Abstract
The pro-inflammatory cytokine macrophage migration inhibitory factor (MIF) stimulates tumor cell proliferation, migration and metastasis, promotes tumor angiogenesis, suppresses p53-mediated apoptosis and inhibits anti-tumor immunity by largely unknown mechanisms. We here describe an overexpression of MIF in ovarian cancer that correlates with malignancy and the presence of ascites. Functionally, we find that MIF may contribute to the immune escape of OvCA by transcriptionally downregulating NKG2D in vitro and in vivo which impairs natural killer (NK) cell cytotoxicity towards tumor cells. Together with MIF's additional tumorigenic properties, this finding provides a rationale for novel small-molecule inhibitors of MIF to be used for the treatment of MIF-secreting cancers.
Keywords: human, natural killer cells, cytokines, tumor immunology
Introduction
Epithelial ovarian carcinoma (OvCA) is the most common cause of death from gynecological malignancy. Even with extended surgery and chemotherapy, 5-year-survival rates do not exceed 20-40% (1). Only non-invasive OvCA of low malignant potential, here designated as Borderline tumors, bear a more favourable prognosis (2). Since long-term survival strongly correlates with favorable immunological parameters (3), immunomodulatory therapies would be of major clinical interest. On the one hand, they should seek to relieve tumor-induced immunosuppression (4, 5), on the other hand, they need to consider inflammation, which is not only central to tumorigenesis (6) but also contributes to the formation of ascites (7) (8), thus correlating with a poor prognosis (9).
Macrophage migration inhibitory factor (MIF)3, a cytokine that promotes both processes, has initially been described as a mediator responsible for lethal shock syndrome after exposure to bacterial endotoxins (10). MIF has also been found to be critical for wound healing (11). A polymorphism in the MIF promoter may lead to high levels of MIF that antagonize glucocorticoid functions and contribute to autoimmune diseases like rheumatoid arthritis, inflammatory bowel disease, systemic lupus erythematosus and atopic dermatitis (12, 13). In addition, MIF can recruit inflammatory cells and promote atherosclerosis via non-cognate binding to chemokine receptors CXCR2 and CXCR4 (14). In normal ovarian tissue, MIF is secreted by granulosa cells and is found in follicular fluids (15). In tumor biology, MIF has been proposed as a biomarker for prostate cancer (16). Further, MIF plays a critical role for angiogenesis, stimulates tumor cell migration, suppresses p53 activity (17, 18) and activates Cyclin D1 and E2F transcription factors (19) (summarized in (20)). The MIF-CD74 ligand-receptor complex (21) activates down-stream non-receptor tyrosine kinases via CD44 (22), a co-receptor that has been proposed as tumor stem cell marker for breast cancer (23), leukemia (24) and other malignancies. The importance of MIF for tumorigenesis is strikingly demonstrated by the resistance of MIF-deficient fibroblasts towards malignant transformation induced by c-myc, H-ras or dominant-negative p53 (25).
MIF also impairs anti-tumor immunity by inhibiting CTL and NK cell responses (26, 27), an effect proposed to be caused by MIF-induced T cell activation followed by activation-induced cell death (28) - a mechanism that might reconcile immune inhibitory with pro-inflammatory potential. However, an activation of NK cells by MIF has not been observed which suggests that the MIF-mediated inhibition of NK cells is effected via a different mechanism (26).
The capacity of NK cells to potentially clear malignant cells depends on the recognition of stress- or transformation-induced molecules, most notably ligands for the activating NK cell receptor NKG2D (natural killer group 2D)4. These ligands (MICA/B, ULBP1-4) are induced by DNA damage (29) and found on virus-infected and tumor cells (30-32). In human cells, ligation of NKG2D leads to the activation of the adaptor protein DAP10. DAP10 signaling initiates a perforin-mediated cytolytic response (30, 32) that can lead to NK cell-mediated tumor clearance without prior activation or antigen-specific tumor cell recognition. CD8+ αβ T cells (CTL), on the other hand, need to be activated by their cognate antigen presented on MHC class I in the presence of costimulation which can also be provided via NKG2D (33). In mice, NK cell activation and T cell costimulation are mediated via two different isoforms of NKG2D that recruit specifically either DAP10 or DAP12. In humans, however, the “NKG2Dshort”-DAP12 pathway does not appear to exist (34). The relevance of NKG2D-mediated reactions against ovarian carcinomas has recently been demonstrated when adoptive transfer of transgenic T cells expressing a chimeric NKG2D-CD3 ζ receptor induced the rejection even of late-stage tumors. What makes this strategy particularly appealing is that it does not depend on the presence of known tumor antigens since NKG2D ligands provide both T cell receptor activation and costimulation (35). However, both murine and human tumors can counteract NKG2D-dependent anti-tumor immune reactions by the shedding of NKG2D ligands (36) or by the secretion of cytokines that induce a down-regulation of NKG2D (37, 38).
We here provide evidence that MIF is significantly overexpressed in ovarian cancer (OvCA) and show that MIF may contribute to the inhibition of antitumoral CD8+ T and NK cells by down-regulation of NKG2D in vitro. These findings may be of particular relevance since small molecule inhibitors of MIF are in pre-clinical development (39).
Materials and Methods
Patient characteristics
All tumor tissues and ascites were obtained in the Department of Obstetrics and Gynecology of the University Hospital in Würzburg, Germany. Patients were aged between 25 and 82 years (median age: 59.5 years). Solid tumor tissues and ascites-derived cells were assessed by at least two experienced pathologists. Of the 38 epithelial ovarian malignancies included, 21 were classified as tumors of the serous-papillary subtype, 11 as mucinous and 6 as endometrioid. Non-invasive OvCA of low malignant potential, so-called ovarian borderline tumors, bear a more favorable prognosis and were therefore regarded separately (n=19). The experiments were approved by the local ethics committee and all patients gave informed written consent.
Cell culture
Primary tumor cells were derived from ascitic fluid from ovarian cancer patients. Cells were centrifuged, washed with PBS and transferred to MCDB105/M199 medium (PAA, Cölbe, Germany) supplemented with 10% heat-inactivated FCS (Biochrom, Berlin, Germany), penicillin (100 IU/ml), streptomycin (100 μg/ml) and 0.02% Na-Pyruvate (all from PAA). Non-adherent cells were removed by washing after 72 h. The immortalized cell lines SK-OV-3 (derived from a 64-year old Caucasian woman diagnosed with ovarian adenocarcinoma, ATCC HTB-77), PA-1 (derived from ascites of a 12-year old Caucasian diagnosed with ovarian teratocarcinoma, ATCC CRL-1572) and OAW-42 (derived from ascites of a patient diagnosed with ovarian cystadenocarcinoma, ECACC 85073102) were cultured in RPMI 1640 with 10% FCS.
Generation of cell culture and tissue supernatants (SN)
For the generation of SNs, 105 cells/well were seeded in a 6 well-plate and adhered overnight before the cell culture medium was removed and replaced by 2 ml of fresh serum-free RPMI1640 medium. SN were collected at 72 h after the medium exchange. Tissue SN were obtained by placing 2 mg of freshly resected tumor for 72 h into 2 ml of fully supplemented RPMI 1640 medium.
Generation of MIF siRNA and pcDNA3-MIF transfectants
The pSUPER plasmid was obtained from R. Agami (Amsterdam, NL) (40). A puromycin cassette was inserted into the Nae1 site. The MIF-specific oligonucleotide sequences GATCCCCTCAACTATTACGACATGAAttcaagagaTTCATGTCGTAATAGTTGATTT TTGGAAA and TCGATTTCCAAAAATCAACTATTACGACATGAAtctcttg aaTTCATGTCGTAATAGTTGAGGG (nucleotides 385-403) were obtained from Sigma Genosys (Deisenhofen, Germany) and cloned into the BglII and SalI sites of pSUPER. The MIF-specific parts of the sequences are bold and underlined. For the generation of stable siMIF transfectants, pSUPERpuro control or siMIF plasmids were introduced using Transfectin transfection reagent (BioRad, Munich, Germany). The cells were selected in medium containing 2 μg/ml puromycin (Carl Roth, Karlsruhe, Germany).
RT-PCR
Total RNA was prepared using the RNeasy system (Quiagen, Hilden, Germany) and transcribed with the RevertAid First-Strand cDNA Synthesis kit (Fermentas, St. Leon-Rot, Germany). For real-time PCR, cDNA amplification was monitored using SybrGreen chemistry on the ABI PRISM 7500 Sequence Detection System (Applied Biosystems, Weiterstadt, Germany). The conditions for these PCR reactions were: 40 cycles 95°C/15 s, 60°C/1 min, using the following specific primers: 18S up: 5‘-CGGCTACCACATCCAAGGAA-3‘ (nucleotides 450-469), 18S down: 5‘-GCTGGAAT TACCGCGGCT-3‘ (nucleotides 636-619); MIF up. 5‘-GCCCGGACAGGGTCTACA-3“ (nucleotides 272-289), MIF down: 5‘-CTTAGGCGAAGGTGGAGTTGTT-3‘ (nucleotides 349-328); NKG2D up: 5′-TCTCGACACAGCTGGGAGATG-3′, NKG2D down: 5′-GACATCTTTGCTTTTGCCATCGTG-3′. Data analysis was done using the ΔΔCT method for relative quantification. Briefly, threshold cycles (CT) for 18S rRNA (reference and MIF) were determined in duplicates and the relative change (rI) in copy numbers was calculated according to the formulae
Dissociation curves and PCR products were analyzed to confirm the presence of a single specific PCR product at the expected size.
Immunohistochemical staining
All tissue specimens were from the tumor bank of the University of Würzburg School of Medicine where they had been evaluated by at least two pathologists in routine diagnostics. Paraffin-embedded tissue samples of ovarian epithelial carcinomas, borderline tumors and normal ovaries were cut at 2 μm, placed on slides (Superfrost, Langenbrinck, Emmendingen, Germany), deparaffinized with xylene and rehydrated in a descending alcohol sequence. Antigens were unmasked in 10 mM sodium citrate buffer in a microwave oven. Endogenous peroxidases were left to react with 3% H202 in methanol for 10 min. Slides were washed in PBS and non-specific binding was blocked with 1% goat serum. Subsequently, slides were incubated for 1h with the monoclonal mouse anti-MIF antibody (Mab289, R&D Systems, Wiesbaden, Germany) diluted in commercial antibody diluent (DakoCytomation, Hamburg, Germany) at 1:100. Biotinylated anti-mouse immunoglobulins and HRP-conjugated streptavidin (both from DakoCytomation) were used according to the manufacturer's protocol. Stainings were developed for 5-10 min with diaminobenzidine. Nuclei were counterstained with haematoxilin. Stained sections were then dehydrated by washing in graded ethanol and embedded in Vitro Clud (Langenbrinck, Emmendingen, Germany).
Immunoblotting
Lysates were prepared from 106 cancer cells or from 10 μm thick slices of snap-frozen tumor or control tissue. Protein lysis buffer contained 50 mM Tris pH 7.4, 0.25 M NaCl, 0.2% Tween, 10 μg/ml leupeptin, 10 μg/ml Phenylmethansulfonylfluorid (PMSF) and 2 μg/ml aprotinin. Protein concentrations were determined using RotiQuant Universal (Roth, Karlsruhe, Germany). 10 μg of lysate/lane were separated. Western Blotting was performed using 15% SDS-polyacrylamide gels in a Mini V8.10 gel chamber (Whatman, Dassel, Germany). Proteins were transferred to nitrocellulose membranes (Whatman) that were then blocked with Roti-Block (Roth), incubated with 0.5 μg/ml Mab289 mouse anti-human MIF antibody (R&D Systems), washed in PBS containing 0.05% Tween-20 and developed with HRP-conjugated goat anti-mouse antibody (Santa Cruz, Santa Cruz, CA) and luminol solution (88.5 mM TRIS-HCl, pH 8.6, 0.2 mg/ml luminol, 1 mg/ml para-hydroxycoumarinic acid and 0.01% H2O2). β-Actin staining (Abcam Ab8226, Cambridge, UK) was performed as loading control.
Enzyme-linked immunosorbent assay (ELISA)
The concentration of soluble MIF was determined by ELISA using mouse anti-human MIF Mab289 as capture and biotinylated goat anti-human MIF BAF289 as detection antibody (both from R&D Systems) in a MaxiSorp 96 well plate (Nunc, Wiesbaden, Germany). Streptavidine-HRP (Immunotools, Friesoythe, Germany) and diaminobenzidine were used for colour development. Absorbance was recorded at 450 nm in a Sunrise microplate reader (Tecan, Crailsheim, Germany) and quantified relative to a standard row. Both samples of ascitic fluids and cell culture SN were examined. IFN-γ and TNF-α levels were also determined by ELISA using matched antibody pairs from Immunotools according to the manufacturer's instructions.
Flow cytometric analysis of NKG2DL expression levels
Ovarian carcinoma cells (106/sample) were detached with Accutase (PAA), blocked and stained with AMO1 IgG1 anti-MICA, BAMO1 IgG1 anti-MICA/B, BMO2 IgG1 anti-MICB, AUMO1 IgG1 anti-ULBP1, BUMO1 IgG1 anti-ULBP2 and CUMO3 IgG1 anti-ULBP3 as described previously (38).
NK cell preparation and lysis assays
PBMC were obtained from healthy volunteers by density gradient centrifugation (Biocoll, Biochrom). Monocytes were depleted by adherence. PBL were cultured on irradiated (30 Gy) RPMI 8866 feeder cells to obtain polyclonal NK cell populations (41). Where indicated, 10 μg/ml blocking anti-MIF antibody Mab289 (42) or irrelevant control IgG (Immunotools) and/or 500 μl of cell culture SN from primary ascites-derived OvCA cells or from MIF-shRNA or control transfected SK-OV-3 ovarian cancer cells were added on day 8. 48 h later, NK cells were harvested and used in different killing assays: Cytotoxicity against primary ascites-derived OvCA cells was assessed in 4 h 51Cr release assays in the absence or presence of mAb used at 10 μg/ml. NK cells were pretreated with normal human IgG to prevent antibody-dependent cellular cytotoxicity before they were co-incubated for 4 h with 1 × 104 51Cr-labeled target cells per well at various effector:target (E:T) ratios. Spontaneous 51Cr release was determined by incubating the target cells with medium alone. Maximum release was determined by adding NP-40 (2%). The percentage of 51Cr release was calculated as follows: 100 × ([experimental release – spontaneous release]/[maximum release – spontaneous release]).
For assays involving SK-OV-3 OvCA cells, NK cells were labeled with PKH-26/Vibrant Dil® solution (Cambrex, Verviers, Belgium) and lytic activity against CFSE-stained (Invitrogen, Karlsruhe, Germany) target cells (100.000 target cells per well) was assessed in modified 4 h FATAL assays (43), using various effector:target (E:T) ratios. Cells were detached and target cell lysis was determined by flow cytometric analysis of 50,000 target cells in a BectonDickinson FACScan flow cytometer (BD Biosciences, Heidelberg, Germany). PKH-26-negative target cells were selected by gating and the percentage of CFSEdim cells within this population was determined. Spontaneous leakage of CFSE was determined by incubating the target cells with medium alone.
For lysis experiments with purified NK cells, SK-OV-3 cells were stably transfected with a firefly luciferase plasmid, kindly provided by Dr. Michael Jensen (Duarte, CA) (44). NK cells were isolated from peripheral blood using a magnetic NK cell isolation kit (Miltenyi Biotech, Bergisch Gladbach, Germany) and stimulated for 48 h with IL-2 (100 IU/ml) in the absence or presence of 5 ng/ml recombinant human MIF (Lot No. 38220000, R&D Systems) or SN of primary ovarian cancer cells as described above for recovery experiments. Target cells were seeded into 96-well plates (104/well) and NK cells were added in triplicates at the indicated E:T ratios. After addition of cell-permeable D-luciferin (PJK, Kleinblittersdorf, Germany) at 0.14 mg/ml, luminescence was recorded at different time points using an Orion II luminometer (Berthold, Pforzheim, Germany). The ATP-dependent conversion of luciferin occurs only in viable luciferase-transfected cells and is thus directly proportional to cell viability (44).
Regulation of NKG2D expression by patient or control serum, ascites, OvCA SN or recombinant human MIF and TGF-β
PBL were obtained from healthy volunteers or OvCA patients and incubated for 48 h with patient or control serum or ascites or with SN generated from primary ascites-derived OvCA cells or from SK-OV-3 control and MIF-knockdown cells. Likewise, recombinant human (rh) MIF or TGF-β1 (Peprotech, London, UK) were applied for 24 h or 48 h. rhMIF was prepared at Yale University by a standardized protocol (45) and found to contain 32 EU/mg recombinant protein. Several lots from a commercial supplier were also tested (R&D Systems, Lot No. FC115011, FC115061, FC115091, FC1107011). The TGF-β receptor I kinase inhibitor SD-208 (46) was kindly provided by Scios Inc., Fremont, CA. Where indicated, MIF was blocked with 10 μg/ml of azide-free Mab289 (R&D Systems). Anti-CXCR2 (MAB331, R&D Systems) and anti-CD74 (clone BU45, Immunotools) were also used at 10 μg/ml. For analysis of MIF mRNA levels, PBL were lysed at 24 h and RNA was extracted and reverse-transcribed as described above. Surface expression levels of different NK cell receptors were analyzed at 48 h. The cells were stained with the following antibodies: MAB139 anti-NKG2D antibody (R&D Systems), BAT221 anti-NKG2D-PE (Miltenyi Biotech), mouse anti-human NKp30-PE (Immunotech, Marseille, France) or a mouse IgG1 isotype control (Immunotools) and washed. Indirect stainings were visualized with goat anti-mouse Cy3 (Dianova, Hamburg, Germany) or goat-anti mouse FITC (Caltag, Hamburg, Germany). After thorough washing, further antibodies (all from Immunotools) were added to enable the discrimination of the various immune cell subpopulations. The following combinations were used: CD3-PeCy5/CD56-PE/NKG2D-FITC, CD3-FITC/CD8PeCy5/NKG2D-Cy3 and CD3-PeCy5/CD4-FITC/NKG2D-Cy3. Cells were analyzed in a Becton Dickinson FACScan, gated for CD4+ T (CD3+CD4+), CD8+ T (CD3+CD8+) and NK (CD3-CD56+) cells and NKG2D expression was determined for each subset. Expression levels are indicated as SFI values (SFI = specific fluorescence intensity)5. These values are obtained by dividing the fluorescence intensity detected with the specific antibody by the fluorescence signal measured with the isotype control antibody. Expression of NKG2DL was assessed as previously described (38).
For recovery experiments, treated immune cells were washed and cultured for another 48 h before being analyzed as above.
Statistics
Experiments were performed at least three times with similar results. Analysis of significance was performed using the two-tailed Student's t-test or, where indicated, ANOVA (analysis of variance) (Excel, Microsoft, Redmond, WA). For the assessment of in vivo expression levels, the scores for the relative staining intensities were compared between the various tumour entities using the Kruskal–Wallis test (P < 0.05* and P < 0.01**). For flow cytometry data, standard deviations are indicated as calculated from the raw data by Summit software (DakoCytomation).
Results
MIF is strongly overexpressed in OvCA
In order to investigate the in vivo expression of MIF in OvCA, paraffin sections from OvCA (n=11, 7 serous, 3 mucinous, 1 endometrioid), borderline tumors (n=10) or healthy control tissue (n=11) were stained with the MIF-specific Mab 289 (Fig. 1A). The percentage of MIF-positive tumor cells and the staining intensities were quantified on a scale ranging from 0 to 5 with 0 indicating the absence of staining and 5 being the maximum as outlined in the Methods section. Average scores were 0.3 ± 0.3 (range: 0-1) for normal ovaries, 1.2 ± 0.8 (range: 0-3) for borderline tumors (p<0.01) and 4.6 ± 0.4 (range: 3-5) for OvCA (p<0.001). No significant differences were found between the various OvCA subtypes.
Figure 1.
Immunohistochemistry shows high MIF expression in OvCA tissue. A, MIF expression in non-malignant and malignant ovarian tissue was analysed by immunohistochemistry using mouse anti-human MIF Mab289. Shown are representative stainings for healthy ovarian epithelium (upper left), a borderline tumor (upper right) and two OvCA (lower panels). To control for specificity, the antibody was pre-incubated with 50 μg/ml rhMIF (insert in the lower right panel). Size-bars correspond to 50 μm. B, Double stainings for MIF and tumor or macrophage markers were performed using Mab289 anti-human MIF antibody in conjunction with goat-anti-mouse Alexa Fluor 555 F(ab′)2 fragments and FITC-conjugated CD68 (left) or CD326/EpCAM antibodies. For double staining of MIF and cytokeratin 8, Mab 289 anti-human MIF (mouse IgG1κ) and CK3-3E4 anti-human cytokeratin 8 (mouse IgG2A) were visualized using isotype-specific, Alexa Fluor 488 and 555 conjugated detection antibodies. The staining shown is representative of four different tumors stained.
In addition, we performed double stainings using anti-MIF Mab289 in combination with the tumor markers EpCAM (CD326) or cytokeratin 8 and with the macrophage marker CD68. Stainings of the tumor stroma showed that moderate MIF expression colocalizes with CD68 or with endothelial vessel structures. However, MIF levels were much higher in EpCAM (CD326) or cytokeratin 8 positive cells in the tumor center (Fig. 1B).
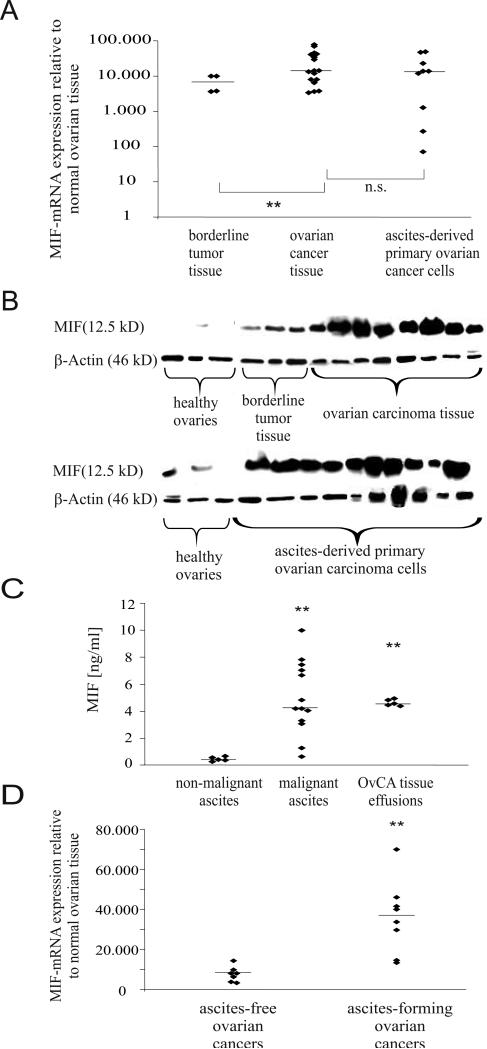
To quantify MIF mRNA expression relative to normal ovarian tissue (n=3), solid tissues from borderline tumors (n=4) and OvCA (n=17, 9 serous-papillary, 5 mucinous, 3 endometrioid) as well as ascites-derived EpCAM-positive primary OvCA cells (n=10, 5 serous-papillary, 3 mucinous, 2 endometrioid) were analyzed by qRT-PCR revealing a highly significant overexpression of MIF mRNA in tissue from borderline tumors (7,000-fold on average, range: 4,000-10,000) and further increased MIF levels in solid OvCA tissue (25,000-fold on average, range: 3,300-70,000) (Fig. 2A). MIF mRNA expression did not differ between solid tumor tissue and purified ascites-derived primary OvCA cells (18,000 fold overexpression on average, range: 70-49,000). cDNA samples prepared from splenocytes of MIF-/--mice served as negative controls which confirmed that the low MIF levels detected in normal ovarian tissue were still >8,000 fold above background.
Figure 2.
MIF mRNA and protein are highly expressed in different OvCA entities. A, MIF mRNA expression was analyzed by quantitative real-time PCR in healthy ovaries (n=3), borderline tumor tissue (n=4), frozen tissue from OvCA (n=17) and in purified ascites-derived primary OvCA cells (n=9). MIF mRNA levels in tumors are expressed relative to the MIF expression found in normal ovaries. Note the logarithmic scale. Student's t-test was used to compare expression levels between tumors and healthy tissue and between tumors of different malignant potential (P < 0.05* and P < 0.01**). B, MIF protein levels were analyzed by Western Blot in 6 normal ovarian tissue samples, 10 lysates from ascites-derived primary OvCA cells, 7 tissue samples of solid OvCA and 3 tissue samples of borderline tumors. Equal loading was verified by b -actin staining. C, Soluble MIF was measured by ELISA in ascitic fluid from OvCA patients (n=13) and from patients with non-malignant ascites (n=5, p<0.01). Effusion of MIF from OvCA tissues was further assessed by placing 2 mg of freshly resected OvCA tissue in 2 ml of medium. Secreted MIF levels were determined by ELISA from the SN at 48 h. D, MIF mRNA levels were compared between ascites-free (n=7) and ascites-forming (n=8) OvCA.
Immunoblotting of protein lysates from normal ovarian tissue (n=6), borderline tumors (n=3), OvCA tissue (n=8, 5 serous-papillary, 2 mucinous, 1 endometrioid) and ascites-derived primary OvCA cells (n=10, 5 serous-papillary, 3 mucinous, 2 endometrioid) confirmed increased MIF protein levels in OvCA compared with borderline tumors and normal ovarian tissues (Fig. 2B).
High MIF levels correlate with the presence of ascites
To obtain data on the presence of secreted soluble MIF, we measured MIF protein levels in ascitic fluids from 13 patients with OvCA and 5 patients with non-malignant ascites by ELISA (Fig. 2C). While MIF levels in malignant OvCA ascites ranged from 0.341 ng/ml to 11.277 ng/ml (median 2.69 ng/ml), ascites from patients with liver cirrhosis or cardiac ascites contained only 0.301 ng/ml to 0.63 ng/ml (median 0.501 ng/ml, p<0.05). To further assess the secretion of MIF by OvCA cells, we placed 2 mg of freshly resected OvCA tissue in 2 ml of medium and measured the resulting MIF concentrations after 72 h of incubation (Fig. 2C). The high amounts of MIF secreted by OvCA tissue (median 4.5 ng/ml, range from 4.3 ng/ml to 4.9 ng/ml, n=5, ) may explain the increase in MIF serum levels already reported for OvCA patients (47).
Having found that MIF levels are high in malignant ascites, we wondered whether the formation of ascites might correlate with MIF expression levels in the primary tumor (Fig. 2D). A two-sided, unpaired t-test revealed that MIF expression levels were significantly higher in patients that had developed ascites (n=8) at the time of tumor surgery than in ascites-free patients (n=7, p<0.01).
MIF inhibits NK cell-mediated killing by decreasing NKG2D expression
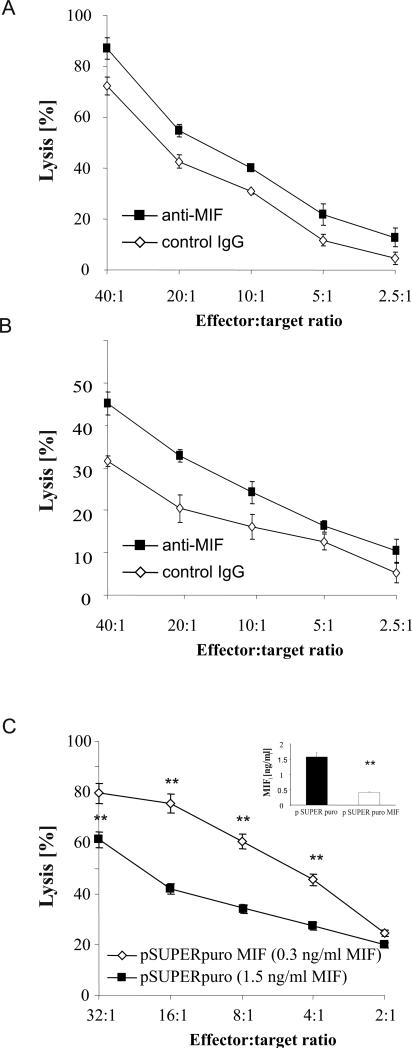
In order to elucidate a functional immunological role for MIF, we investigated whether a blockade of MIF would affect the NK cell-mediated killing of OvCA cells. To this aim, polyclonal NK cell cultures were generated and used as effector cells against 51Cr-labeled primary ascites-derived OvCA cells. Inclusion of a blocking anti-MIF antibody resulted in a moderate but significant increase in target cell lysis (Fig. 3A). In an alternative experimental setup, polyclonal NK cells were treated for 48 h with MIF-containing SN from primary ascites-derived OvCA cells before being used as effector cells against the same primary OvCA targets (Fig. 3B). A comparison with the previous experiment showed that target cell lysis is > 50% inhibited by the tumor cell SN. However, this inhibition is greatly relieved when a blocking anti-MIF antibody is present during the pre-incubation period. To confirm these data with an established permanent cell line, we stably down-regulated MIF in SK-OV-3 cells. Next, we pre-incubated polyclonal human NK cell cultures with SN of SK-OV-3 pSUPERpuro control (1.5 ng/ml MIF) or MIF-depleted SK-OV-3 pSUPERpuroMIF (0.3 ng/ml MIF) cells. In line with the results obtained with primary cells, we also found the subsequent lytic activity of NK cells against SK-OV-3 WT cells to be significantly lower when they had been pre-treated with MIF-containing SN than when they had been exposed to SN of pSUPERpuroMIF cells (Fig. 3C).
Figure 3.
Tumor-derived MIF suppresses lytic activity of NK cells. A, Primary human ascites-derived OvCA cells were labeled with 51Cr (NaCrO4), washed and used as targets (104/well) in a standard 4 h 51Cr-release assay with polyclonal NK cells as effector cells. Blocking anti-MIF antibody Mab289 or an isotype control antibody were used at 10 μg/ml. A representative of three assays performed in triplicates is shown (p<0.05 by ANOVA). B, SN were generated from 105 primary ascites-derived OvCA cells cultured for 72 h in 2 ml RPMI1640 medium with FCS and antibiotics. Polyclonal NK cells were pre-treated with these SN for 48 h in the presence of either blocking anti-MIF antibody Mab289 or an irrelevant isotype control (both used at 10 μg/ml). Subsequently, the NK cells were used in a 4 h 51Cr-release assay against the primary targets that had been used to generate the SN. A representative of 3 experiments is shown (p<0.01 by ANOVA) C, SK-OV-3 cells were transfected with a MIF shRNA plasmid (pSUPERpuroMIF) or the respective control vector (pSUPERpuro). Downregulation of MIF secretion was confirmed by ELISA (n=3). SN were added for 48 h to polyclonal human NK cells before these were used as effectors in a 4 h lysis assay against SK-OV-3 wild-type targets. Target cell lysis was determined by flow cytometric analysis of PKH-26 and CFSE-stained SK-OV-3 cells. A representative of 3 experiments is shown (p=0.01 by ANOVA).
Accordingly, we hypothesized that MIF might down-regulate a receptor involved in the recognition of tumor cells by NK cells. One obvious candidate molecule in this context was NKG2D (30, 31), an activating receptor on NK and a co-stimulatory receptor on CD8+ T cells. In fact, NKG2D levels on CD8+ T cells and NK cells were substantially reduced by MIF-containing SN. This effect was abrogated when SN from MIF-depleted cells were used (Fig. 3B).
Since TGF-β exerts similar effects on the NKG2D system (38), we assessed possible interrelations between TGF-β and MIF. TGF-β levels were unaltered in MIF-depleted cells, as assessed by the TGF-β–specific CCL-64 bioassay (46) (data not shown). Both a blocking anti-MIF antibody (Mab289) or the TGF-β receptor I kinase inhibitor, SD-208 (46), partly prevented the reduction of NKG2D levels mediated by the SK-OV-3 SN (Fig. 3C). While concentration escalation experiments did not enhance the effect of either reagent alone (data not shown), the combination of SD-208 and Mab289 rescued NKG2D surface expression levels completely. Thus, MIF and TGF-β down-regulate NKG2D levels in an additive and therefore presumably independent manner.
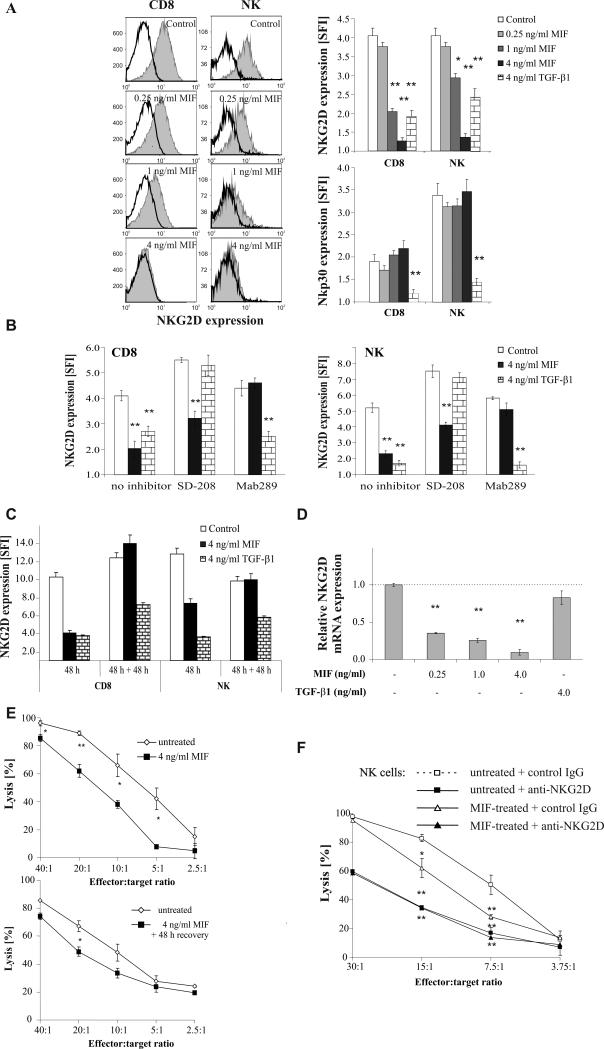
In human ovarian cancer patients, both MIF and TGF-β may affect NKG2D expression in vivo. Consequently, we found NKG2D surface expression levels to be greatly reduced in these patients (Fig. 4C). In order to get more information on the role of MIF in this context, we incubated PBL from ovarian cancer patients (n=3) or healthy donors (n=5) with OvCA ascites (n=5) or serum (n=3) in the absence or presence of the blocking anti-MIF or an isotype control antibody. Serum from healthy donors (n=4) and RPMI1640 medium with FCS were also included as controls. These experiments showed that NKG2D expression is drastically reduced on freshly isolated PBL or ascites-derived immune cells (TIL) from tumor patients (Fig. 4 C-E). However, NKG2D expression recovers upon brief (48 h) in vitro culture in the absence of tumor-derived factors (Fig. 4 D,E). In contrast, NKG2D expression on immune cells from healthy donors is decreased by ascites or serum from tumor patients (Fig. 4 D,E). Moreover, when PBL from either donor are cultured in the presence of ascites or serum, addition of a neutralizing anti-MIF antibody partly rescues NKG2D expression. This effect is more pronounced with serum from tumor patients than from healthy controls (Fig. 4E). This confirms that MIF contained in serum or ascites from OvCA patients is partly responsible for the observed down-regulation of NKG2D, at least under ex vivo conditions. Another part of the effect may be due to TGF-β which was also detected in SN from primary OvCA cells (median concentration for TGF-β1 = 0.5 ng/ml, range from 0.08 ng/ml to 1.25 ng/ml, n=7).
Figure 4.
OvCA-derived MIF induces a downregulation of NKG2D. A, PBL were incubated with control medium or with SN of SK-OV-3 pSUPERpuro control (1.6 ng/ml MIF) or SK-OV-3 pSUPERpuroMIF (0.4 ng/ml MIF) cells. At 48 h, the cells were stained with anti-NKG2D antibody or the respective isotype control. Antibodies directed against CD3, CD4, CD8, CD56 were used to discriminate the various immune cell subsets. Cells were analysed in a Becton Dickinson FACScan, gated for CD8+ T (CD3+ CD8+) and NK (CD3-CD56+) cells and the NKG2D expression was determined for each population. Expression levels are indicated as SFI values (n=5). B, PBL were incubated with SN of SK-OV-3 WT cells (1.3 ng/ml MIF) in the presence or absence of an isotype control or a blocking anti-MIF antibody (10 μg/ml) or the TGF-β receptor I kinase inhibitor SD-208 (1 μM). NKG2D expression on the various immune cell subsets was determined as in B. A representative of 3 experiments is shown. C, NKG2D expression was analyzed as in 4A, using freshly isolated CD8+ T cells and NK cells from OvCA patients and healthy donors. Mean values and standard deviations are shown for 4 OvCA patients and 5 healthy individuals. Differences between the groups were assessed by two-sided, unpaired t-test (*p<0.05, **p<0.01). D, PBL were isolated from OvCA patients (n=3) and healthy donors (n=5) and incubated for 48 h in vitro using either normal culture medium (RPMI1640 with FCS) or cell culture medium with 50% ascites and 10 μg/ml of a blocking anti-MIF antibody or an isotype control. NKG2D expression on CD8+ T cells and NK cells was determined by flow cytometry right after isolation and after 48 h of in vitro culture. A representative experiment is shown. E, PBL were isolated from OvCA patients (n=3) and healthy donors (n=3) and incubated for 48 h in vitro using either normal culture medium (RPMI1640 with 10% FCS) or cell culture medium with 50% serum from a healthy individual or a tumor patient. To assess MIF-dependent effects, a blocking anti-MIF antibody or an isotype control antibody was added at 10 μg/ml. NKG2D expression on CD8+ T cells and NK cells was determined right after isolation or after 48 h of in vitro culture under the described conditions. Afterwards, the remaining cells were washed and maintained for another 48 h in RPMI 1640 with 10% FCS before they were also analyzed by flow cytometry. A representative experiment is shown.
Importantly, the effect of MIF-containing SN or ascites on NKG2D expression was mimicked by rhMIF, which induced a concentration-dependent down-regulation of NKG2D on CD8+ T cells and NK cells. Surface levels of NKp30 were also reduced by treatment with TGF-β 1 (37), but not by MIF (Fig. 5A, lower right). Neither did MIF (4 ng/ml, 48 h) affect the expression of NKG2A, NKG2C, NKp44, NKp46 or NKp80 (data not shown).
Figure 5.
rhMIF inhibits NK cell activity towards OvCA targets through a transcriptional downregulation of NKG2D. A, PBL were incubated for 48 h with different concentrations of rh MIF or TGF-β 1 (4 ng/ml) before NKG2D and NKp30 expression levels were quantified as in Fig. 4A. Shown are histograms for NKG2D in MIF-treated CD8+ T and NK cells and a summary of the findings on NKG2D and NKp30 regulation (n=5 for NKG2D, n=3 for NKp30). B, PBL were pre-incubated with SD-208 (1 μM), blocking anti-MIF Mab 289 (10 μg/ml) or control medium for 1 h before they were treated with 4 ng/ml of human recombinant MIF or TGF-β 1 for 48 h. SD-208 or the antibody were present during the whole course of the experiment. NKG2D expression levels were quantified as in (A) (n=3, a representative experiment is shown). C, PBL were treated as indicated with rhMIF or TGF-β1. After 48 h, about half of the cells were used to determine NKG2D expression as in Fig. 4A. The remaining cells were washed and cultured for further 48 h in RPMI 1640 with 10% FCS before they were also subjected to FACS analysis. D, PBL were incubated for 24 h with different concentrations of rhMIF or TGF-β 1 before total cellular RNA was extracted. The RNA was reverse-transcribed by standard techniques and analyzed for NKG2D expression levels using a SybrGreen-based Taqman assay (n=3). E, Magnetically sorted NK cells were activated for 48 h with 100 IU/ml of IL-2 in the absence or presence of rhMIF (4 ng/ml). When half of the cells were now used as effectors in a 4 h biophotonic lysis assay against SK-OV-3-fLuc cells, the MIF-treated cells showed clearly reduced lytic capacity. However, the remaining cells were cultured for further 48 h in medium low-dose IL-2 (10 IU/ml), but no MIF. Then they were used in a subsequent lysis assay against SK-OV-3-fLuc targets. A representative of three experiments performed in triplicates is shown. F, Purified NK cells were activated for 48 h with 100 U/ml IL-2 in the absence or presence of rh MIF (4 ng/ml), before they were incubated for 30 min with a blocking anti-NKG2D antibody or an isotype control antibody (both used at 10 μg/ml). MIF-treated (triangles) and untreated (squares) NK cells with (filled symbols) and without (open symbols) blockade of NKG2D were then used as effector cells in a 4 h biophotonic lysis assay directed against luc-transfected SK-OV-3 target cells. A representative of 3 experiments performed in triplicates is shown.
To further dissociate MIF-dependent from TGF-β-mediated effects, we incubated PBL with rhMIF or TGF-β 1 (4 ng/ml each) for 48 h in the presence or absence of 1 μM SD-208 or the blocking anti-MIF antibody Mab289 used at 10 μg/ml. For each treatment, only the specific antagonist prevented the reduction of NKG2D surface levels on CD8+ T and NK cells (Fig. 5B). The increased NKG2D surface expression on SD-208-treated cells can be explained by SD-208 blocking auto- and paracrine effects of lymphocyte-derived TGF-β.
Another difference between MIF and TGF-β became apparent when we investigated the recovery of NKG2D expression on cytokine-treated cells: The effect of MIF was reversed 48 h after the cells had been switched to RPMI 1640 medium with 10% FCS + 100 IU/ml IL-2. In contrast, the effect of TGF-β largely persisted even 48 h after the medium exchange (Fig. 5C). This suggests that MIF and TGF-β may exert their effects on NKG2D via different mechanisms. In fact, PBL that had been treated with MIF showed a greatly reduced number of NKG2D transcripts at 24 h (Fig. 5D), whereas NKG2D mRNA was only slightly down-regulated by TGF-β 1 (p=0.17). Accordingly, MIF seems to have a strong transcriptional effect on NKG2D whereas TGF-β appears to modulate NKG2D expression levels largely by post-transcriptional mechanisms.
Impaired cytotoxic capacity of MIF-treated NK cells is due to a MIF-dependent reduction in NKG2D expression
To confirm the functional significance of NKG2D levels at the surface of NK cells, we first characterized the expression of NKG2D ligands in SK-OV-3 cells. SFI values were 1.4 ± 0.2 for MICA, 1.1 ± 0.04 for MICB, 1.1 ± 0.1 for ULBP1, 1.9 ± 0.3 for ULBP2 and 1.7 ± 0.1 for ULBP3. Autocrine regulations of NKG2D ligands (38) were not observed between control-transfected and pSUPERpuroMIF-transfected SK-OV-3 cells (data not shown). Having confirmed that ligands for NKG2D are expressed by OvCA cells, we pre-incubated magnetically sorted NK cells for 48 h with 100 IU/ml of IL-2 (to activate them) or with 100 IU/ml of IL-2 + 4 ng/ml rhMIF or with 500 μl of tumor cell SN in the absence or presence of 10 μg/ml anti-MIF antibody or isotype control. When half of the cells were now used as effectors in a 4 h biophotonic lysis assay against SK-OV-3-fLuc cells, the MIF-treated cells showed clearly reduced lytic capacity (p<0.05, Fig. 5E, upper panel). However, the remaining cells were cultured for further 48 h in medium low-dose IL-2 (10 IU/ml), but no MIF. Then a subsequent lysis assay confirmed that the recovery of NKG2D surface expression coincided with an almost complete restoration of the killing capacity of MIF-treated NK cells (p>0.05, Fig. 5E, lower panel). Likewise, when NK cells had been treated for 48 h with tumor cell SN (in the absence or presence of MIF antibody), MIF-dependent differences in target cell lysis almost disappeared after 48 h of recovery (data not shown). However, SN from primary ascites-derived OvCA cells also induced long-term effects, such as reduced NK cells viability and diminished killing capacity that did not depend on MIF.
While these data show that the down-regulation of NKG2D expression correlates with the impaired lytic activity of MIF-treated NK cells, we also sought to prove a functional relationship between reduced NKG2D levels and killing capacity: Toward this aim, we repeated the 4 h killing assay with MIF-treated NK cells in the absence or presence of a blocking anti-NKG2D antibody (10 μg/ml) or the respective isotype control (Fig. 5F). Again, MIF-treated polyclonal NK cells displayed reduced lytic capacity when compared with untreated NK cells (p<0.05). The addition of anti-NKG2D inhibited target cell lysis by both untreated (p<0.01) and MIF-treated NK cells (p<0.05), which still displayed residual NKG2D expression. Importantly, pretreatment with MIF showed no additional effect when NKG2D was blocked (Fig. 5F), indicating that MIF does not affect the NKG2D-independent lytic activity of the NK effector cells. These data therefore lead to the conclusion that the MIF effect on NKG2D expression is directly responsible for the MIF-induced inhibition of lytic activity.
Discussion
MIF was the first cytokine to be discovered (10), but its diverse role in immunity has only recently been elucidated (12). MIF-knockout mice display no obvious phenotype (48) apart from p53-dependent growth alterations (18). Nevertheless gene array analysis of MIF-knockdown neuroblastoma cells revealed no less than 166 different genes regulated in an autocrine fashion by MIF (49). Its most important physiological functions may be the sustainment of inflammatory reactions (50) and its promotion of tissue repair (11). A role for MIF in autoimmunity has been confirmed since MIF-knockout mice are resistant towards experimentally induced arthritis and autoimmune encephalomyelitis (51, 52). A critical role for MIF in tumor biology is strongly supported by the finding that fibroblasts from MIF-knockout mice cannot be transformed by various oncogenes (25). The inactivating effect of MIF on p53 has been demonstrated in a knock-out mouse model (17, 18). An effect of MIF on tumor angiogenesis, proliferation and metastasis was shown in vivo using MIF-knockdown neuroblastoma cells (53). In addition, MIF inhibits tumor-cell specific cytolytic responses (27, 28) and induces TLR4 (54) that is found on OvCA cells and associated with an inflammatory, chemoresistance-promoting tumor milieu (7). Given these manifold tumorigenic effects of MIF, its overexpression in various human neoplasias including prostate, bladder, breast, colon, brain, skin and lung-derived tumors (20) comes as little surprise.
We have analyzed MIF in OvCA and found a significant overexpression in vitro and in vivo (Fig. 1A, 2). MIF levels were similar in solid tissue from the primary tumor and in purified ascites-derived tumor cells, suggesting that MIF expression remains unchanged when cells dissociate from the primary tumor and migrate towards the peritoneum. Immunohistochemical double staining of primary tumors revealed that MIF overexpression may largely be ascribed to EpCAM- and cytokeratin 8-positive OvCA cells while the contribution from tumor-infiltrating macrophages and endothelial cells appears to be small in comparison (Fig. 1B).
The high levels of tumor-derived MIF were also reflected by an overexpression of MIF in malignant ascites as compared with non-malignant hepatic and cardiac ascites (Fig. 2C). Of note, MIF expression levels correlate with malignancy: Whereas borderline tumors that are characterized by a non-invasive growth pattern overexpress MIF to a much lesser extent (Fig. 1A, 2), we found the highest MIF mRNA expression in ascites-forming OvCA that are associated with the poorest prognosis (9) (Fig. 2D). It may be speculated whether pro-inflammatory effects exerted by MIF contribute to the formation of malignant ascites. Alternatively, lysophosphatidic acid (LPA) contained in malignant ascites may superinduce MIF levels.
To address a functional immunological role for MIF in OvCA, we generated a SK-OV-3 MIF-knockdown subline that secreted about 80% less MIF than control cells. Viability, proliferation and morphological appearance of the MIF-depleted cells were unaltered (data not shown). SN from MIF-knockdown cells was considerably less suppressive for NK cytotoxicity than SN derived from control transfectants (Fig. 3C). Likewise, inclusion of a blocking anti-MIF antibody enhanced the NK cell mediated lysis of primary ascites-derived OvCA cells (Fig. 3A). Moreover, the inhibitory effect of SN from primary ascites-derived OvCA cells could also be relieved by the blockade of MIF (Fig. 3B). Nevertheless, the lytic activity of polyclonal NK cells was still inhibited by OvCA SN even when a blocking anti-MIF antibody was included.
Since NK cell activation depends on the balance between activating and inhibitory signals transmitted by various NK receptors, we hypothesized that MIF might induce the down-regulation of an activating receptor. Flow cytometry and qRT-PCR revealed that MIF decreases NKG2D expression in NK and CD8+ T cells (Fig. 4 and 5A,B). Importantly, this effect was not restricted to recombinant human MIF or MIF contained in the SN from SK-OV-3 cells. Serum or ascites from OvCA patients also decreased the NKG2D surface expression on CD8+ T and NK cells from healthy donors in a MIF-dependent manner. In contrast, serum from control persons had a much weaker effect which corresponds to the lower MIF levels described in the sera of healthy individuals (Fig. 4D,E). This correlates with reduced NKG2D levels on tumor-infiltrating lymphocytes or PBL from OvCA patients (Fig. 4C-E). Importantly, the reduced NKG2D expression levels on immune cells from tumor patients recover to normal levels when the PBL are cultured in normal growth medium in vitro. Accordingly, the reduced NKG2D expression levels observed in vivo do not seem to be due to T or NK cell-intrinsic deficiencies in OvCA patients but rather to the presence of OvCA-derived factors including MIF. Not surprisingly, however, immune cells that are treated with tumor ascites or serum still show a moderate downregulation of NKG2D when MIF is antagonized which indicates that further tumor-associated immunosuppressive cytokines also contribute to the observed effect. Since TGF-β has so far been the only cytokine known to affect NKG2D expression, we confirmed the presence of low to moderate levels of TGF-β1 by ELISA (median concentration; 0.5 ng/ml) and sought to differentiate between effects caused by TGF-β and MIF.
One difference appears to be a much higher specificity of MIF for NKG2D: Whereas NKG2A, NKG2C, NKp30, NKp44, NKp46 and NKp80 expression levels on NK cells were not affected by MIF, TGF-β also also induces a strong downregulation of NKp30 (37) as well as an upregulation of NKG2A (Fig. 5A and data not shown). The recovery of NKG2D levels on cytokine-treated cells also seems to follow a different kinetics: Whereas NKG2D expression on MIF-treated cells is restored to control levels within 48 h after withdrawal of the cytokine, TGF-β shows a more persistent effect (Fig. 5C). Mechanistically, we show that MIF inhibits the transcription of NKG2D mRNA, whereas TGF-β appears to exert its effect on NKG2D mainly via post-transcriptional mechanisms (Fig. 5D). In accordance with these data, both a blocking anti-MIF antibody and the TGF-β receptor kinase inhibitor SD-208 partly prevented the down-regulation of NKG2D by tumor cell SN, with additive effects (Fig. 4B) (46). SD-208, however, did not prevent the reduction of NKG2D levels when PBL were treated with rhMIF (Fig. 5B). Experiments involving MIF and a blocking antibody against one of its receptors (CXCR2) showed that MIF signaling via CXCR2 cannot fully account for the effect of MIF on NKG2D expression (data not shown). However, due to the lack of reliable blocking antibodies, the contribution from other receptors (CD74, CXCR4) could not be easily evaluated (14, 21, 22).
To test whether NKG2D expression on NK cells really correlates with lytic activity against OvCA targets, we performed killing assays using MIF-treated IL-2 activated NK cells either immediately after 48 h of exposure to the cytokine or after an additional 48 h of recovery in medium with IL-2 (100 IU/ml). As predicted, the initially reduced killing capacity of MIF-treated NK cells was largely restored after the recovery period (Fig. 5E). When (instead of the recombinant cytokine) MIF-containing tumor cell SN was used, addition of anti-MIF antibody also attenuated the inhibition of NK cell lytic activity at 48 h. Again, the MIF-dependent differences in target cell lysis lost their significance after additional 48 h of culture in fully supplemented RPMI 1640 medium + IL-2. However, OvCA SN also induced MIF-independent long-term effects including significant cell death and reduced lytic capacity (data not shown).
To further confirm the functional importance of MIF-induced downregulation of NKG2D, we treated purified NK cells with MIF and performed a lysis assay in the presence of either a control or a blocking anti-NKG2D antibody (Fig. 5F). This showed that the blocking of NKG2D by antibody represses the cytolytic activity of NK cells even more efficiently than treatment with MIF, suggesting that the low levels of NKG2D remaining on the surface of MIF-treated cells are still sufficient to mediate some activation and ensuing killing activity. An important observation, however, was that there was no remaining MIF-dependent effect when NKG2D was blocked by neutralizing antibodies. Accordingly, MIF does not seem to affect the residual NKG2D-independent lytic activity of NKG2D-blocked NK cells which illustrates that the effect of MIF on lytic activity is mediated by the induced loss of NKG2D.
Taken together, our study is the first to demonstrate that MIF expression in OvCA increases with the grade of malignancy. Functionally, we demonstrate that MIF inhibits anti-tumor immunity by down-regulating NKG2D on NK and CD8+ T cells. Autocrine effects of MIF on the progression of OvCA have not been investigated, but are likely. Thus, MIF inhibitors that are currently being developed for the treatment of rheumatoid arthritis (39) may become promising agents also for the treatment of OvCA.
Non-standard abbreviations
- MIF
macrophage migration inhibitory factor
- NKG2D
natural killer group 2D
- SFI
specific fluorescence intensity
Footnotes
Grant support: This work was supported by grants from the IZKF Würzburg (JW) and the NIH (LL, RB).
References
- 1.Naora H, Montell DJ. Ovarian cancer metastasis: integrating insights from disparate model organisms. Nat. Rev. Cancer. 2005;5:355–366. doi: 10.1038/nrc1611. [DOI] [PubMed] [Google Scholar]
- 2.Ozols R, Rubin S, Thomas G, Robboy S. Epithelial ovarian cancer. In Principles and Practice of Gynecologic Oncology. In: Hoskins W, Perez C, Young R, editors. Lippincott-Raven; Philadelphia: 1997. pp. 936–986. [Google Scholar]
- 3.Curiel TJ, Coukos G, Zou L, Alvarez X, Cheng P, Mottram P, Evdemon-Hogan M, Conejo-Garcia JR, Zhang L, Burow M, Zhu Y, Wei S, Kryczek I, Daniel B, Gordon A, Myers L, Lackner A, Disis ML, Knutson KL, Chen L, Zou W. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat. Med. 2004;10:942–949. doi: 10.1038/nm1093. [DOI] [PubMed] [Google Scholar]
- 4.Haskill S, Becker S, Fowler W, Walton L. Mononuclear-cell infiltration in ovarian cancer. I. Inflammatory-cell infiltrates from tumour and ascites material. Br. J. Cancer. 1982;45:728–736. doi: 10.1038/bjc.1982.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.de Visser KE, Eichten A, Coussens LM. Paradoxical roles of the immune system during cancer development. Nat. Rev. Cancer. 2006;6:24–37. doi: 10.1038/nrc1782. [DOI] [PubMed] [Google Scholar]
- 6.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kelly MG, Alvero AB, Chen R, Silasi DA, Abrahams VM, Chan S, Visintin I, Rutherford T, Mor G. TLR-4 signaling promotes tumor growth and paclitaxel chemoresistance in ovarian cancer. Cancer Res. 2006;66:3859–3868. doi: 10.1158/0008-5472.CAN-05-3948. [DOI] [PubMed] [Google Scholar]
- 8.Wang X, Wang E, Kavanagh JJ, Freedman RS. Ovarian cancer, the coagulation pathway, and inflammation. J. Transl. Med. 2005;3:25. doi: 10.1186/1479-5876-3-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Omura GA, Brady MF, Homesley HD, Yordan E, Major FJ, Buchsbaum HJ, Park RC. Long-term follow-up and prognostic factor analysis in advanced ovarian carcinoma: the Gynecologic Oncology Group experience. J. Clin. Oncol. 1991;9:1138–1150. doi: 10.1200/JCO.1991.9.7.1138. [DOI] [PubMed] [Google Scholar]
- 10.Bloom BR, Bennett B. Mechanism of a reaction in vitro associated with delayed-type hypersensitivity. Science. 1966;153:80–82. doi: 10.1126/science.153.3731.80. [DOI] [PubMed] [Google Scholar]
- 11.Hardman MJ, Waite A, Zeef L, Burow M, Nakayama T, Ashcroft GS. Macrophage migration inhibitory factor: a central regulator of wound healing. Am. J. Pathol. 2005;167:1561–1574. doi: 10.1016/S0002-9440(10)61241-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bernhagen J, Calandra T, Mitchell RA, Martin SB, Tracey KJ, Voelter W, Manogue KR, Cerami A, Bucala R. MIF is a pituitary-derived cytokine that potentiates lethal endotoxaemia. Nature. 1993;365:756–759. doi: 10.1038/365756a0. [DOI] [PubMed] [Google Scholar]
- 13.Bucala R, Lolis E. Macrophage migration inhibitory factor: a critical component of autoimmune inflammatory diseases. Drug News Perspect. 2005;18:417–426. doi: 10.1358/dnp.2005.18.7.939345. [DOI] [PubMed] [Google Scholar]
- 14.Bernhagen J, Krohn R, Lue H, Gregory JL, Zernecke A, Koenen RR, Dewor M, Georgiev I, Schober A, Leng L, Kooistra T, Fingerle-Rowson G, Ghezzi P, Kleemann R, McColl SR, Bucala R, Hickey MJ, Weber C. MIF is a noncognate ligand of CXC chemokine receptors in inflammatory and atherogenic cell recruitment. Nat. Med. 2007;13:587–596. doi: 10.1038/nm1567. [DOI] [PubMed] [Google Scholar]
- 15.Wada S, Fujimoto S, Mizue Y, Nishihira J. Macrophage migration inhibitory factor in the human ovary: presence in the follicular fluids and production by granulosa cells. Biochem. Mol. Biol. Int. 1997;41:805–814. doi: 10.1080/15216549700201841. [DOI] [PubMed] [Google Scholar]
- 16.Meyer-Siegler KL, Bellino MA, Tannenbaum M. Macrophage migration inhibitory factor evaluation compared with prostate specific antigen as a biomarker in patients with prostate carcinoma. Cancer. 2002;94:1449–1456. doi: 10.1002/cncr.10354. [DOI] [PubMed] [Google Scholar]
- 17.Hudson JD, Shoaibi MA, Maestro R, Carnero A, Hannon GJ, Beach DH. A proinflammatory cytokine inhibits p53 tumor suppressor activity. J. Exp. Med. 1999;190:1375–1382. doi: 10.1084/jem.190.10.1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fingerle-Rowson G, Petrenko O, Metz CN, Forsthuber TG, Mitchell R, Huss R, Moll U, Müller W, Bucala R. The p53-dependent effects of macrophage migration inhibitory factor revealed by gene targeting. Proc. Natl. Acad. Sci. U S A. 2003;100:9354–9359. doi: 10.1073/pnas.1533295100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liao H, Bucala R, Mitchell RA. Adhesion-dependent signaling by macrophage migration inhibitory factor (MIF). J. Biol. Chem. 2003;278:76–81. doi: 10.1074/jbc.M208820200. [DOI] [PubMed] [Google Scholar]
- 20.Mitchell RA. Mechanisms and effectors of MIF-dependent promotion of tumourigenesis. Cell Signal. 2004;16:13–19. doi: 10.1016/j.cellsig.2003.07.002. [DOI] [PubMed] [Google Scholar]
- 21.Leng L, Metz CN, Fang Y, Xu J, Donnelly S, Baugh J, Delohery T, Chen Y, Mitchell RA, Bucala R. MIF signal transduction initiated by binding to CD74. J. Exp. Med. 2003;197:1467–1476. doi: 10.1084/jem.20030286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shi X, Leng L, Wang T, Wang W, Du X, Li J, McDonald C, Chen Z, Murphy JW, Lolis E, Noble P, Knudson W, Bucala R. CD44 Is the Signaling Component of the Macrophage Migration Inhibitory Factor-CD74 Receptor Complex. Immunity. 2006;25:595–606. doi: 10.1016/j.immuni.2006.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc. Natl. Acad. Sci. U S A. 2003;100:3983–3988. doi: 10.1073/pnas.0530291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jin L, Hope KJ, Zhai Q, Smadja-Joffe F, Dick JE. Targeting of CD44 eradicates human acute myeloid leukemic stem cells. Nat. Med. 2006;12:1167–1174. doi: 10.1038/nm1483. [DOI] [PubMed] [Google Scholar]
- 25.Petrenko O, Fingerle-Rowson G, Peng T, Mitchell RA, Metz CN. Macrophage migration inhibitory factor deficiency is associated with altered cell growth and reduced susceptibility to Ras-mediated transformation. J. Biol. Chem. 2003;278:11078–11085. doi: 10.1074/jbc.M211985200. [DOI] [PubMed] [Google Scholar]
- 26.Apte RS, Sinha D, Mayhew E, Wistow GJ, Niederkorn JY. Cutting edge: role of macrophage migration inhibitory factor in inhibiting NK cell activity and preserving immune privilege. J. Immunol. 1998;160:5693–5696. [PubMed] [Google Scholar]
- 27.Repp AC, Mayhew ES, Apte S, Niederkorn JY. Human uveal melanoma cells produce macrophage migration-inhibitory factor to prevent lysis by NK cells. J. Immunol. 2000;165:710–715. doi: 10.4049/jimmunol.165.2.710. [DOI] [PubMed] [Google Scholar]
- 28.Yan X, Orentas RJ, Johnson BD. Tumor-derived macrophage migration inhibitory factor (MIF) inhibits T lymphocyte activation. Cytokine. 2006;33:188–198. doi: 10.1016/j.cyto.2006.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gasser S, Orsulic S, Brown EJ, Raulet DH. The DNA damage pathway regulates innate immune system ligands of the NKG2D receptor. Nature. 2005;436:1186–1190. doi: 10.1038/nature03884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bauer S, Groh V, Wu J, Steinle A, Phillips JH, Lanier LL, Spies T. Activation of NK cells and T cells by NKG2D, a receptor for stress-inducible MICA. Science. 1999;285:727–729. doi: 10.1126/science.285.5428.727. [DOI] [PubMed] [Google Scholar]
- 31.Groh V, Rhinehart R, Secrist H, Bauer S, Grabstein KH, Spies T. Broad tumor-associated expression and recognition by tumor-derived gamma delta T cells of MICA and MICB. Proc. Natl. Acad. Sci. U S A. 1999;96:6879–6884. doi: 10.1073/pnas.96.12.6879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pende D, Rivera P, Marcenaro S, Chang CC, Biassoni R, Conte R, Kubin M, Cosman D, Ferrone S, Moretta L, Moretta A. Major histocompatibility complex class I-related chain A and UL16-binding protein expression on tumor cell lines of different histotypes: analysis of tumor susceptibility to NKG2D-dependent natural killer cell cytotoxicity. Cancer Res. 2002;62:6178–6186. [PubMed] [Google Scholar]
- 33.Groh V, Rhinehart R, Randolph-Habecker J, Topp MS, Riddell SR, Spies T. Costimulation of CD8alphabeta T cells by NKG2D via engagement by MIC induced on virus-infected cells. Nat. Immunol. 2001;2:255–260. doi: 10.1038/85321. [DOI] [PubMed] [Google Scholar]
- 34.Rosen DB, Araki M, Hamerman JA, Chen T, Yamamura T, Lanier LL. A Structural basis for the association of DAP12 with mouse, but not human, NKG2D. J. Immunol. 2004;173:2470–2478. doi: 10.4049/jimmunol.173.4.2470. [DOI] [PubMed] [Google Scholar]
- 35.Barber A, Zhang T, Sentman CL. Immunotherapy with Chimeric NKG2D Receptors Leads to Long-Term Tumor-Free Survival and Development of Host Antitumor Immunity in Murine Ovarian Cancer. J. Immunol. 2008;180:72–78. doi: 10.4049/jimmunol.180.1.72. [DOI] [PubMed] [Google Scholar]
- 36.Groh V, Wu J, Yee C, Spies T. Tumour-derived soluble MIC ligands impair expression of NKG2D and T-cell activation. Nature. 2002;419:734–738. doi: 10.1038/nature01112. [DOI] [PubMed] [Google Scholar]
- 37.Castriconi R, Cantoni C, Della Chiesa M, Vitale M, Marcenaro E, Conte R, Biassoni R, Bottino C, Moretta L, Moretta A. Transforming growth factor beta 1 inhibits expression of NKp30 and NKG2D receptors: consequences for the NK-mediated killing of dendritic cells. Proc. Natl. Acad. Sci. U S A. 2003;100:4120–4125. doi: 10.1073/pnas.0730640100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Eisele G, Wischhusen J, Mittelbronn M, Meyermann R, Waldhauer I, Steinle A, Weller M, Friese MA. TGF-beta and metalloproteinases differentially suppress NKG2D ligand surface expression on malignant glioma cells. Brain. 2006;129:2416–2425. doi: 10.1093/brain/awl205. [DOI] [PubMed] [Google Scholar]
- 39.Morand EF, Leech M, Bernhagen J. MIF: a new cytokine link between rheumatoid arthritis and atherosclerosis. Nat. Rev. Drug Discov. 2006;5:399–410. doi: 10.1038/nrd2029. [DOI] [PubMed] [Google Scholar]
- 40.Brummelkamp TR, Bernards R, Agami R. A system for stable expression of short interfering RNAs in mammalian cells. Science. 2002;296:550–553. doi: 10.1126/science.1068999. [DOI] [PubMed] [Google Scholar]
- 41.Valiante NM, Rengaraju M, Trinchieri G. Role of the production of natural killer cell stimulatory factor (NKSF/IL-12) in the ability of B cell lines to stimulate T and NK cell proliferation. Cell. Immunol. 1992;145:187–198. doi: 10.1016/0008-8749(92)90322-g. [DOI] [PubMed] [Google Scholar]
- 42.Meyer-Siegler KL, Leifheit EC, Vera PL. Inhibition of macrophage migration inhibitory factor decreases proliferation and cytokine expression in bladder cancer cells. BMC Cancer. 2004;4:34. doi: 10.1186/1471-2407-4-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sheehy ME, McDermott AB, Furlan SN, Klenerman P, Nixon DF. A novel technique for the fluorometric assessment of T lymphocyte antigen specific lysis. J. Immunol. Methods. 2001;249:99–110. doi: 10.1016/s0022-1759(00)00329-x. [DOI] [PubMed] [Google Scholar]
- 44.Brown CE, Wright CL, Naranjo A, Vishwanath RP, Chang WC, Olivares S, Wagner JR, Bruins L, Raubitschek A, Cooper LJ, Jensen MC. Biophotonic cytotoxicity assay for high-throughput screening of cytolytic killing. J. Immunol. Methods. 2005;297:39–52. doi: 10.1016/j.jim.2004.11.021. [DOI] [PubMed] [Google Scholar]
- 45.Bernhagen J, Mitchell RA, Calandra T, Voelter W, Cerami A, Bucala R. Purification, bioactivity, and secondary structure analysis of mouse and human macrophage migration inhibitory factor (MIF). Biochemistry. 1994;33:14144–14155. doi: 10.1021/bi00251a025. [DOI] [PubMed] [Google Scholar]
- 46.Uhl M, Aulwurm S, Wischhusen J, Weiler M, Ma JY, Almirez R, Mangadu R, Liu YW, Platten M, Herrlinger U, Murphy A, Wong DH, Wick W, Higgins LS, Weller M. SD-208, a novel transforming growth factor beta receptor I kinase inhibitor, inhibits growth and invasiveness and enhances immunogenicity of murine and human glioma cells in vitro and in vivo. Cancer Res. 2004;64:7954–7961. doi: 10.1158/0008-5472.CAN-04-1013. [DOI] [PubMed] [Google Scholar]
- 47.Agarwal R, Whang DH, Alvero AB, Visintin I, Lai Y, Segal EA, Schwartz P, Ward D, Rutherford T, Mor G. Macrophage migration inhibitory factor expression in ovarian cancer. Am. J. Obstet. Gynecol. 2007;196:348, e341–345. doi: 10.1016/j.ajog.2006.12.030. [DOI] [PubMed] [Google Scholar]
- 48.Bozza M, Satoskar AR, Lin G, Lu B, Humbles AA, Gerard C, David JR. Targeted disruption of migration inhibitory factor gene reveals its critical role in sepsis. J. Exp. Med. 1999;189:341–346. doi: 10.1084/jem.189.2.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fan J, Chen Y, Chan HM, Tam PK, Ren Y. Removing intensity effects and identifying significant genes for Affymetrix arrays in macrophage migration inhibitory factor-suppressed neuroblastoma cells. Proc. Natl. Acad. Sci. U S A. 2005;102:17751–17756. doi: 10.1073/pnas.0509175102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mitchell RA, Liao H, Chesney J, Fingerle-Rowson G, Baugh J, David J, Bucala R. Macrophage migration inhibitory factor (MIF) sustains macrophage proinflammatory function by inhibiting p53: regulatory role in the innate immune response. Proc. Natl. Acad. Sci. U S A. 2002;99:345–350. doi: 10.1073/pnas.012511599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Leech M, Lacey D, Xue JR, Santos L, Hutchinson P, Wolvetang E, David JR, Bucala R, Morand EF. Regulation of p53 by macrophage migration inhibitory factor in inflammatory arthritis. Arthritis Rheum. 2003;48:1881–1889. doi: 10.1002/art.11165. [DOI] [PubMed] [Google Scholar]
- 52.Powell ND, Papenfuss TL, McClain MA, Gienapp IE, Shawler TM, Satoskar AR, Whitacre CC. Cutting edge: macrophage migration inhibitory factor is necessary for progression of experimental autoimmune encephalomyelitis. J. Immunol. 2005;175:5611–5614. doi: 10.4049/jimmunol.175.9.5611. [DOI] [PubMed] [Google Scholar]
- 53.Ren Y, Chan HM, Fan J, Xie Y, Chen YX, Li W, Jiang GP, Liu Q, Meinhardt A, Tam PK. Inhibition of tumor growth and metastasis in vitro and in vivo by targeting macrophage migration inhibitory factor in human neuroblastoma. Oncogene. 2006;25:3501–3508. doi: 10.1038/sj.onc.1209395. [DOI] [PubMed] [Google Scholar]
- 54.Roger T, David J, Glauser MP, Calandra T. MIF regulates innate immune responses through modulation of Toll-like receptor 4. Nature. 2001;414:920–924. doi: 10.1038/414920a. [DOI] [PubMed] [Google Scholar]