Abstract
Purpose
To quantify the effects of barrier precautions and antibiotic mixing on prevalence and acquisition of five drug-resistant microorganisms within a single tetanus intensive care unit at a tertiary referral hospital in Ho Chi Minh City, Vietnam.
Methods
All patients admitted within the study period were included. After a 1-year baseline period, barrier precautions were implemented and the single empirical treatment ceftazidime was changed to mixing (per consecutive patient) of three different regimens (ceftazidime, ciprofloxacin, piperacillin–tazobactam). Markov chain modeling and genotyping were used to determine the effects of interventions on prevalence levels and the relative importance of cross-transmission and antibiotic-associated selection.
Results
A total of 190 patients were included in year 1 (2,708 patient days, 17,260 cultures) and 167 patients in year 2 (3,384 patient days, 20,580 cultures). In year 1, average daily prevalence rates for methicillin-resistant Staphylococcus aureus (MRSA), extended spectrum beta-lactamase (ESBL)-producing Enterobacteriaceae (excluding Klebsiella pneumoniae), Pseudomonas aeruginosa, gentamicin-resistant K. pneumoniae, and amikacin-resistant Acinetobacter species were 34.0, 61.3, 53.4, 65.7 and 57.1 %. After intervention, ceftazidime usage decreased by 53 %; the use of piperacillin–tazobactam and ciprofloxacin increased 7.2-fold and 4.5-fold, respectively. Adherence to hand hygiene after patient contact was 54 %. These measures were associated with a reduction of MRSA prevalence by 69.8 % (to 10.3 %), mainly because of less cross-transmission (88 % reduction), and of ESBL-producing Enterobacteriaceae prevalence by 10.3 % (non-significantly). In contrast, prevalence levels of the other three pathogens remained unaffected.
Conclusion
The combination of simple infection control measures and antibiotic mixing was highly effective in reducing the prevalence of MRSA, but not of Gram-negative microorganisms.
Electronic supplementary material
The online version of this article (doi:10.1007/s00134-012-2771-1) contains supplementary material.
Keywords: Antimicrobial drug resistance, Intensive care, Mathematical modeling, MRSA, ESBL, Vietnam
Introduction
Nosocomial infections and health-care-associated transmission of pathogens are increasingly recognized as challenges to health care and public health in developing countries [1–3]. Although hospital infection control activities have increased [4], many countries struggle to implement control policies [5]. Nevertheless, with increased availability of intensive care unit (ICU) facilities, infection control is becoming increasingly important [6].
Nosocomial infection is usually preceded by colonization of the patient with the infecting microorganism. As only a minority of the colonized patients will develop overt infections, colonization remains unnoticed in most cases. Patients are considered to be the major exogenous source of microorganisms, with patient to patient transmission occurring through transiently colonized hands of health-care workers. Exogenous colonization typically is affected by infection control measures such as barrier precautions and hand disinfection. Colonization is considered to have originated from an endogenous source when microorganisms that are present but undetectable on admission are allowed to grow above a certain detection limit as a result of selective growth advantages during antimicrobial therapy [7].
Assessing the acquisition routes of microorganisms is essential for the design of appropriate infection control measures. However, determination of the transmission route requires microbiological surveillance, including genotyping of isolates, which is costly and laborious and requires technical expertise. In addition, application of costly infection control measures may not be feasible due to limited resources.
We performed a prospective study to determine the predominant routes of acquisition of five prevalent nosocomial pathogens and to determine the effects of infection control measures on the respective rates and routes of acquisition, after a 1-year baseline surveillance period, in a dedicated tetanus ICU of the Hospital for Tropical Diseases (HTD) in Ho Chi Minh City (HCMC), Vietnam. To circumvent the well-known methodological pitfalls associated with such a quasi-experimental approach executed on a ward with a relatively limited number of patients [8], we used a mathematical modeling approach to assess the relative importance of infection routes [9] and validated the results of the model by genotyping of cultured microorganisms.
Materials and methods
Study setting
The HTD is a 500-bed infectious diseases hospital. The hospital has a 15-bed ward dedicated to tetanus patients, admitting approximately 200 patients each year. The ward consists of a single large room. Facilities for isolation of patients are not available.
One nurse is responsible for the care of two patients and on average three nursing aids are present to clean the ward and to assist nurses with procedures such as washing of patients. Hand washing facilities consist of four sinks on the ward and hand rub alcohol dispensers at the end of each bed. Gloves are only used when contact with secretions is likely to occur, e.g., during cleaning of dirty beds. A single glove is used during suction of a tracheostoma. Gowns are not available and masks are not used. The ward is cleaned twice daily by mopping the floor and disinfection of horizontal surfaces using a quaternary ammonium compound solution (HexaniosR).
Tetanus treatment includes wound cleaning and administration of intravenous penicillin or oral metronidazole, equine antitoxin, and benzodiazepines. Tracheostomy is performed in case of acute airway obstruction, or to facilitate mechanical ventilation. Pneumonia, as diagnosed on the basis of increased oxygen requirements, fever, new chest X-ray abnormalities, and elevated white cell count, is treated with empirical antimicrobial therapy. Therapy is adjusted on an individual patient basis if required, following semi-quantitative culture of tracheobronchial aspirates and sensitivity testing of isolated microorganisms.
Study design
This study was designed as a prospective before–after study over 2 years, with a baseline surveillance period from 1 May 2004 until 30 April 2005 and an intervention period from 1 May 2005 until 30 April 2006. While the options for infection control measures were discussed in the design phase of the study, the actual measures to implement were decided upon during the second half of the baseline period, when the surveillance and modeling results of the first half year were available. The study was approved by the Scientific and Ethical Committee of the HTD who waived the need for informed consent.
Patient characteristics and clinical data
All patients admitted to the tetanus ICU were eligible for study inclusion. Demographic data, admission data including prior hospitalization and tetanus severity score [10], and clinical data, including the use of devices (tracheostoma, urinary catheter, central venous line) and the presence of nosocomial infections, were recorded for all patients in the medical file and on study record forms.
Microbiological investigations
Surveillance cultures were taken from all patients on admission and then twice weekly on Monday and Thursday. Swab samples were taken from anterior nares, sputum or tracheostomy site or interior canula, axilla, and anus and all were inoculated onto selective agars for detection of Pseudomonas aeruginosa (all), gentamicin-resistant Klebsiella pneumoniae (GRKpn), amikacin-resistant Acinetobacter spp. (ARAc), Enterobacteriaceae producing extended spectrum beta-lactamases (ESBL), excluding K. pneumoniae (ESBLE), and methicillin-resistant Staphylococcus aureus (MRSA) (Online Resource 1). K. pneumoniae were excluded from the surveillance of ESBLE, because GRKpn, which are often also ESBL-positive, were reported frequently in diagnostic broncheal aspirate cultures. To obtain better insight into the acquisition routes of these isolates, GRKpn were studied separate from other ESBLE.
Genotyping of isolates was performed after completion of the study (Online Resource 1).
Infection control measures
On the basis of the surveillance data obtained during the first study year, a set of infection control measures, which were considered rational, feasible, and affordable, was implemented (Table 1).
Table 1.
Infection control measures implemented at the start of study year 2
| Target | Infection control measures |
|---|---|
| Exogenous acquisition of microorganisms |
Reinforcement of hand hygiene Limit exchange of equipment, materials, and staff between patients by allocating dedicated materials to individual patients at bedside Revise washing procedures: allocate wash bowls to individual patients Measure compliance by direct observations |
| Exogenous acquisition of MRSA |
Reporting of results of surveillance cultures Sign indicating positivity at the bedside |
| Endogenous acquisition of ESBL-positive Enterobacteriaceae | Change in empirical antimicrobial therapy resulting in three regimens applied simultaneously (“antibiotic mixing”) |
Measures were targeted at reducing colonization by exogenous or endogenous acquisition. The target of each (set of) measure(s) is indicated
To prevent exogenous transmission of microorganisms, exchange of equipment, materials, and staff between patients was limited by reallocation of equipment and materials, and procedures for washing of patients were revised to include disinfection of washing bowls after each patient. Appropriate use of gloves and hand washing or disinfection before and after caring for a patient was reinforced. Teaching sessions were given by the nurse supervisor and medical staff to all doctors, nurses, and nurse aids in May and June 2005. Compliance with the new measures was assessed by direct observations, recorded on data forms, by the nurse supervisor and one of the staff doctors in August 2005 and February 2006. Other staff were unaware of these assessments. The results were reported to all staff and additional teaching sessions were given in September 2005.
At the request of the clinicians, results of MRSA surveillance cultures were reported to the ward, but not for the other microorganisms studied. MRSA-positive patients had a sign displayed on their bed.
To prevent colonization with ESBLE, the empirical antimicrobial therapy for suspected pneumonia and sepsis was changed to include multiple classes of antimicrobial drugs which were available in Vietnam, to reduce selective pressure of a single antimicrobial drug regimen. Empirical treatment consisted of ceftazidime with amikacin in the first year, which was changed to a per patient rotational regimen consisting of ceftazidime, or ciprofloxacin, or piperacillin–tazobactam, all in combination with amikacin, because dual empirical treatment was preferred by the treating physicians. Imipenem was used for second-line therapy only. Antimicrobial therapy was recorded for all patients during the study period.
Daily ward rounds continued during the first and second study year without changes. There were no major changes in staff numbers in the study period.
Data analysis
Because of the typically small patient populations in ICUs (usually less than 20) and the rapid patient turnover, large fluctuations in proportions of colonized patients occur naturally. In addition, the dependence created by cross-transmission of microorganisms between patients (the risk of acquisition is influenced by the colonization status of other patients) precludes comparison of colonization rates before and after application of infection control measures using statistical methods that assume that cross-transmission events occur independently (e.g., χ 2 test) [11]. In order to avoid these pitfalls in statistical analysis, we used an algorithm based on a Markov model [9] (Online Resource 2). On the basis of days of admission and discharge, days on which cultures are taken, and results of these surveillance cultures, maximum likelihood estimators (MLEs) and confidence intervals of the acquisition parameters for endogenous (α) and exogenous (β) acquisition are calculated. In addition, the daily prevalence, number of acquisitions, and fraction of acquisitions ascribed to each acquisition route can be estimated. A likelihood ratio test (χ 2 test) was used to test whether the acquisition parameters were significantly different between both periods (Online Resource 2). We compared the results of modeling with the results obtained using conventional analysis methods. The time to acquisition of each of the five pathogens was compared for all patients admitted in the first (before intervention) and second study year by constructing multivariable Cox regression models adjusted for individual patient characteristics (Online Resource 2).
Categorical values were compared using χ 2 test. Continuous variables were expressed as medians (range) and compared using Wilcoxon-rank sum test. For calculation of infection rates, patients admitted in the first study year but discharged in the second study year were included in the first year analysis. Infection rates were calculated only for patients whose full admission period was within the study period. Infections were considered hospital-acquired if they occurred more than 2 days after admission to the ICU. Device days at risk were calculated by adding the device days until the first infection or total number of device days if no infection occurred.
Results
All 190 patients with tetanus were included in the baseline surveillance period (year 1) and 167 patients in the intervention period (year 2) with a total of 2,708 patient days in year 1 and 3,384 in year 2. A total of 174 patients in year 1 (2,527 patient days) and 161 patients in year 2 (3,235 patient days) had the complete admission within the study period. Characteristics of these patients on admission and during treatment are presented in Table 2. A total of 863 culture sets were obtained in year 1 and 1,029 in year 2. Forty-one culture sets (4.5 %) were missing and 18 cultures failed for technical reasons in year 1; 74 culture sets (6.7 %) were missing and 6 cultures failed in year 2.
Table 2.
Patient characteristics on admission and during intensive care stay, in year 1 (baseline) and year 2 (infection control measures implemented) of study (includes only patients with admission and discharge within total study period)
| Characteristica | Year 1 | Year 2 | P valueb |
|---|---|---|---|
| Age, years (excluding neonates) | |||
| No. of patientsc | 167 | 150 | |
| Median | 38 | 42 | 0.23 |
| Range | 5–88 | 2–84 | |
| Neonates no. | 5 (2.9) | 5 (3.1) | |
| Male sex—no./total no. (%)c | 124/174 (71.3) | 120/157 (76.4) | |
| Admission from other hospital—no./total no. (%) | 149/174 (85.6) | 137/157 (87.3) | 0.67 |
| >48 h in other hospital—no./total no. (%)c | 27/144 (18.8) | 26/128 (20.3) | |
| Tetanus Severity Scored | |||
| No. of patientsc | 166 | 151 | |
| Median | 0.0 | 0.0 | 0.007 |
| Range | −6 to 17 | −6 to 20 | |
| Duration of stay (days) | |||
| No. of patients | 174 | 161 | |
| Median | 12 | 17 | 0.004 |
| Range | 1–66 | 1–148 | |
| No. of patient days | 2,527 | 3,235 | |
| Patients with tracheostomy—no./total no. (%) | 83/174 (47.7) | 97/159 (61.0) | 0.015 |
| Total no. of tracheostomy days | 1,651 | 2,350 | |
| No. of patients | 83 | 97 | |
| Median | 20 | 21 | 0.25 |
| Range | 1–57 | 1–145 | |
| Patients ventilated—no./total no. (%) | 71/174 (40.8) | 97/159 (61.0) | 0.0002 |
| Total no. of ventilation days | 1,089 | 1,713 | |
| No. of patientsc | 70 | 96 | |
| Median | 16 | 18 | 0.36 |
| Range | 1–41 | 1–88 | |
| No. nosocomial pneumonia/1,000 ventilation days at risk | 56 | 40 | 0.17 |
| Patients with urinary catheter—no./total no. (%)c | 100/165 (60.6) | 109/159 (68.6) | 0.14 |
| Total no. of catheter days | 1,419 | 1,859 | |
| No. of patientsc | 94 | 108 | |
| Median | 13.5 | 14.5 | 0.54 |
| Range | 2–63 | 2–85 | |
| No. urinary tract infection/1,000 catheter days at risk | 12.8 | 15.1 | 0.61 |
aClinical files were missing for two patients in year 2
bDetermined using χ 2 test or Wilcoxon-rank test
cNumber of patients for whom data were available
dThe Tetanus Severity Score is a composite score calculated from the total of individual scores of age, time from first symptom to admission, difficulty breathing on admission, co-existing medical conditions, entry site, highest blood pressure recorded during first day in hospital, highest heart rate recorded during first day in hospital, lowest heart rate recorded during first day in hospital, and highest temperature recorded during first day in hospital. The score ranges between −8 and 48 and a score ≥8 is predictive of death. It is not applicable to neonates [10]
Compliance with hand hygiene and glove use was assessed in year 2 during 43 observation sessions with a total of 311 interactions between staff and patients. Adherence to hand hygiene prior to and after patient contact was 54 %. Adherence to glove use, including removal of gloves, was 70 %. Staff left the patient during an activity in only 6 % of interactions.
Ceftazidime usage decreased by 53 % (95 % CI 45–60 %) in year 2. The use of piperacillin–tazobactam and ciprofloxacin increased 7.2-fold (95 % CI 4.6–11.8) and 4.5-fold (95 % CI 3.1–6.6), respectively (Table 3). The use of amikacin remained unchanged. Imipenem usage decreased by 40 % (95 % CI 26–52 %). Penicillin was used more often for tetanus treatment in year 1 compared to year 2 (36 % decrease in year 2, 95 % CI 29–43 %), whereas metronidazole use increased in year 2 (131 %, 95 % CI 117–148 %).
Table 3.
Antibiotic usage in study years 1 and 2
| Agent | Defined daily dosages per 1,000 patient days |
Rate (year 2 vs. year 1) |
95 % CI | |
|---|---|---|---|---|
| Year 1 | Year 2 | |||
| Ceftazidime | 173 | 81 | 0.47 | 0.40–0.55 |
|
All 3rd-generation cephalosporines |
179 | 85 | 0.48 | 0.41–0.55 |
| Ciprofloxacine | 13 | 59 | 4.46 | 3.10–6.59 |
| All fluoroquinolones | 81 | 99 | 1.22 | 1.02–1.46 |
| Piperacillin–tazobactam | 8 | 59 | 7.2 | 4.63–11.76 |
| Amikacin | 207 | 205 | 0.99 | 0.88–1.11 |
| Imipenem | 80 | 48 | 0.60 | 0.48–0.74 |
| Penicillin | 261 | 167 | 0.64 | 0.57–0.71 |
| Metronidazole | 165 | 217 | 1.31 | 1.17–1.48 |
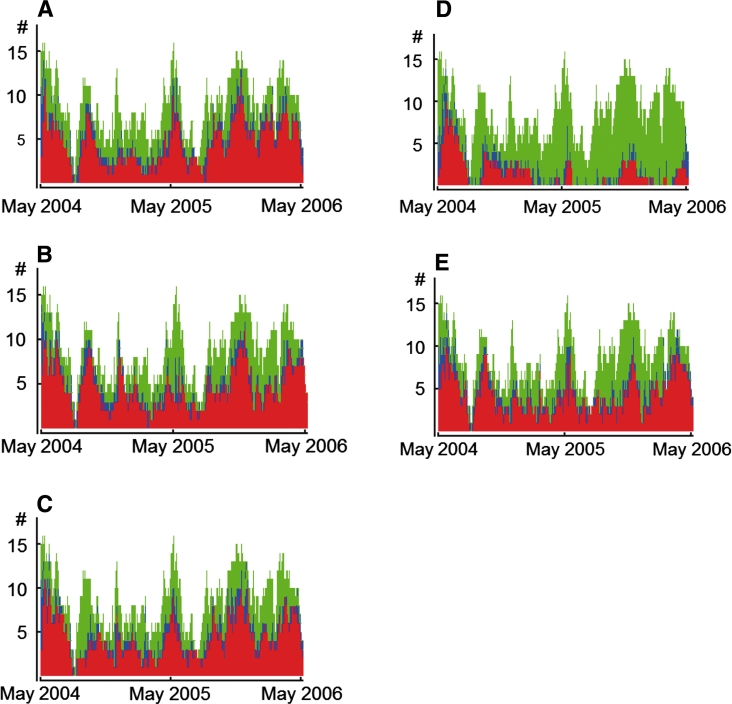
The prevalence data of the five pathogens under investigation for the entire study period are shown in Fig. 1.
Fig. 1.
Estimated prevalence of Pseudomonas aeruginosa (a), gentamicin-resistant Klebsiella pneumoniae (b), amikacin-resistant Acinetobacter spp. (c), methicillin-resistant Staphylococcus aureus (d), extended spectrum beta-lactamase-producing Enterobacteriaceae (excl. K. pneumoniae) (e) on tetanus intensive care unit during year 1 (May 2004–April 2005) and year 2 (May 2005–April 2006). Red indicates the number of patients colonized at a given time point, green the number of patients uncolonized, and blue the number of patients with unknown colonization status, i.e., patient days between the last negative culture result and the first positive culture result, or patient days between the last negative result and discharge
MRSA
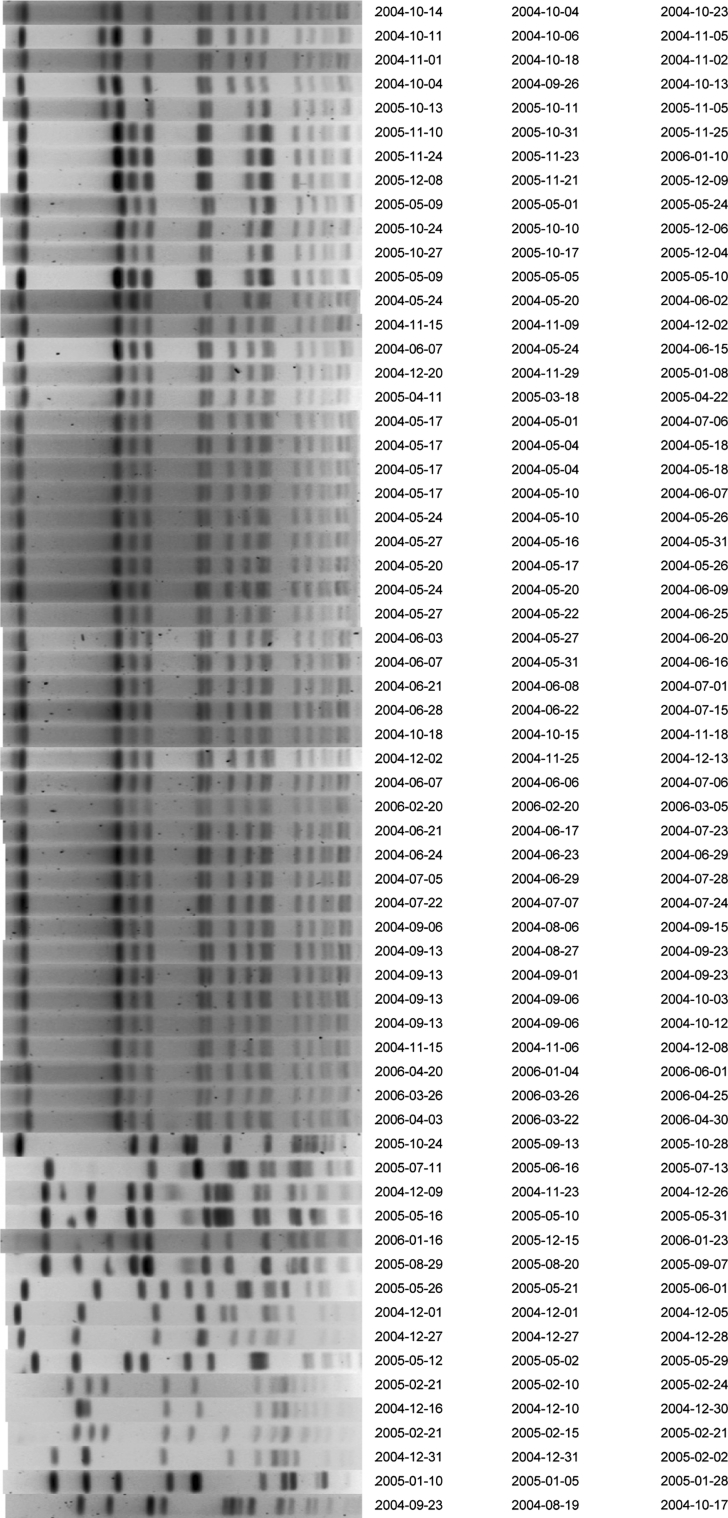
The estimated daily prevalence of MRSA decreased by 69.8 % from 34.0 to 10.3 % (Table 4; Fig. 1d), mainly because of a reduction in exogenous acquisition of MRSA. According to model predictions 84 % (95 % CI 69–91 %) of the MRSA acquisitions occurring during year 1, were from exogenous origin, which reduced to 22 % (95 % CI 0–56 %) in year 2, but the endogenous acquisition risk per uncolonized patient per day remained unchanged (Fig. 2). Consequently, the number of acquisitions decreased from 54 in year 1 to 19 in year 2 (Table 4). Genotyping of all MRSA strains confirmed the modeling results showing a clonal distribution of strains cultured in year 1 compared to variable genotypes of strains cultured in year 2 (Fig. 3).
Table 4.
Number of patients colonized (includes only patients with admission in study period) and maximum likelihood estimators for the acquisition parameters α (endogenous acquisition) and β (exogenous acquisition) and related quantities, for all pathogens under study (using data obtained from all patients present on ward during study period), in year 1 and year 2
| Year 1 N = 190 |
Year 2 N = 167 |
P valuea | |
|---|---|---|---|
|
Methicillin-resistant Staphylococcus aureus | |||
| Patients with a positive culture—no./total no. (%) | 44/174 (25.3) | 19/161 (11.8) | |
| Patients with a positive culture on admission—no./total no. (%) | 5/174 (2.9) | 4/161 (2.5) | 0.83 |
| Acquisition parameter α—MLE (95 % CI) | 0.005 (0.002; 0.012) | 0.005 (0.002; 0.009) | |
| Acquisition parameter β—MLE (95 % CI) | 0.117 (0.079; 0.162) | 0.015 (0.0; 0.055) | |
| Estimated daily prevalence—% (95 % CI) | 34.0 (33.9; 34.2) | 10.3 (10.26; 10.31) | |
| Acquisitions—estimated no. (95 % CI) | 53.7 (51.8; 55.8) | 18.8 (18.1; 19.8) | |
| Exogenous acquisitions—estimated % of total no. of acquisitions (95 % CI) | 83.7 (69.1; 90.9) | 21.7 (0; 55.5) | |
| ESBL-positive Enterobacteriaceae (excl. K. pneumoniae) | |||
| Patients with a positive culture–no./total no. (%) | 94/174 (54.0) | 90/161 (55.9) | |
| Patients with a positive culture on admission—no./total no. (%) | 22/174 (12.6) | 10/161 (6.2) | 0.04 |
| Acquisition parameter α—MLE (95 % CI) | 0.086 (0.043; 0.107) | 0.020 (0; 0.059) | |
| Acquisition parameter β—MLE (95 % CI) | 0 (0.0; 0.085) | 0.105 (0.024; 0.0173) | |
| Estimated daily prevalence—% (95 % CI) | 61.3 (61.1; 61.5) | 55.0 (54.9; 55.0) | |
| Acquisitions—estimated no. (95 % CI) | 86.5 (84.4; 88.7) | 100.2 (98.8; 101.8) | |
| Exogenous acquisitions—estimated % of total no. of acquisitions (95 % CI) | 0 (0; 48.2) | 70.8 (17.0; 100.0) | |
| Pseudomonas aeruginosa | |||
| Patients with a positive culture—no./total no. (%) | 66/174 (37.9) | 95/161 (59) | |
| Patients with a positive culture on admission—no./total no. (%) | 7/174 (4.0) | 9/161 (5.6) | 0.5 |
| Acquisition parameter α—MLE (95 % CI) | 0.042 (0.01; 0.073) | 0.085 (0.026; 0.119) | |
| Acquisition parameter β—MLE (95 % CI) | 0.037 (0; 0.114) | 0.026 (0; 0.132) | |
| Estimated daily prevalence—% (95 % CI) | 53.4 (53.2; 53.7) | 66.4 (66.3; 66.5) | |
| Acquisitions—estimated no. (95 % CI) | 71.3 (68.8; 74.1) | 107.6 (105.8; 109.6) | |
| Exogenous acquisitions—estimated % of total no. of acquisitions (95 % CI) | 28.8 (0; 81.5) | 15.1 (0; 74.0) | |
|
Gentamicin-resistant Klebsiella pneumoniae | |||
| Patients with a positive culture—no./total no. (%) | 86/174 (49.4) | 92/161 (57.1) | |
| Patients with a positive culture on admission—no./total no. (%) | 19/174 (10.9) | 15/161 (9.3) | 0.63 |
| Acquisition parameter α—MLE (95 % CI) | 0.102 (0.049; 0.119) | 0.075 (0.031; 0.091) | |
| Acquisition parameter β—MLE (95 % CI) | 0 (0; 0.094) | 0 (0; 0.087) | |
| Estimated daily prevalence—% (95 % CI) | 65.7 (65.4; 65.9) | 58.4 (58.4; 58.5) | |
| Acquisitions—estimated no. (95 % CI) | 90.3 (87.7; 93.1) | 100.9 (99.6; 102.5) | |
| Exogenous acquisitions—estimated % of total no. of acquisitions (95 % CI) | 0 (0; 49.8) | 0 (0; 57.7) | |
| Amikacin-resistant Acinetobacter species | |||
| Patients with a positive culture—no./total no. (%) | 85/174 (48.9) | 89/161 (55.3) | |
| Patients with a positive culture on admission—no./total no. (%) | 21/174 (12.1) | 7/161 (4.3) | 0.01 |
| Acquisition parameter α—MLE (95 % CI) | 0.047 (0.014; 0.090) | 0.066 (0.005; 0.096) | |
| Acquisition parameter β—MLE (95 % CI) | 0.067 (0; 0.147) | 0.025 (0; 0.143) | |
| Estimated daily prevalence—% (95 % CI) | 57.1 (56.9.7; 57.2) | 60.2 (60.1; 60.3) | |
| Acquisitions—estimated no. (95 % CI) | 86.9 (84.8; 89.4) | 102.5 (100.8; 104.3) | |
| Exogenous acquisitions—estimated % of total no. of acquisitions (95% CI) | 39.8 (0; 80.4) | 17.0 (0; 93.6) | |
MLE maximum likelihood estimate
aDetermined using χ 2 test for independent observations only
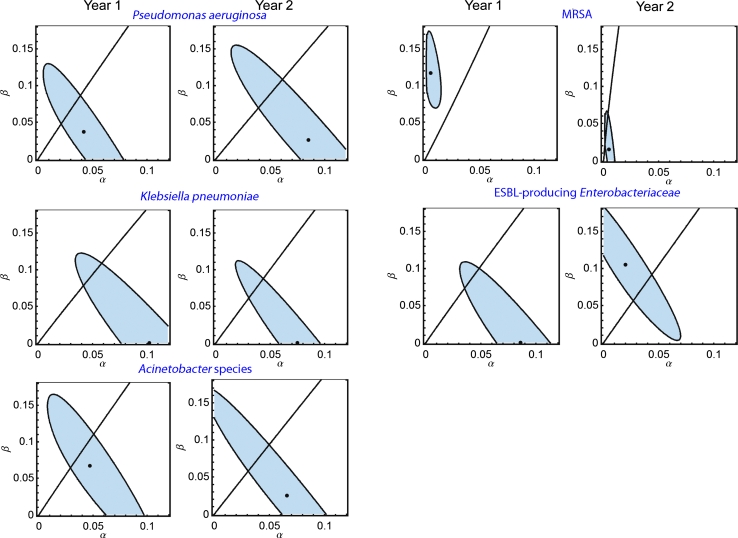
Fig. 2.
Contour plots of the likelihood of the acquisition parameters α (endogenous acquisition, horizontal axis) and β (exogenous acquisition, vertical axis) for Pseudomonas aeruginosa, gentamicin-resistant Klebsiella pneumoniae, amikacin-resistant Acinetobacter spp., methicillin-resistant Staphylococcus aureus (MRSA), extended spectrum beta-lactamase-producing Enterobacteriaceae (excl. K. pneumoniae) (ESBL-producing Enterobacteriaceae) in year 1 and year 2. The black dot represents the maximum likelihood estimate (MLE), the shaded area represents the corresponding 95 % confidence interval (CI). The line represents the parameters for which the endogenous route and the exogenous route are equally important. For example, the MLE of exogenous acquisition parameter β of MRSA was 0.117 (95 % CI 0.079; 0.162) in year 1 and 0.015 (0.0; 0.055) in year 2, indicating that the probability of exogenous acquisition of MRSA per unit of time for a given prevalence of MRSA on the ward was 8 (0.117/0.015) times lower in year 2 compared to year 1. In contrast, the MLEs of endogenous acquisition parameter α were both 0.005 (95 % CI 0.002; 0.012 and 0.002; 0.009) in year 1 and year 2, indicating that the daily probability of endogenous acquisition of MRSA remained 0.5 % throughout the entire study period
Fig. 3.
Pulse field gel electrophoreses analysis of all first methicillin-resistant Staphylococcus aureus (MRSA) strains, isolated from patients admitted to the tetanus ICU during the entire study period (May 2004–April 2006). Band patterns were generated by restriction enzyme digestion of total bacterial DNA. Each row depicts the band pattern of a single isolate and the date of sample collection (first column), date of admission (second column), and date of discharge (third column) of the patient the isolate was cultured from. Identical band patterns indicate clonality as can be expected after exogenous transmission of MRSA
ESBLE
The estimated daily prevalence of ESBLE decreased from 61.3 to 55.0 %, but the estimated number of acquisitions per year increased from 87 to 100. The predominance of the endogenous acquisition route in year 1 changed to predominance of exogenous acquisitions in year 2, at approaching statistical significance (p = 0.06, χ 2 test) (Table 4; Fig. 2). Genotyping of strains (approximately 70 % were Escherichia coli in both years) isolated in the first 6 months of year 1 showed a ratio between endogenous and exogenous acquisition of 10.5:1, whereas typing of strains isolated in the first half of the second year revealed a ratio of 2:1 (see Online Resource 1 for criteria used).
GRKpn, P. aeruginosa, and ARAc
Analysis of GRKpn indicated endogenous acquisition as the predominant colonization route (Table 4). The daily prevalence and acquisition rates did not differ between year 1 and year 2 (p = 0.14, χ 2 test). Analysis of P. aeruginosa and ARAc also indicated endogenous acquisition as the predominant colonization route, which increased for both in year 2 (Fig. 2; Table 4). Acquisition parameters changed for P. aeruginosa when comparing year 1 and year 2 by χ 2 test (p = 0.02) but they did not for ARAc (p = 0.14). However, the 95 % confidence intervals for the estimates of α and β were large for all three pathogens in both years (Fig. 2), and these estimates should therefore be interpreted with caution.
Comparison of modeling results with Cox regression analysis
Multivariate regression analysis indicated a significant increase in time to colonization in year 2 for MRSA (p < 0.0001) and GRKpn (p = 0.046). For MRSA this could correctly be interpreted as an effect of enhanced hygiene. However, Markov modeling results for GRKPn indicated predominantly endogenous acquisition in both year 1 and year 2, suggesting that the observed increased time to colonization with GRKpn is unlikely to be the result of prevention of exogenous transmission due to improved hygiene measures. Instead, differences between year 1 and year 2 may be the result of an increased median length of stay (Table 2) combined with a lower endogenous acquisition parameter in year 2 (Table 4). No significant differences between year 1 and year 2 were observed for the other pathogens after multivariate regression analysis (data not shown).
Infection rates
Rates of nosocomial pneumonia and urinary tract infection were similar in year 1 and year 2 (Table 2) despite more severe illness on admission, more device days, and longer duration of stay, of patients in year 2 (Table 2).
Discussion
In a tetanus ICU in Vietnam with extremely high prevalence of a variety of antibiotic-resistant pathogens, simple infection control measures and rotational antibiotic use had markedly different effects on different pathogens. The combined measures were highly effective in reducing exogenous MRSA transmission, but failed to reduce the prevalence of drug-resistant Gram-negative bacteria. Using Markov chain modeling, we observed clear differences in the predominant acquisition routes between MRSA and Gram-negative microorganisms. MRSA was acquired predominantly through exogenous colonization during the baseline period, which was almost completely controlled by the relatively simple infection control measures installed, which did not include widely considered key measures such as patient isolation and the use of gowns [12, 13], as these were unavailable in our setting. This conclusion is based on the observed decrease in the estimated percentage of total number of acquisitions which were exogenous, between estimates for year 1 and year 2. Despite the fact that the confidence intervals are large, they are non-overlapping indicating that this difference can be considered significant. It is difficult to assign this beneficial effect to a single control measure, because a set of measures was introduced, including the reporting of MRSA-positive cultures (Table 1). Unfortunately, we did not measure adherence to routine hygiene procedures, such as hand hygiene, in the first study year. However, it is the view of the medical and nursing team that performing observations to measure adherence followed by feedback of data, and active involvement in discussions, substantially contributed to increased compliance to infection control measures of all staff during the second study year, which is confirmed by the reasonably high compliance with hand hygiene and glove use [14] observed in year 2.
Several strategies aimed at reducing selection of ESBL-positive and multidrug-resistant bacteria by changing treatment regimens have been studied, including scheduled changes to regimens considered to be less selective [15–18], antibiotic cycling [19–21], and antibiotic mixing [19]. The optimal strategy is unknown because most of these studies did not take acquisition routes into account [11, 22]. The empirical antimicrobial treatment regimen was changed to induce mixing of different antibiotic classes, aimed at reducing endogenous colonization by reducing selective pressure. Although we observed a reduction in endogenous ESBLE acquisition in year 2, this was counterbalanced by a higher exogenous acquisition rate, despite lower admission rates of ESBLE and infection control measures targeted at reducing exogenous colonization. As a result, only a small decrease in the estimated daily prevalence of ESBLE was observed despite a 50 % reduction in cephalosporin use. We hypothesize that the increase in exogenous acquisition of ESBLE may be related to the increased proportion of patients with tracheostomy and receiving ventilation, associated with admission of patients with more severe disease in year 2 (Table 2). Nevertheless, this observation clearly demonstrates that measures which are effective in preventing exogenous acquisition of MRSA may not be effective in preventing exogenous acquisition of ESBLE.
Acquisition may also result from a constant background source. One drawback of our modeling approach is that in such a scenario acquisition would be considered as endogenous because it would occur independently of the prevalence of other colonized patients. However, genotyping isolates originating from a common source typically yields highly similar patterns and this was not the case when K. pneumoniae strains were typed (data not shown).
The very high prevalence of multidrug-resistant Gram-negative microorganisms acquired through endogenous colonization suggests high colonization rates of such bacteria in the community, still undetected at the time of admission to ICU. Indeed, we have observed carriage rates of Enterobacteriaceae resistant to third-generation cephalosporins and/or gentamicin in stool samples from up to 90 % of healthy people in HCMC [23].
Our modeling approach enabled assessment of the effect of infection control measures, without the need for genotyping. Performing surveillance cultures for five pathogens simultaneously during 2 years resulted in a very large number of cultures. In a clinical routine setting, such extensive assessment will rarely be indicated. Exchange of culture and modeling results by email contributed to feasibility. Our approach deserves wider application, e.g., in settings where genotyping is not available.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
We thank all the nurses and doctors of the tetanus ICU of the Hospital for Tropical Diseases for their contribution and dedication to the study. We thank Menno de Jong for critically reviewing the manuscript. This study was supported by the Wellcome Trust, UK. MCJB was supported by the Nederlandse Organisatie voor Wetenschappelijk Onderzoek Grant CLS 635.100.002 and VENI 916.86.128.
Conflicts of interest
We declare that we have no conflict of interest.
References
- 1.Rosenthal VD, Maki DG, Salomao R, Moreno CA, Mehta Y, Higuera F, Cuellar LE, Arikan OA, Abouqal R, Leblebicioglu H. Device-associated nosocomial infections in 55 intensive care units of 8 developing countries. Ann Intern Med. 2006;145(8):582–591. doi: 10.7326/0003-4819-145-8-200610170-00007. [DOI] [PubMed] [Google Scholar]
- 2.Zaidi AK, Huskins WC, Thaver D, Bhutta ZA, Abbas Z, Goldmann DA. Hospital-acquired neonatal infections in developing countries. Lancet. 2005;36:1175–1188. doi: 10.1016/S0140-6736(05)71881-X. [DOI] [PubMed] [Google Scholar]
- 3.Basu S, Andrews JR, Poolman EM, Gandhi NR, Shah NS, Moll A, Moodley P, Galvani AP, Friedland GH. Prevention of nosocomial transmission of extensively drug-resistant tuberculosis in rural South African district hospitals: an epidemiological modelling study. Lancet. 2007;370:1500–1507. doi: 10.1016/S0140-6736(07)61636-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Starling C. Infection control in developing countries. Curr Opin Infect Dis. 2001;14:461–466. doi: 10.1097/00001432-200108000-00011. [DOI] [PubMed] [Google Scholar]
- 5.Raza MW, Kazi BM, Mustafa M, Gould FK. Developing countries have their own characteristic problems with infection control. J Hosp Infect. 2004;57:294–299. doi: 10.1016/j.jhin.2004.03.019. [DOI] [PubMed] [Google Scholar]
- 6.Vincent J-L, Rello J, Marshall J, Silva E, Anzueto A, Martin CD, Moreno R, Lipman J, Gomersall C, Sakr Y, Reinhart K, for the EIIGoI International study of the prevalence and outcomes of infection in intensive care units. JAMA. 2009;302:2323–2329. doi: 10.1001/jama.2009.1754. [DOI] [PubMed] [Google Scholar]
- 7.Lipsitch M, Samore MH. Antimicrobial use and antimicrobial resistance: a population perspective. Emerg Infect Dis. 2002;8:347–354. doi: 10.3201/eid0804.010312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harris AD, Lautenbach E, Perencevich E. A systematic review of quasi-experimental study designs in the fields of infection control and antibiotic resistance. Clin Infect Dis. 2005;41:77–82. doi: 10.1086/430713. [DOI] [PubMed] [Google Scholar]
- 9.Bootsma MC, Bonten MJ, Nijssen S, Fluit AC, Diekmann O. An algorithm to estimate the importance of bacterial acquisition routes in hospital settings. Am J Epidemiol. 2007;166:841–851. doi: 10.1093/aje/kwm149. [DOI] [PubMed] [Google Scholar]
- 10.Thwaites CL, Yen LM, Glover C, Tuan PQ, Nga NT, Parry J, Loan HT, Bethell D, Day NP, White NJ, Soni N, Farrar JJ. Predicting the clinical outcome of tetanus: the Tetanus Severity Score. Trop Med Int Health. 2006;11:279–287. doi: 10.1111/j.1365-3156.2006.01562.x. [DOI] [PubMed] [Google Scholar]
- 11.Nijssen S, Bootsma M, Bonten M. Potential confounding in evaluating infection-control interventions in hospital settings: changing antibiotic prescription. Clin Infect Dis. 2006;43:616–623. doi: 10.1086/506438. [DOI] [PubMed] [Google Scholar]
- 12.Muto CA, Jernigan JA, Ostrowsky BE, Richet HM, Jarvis WR, Boyce JM, Farr BM. SHEA guideline for preventing nosocomial transmission of multidrug-resistant strains of Staphylococcus aureus and enterococcus. Infect Control Hosp Epidemiol. 2003;24:362–386. doi: 10.1086/502213. [DOI] [PubMed] [Google Scholar]
- 13.Cooper BS, Stone SP, Kibbler CC, Cookson BD, Roberts JA, Medley GF, Duckworth G, Lai R, Ebrahim S. Isolation measures in the hospital management of methicillin resistant Staphylococcus aureus (MRSA): systematic review of the literature. BMJ. 2004;329:533. doi: 10.1136/bmj.329.7465.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Allegranzi B, Pittet D. Role of hand hygiene in healthcare-associated infection prevention. J Hosp Infect. 2009;73:305–315. doi: 10.1016/j.jhin.2009.04.019. [DOI] [PubMed] [Google Scholar]
- 15.de Man P, Verhoeven BA, Verbrugh HA, Vos MC, van den Anker JN. An antibiotic policy to prevent emergence of resistant bacilli. Lancet. 2000;355:973–978. doi: 10.1016/S0140-6736(00)90015-1. [DOI] [PubMed] [Google Scholar]
- 16.Bantar C, Vesco E, Heft C, Salamone F, Krayeski M, Gomez H, Coassolo MA, Fiorillo A, Franco D, Arango C, Duret F, Oliva ME. Replacement of broad-spectrum cephalosporins by piperacillin-tazobactam: impact on sustained high rates of bacterial resistance. Antimicrob Agents Chemother. 2004;48:392–395. doi: 10.1128/AAC.48.2.392-395.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee J, Pai H, Kim YK, Kim NH, Eun BW, Kang HJ, Park KH, Choi EH, Shin HY, Kim EC, Lee HJ, Ahn HS. Control of extended-spectrum beta-lactamase-producing Escherichia coli and Klebsiella pneumoniae in a children’s hospital by changing antimicrobial agent usage policy. J Antimicrob Chemother. 2007;60:629–637. doi: 10.1093/jac/dkm225. [DOI] [PubMed] [Google Scholar]
- 18.Rahal JJ, Urban C, Horn D, Freeman K, Segal-Maurer S, Maurer J, Mariano N, Marks S, Burns JM, Dominick D, Lim M. Class restriction of cephalosporin use to control total cephalosporin resistance in nosocomial Klebsiella. JAMA. 1998;280:1233–1237. doi: 10.1001/jama.280.14.1233. [DOI] [PubMed] [Google Scholar]
- 19.Martinez JA, Nicolas JM, Marco F, Horcajada JP, Garcia-Segarra G, Trilla A, Codina C, Torres A, Mensa J. Comparison of antimicrobial cycling and mixing strategies in two medical intensive care units. Crit Care Med. 2006;34:329–336. doi: 10.1097/01.CCM.0000195010.63855.45. [DOI] [PubMed] [Google Scholar]
- 20.van Loon HJ, Vriens MR, Fluit AC, Troelstra A, van der Werken C, Verhoef J, Bonten MJ. Antibiotic rotation and development of gram-negative antibiotic resistance. Am J Respir Crit Care Med. 2005;171:480–487. doi: 10.1164/rccm.200401-070OC. [DOI] [PubMed] [Google Scholar]
- 21.Kollef MH. Is antibiotic cycling the answer to preventing the emergence of bacterial resistance in the intensive care unit? Clin Infect Dis. 2006;43(Suppl 2):S82–S88. doi: 10.1086/504484. [DOI] [PubMed] [Google Scholar]
- 22.Bonten MJ, Weinstein RA. Antibiotic cycling in intensive care units: the value of organized chaos? Crit Care Med. 2006;34:549–551. doi: 10.1097/01.CCM.0000196090.92997.A7. [DOI] [PubMed] [Google Scholar]
- 23.Le TM, Baker S, Le TP, Le TP, Cao TT, Tran TT, Nguyen VM, Campbell JI, Lam MY, Nguyen TH, Nguyen VV, Farrar J, Schultsz C. High prevalence of plasmid-mediated quinolone resistance determinants in commensal members of the Enterobacteriaceae in Ho Chi Minh City Vietnam. J Med Microbiol. 2009;58:1585–1592. doi: 10.1099/jmm.0.010033-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.