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Jakubowiak A, Janecki A, Steinberger A. Similar effects of inhibin and cycloheximide on gonadotropin release in superfused pituitary cell cultures. Biol Reprod. 1989;41:454–463 [DOI] [PubMed] [Google Scholar]
- 27. Haisenleder DJ, Burger LL, Aylor KW, Dalkin AC, Marshall JC. Gonadotropin-releasing hormone stimulation of gonadotropin subunit transcription: evidence for the involvement of calcium/calmodulin-dependent kinase II (Ca/CAMK II) activation in rat pituitaries. Endocrinology. 2003;144:2768–2774 [DOI] [PubMed] [Google Scholar]
- 28. Haisenleder DJ, Ferris HA, Shupnik MA. The calcium component of gonadotropin-releasing hormone-stimulated luteinizing hormone subunit gene transcription is mediated by calcium/calmodulin-dependent protein kinase type II. Endocrinology. 2003;144:2409–2416 [DOI] [PubMed] [Google Scholar]
- 29. Duan WR, Ito M, Park Y, Maizels ET, Hunzicker-Dunn M, Jameson JL. GnRH regulates early growth response protein 1 transcription through multiple promoter elements. Mol Endocrinol. 2002;16:221–233 [DOI] [PubMed] [Google Scholar]
- 30. Grafer CM, Thomas R, Lambrakos L, Montoya I, White S, Halvorson LM. GnRH stimulates expression of PACAP in the pituitary gonadotropes via both the PKA and PKC signaling systems. Mol Endocrinol. 2009;23:1022–1032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kanasaki H, Bedecarrats GY, Kam KY, Xu S, Kaiser UB. Gonadotropin-releasing hormone pulse frequency-dependent activation of extracellular signal-regulated kinase pathways in perifused LβT2 cells. Endocrinology. 2005;146:5503–5513 [DOI] [PubMed] [Google Scholar]
- 32. Call GB, Wolfe MW. Gonadotropin-releasing hormone activates the equine luteinizing hormone β promoter through a protein kinase C/mitogen-activated protein kinase pathway. Biol Reprod. 1999;61:715–723 [DOI] [PubMed] [Google Scholar]
- 33. Yokoi T, Ohmichi M, Tasaka K, et al. Activation of the luteinizing hormone β promoter by gonadotropin-releasing hormone requires c-Jun NH2-terminal protein kinase. J Biol Chem. 2000;275:21639–21647 [DOI] [PubMed] [Google Scholar]
- 34. Mulvaney JM, Zhang T, Fewtrell C, Roberson MS. Calcium influx through L-type channels is required for selective activation of extracellular signal-regulated kinase by gonadotropin-releasing hormone. J Biol Chem. 1999;274:29796–29804 [DOI] [PubMed] [Google Scholar]
- 35. Armstrong SP, Caunt CJ, Fowkes RC, Tsaneva-Atanasova K, McArdle CA. Pulsatile and sustained gonadotropin-releasing hormone (GnRH) receptor signaling: does the ERK signaling pathway decode GnRH pulse frequency? J Biol Chem. 2010;285:24360–24371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Naor Z, Harris D, Shacham S. Mechanism of GnRH receptor signaling: combinatorial cross-talk of Ca2+ and protein kinase C. Front Neuroendocrinol. 1998;19:1–19 [DOI] [PubMed] [Google Scholar]
- 37. Halvorson LM, Kaiser UB, Chin WW. The protein kinase C system acts through the early growth response protein 1 to increase LHβ gene expression in synergy with steroidogenic factor-1. Mol Endocrinol. 1999;13:106–116 [DOI] [PubMed] [Google Scholar]
- 38. Klausen C, Booth M, Habibi HR, Chang JP. Extracellular signal-regulated kinase mediates gonadotropin subunit gene expression and LH release responses to endogenous gonadotropin-releasing hormones in goldfish. Gen Comp Endocrinol. 2008;158:36–46 [DOI] [PubMed] [Google Scholar]
- 39. Weck J, Fallest PC, Pitt LK, Shupnik MA. Differential gonadotropin-releasing hormone stimulation of rat luteinizing hormone subunit gene transcription by calcium influx and mitogen-activated protein kinase-signaling pathways. Mol Endocrinol. 1998;12:451–457 [DOI] [PubMed] [Google Scholar]
- 40. Duan WR, Shin JL, Jameson JL. Estradiol suppresses phosphorylation of cyclic adenosine 3′,5′-monophosphate response element binding protein (CREB) in the pituitary: evidence for indirect action via gonadotropin-releasing hormone. Mol Endocrinol. 1999;13:1338–1352 [DOI] [PubMed] [Google Scholar]
- 41. Woodford TA, Correll LA, McKnight GS, Corbin JD. Expression and characterization of mutant forms of the type I regulatory subunit of cAMP-dependent protein kinase. The effect of defective cAMP binding on holoenzyme activation. J Biol Chem. 1989;264:13321–13328 [PubMed] [Google Scholar]
- 42. Liu F, Usui I, Evans LG, et al. Involvement of both G(q/11) and G(s) proteins in gonadotropin-releasing hormone receptor-mediated signaling in L β T2 cells. J Biol Chem. 2002;277:32099–32108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Lariviere S, Garrel G, Simon V, et al. Gonadotropin-releasing hormone couples to 3′,5′-cyclic adenosine-5′-monophosphate pathway through novel protein kinase Cdelta and -epsilon in LβT2 gonadotrope cells. Endocrinology. 2007;148:1099–1107 [DOI] [PubMed] [Google Scholar]
- 44. Garrel G, McArdle CA, Hemmings BA, Counis R. Gonadotropin-releasing hormone and pituitary adenylate cyclase-activating polypeptide affect levels of cyclic adenosine 3′,5′-monophosphate-dependent protein kinase A (PKA) subunits in the clonal gonadotrope αT3–1 cells: evidence for cross-talk between PKA and protein kinase C pathways. Endocrinology. 1997;138:2259–2266 [DOI] [PubMed] [Google Scholar]
- 45. Borgeat P, Chavancy G, Dupont A, Labrie F, Arimura A, Schally AV. Stimulation of adenosine 3′:5′-cyclic monophosphate accumulation in anterior pituitary gland in vitro by synthetic luteinizing hormone-releasing hormone. Proc Natl Acad Sci USA. 1972;69:2677–2681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Burger LL, Haisenleder DJ, Aylor KW, Marshall JC. Regulation of intracellular signaling cascades by GNRH pulse frequency in the rat pituitary: roles for CaMK II, ERK, and JNK activation. Biol Reprod. 2008;79:947–953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Burger LL, Haisenleder DJ, Aylor KW, Marshall JC. Regulation of Lhb and Egr1 gene expression by GNRH pulses in rat pituitaries is both c-Jun N-terminal kinase (JNK)- and extracellular signal-regulated kinase (ERK)-dependent. Biol Reprod. 2009;81:1206–1215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Haisenleder DJ, Workman LJ, Burger LL, Aylor KW, Dalkin AC, Marshall JC. Gonadotropin subunit transcriptional responses to calcium signals in the rat: evidence for regulation by pulse frequency. Biol Reprod. 2001;65:1789–1793 [DOI] [PubMed] [Google Scholar]
- 49. Burger LL, Dalkin AC, Aylor KW, Haisenleder DJ, Marshall JC. GnRH pulse frequency modulation of gonadotropin subunit gene transcription in normal gonadotropes-assessment by primary transcript assay provides evidence for roles of GnRH and follistatin. Endocrinology. 2002;143:3243–3249 [DOI] [PubMed] [Google Scholar]
- 50. Cattanach BM, Iddon CA, Charlton HM, Chiappa SA, Fink G. Gonadotrophin-releasing hormone deficiency in a mutant mouse with hypogonadism. Nature. 1977;269:338–340 [DOI] [PubMed] [Google Scholar]
- 51. Charlton HM, Halpin DM, Iddon C, et al. The effects of daily administration of single and multiple injections of gonadotropin-releasing hormone on pituitary and gonadal function in the hypogonadal (hpg) mouse. Endocrinology. 1983;113:535–544 [DOI] [PubMed] [Google Scholar]
- 52. Alarid ET, Windle JJ, Whyte DB, Mellon PL. Immortalization of pituitary cells at discrete stages of development by directed oncogenesis in transgenic mice. Development. 1996;122:3319–3329 [DOI] [PubMed] [Google Scholar]
- 53. Windle JJ, Weiner RI, Mellon PL. Cell lines of the pituitary gonadotrope lineage derived by targeted oncogenesis in transgenic mice. Mol Endocrinol. 1990;4:597–603 [DOI] [PubMed] [Google Scholar]
- 54. Harris D, Chuderland D, Bonfil D, Kraus S, Seger R, Naor Z. Extracellular signal-regulated kinase and c-Src, but not Jun N-terminal kinase, are involved in basal and gonadotropin-releasing hormone-stimulated activity of the glycoprotein hormone α-subunit promoter. Endocrinology. 2003;144:612–622 [DOI] [PubMed] [Google Scholar]
- 55. Liu F, Austin DA, Mellon PL, Olefsky JM, Webster NJ. GnRH activates ERK1/2 leading to the induction of c-fos and LHβ protein expression in LβT2 cells. Mol Endocrinol. 2002;16:419–434 [DOI] [PubMed] [Google Scholar]
- 56. Stojilkovic SS, Catt KJ. Expression and signal transduction pathways of gonadotropin-releasing hormone receptors. Recent Prog Horm Res. 1995;50:161–205 [DOI] [PubMed] [Google Scholar]
- 57. Stojilkovic SS, Catt KJ. Novel aspects of GnRH-induced intracellular signaling and secretion in pituitary gonadotrophs. J Neuroendocrinol. 1995;7:739–757 [DOI] [PubMed] [Google Scholar]
- 58. Ando H, Hew CL, Urano A. Signal transduction pathways and transcription factors involved in the gonadotropin-releasing hormone-stimulated gonadotropin subunit gene expression. Comp Biochem Physiol B Biochem Mol Biol. 2001;129:525–532 [DOI] [PubMed] [Google Scholar]
- 59. Lawson MA, Tsutsumi R, Zhang H, et al. Pulse sensitivity of the luteinizing hormone β promoter is determined by a negative feedback loop Involving early growth response-1 and Ngfi-A binding protein 1 and 2. Mol Endocrinol. 2007;21:1175–1191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Mistry DS, Tsutsumi R, Fernandez M, et al. Gonadotropin-releasing hormone pulse sensitivity of follicle-stimulating hormone-β gene is mediated by differential expression of positive regulatory activator protein 1 factors and corepressors SKIL and TGIF1. Mol Endocrinol. 2011;25:1387–1403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Haisenleder DJ, Burger LL, Walsh HE, et al. Pulsatile gonadotropin-releasing hormone stimulation of gonadotropin subunit transcription in rat pituitaries: evidence for the involvement of Jun N-terminal kinase but not p38. Endocrinology. 2008;149:139–145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Kwok RP, Lundblad JR, Chrivia JC, et al. Nuclear protein CBP is a coactivator for the transcription factor CREB. Nature. 1994;370:223–226 [DOI] [PubMed] [Google Scholar]
- 63. Halvorson LM, Ito M, Jameson JL, Chin WW. Steroidogenic factor-1 and early growth response protein 1 act through two composite DNA binding sites to regulate luteinizing hormone β-subunit gene expression. J Biol Chem. 1998;273:14712–14720 [DOI] [PubMed] [Google Scholar]
- 64. Kaiser UB, Halvorson LM, Chen MT. Sp1, steroidogenic factor 1 (SF-1), and early growth response protein 1 (egr-1) binding sites form a tripartite gonadotropin-releasing hormone response element in the rat luteinizing hormone-β gene promoter: an integral role for SF-1. Mol Endocrinol. 2000;14:1235–1245 [DOI] [PubMed] [Google Scholar]
- 65. Tremblay JJ, Drouin J. Egr-1 is a downstream effector of GnRH and synergizes by direct interaction with Ptx1 and SF-1 to enhance luteinizing hormone β gene transcription. Mol Cell Biol. 1999;19:2567–2576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Dorn C, Ou Q, Svaren J, Crawford PA, Sadovsky Y. Activation of luteinizing hormone β gene by gonadotropin-releasing hormone requires the synergy of early growth response-1 and steroidogenic factor-1. J Biol Chem. 1999;274:13870–13876 [DOI] [PubMed] [Google Scholar]
- 67. Wolfe MW, Call GB. Early growth response protein 1 binds to the luteinizing hormone-β promoter and mediates gonadotropin-releasing hormone-stimulated gene expression. Mol Endocrinol. 1999;13:752–763 [DOI] [PubMed] [Google Scholar]
- 68. Lee SL, Sadovsky Y, Swirnoff AH, et al. Luteinizing hormone deficiency and female infertility in mice lacking the transcription factor NGFI-A (Egr-1). Science. 1996;273:1219–1221 [DOI] [PubMed] [Google Scholar]
- 69. Topilko P, Schneider-Maunoury S, Levi G, et al. Multiple pituitary and ovarian defects in Krox-24 (NGFI-A, Egr-1)-targeted mice. Mol Endocrinol. 1998;12:107–122 [DOI] [PubMed] [Google Scholar]
- 70. Tsutsumi R, Mistry D, Webster NJ. Signaling responses to pulsatile gonadotropin-releasing hormone in LβT2 gonadotrope cells. J Biol Chem. 2010;285:20262–20272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Garrel G, Simon V, Thieulant ML, et al. Sustained gonadotropin-releasing hormone stimulation mobilizes the cAMP/PKA pathway to induce nitric oxide synthase type 1 expression in rat pituitary cells in vitro and in vivo at proestrus. Biol Reprod. 2010;82:1170–1179 [DOI] [PubMed] [Google Scholar]
- 72. Winters SJ, Ghooray D, Fujii Y, Moore JP, Jr., Nevitt JR, Kakar SS. Transcriptional regulation of follistatin expression by GnRH in mouse gonadotroph cell lines: evidence for a role for cAMP signaling. Mol Cell Endocrinol. 2007;271:45–54 [DOI] [PubMed] [Google Scholar]
- 73. Mulvaney JM, Roberson MS. Divergent signaling pathways requiring discrete calcium signals mediate concurrent activation of two mitogen-activated protein kinases by gonadotropin-releasing hormone. J Biol Chem. 2000;275:14182–14189 [DOI] [PubMed] [Google Scholar]
- 74. Armstrong SP, Caunt CJ, Fowkes RC, Tsaneva-Atanasova K, McArdle CA. Pulsatile and sustained gonadotropin-releasing hormone (GnRH) receptor signaling: does the Ca2+/NFAT signaling pathway decode GnRH pulse frequency? J Biol Chem. 2009;284:35746–35757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Tsaneva-Atanasova K, Caunt CJ, Armstrong SP, Perrett RM, McArdle CA. Decoding neurohormone pulse frequency by convergent signalling modules. Biochem Soc Trans. 2012;40:273–278 [DOI] [PubMed] [Google Scholar]
- 76. Tsaneva-Atanasova K, Mina P, Caunt CJ, Armstrong SP, McArdle CA. Decoding GnRH neurohormone pulse frequency by convergent signalling modules. J R Soc Interface /R Soc. 2012;9:170–182 [DOI] [PMC free article] [PubMed] [Google Scholar]