Abstract
Portal hypertension (PHT) is defined as a pathological increase in portal venous pressure and frequently accompanies cirrhosis. Portal pressure can be increased by a rise in portal blood flow, an increase in vascular resistance, or the combination. In cirrhosis, the primary factor leading to PHT is an increase in intra-hepatic resistance to blood flow. Although much of this increase is a mechanical consequence of architectural disturbances, there is a dynamic and reversible component that represents up to a third of the increased vascular resistance in cirrhosis. Many vasoactive substances contribute to the development of PHT. Among these, nitric oxide (NO) is the key mediator that paradoxically regulates the sinusoidal (intra-hepatic) and systemic/splanchnic circulations. NO deficiency in the liver leads to increased intra-hepatic resistance while increased NO in the circulation contributes to the hyperdynamic systemic/splanchnic circulation. NO mediated-angiogenesis also plays a role in splanchnic vasodilation and collateral circulation formation. NO donors reduce PHT in animals models but the key clinical challenge is the development of an NO donor or drug delivery system that selectively targets the liver.
Keywords: Nitric oxide, Portal hypertension, Hepatic stellate cell, Liver cirrhosis
INTRODUCTION
Portal hypertension (PHT) is a common clinical consequence of chronic liver disease that is associated with significant morbidity and mortality. PHT is classified as either pre-hepatic, intra-hepatic or post-hepatic, with intra-hepatic PHT being the form most often caused by cirrhosis, irrespective of etiology[1]. The extent of PHT is quantified in clinical practice by measuring the hepatic portal vein pressure gradient (HPVG)[2], representing the difference between the wedged hepatic vein pressure (a measure of pressure at the level of the hepatic sinusoid), and the free hepatic vein pressure. Thus, HPVG is often used to assess the effects of pharmacological therapy in reducing portal pressure[3].
Based on hydromechanics, fluid pressure in a hollow tube is determined by fluid resistance and flow. In PHT, therefore, the intra-hepatic vascular resistance (IHVR) and splanchnic blood flow are the two main contributors to portal pressure[4]. Under normal circumstances, postprandial increases in splanchnic blood flow is always associated with an autonomous down-regulation of IHVR, leading to no alteration in portal pressure. In contrast, IHVR is significantly up-regulated by mechanical and hemodynamic factors in the setting of cirrhosis, which is further aggravated by splanchnic vasodilation[5]. Clinically, this increase in portal pressure is the antecedent to variceal bleeding with its associated morbidity and high mortality[6,7].
IHVR is influenced by both hepatic fibrotic architectural distortion in cirrhosis leading to obstruction to blood flow, as well as by dynamic hepatic stellate cell (HSC) contraction around sinusoidal blood vessels. Angiogenesis, or the formation of new blood vessels, is also an important component of the pathophysiology of PHT. The resulting alterations in vascular contractility and angiogenesis contribute to PHT in both the intra-hepatic and splanchnic circulation.
Endothelin 1 (ET-1), angiotensin II, norepinephrine, prostaglandin F2, thromboxane A2, and thrombin can trigger liver sinusoidal contraction. In contrast, substances such as acetylcholine, vasointestinal peptides, nitric oxide (NO), carbon monoxide, prostaglandin E2, and adrenomedullin relax the sinusoidal vasculature[8,9]. Among these agents, ET-1 and NO are the most important regulators of the sinusoidal microcirculation[8,9]. In PHT, an insufficient release of vasodilators particularly NO from endothelial cells is critical to the genesis of the dynamic and modifiable component of increased vascular resistance[8,9]. Consistent with this, improvements in intra-hepatic NO availability is beneficial for the treatment of PHT in animals and patients[10-14]. Hence, this review will focus on an update on the mechanisms whereby NO mediates PHT and on the potential to modulate this system to reduce portal pressure.
SYNTHESIS AND FUNCTION OF NO
NO is synthesized by nitric oxide synthase (NOS) through a series of redox reactions involving L-arginine (the main substrate), oxygen and nicotinamide adenine dinucleotide phosphate. There are 4 major isoforms of NOS: endothelial nitric oxide synthase (eNOS), inducible nitric oxide synthase (iNOS), neuronal nitric oxide synthase (nNOS) and mitochondrial nitric oxide synthase[15]. Following synthesis by NOS, the half-life of endogenously generated NO is extremely short, about 1 s. Thus, endogenous NO production is intimately regulated by the activity of NOS.
The generated NO molecule has a large diffusion coefficient and can therefore freely penetrate cellular membranes in an autocrine or paracrine manner. Within the cell, NO stimulates the conversion of guanosine 5’-triphosphate (GTP) to cyclic guanosine 3’-5’-monophosphate (cGMP), thereby regulating calcium balance through the cGMP-dependent protein kinase pathway (Figure 1). This leads to vasodilatation[16]. The end products of NO metabolism in vivo are nitrate (NO3-) and nitrite (NO2-) that are an indirect measure of the total NO concentration[17].
Figure 1.
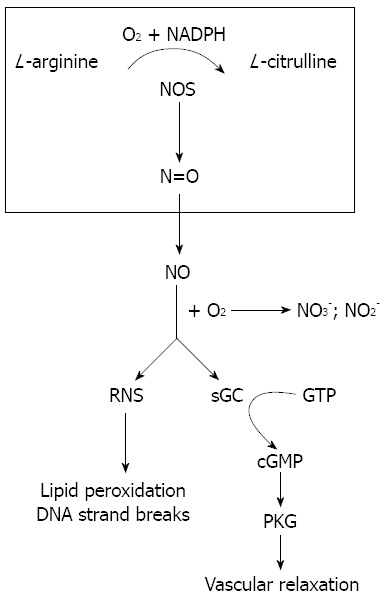
Nitric oxide formation and function. Nitric oxide synthase (NOS) catalyzes the biosynthesis of nitric oxide (NO) from L-arginine, nicotinamide adenine dinucleotide phosphate (NADPH) and O2•NO freely diffuses into cells where it mediates vascular relaxation by stimulating the cyclic guanosine 3’-5’-monophosphate (cGMP)/cGMP-dependent protein kinase G (PKG) pathway. It also forms reactive nitrogen species (RNS) which leads to many damaging reactions including lipid peroxidation and DNA strand breaks. GTP: Guanosine 5’-triphosphate; sGC: Soluble guanylyl cyclase.
NO is also highly reactive with other molecules including superoxide anion (O2-), oxygen (O2) and hemoproteins such as hemoglobin and myoglobin. The intermediate products of these reactions are known as reactive nitrogen species, which promotes many pathophysiologically damaging reactions including lipid peroxidation, DNA strand breaks, and the generation of nitrosamines, nitrotyrosine and nitro guanosine.
MOLECULAR MECHANISMS REGULATING NOS
eNOS serves a key role in maintaining circulatory homeostasis and is expressed mainly in endothelial cells and to a lesser extent in cardiac myocytes and platelets[15]. The enzyme localizes to small invaginations of the plasma membrane named caveolae in quiescent cells. eNOS protein is constitutively expressed in the cell and activation mostly comprises post-translational regulation and modifications in its subcellular localization[18].
Within cells, eNOS closely associates with several proteins that impact on its function, including caveolin. Caveolin negatively regulates eNOS by directly abrogating the enzyme’s activation and blocking the binding site for calmodulin[19]. In contrast, calmodulin acts as an indispensable protein competing with caveolin for binding with, and activating eNOS[20,21]. Other relevant proteins in relation to NO production include heat shock protein 90 and tetrahydrobiopterin (BH4) that are positive regulators of eNOS[22-24]. Finally, eNOS interacting protein and eNOS trafficking inducer protein participate in the sub-cellular trafficking of eNOS when eNOS translocates from caveolae into the cytoplasm[25-27].
Phosphorylation at key serine residues is the major post-translational modification that is required for eNOS function. Phosphorylation of Ser 1177, Ser 635 and Ser 617 activates eNOS whereas phosphorylation of Thr 495 and Ser 116 inhibits eNOS activity[28]. Phosphorylation at Ser 1177 can be initiated by activation of several intracellular pathways including phosphatidylinositol-3-kinase (PI3K/AKT), cAMP-dependent protein kinase A (PKA), adenosine monophosphate-activated protein kinase, cGMP-dependent protein kinase G (PKG) and Calmodulin Kinase II-dependent pathway (CaM kinase II)[29-32], while Ser 635 and Ser 116 is activated via a PKA-dependent pathway[33,34]. Additionally, shear stress, vascular endothelial growth factor (VEGF) and high-density fatty acids can phosphorylate and activate eNOS (Figure 2)[35]. In contrast, phosphatases like protein phosphatase 2 dephosphorylates and inactivates eNOS[36]. S-Nitrosylation inhibits eNOS activity by modifying its steric configuration, whereas de-nitrosylation is associated with an increase in eNOS activity[37,38].
Figure 2.

The molecular regulation of endothelial nitric oxide synthase activity. Endothelial nitric oxide synthase (eNOS) phosphorylation can be triggered by shear stress, vascular endothelial growth factor (VEGF), endothelin 1 (ET-1) and other factors though adenosine monophosphate-activated protein kinase (AMPK), protein kinase B (AKT) and protein kinase A (PKA) pathways, whereas protein phosphatase 2 (PP2A) de-phosphorylates eNOS. In addition, S-nitrosylation (SNOs) by eNOS-derived nitric oxide (NO) inhibits eNOS activity. Endothelial nitric oxide synthase interacting protein (NOSIP) and endothelial nitric oxide synthase trafficking inducer protein (NOSTRIN) regulate the sub-cellular location of eNOS protein between the caveolae and cytoplasm. The principal location of eNOS is in caveolae where its function is inhibited by binding to caveolin (Cav). HSP90, calmodulin (Calm) and tetrahydrobiopterin (BH4) are indispensable proteins and cofactors for catalyzing NO production. PI3K: Phosphatidylinositol-3-kinase; GPCR: G protein-coupled receptor.
Unlike eNOS, iNOS is more widely expressed, including in macrophages, vascular smooth muscle cells, HSCs and Kupffer cells after stimulated by lipopolysaccharide (LPS) or inflammatory cytokines. iNOS produces a relatively high level of NO compared to eNOS[39]. In contrast to eNOS, iNOS expression is principally modulated by transcriptional mechanisms. Many transcription factors regulate the expression of iNOS including nuclear factor-κB (NF-κB), activator protein, signal transduction and activation of transcription 1a, specificity protein 1, CCAAT/enhancer-binding protein (C/EBP), cAMP response element-binding, GATA binding transcription factor, hypoxia-inducible factor, interferon regulatory transcription factor, nuclear factor of activated T-cells, nuclear factor-interleukin 6, octamer-1 transcription factor, poly [ADP-ribose] polymerase 1, polyomavirus enhancer activator 3, tumor protein 53 and serum response factor[40]. Among these, NF-κB is considered the primary mediator for iNOS induction. In turn, NF-κB can be activated by a range of stimuli including LPS, interleukin-1β, tumor necrosis factor (TNF)-α and oxidative stress[41,42]. iNOS can also be post-transcriptionally regulated through mRNA stabilisation by RNA-binding proteins such as A+U rich RNA binding factor, human antigen R, K-homology splicing regulator protein, polypyrimidine tract-binding protein and tristetraprolin[40].
nNOS is principally expressed in neurons localized to the nervous system including the brain, the autonomic nervous system and neurons around interlobular arteries. The portal vein endothelial cells also expresses nNOS[43]. Like eNOS, nNOS is regulated by post-translational mechanisms and both Hsp90 and calmodulin are involved in the process of nNOS activation[44-46].
MOLECULAR REGULATION OF NOS IN LIVER CIRRHOSIS AND PHT
Regulation of intra-hepatic eNOS
In cirrhosis and PHT, there is reduced NO production by hepatic endothelial cells that is attributed to dysfunction of the eNOS system[47-50]. Many factors contribute to intra-hepatic eNOS dysfunction/reduced eNOS activity. These include increases in oxidative stress, caveolin-1, RhoA, thromboxane A2 (TXA2), G-protein-coupled receptor kinase-2 (GRK2) and asymmetric dimethylarginine (ADMA) as well as decreased AKT and BH4 activity.
Reduced AKT activity and increased binding ability of caveolin-1 to eNOS in cirrhosis attenuates eNOS expression[51,52]. Liu et al[51], reported that ET-1 activates G-protein-coupled receptor kinase-2 (GRK2) which directly interacts with and inhibits AKT phosphorylation. They also noted that the IHVR was significantly reduced in bile duct ligation (BDL) mice genetically deficient in GRK2[52]. In another study of eNOS expression during BDL, Morvarid et al[53], noted that total eNOS protein was unchanged, but that functional, phosphorylated eNOS protein was decreased. Similarly, AKT expression was down-regulated in a time dependent manner. In contrast, caveolin-1 was increased[53].
Intrahepatic oxidative stress is a key mediator of sinusoidal endothelial dysfunction and impairment of eNOS/NO expression[54-57]. For example, Gracia et al[58], noted that increased intrahepatic oxidative stress (increased ROS and O2-) was associated with reduced NO production and NO bioavailability. The authors went on to demonstrate that cyclooxygenase (COX) attenuated eNOS activation by stimulating TXA2 which inhibits AKT phosphorylation in endothelial cells[59]. A superoxide dismutase mimetic, Tempol significantly decreased superoxide, and increased NO in cultured hepatic endothelial cells. As expected, Tempol administration also resulted in a decline of portal pressure[60].
ADMA, an endogenous inhibitor of NOS, causes uncoupling of NOS leading to generation of RNS, such as peroxynitrite. In BDL rats, a higher serum ADMA level was observed[61]. Further, impaired endothelial cell-mediated relaxation in perfused livers of BDL rats was exacerbated by ADMA and was associated with a decreased rate of ADMA removal[61,62].
BH4, a cofactor of eNOS, has been reported to be associated with dysfunction of the NO system. BH4 expression is down-regulated in liver cirrhosis and can further be oxidized and inactivated by O2-. In the absence of BH4, eNOS cannot generate NO but instead produces O2-, thereby leading to further decreases in NO production[24,63]. In an in vivo study, Matei et al, observed that in rats rendered cirrhotic after the administration of carbon tetrachloride (CCl4), exogenous BH4 resulted in hepatic NOS and cGMP activation and a reduction in portal pressure[64].
Rho-associated protein kinase (ROCK) is a kinase belonging to the AGC (PKA/PKG/PKC) family of serine-threonine kinases. It is mainly involved in regulating the shape and movement of cells by acting on the cytoskeleton. Rho-kinase is substantially involved in the contraction of activated HSCs[65,66]. In BDL rats, fasudil (a potent Rho-kinase inhibitor) significantly suppressed liver Rho-kinase activity and increased eNOS phosphorylation compared with controls[67]. Fasudil also reduced the binding of the serine/threonine AKT to Rho-kinase and increased the binding of AKT to eNOS[67].
Regulation of extra-hepatic vascular eNOS, iNOS and nNOS in cirrhosis
In contrast to the hypoactive SECs in the intrahepatic microcirculation, hyperactive endothelial cells with increased NO production play a critical role in modulating the vascular changes observed in the splanchnic and systemic circulation. For example, increased activity of peripheral vascular AKT signaling is noted, while constitutive AKT inhibition by an inactive mutant decreases aortic eNOS and improves systemic hemodynamics, splanchnic perfusion pressure and renal excretory function without affecting portal pressure[68]. Other studies reported that VEGF induces NO production by activation of eNOS protein expression and activity[69,70]. Likewise, in portal hypertensive rats, NO production is increased in response to shear stress[71]. LPS detoxification is limited in liver with PHT thereby increasing plasma LPS. Resident macrophages in the splanchnic circulation respond to this circulating LPS with the production of proinflammatory cytokines, such as TNF-α[72] that then induces iNOS in extrahepatic vasculature[73-75]. Bacteria-derived TNF-α also triggers the expression and activity of the key enzyme involved in the regulation of BH4, GTP-cyclohydrolase I, thereby increasing eNOS-derived NO in the mesenteric vasculature[76,77]. Finally, nNOS expression is augmented in mesenteric nerves in portal hypertensive rats (portal vein ligation), an effect mediated by HSP-90[46,78,79].
THE ROLE OF NO/NOS IN THE REGULATION OF IHVR
An increase in IHVR can be induced by reversible hemodynamic modifications to vascular tone which may represent 28%-40% of the increase in portal pressure in cirrhosis[80-82]. Anatomic structures leading to this change include vascular smooth muscle cells surrounding branches of the portal vein, and HSCs located in the space of Disse. Both cells types have contractile properties and thus modulate IHVR[82-84].
The role of NO in the modulation of IHVR has been well documented[85-87]. eNOS dysfunction in sinusoidal endothelial cells and consequent reduction in NO production (or bioavailability) plays an essential role[51]. This results in reduced vasodilation and a decreased capacity for antagonizing contractile factors such as ET-1, angiotensin II, norepinephrine, prostaglandin F2, and thromboxane A2[83,88].
Recently, gene delivery techniques have been used to increase NOS (eNOS or nNOS) delivery to the liver of CCl4 treated mice. In one study, a plasmid eukaryotic expression vector (liposome-pcDNA3/eNOS) or control vector was injected into rat portal vein, leading to increased eNOS mRNA and protein in liver. Hepatic NO production was enhanced and IHVR and portal vein pressure (PVP) reduced[89]. In another study, recombinant adenovirus carrying the nNOS gene (Ad.nNOS) or control vector was administered via the femoral vein to rats. Again, Ad.nNOS reduced IHVR and portal pressure[90]. These data indicate that NO deficiency in cirrhotic liver contributes to the elevation in IHVR and conversely that NO delivery may play a therapeutic role[89-92].
Activation and contraction of HSCs also contributes significantly to the dynamic and reversible component of IHVR. Indeed, activated HSCs are more susceptible to vasoconstrictor substances than quiescent cells[83,92,93]. Under physiological conditions, NO produced by hepatic endothelial cells inhibits the growth, migration and contraction of HSCs through paracrine pathways[94,95]. However, reduced NO production and/or impaired NO bioavailability in cirrhosis promotes HSCs activation and contraction, leading to sinusoidal remodeling and elevation of the IHVR.
iNOS has also been suggested to contribute to the hyperdynamic status seen in PHT. However, its role in mediating IHVR is unclear. In one study, liver iNOS was increased in BDL rats and reduction of portal pressure by ursodeoxycholic acid was associated with iNOS down-regulation[96,97].
ROLE OF NO/NOS IN THE REGULATION OF SPLANCHNIC BLOOD FLOW
A hyperdynamic splanchnic circulatory state is a major accompaniment of PHT. The increase in splanchnic blood flow and the subsequent increase in portal venous inflow aggravates and perpetuates PHT. The mechanisms underlying this phenomenon are not fully understood, but overproduction of endogenous vasodilators and decreased vascular reactivity to vasoconstrictors has been suggested[98].
Overproduction of NO in the splanchnic and systemic circulation contributes to this phenomenon as NOS inhibition effectively ameliorates splanchnic hyperemia[99,100]. eNOS up-regulation and increased NO release by the superior mesenteric arteries endothelium occur before the development of the hyperdynamic splanchnic circulation[101]. Juan et al[70], noted increased eNOS expression in portal-hypertensive rats with even mild increases in portal pressure. In another study, phosphorylated eNOS protein was increased, whereas caveolin-1 was decreased in the aorta of BDL rats[52]. In contrast, in eNOS knockout mice injected with CCL4, attenuated splanchnic blood flow was observed. However, this was associated with an increase in IHVR, presumably due to the reduced NO within the liver[102]. Taken together, these results suggest up-regulated eNOS expression during splanchnic hyperemia, contrasts with the relative eNOS deficiency in liver.
There are also several studies demonstrating the importance of iNOS in the hyperdynamic circulation of cirrhosis[64,72,73,103,104]. In cirrhosis, endotoxins, cytokines and bacterial infection promote iNOS formation and overproduction of NO[64,105-107]. The increased splanchnic iNOS appears to reside in resident macrophages of the superior mesenteric artery[73,108]. Supporting this concept, Ferguson et al[64], observed that a selective iNOS inhibitor, N-[3-(aminomethyl) benzyl]acetamidine, caused peripheral vasoconstriction in patients with cirrhosis. It is interesting to note that there also exists an interaction between eNOS and iNOS in the vasculature. For example, in cirrhosis, increased and dominant expression of eNOS in large arteries results in systemic hypotension and increased blood flow. These effects could be abrogated by activated iNOS in the small vessels of the splanchnic circulation as iNOS activation inhibited eNOS expression in the small vessels[109]. nNOS may likewise promote vasodilation of the splanchnic circulation, though its contribution is overall less significant[110,111].
NO AND ANGIOGENESIS IN PHT
It is now established that angiogenesis is associated with the progression of PHT[112,113]. Angiogenic factors stimulate collateral vessel formation both in the liver and in extrahepatic locations, manifesting as the reopening of pre-existing shunts[114,115]. This pathological angiogenesis may directly participate in the development of liver fibrosis[56,116,117].
Again, NO is an important mediator of intrahepatic microcirculatory remodeling[114,115]. Thus, NO inhibition prevents angiogenesis and diminishes mesenteric vascular proliferation in animals with PHT[118,119]. Shaki et al[120], found that NO-mediated angiogenesis was mediated by endothelial VEGF and VEGF receptor-1. Most recently, Huang et al[121], reported that through mesenteric eNOS and COX1 down-regulation, the cannabinoid receptor 2 agonist JWH 015, could alleviate mesenteric and intrahepatic angiogenesis, PHT, the severity of portosystemic collaterals and the extent of fibrosis in BDL cirrhotic rats.
NO-BASED PHARMACOTHERAPY
As discussed, NO is paradoxically regulated in PHT. There is excessive production of NO in the splanchnic circulation (thereby leading to vasodilation), while in the intra-hepatic microcirculation, a deficit of NO production is associated with increased IHVR. These paradoxical roles of NO initially raised concerns about the use of NO inhibitors or donors as therapy for PHT. However, inhibition of NO release has been shown in animals and humans to attenuate the hyperdynamic circulation of cirrhosis[122-125]. No significant reduction in portal pressure was achieved[122-125]. This is likely a consequence of reductions in portal venous inflow induced by the NO inhibitors being offset by an increase in intra-hepatic resistance.
In recent years, many animal and clinical studies have demonstrated that NO donors result in a substantial reduction in portal pressure[10-14]. These agents could theoretically aggravate the cirrhotic vasodilatory syndrome leading to harmful effects such as systemic hypotension and renal dysfunction[126,127]. For these reasons, the ideal NO drug for the treatment of PHT should act to decrease IHVR without worsening splanchnic/systemic vasodilatation[128].
NCX-1000 is a drug synthesized by adding an NO-releasing moiety to ursodeoxycholic acid. The compound is selectively metabolized by hepatocytes to release NO in the liver[129,130]. Animal studies demonstrate that this drug alleviates IHVR and portal pressure without changes in systemic hemodynamics[129-131]. However, human clinic trials were disappointing as NCX-1000 failed to decrease HVPG, there were postprandial increases in portal pressure and systolic blood pressure was reduced in a dose-dependent manner[132].
O2-vinyl1-(pyrrolidin-1-yl)diazen-1-ium-1,2-diolate (V-PYRRO/NO) was designed as a liver-selective NO-producing pro-drug activated by hepatic P450s[133]. The drug has a short half-life and may additionally alleviate liver injury by NO-mediated protection of hepatocytes[134-136]. Continuous administration of V-PYRRO/NO to BDL rats was shown to improve liver fibrosis and splanchnic hemodynamics without adverse systemic effects[137]. However, in another study in mice using the CCl4 model, V-PYRRO/NO significantly lowered mean arterial pressure making it less suitable for use in humans[138].
AVE-9488(4-fluoro-N-indan-2-yl-benzamide) is a novel agent that up-regulates eNOS expression[139]. Biecker et al[139], reported that oral application of AVE 9488 ameliorated portal pressure by 24% in BDL rats, without any impact on the mean arterial pressure. Additional experiments confirmed that AVE 9488 increased hepatic eNOS protein synthesis, but not in the aortic and superior mesenteric artery[139]. However, following 3-d use, AVE 9488 increased blood flow in the collateral circulation[139].
Recently, an inorganic gold and silica nanoparticle mediated drug delivery system using SNAP (S-nitroso-N-acetyl-DL-penicillamine), an NO donor was reported[140]. This system inhibited HSC proliferation and HSC tube formation, though the relevance of the latter to the situation in vivo is unclear. The methodology described however, does provide a novel approach to deliver NO into specific liver cell types. Whether this drug modulates PHT in vivo is unclear. Taken together, the data presented indicates that there are no liver-selective NO donors/drugs with demonstrated efficacy for the treatment of PHT.
CONCLUSION
NO plays a pivotal role in the pathogenesis of PHT. NO levels are differentially altered in cirrhosis, with reduced production in the intrahepatic circulation and increased NO production in the splanchnic bed. Ideally, a NO donor or drug delivery system that selectively targets liver cells (HSCs or SECs) without actions on the systemic circulation is required to reduce PHT without adverse systemic effects.
Footnotes
P- Reviewer Hartleb M S- Editor Wen LL L- Editor A E- Editor Zhang DN
References
- 1.Buob S, Johnston AN, Webster CR. Portal hypertension: pathophysiology, diagnosis, and treatment. J Vet Intern Med. 2011;25:169–186. doi: 10.1111/j.1939-1676.2011.00691.x. [DOI] [PubMed] [Google Scholar]
- 2.Burroughs AK, Thalheimer U. Hepatic venous pressure gradient in 2010: optimal measurement is key. Hepatology. 2010;51:1894–1896. doi: 10.1002/hep.23710. [DOI] [PubMed] [Google Scholar]
- 3.Garcia-Tsao G, Bosch J, Groszmann RJ. Portal hypertension and variceal bleeding--unresolved issues. Summary of an American Association for the study of liver diseases and European Association for the study of the liver single-topic conference. Hepatology. 2008;47:1764–1772. doi: 10.1002/hep.22273. [DOI] [PubMed] [Google Scholar]
- 4.Cichoz-Lach H, Celiński K, Słomka M, Kasztelan-Szczerbińska B. Pathophysiology of portal hypertension. J Physiol Pharmacol. 2008;59 Suppl 2:231–238. [PubMed] [Google Scholar]
- 5.Moneta GL, Taylor DC, Helton WS, Mulholland MW, Strandness DE. Duplex ultrasound measurement of postprandial intestinal blood flow: effect of meal composition. Gastroenterology. 1988;95:1294–1301. doi: 10.1016/0016-5085(88)90364-2. [DOI] [PubMed] [Google Scholar]
- 6.Albillos A, Bañares R, González M, Catalina MV, Pastor O, Gonzalez R, Ripoll C, Bosch J. The extent of the collateral circulation influences the postprandial increase in portal pressure in patients with cirrhosis. Gut. 2007;56:259–264. doi: 10.1136/gut.2006.095240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bellis L, Berzigotti A, Abraldes JG, Moitinho E, García-Pagán JC, Bosch J, Rodés J. Low doses of isosorbide mononitrate attenuate the postprandial increase in portal pressure in patients with cirrhosis. Hepatology. 2003;37:378–384. doi: 10.1053/jhep.2003.50053. [DOI] [PubMed] [Google Scholar]
- 8.Rockey DC. Hepatic fibrosis, stellate cells, and portal hypertension. Clin Liver Dis. 2006;10:459–79, vii-viii. doi: 10.1016/j.cld.2006.08.017. [DOI] [PubMed] [Google Scholar]
- 9.Iwakiri Y, Groszmann RJ. Vascular endothelial dysfunction in cirrhosis. J Hepatol. 2007;46:927–934. doi: 10.1016/j.jhep.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 10.Laleman W, Van Landeghem L, Van der Elst I, Zeegers M, Fevery J, Nevens F. Nitroflurbiprofen, a nitric oxide-releasing cyclooxygenase inhibitor, improves cirrhotic portal hypertension in rats. Gastroenterology. 2007;132:709–719. doi: 10.1053/j.gastro.2006.12.041. [DOI] [PubMed] [Google Scholar]
- 11.Abraldes JG, Rodríguez-Vilarrupla A, Graupera M, Zafra C, García-Calderó H, García-Pagán JC, Bosch J. Simvastatin treatment improves liver sinusoidal endothelial dysfunction in CCl4 cirrhotic rats. J Hepatol. 2007;46:1040–1046. doi: 10.1016/j.jhep.2007.01.020. [DOI] [PubMed] [Google Scholar]
- 12.Trebicka J, Hennenberg M, Laleman W, Shelest N, Biecker E, Schepke M, Nevens F, Sauerbruch T, Heller J. Atorvastatin lowers portal pressure in cirrhotic rats by inhibition of RhoA/Rho-kinase and activation of endothelial nitric oxide synthase. Hepatology. 2007;46:242–253. doi: 10.1002/hep.21673. [DOI] [PubMed] [Google Scholar]
- 13.Abraldes JG, Albillos A, Bañares R, Turnes J, González R, García-Pagán JC, Bosch J. Simvastatin lowers portal pressure in patients with cirrhosis and portal hypertension: a randomized controlled trial. Gastroenterology. 2009;136:1651–1658. doi: 10.1053/j.gastro.2009.01.043. [DOI] [PubMed] [Google Scholar]
- 14.Zafra C, Abraldes JG, Turnes J, Berzigotti A, Fernández M, Garca-Pagán JC, Rodés J, Bosch J. Simvastatin enhances hepatic nitric oxide production and decreases the hepatic vascular tone in patients with cirrhosis. Gastroenterology. 2004;126:749–755. doi: 10.1053/j.gastro.2003.12.007. [DOI] [PubMed] [Google Scholar]
- 15.Dudzinski DM, Igarashi J, Greif D, Michel T. The regulation and pharmacology of endothelial nitric oxide synthase. Annu Rev Pharmacol Toxicol. 2006;46:235–276. doi: 10.1146/annurev.pharmtox.44.101802.121844. [DOI] [PubMed] [Google Scholar]
- 16.Wiest R, Groszmann RJ. The paradox of nitric oxide in cirrhosis and portal hypertension: too much, not enough. Hepatology. 2002;35:478–491. doi: 10.1053/jhep.2002.31432. [DOI] [PubMed] [Google Scholar]
- 17.Miles AM, Chen Y, Owens MW, Grisham MB. Fluorometric determination of nitric oxide. Methods. 1995;7:40–47. [Google Scholar]
- 18.Dudzinski DM, Michel T. Life history of eNOS: partners and pathways. Cardiovasc Res. 2007;75:247–260. doi: 10.1016/j.cardiores.2007.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Minshall RD, Sessa WC, Stan RV, Anderson RG, Malik AB. Caveolin regulation of endothelial function. Am J Physiol Lung Cell Mol Physiol. 2003;285:L1179–L1183. doi: 10.1152/ajplung.00242.2003. [DOI] [PubMed] [Google Scholar]
- 20.Shah V, Cao S, Hendrickson H, Yao J, Katusic ZS. Regulation of hepatic eNOS by caveolin and calmodulin after bile duct ligation in rats. Am J Physiol Gastrointest Liver Physiol. 2001;280:G1209–G1216. doi: 10.1152/ajpgi.2001.280.6.G1209. [DOI] [PubMed] [Google Scholar]
- 21.Chen PF, Wu KK. Characterization of the roles of the 594-645 region in human endothelial nitric-oxide synthase in regulating calmodulin binding and electron transfer. J Biol Chem. 2000;275:13155–13163. doi: 10.1074/jbc.275.17.13155. [DOI] [PubMed] [Google Scholar]
- 22.Shah V, Wiest R, Garcia-Cardena G, Cadelina G, Groszmann RJ, Sessa WC. Hsp90 regulation of endothelial nitric oxide synthase contributes to vascular control in portal hypertension. Am J Physiol. 1999;277:G463–G468. doi: 10.1152/ajpgi.1999.277.2.G463. [DOI] [PubMed] [Google Scholar]
- 23.Matei V, Rodríguez-Vilarrupla A, Deulofeu R, Colomer D, Fernández M, Bosch J, Garcia-Pagán JC. The eNOS cofactor tetrahydrobiopterin improves endothelial dysfunction in livers of rats with CCl4 cirrhosis. Hepatology. 2006;44:44–52. doi: 10.1002/hep.21228. [DOI] [PubMed] [Google Scholar]
- 24.Ghafourifar P, Sen CK. Mitochondrial nitric oxide synthase. Front Biosci. 2007;12:1072–1078. doi: 10.2741/2127. [DOI] [PubMed] [Google Scholar]
- 25.Dedio J, König P, Wohlfart P, Schroeder C, Kummer W, Müller-Esterl W. NOSIP, a novel modulator of endothelial nitric oxide synthase activity. FASEB J. 2001;15:79–89. doi: 10.1096/fj.00-0078com. [DOI] [PubMed] [Google Scholar]
- 26.Zimmermann K, Opitz N, Dedio J, Renne C, Muller-Esterl W, Oess S. NOSTRIN: a protein modulating nitric oxide release and subcellular distribution of endothelial nitric oxide synthase. Proc Natl Acad Sci USA. 2002;99:17167–17172. doi: 10.1073/pnas.252345399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schilling K, Opitz N, Wiesenthal A, Oess S, Tikkanen R, Müller-Esterl W, Icking A. Translocation of endothelial nitric-oxide synthase involves a ternary complex with caveolin-1 and NOSTRIN. Mol Biol Cell. 2006;17:3870–3880. doi: 10.1091/mbc.E05-08-0709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bauer PM, Fulton D, Boo YC, Sorescu GP, Kemp BE, Jo H, Sessa WC. Compensatory phosphorylation and protein-protein interactions revealed by loss of function and gain of function mutants of multiple serine phosphorylation sites in endothelial nitric-oxide synthase. J Biol Chem. 2003;278:14841–14849. doi: 10.1074/jbc.M211926200. [DOI] [PubMed] [Google Scholar]
- 29.Fulton D, Gratton JP, Sessa WC. Post-translational control of endothelial nitric oxide synthase: why isn't calcium/calmodulin enough? J Pharmacol Exp Ther. 2001;299:818–824. [PubMed] [Google Scholar]
- 30.Michell BJ, Chen Zp T, Stapleton D, Katsis F, Power DA, Sim AT, Kemp BE. Coordinated control of endothelial nitric-oxide synthase phosphorylation by protein kinase C and the cAMP-dependent protein kinase. J Biol Chem. 2001;276:17625–17628. doi: 10.1074/jbc.C100122200. [DOI] [PubMed] [Google Scholar]
- 31.Chen ZP, Mitchelhill KI, Michell BJ, Stapleton D, Rodriguez-Crespo I, Witters LA, Power DA, Ortiz de Montellano PR, Kemp BE. AMP-activated protein kinase phosphorylation of endothelial NO synthase. FEBS Lett. 1999;443:285–289. doi: 10.1016/s0014-5793(98)01705-0. [DOI] [PubMed] [Google Scholar]
- 32.Fleming I, Fisslthaler B, Dimmeler S, Kemp BE, Busse R. Phosphorylation of Thr(495) regulates Ca(2+)/calmodulin-dependent endothelial nitric oxide synthase activity. Circ Res. 2001;88:E68–E75. doi: 10.1161/hh1101.092677. [DOI] [PubMed] [Google Scholar]
- 33.Michell BJ, Harris MB, Chen ZP, Ju H, Venema VJ, Blackstone MA, Huang W, Venema RC, Kemp BE. Identification of regulatory sites of phosphorylation of the bovine endothelial nitric-oxide synthase at serine 617 and serine 635. J Biol Chem. 2002;277:42344–42351. doi: 10.1074/jbc.M205144200. [DOI] [PubMed] [Google Scholar]
- 34.Boo YC, Hwang J, Sykes M, Michell BJ, Kemp BE, Lum H, Jo H. Shear stress stimulates phosphorylation of eNOS at Ser(635) by a protein kinase A-dependent mechanism. Am J Physiol Heart Circ Physiol. 2002;283:H1819–H1828. doi: 10.1152/ajpheart.00214.2002. [DOI] [PubMed] [Google Scholar]
- 35.Shaul PW. Regulation of endothelial nitric oxide synthase: location, location, location. Annu Rev Physiol. 2002;64:749–774. doi: 10.1146/annurev.physiol.64.081501.155952. [DOI] [PubMed] [Google Scholar]
- 36.Wei Q, Xia Y. Proteasome inhibition down-regulates endothelial nitric-oxide synthase phosphorylation and function. J Biol Chem. 2006;281:21652–21659. doi: 10.1074/jbc.M602105200. [DOI] [PubMed] [Google Scholar]
- 37.Erwin PA, Lin AJ, Golan DE, Michel T. Receptor-regulated dynamic S-nitrosylation of endothelial nitric-oxide synthase in vascular endothelial cells. J Biol Chem. 2005;280:19888–19894. doi: 10.1074/jbc.M413058200. [DOI] [PubMed] [Google Scholar]
- 38.Erwin PA, Mitchell DA, Sartoretto J, Marletta MA, Michel T. Subcellular targeting and differential S-nitrosylation of endothelial nitric-oxide synthase. J Biol Chem. 2006;281:151–157. doi: 10.1074/jbc.M510421200. [DOI] [PubMed] [Google Scholar]
- 39.MacMicking J, Xie QW, Nathan C. Nitric oxide and macrophage function. Annu Rev Immunol. 1997;15:323–350. doi: 10.1146/annurev.immunol.15.1.323. [DOI] [PubMed] [Google Scholar]
- 40.Pautz A, Art J, Hahn S, Nowag S, Voss C, Kleinert H. Regulation of the expression of inducible nitric oxide synthase. Nitric Oxide. 2010;23:75–93. doi: 10.1016/j.niox.2010.04.007. [DOI] [PubMed] [Google Scholar]
- 41.Mankan AK, Lawless MW, Gray SG, Kelleher D, McManus R. NF-kappaB regulation: the nuclear response. J Cell Mol Med. 2009;13:631–643. doi: 10.1111/j.1582-4934.2009.00632.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Basak S, Hoffmann A. Crosstalk via the NF-kappaB signaling system. Cytokine Growth Factor Rev. 2008;19:187–197. doi: 10.1016/j.cytogfr.2008.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Esteban FJ, Pedrosa JA, Jiménez A, Fernández AP, Bentura ML, Martínez-Murillo R, Rodrigo J, Peinado MA. Distribution of neuronal nitric oxide synthase in the rat liver. Neurosci Lett. 1997;226:99–102. doi: 10.1016/s0304-3940(97)00262-0. [DOI] [PubMed] [Google Scholar]
- 44.Billecke SS, Draganov DI, Morishima Y, Murphy PJ, Dunbar AY, Pratt WB, Osawa Y. The role of hsp90 in heme-dependent activation of apo-neuronal nitric-oxide synthase. J Biol Chem. 2004;279:30252–30258. doi: 10.1074/jbc.M403864200. [DOI] [PubMed] [Google Scholar]
- 45.Song Y, Zweier JL, Xia Y. Heat-shock protein 90 augments neuronal nitric oxide synthase activity by enhancing Ca2+/calmodulin binding. Biochem J. 2001;355:357–360. doi: 10.1042/0264-6021:3550357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Moleda L, Jurzik L, Froh M, Gäbele E, Hellerbrand C, Straub RH, Schölmerich J, Wiest R. Role of HSP-90 for increased nNOS-mediated vasodilation in mesenteric arteries in portal hypertension. World J Gastroenterol. 2010;16:1837–1844. doi: 10.3748/wjg.v16.i15.1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bates TE, Loesch A, Burnstock G, Clark JB. Mitochondrial nitric oxide synthase: a ubiquitous regulator of oxidative phosphorylation? Biochem Biophys Res Commun. 1996;218:40–44. doi: 10.1006/bbrc.1996.0008. [DOI] [PubMed] [Google Scholar]
- 48.Gupta TK, Toruner M, Chung MK, Groszmann RJ. Endothelial dysfunction and decreased production of nitric oxide in the intrahepatic microcirculation of cirrhotic rats. Hepatology. 1998;28:926–931. doi: 10.1002/hep.510280405. [DOI] [PubMed] [Google Scholar]
- 49.Petermann H, Vogl S, Schulze E, Dargel R. Chronic liver injury alters basal and stimulated nitric oxide production and 3H-thymidine incorporation in cultured sinusoidal endothelial cells from rats. J Hepatol. 1999;31:284–292. doi: 10.1016/s0168-8278(99)80226-8. [DOI] [PubMed] [Google Scholar]
- 50.Shah V, Toruner M, Haddad F, Cadelina G, Papapetropoulos A, Choo K, Sessa WC, Groszmann RJ. Impaired endothelial nitric oxide synthase activity associated with enhanced caveolin binding in experimental cirrhosis in the rat. Gastroenterology. 1999;117:1222–1228. doi: 10.1016/s0016-5085(99)70408-7. [DOI] [PubMed] [Google Scholar]
- 51.Liu S, Premont RT, Kontos CD, Zhu S, Rockey DC. A crucial role for GRK2 in regulation of endothelial cell nitric oxide synthase function in portal hypertension. Nat Med. 2005;11:952–958. doi: 10.1038/nm1289. [DOI] [PubMed] [Google Scholar]
- 52.Mohammadi MS, Thabut D, Cazals-Hatem D, Galbois A, Rudler M, Bonnefont-Rousselot D, Moreau R, Lebrec D, Tazi KA. Possible mechanisms involved in the discrepancy of hepatic and aortic endothelial nitric oxide synthases during the development of cirrhosis in rats. Liver Int. 2009;29:692–700. doi: 10.1111/j.1478-3231.2008.01909.x. [DOI] [PubMed] [Google Scholar]
- 53.Matveev S, Uittenbogaard A, van Der Westhuyzen D, Smart EJ. Caveolin-1 negatively regulates SR-BI mediated selective uptake of high-density lipoprotein-derived cholesteryl ester. Eur J Biochem. 2001;268:5609–5616. doi: 10.1046/j.1432-1033.2001.02496.x. [DOI] [PubMed] [Google Scholar]
- 54.Sanyal AJ. Mechanisms of Disease: pathogenesis of nonalcoholic fatty liver disease. Nat Clin Pract Gastroenterol Hepatol. 2005;2:46–53. doi: 10.1038/ncpgasthep0084. [DOI] [PubMed] [Google Scholar]
- 55.Cederbaum AI, Lu Y, Wu D. Role of oxidative stress in alcohol-induced liver injury. Arch Toxicol. 2009;83:519–548. doi: 10.1007/s00204-009-0432-0. [DOI] [PubMed] [Google Scholar]
- 56.Bataller R, Brenner DA. Liver fibrosis. J Clin Invest. 2005;115:209–218. doi: 10.1172/JCI24282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ashton AW, Ware JA. Thromboxane A2 receptor signaling inhibits vascular endothelial growth factor-induced endothelial cell differentiation and migration. Circ Res. 2004;95:372–379. doi: 10.1161/01.RES.0000138300.41642.15. [DOI] [PubMed] [Google Scholar]
- 58.García-Calderó H, Rodríguez-Vilarrupla A, Gracia-Sancho J, Diví M, Laviña B, Bosch J, García-Pagán JC. Tempol administration, a superoxide dismutase mimetic, reduces hepatic vascular resistance and portal pressure in cirrhotic rats. J Hepatol. 2011;54:660–665. doi: 10.1016/j.jhep.2010.07.034. [DOI] [PubMed] [Google Scholar]
- 59.Gao Y, Yokota R, Tang S, Ashton AW, Ware JA. Reversal of angiogenesis in vitro, induction of apoptosis, and inhibition of AKT phosphorylation in endothelial cells by thromboxane A(2) Circ Res. 2000;87:739–745. doi: 10.1161/01.res.87.9.739. [DOI] [PubMed] [Google Scholar]
- 60.Landmesser U, Dikalov S, Price SR, McCann L, Fukai T, Holland SM, Mitch WE, Harrison DG. Oxidation of tetrahydrobiopterin leads to uncoupling of endothelial cell nitric oxide synthase in hypertension. J Clin Invest. 2003;111:1201–1209. doi: 10.1172/JCI14172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Laleman W, Omasta A, Van de Casteele M, Zeegers M, Vander Elst I, Van Landeghem L, Severi T, van Pelt J, Roskams T, Fevery J, et al. A role for asymmetric dimethylarginine in the pathophysiology of portal hypertension in rats with biliary cirrhosis. Hepatology. 2005;42:1382–1390. doi: 10.1002/hep.20968. [DOI] [PubMed] [Google Scholar]
- 62.Yang YY, Lee TY, Huang YT, Chan CC, Yeh YC, Lee FY, Lee SD, Lin HC. Asymmetric dimethylarginine (ADMA) determines the improvement of hepatic endothelial dysfunction by vitamin E in cirrhotic rats. Liver Int. 2012;32:48–57. doi: 10.1111/j.1478-3231.2011.02651.x. [DOI] [PubMed] [Google Scholar]
- 63.Matei V, Rodríguez-Vilarrupla A, Deulofeu R, García-Calderó H, Fernández M, Bosch J, Garcia-Pagán JC. Three-day tetrahydrobiopterin therapy increases in vivo hepatic NOS activity and reduces portal pressure in CCl4 cirrhotic rats. J Hepatol. 2008;49:192–197. doi: 10.1016/j.jhep.2008.04.014. [DOI] [PubMed] [Google Scholar]
- 64.Ferguson JW, Dover AR, Chia S, Cruden NL, Hayes PC, Newby DE. Inducible nitric oxide synthase activity contributes to the regulation of peripheral vascular tone in patients with cirrhosis and ascites. Gut. 2006;55:542–546. doi: 10.1136/gut.2005.076562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hennenberg M, Biecker E, Trebicka J, Jochem K, Zhou Q, Schmidt M, Jakobs KH, Sauerbruch T, Heller J. Defective RhoA/Rho-kinase signaling contributes to vascular hypocontractility and vasodilation in cirrhotic rats. Gastroenterology. 2006;130:838–854. doi: 10.1053/j.gastro.2005.11.029. [DOI] [PubMed] [Google Scholar]
- 66.Rockey D. The cellular pathogenesis of portal hypertension: stellate cell contractility, endothelin, and nitric oxide. Hepatology. 1997;25:2–5. doi: 10.1053/jhep.1997.v25.ajhep0250002. [DOI] [PubMed] [Google Scholar]
- 67.Anegawa G, Kawanaka H, Yoshida D, Konishi K, Yamaguchi S, Kinjo N, Taketomi A, Hashizume M, Shimokawa H, Maehara Y. Defective endothelial nitric oxide synthase signaling is mediated by rho-kinase activation in rats with secondary biliary cirrhosis. Hepatology. 2008;47:966–977. doi: 10.1002/hep.22089. [DOI] [PubMed] [Google Scholar]
- 68.Fernández-Varo G, Melgar-Lesmes P, Casals G, Pauta M, Arroyo V, Morales-Ruiz M, Ros J, Jiménez W. Inactivation of extrahepatic vascular Akt improves systemic hemodynamics and sodium excretion in cirrhotic rats. J Hepatol. 2010;53:1041–1048. doi: 10.1016/j.jhep.2010.05.031. [DOI] [PubMed] [Google Scholar]
- 69.Fukumura D, Gohongi T, Kadambi A, Izumi Y, Ang J, Yun CO, Buerk DG, Huang PL, Jain RK. Predominant role of endothelial nitric oxide synthase in vascular endothelial growth factor-induced angiogenesis and vascular permeability. Proc Natl Acad Sci USA. 2001;98:2604–2609. doi: 10.1073/pnas.041359198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Abraldes JG, Iwakiri Y, Loureiro-Silva M, Haq O, Sessa WC, Groszmann RJ. Mild increases in portal pressure upregulate vascular endothelial growth factor and endothelial nitric oxide synthase in the intestinal microcirculatory bed, leading to a hyperdynamic state. Am J Physiol Gastrointest Liver Physiol. 2006;290:G980–G987. doi: 10.1152/ajpgi.00336.2005. [DOI] [PubMed] [Google Scholar]
- 71.Tazi KA, Barrière E, Moreau R, Heller J, Sogni P, Pateron D, Poirel O, Lebrec D. Role of shear stress in aortic eNOS up-regulation in rats with biliary cirrhosis. Gastroenterology. 2002;122:1869–1877. doi: 10.1053/gast.2002.33586. [DOI] [PubMed] [Google Scholar]
- 72.Kajita M, Murata T, Horiguchi K, Iizuka M, Hori M, Ozaki H. iNOS expression in vascular resident macrophages contributes to circulatory dysfunction of splanchnic vascular smooth muscle contractions in portal hypertensive rats. Am J Physiol Heart Circ Physiol. 2011;300:H1021–H1031. doi: 10.1152/ajpheart.00563.2009. [DOI] [PubMed] [Google Scholar]
- 73.Moreau R, Barrière E, Tazi KA, Lardeux B, Dargère D, Urbanowicz W, Poirel O, Chauvelot-Moachon L, Guimont MC, Bernuau D, et al. Terlipressin inhibits in vivo aortic iNOS expression induced by lipopolysaccharide in rats with biliary cirrhosis. Hepatology. 2002;36:1070–1078. doi: 10.1053/jhep.2002.36501. [DOI] [PubMed] [Google Scholar]
- 74.Malyshev E, Tazi KA, Moreau R, Lebrec D. Discrepant effects of inducible nitric oxide synthase modulation on systemic and splanchnic endothelial nitric oxide synthase activity and expression in cirrhotic rats. J Gastroenterol Hepatol. 2007;22:2195–2201. doi: 10.1111/j.1440-1746.2006.04608.x. [DOI] [PubMed] [Google Scholar]
- 75.Huang HC, Wang SS, Chang CC, Lee FY, Lin HC, Hou MC, Teng TH, Chen YC, Lee SD. Evolution of portal-systemic collateral vasopressin response in endotoxemic portal hypertensive rats. Shock. 2009;32:503–508. doi: 10.1097/SHK.0b013e3181a1bf86. [DOI] [PubMed] [Google Scholar]
- 76.Wiest R, Cadelina G, Milstien S, McCuskey RS, Garcia-Tsao G, Groszmann RJ. Bacterial translocation up-regulates GTP-cyclohydrolase I in mesenteric vasculature of cirrhotic rats. Hepatology. 2003;38:1508–1515. doi: 10.1016/j.hep.2003.09.039. [DOI] [PubMed] [Google Scholar]
- 77.Wiest R, Das S, Cadelina G, Garcia-Tsao G, Milstien S, Groszmann RJ. Bacterial translocation in cirrhotic rats stimulates eNOS-derived NO production and impairs mesenteric vascular contractility. J Clin Invest. 1999;104:1223–1233. doi: 10.1172/JCI7458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sastre E, Balfagón G, Revuelta-López E, Aller MÁ, Nava MP, Arias J, Blanco-Rivero J. Effect of short- and long-term portal hypertension on adrenergic, nitrergic and sensory functioning in rat mesenteric artery. Clin Sci (Lond) 2012;122:337–348. doi: 10.1042/CS20110303. [DOI] [PubMed] [Google Scholar]
- 79.Jurzik L, Froh M, Straub RH, Schölmerich J, Wiest R. Up-regulation of nNOS and associated increase in nitrergic vasodilation in superior mesenteric arteries in pre-hepatic portal hypertension. J Hepatol. 2005;43:258–265. doi: 10.1016/j.jhep.2005.02.036. [DOI] [PubMed] [Google Scholar]
- 80.Kaneda K, Ekataksin W, Sogawa M, Matsumura A, Cho A, Kawada N. Endothelin-1-induced vasoconstriction causes a significant increase in portal pressure of rat liver: localized constrictive effect on the distal segment of preterminal portal venules as revealed by light and electron microscopy and serial reconstruction. Hepatology. 1998;27:735–747. doi: 10.1002/hep.510270315. [DOI] [PubMed] [Google Scholar]
- 81.Zhang JX, Pegoli W, Clemens MG. Endothelin-1 induces direct constriction of hepatic sinusoids. Am J Physiol. 1994;266:G624–G632. doi: 10.1152/ajpgi.1994.266.4.G624. [DOI] [PubMed] [Google Scholar]
- 82.Kawada N, Tran-Thi TA, Klein H, Decker K. The contraction of hepatic stellate (Ito) cells stimulated with vasoactive substances. Possible involvement of endothelin 1 and nitric oxide in the regulation of the sinusoidal tonus. Eur J Biochem. 1993;213:815–823. doi: 10.1111/j.1432-1033.1993.tb17824.x. [DOI] [PubMed] [Google Scholar]
- 83.Mittal MK, Gupta TK, Lee FY, Sieber CC, Groszmann RJ. Nitric oxide modulates hepatic vascular tone in normal rat liver. Am J Physiol. 1994;267:G416–G422. doi: 10.1152/ajpgi.1994.267.3.G416. [DOI] [PubMed] [Google Scholar]
- 84.Zhang B, Borderie D, Sogni P, Soubrane O, Houssin D, Calmus Y. NO-mediated vasodilation in the rat liver. Role of hepatocytes and liver endothelial cells. J Hepatol. 1997;26:1348–1355. doi: 10.1016/s0168-8278(97)80471-0. [DOI] [PubMed] [Google Scholar]
- 85.Ming Z, Han C, Lautt WW. Nitric oxide mediates hepatic arterial vascular escape from norepinephrine-induced constriction. Am J Physiol. 1999;277:G1200–G1206. doi: 10.1152/ajpgi.1999.277.6.G1200. [DOI] [PubMed] [Google Scholar]
- 86.Loureiro-Silva MR, Cadelina G, Groszmann R. Nitric oxide modulates both pre-sinusoidal and sinusoidal responses to phenylephrine in normal rat liver. Gastroenterology. 2001;120:A9. [Google Scholar]
- 87.Zhang ZQ, Qiu JF, Luo M, Sun YW, Zhao G, Chen W, Liu H, Wu ZY. Liposome-mediated gene transfer of endothelial nitric oxide synthase to cirrhotic rat liver decreases intrahepatic vascular resistance. J Gastroenterol Hepatol. 2008;23:e487–e493. doi: 10.1111/j.1440-1746.2007.05244.x. [DOI] [PubMed] [Google Scholar]
- 88.Yu Q, Shao R, Qian HS, George SE, Rockey DC. Gene transfer of the neuronal NO synthase isoform to cirrhotic rat liver ameliorates portal hypertension. J Clin Invest. 2000;105:741–748. doi: 10.1172/JCI7997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Van de Casteele M, Omasta A, Janssens S, Roskams T, Desmet V, Nevens F, Fevery J. In vivo gene transfer of endothelial nitric oxide synthase decreases portal pressure in anaesthetised carbon tetrachloride cirrhotic rats. Gut. 2002;51:440–445. doi: 10.1136/gut.51.3.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Rockey DC, Chung JJ. Inducible nitric oxide synthase in rat hepatic lipocytes and the effect of nitric oxide on lipocyte contractility. J Clin Invest. 1995;95:1199–1206. doi: 10.1172/JCI117769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Rockey DC, Housset CN, Friedman SL. Activation-dependent contractility of rat hepatic lipocytes in culture and in vivo. J Clin Invest. 1993;92:1795–1804. doi: 10.1172/JCI116769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Perri RE, Langer DA, Chatterjee S, Gibbons SJ, Gadgil J, Cao S, Farrugia G, Shah VH. Defects in cGMP-PKG pathway contribute to impaired NO-dependent responses in hepatic stellate cells upon activation. Am J Physiol Gastrointest Liver Physiol. 2006;290:G535–G542. doi: 10.1152/ajpgi.00297.2005. [DOI] [PubMed] [Google Scholar]
- 93.Failli P, DeFRANCO RM, Caligiuri A, Gentilini A, Romanelli RG, Marra F, Batignani G, Guerra CT, Laffi G, Gentilini P, et al. Nitrovasodilators inhibit platelet-derived growth factor-induced proliferation and migration of activated human hepatic stellate cells. Gastroenterology. 2000;119:479–492. doi: 10.1053/gast.2000.9354. [DOI] [PubMed] [Google Scholar]
- 94.Vorobioff J, Bredfeldt JE, Groszmann RJ. Increased blood flow through the portal system in cirrhotic rats. Gastroenterology. 1984;87:1120–1126. [PubMed] [Google Scholar]
- 95.Groszmann RJ. Hyperdynamic circulation of liver disease 40 years later: pathophysiology and clinical consequences. Hepatology. 1994;20:1359–1363. [PubMed] [Google Scholar]
- 96.Yang YY, Huang YT, Lee KC, Lee FY, Lee TY, Hou MC, Lin HC, Lee SD. Chronic administration of ursodeoxycholic acid decreases portal pressure in rats with biliary cirrhosis. Clin Sci (Lond) 2009;116:71–79. doi: 10.1042/CS20080075. [DOI] [PubMed] [Google Scholar]
- 97.Leifeld L, Fielenbach M, Dumoulin FL, Speidel N, Sauerbruch T, Spengler U. Inducible nitric oxide synthase (iNOS) and endothelial nitric oxide synthase (eNOS) expression in fulminant hepatic failure. J Hepatol. 2002;37:613–619. doi: 10.1016/s0168-8278(02)00271-4. [DOI] [PubMed] [Google Scholar]
- 98.Rodríguez-Vilarrupla A, Fernández M, Bosch J, García-Pagán JC. Current concepts on the pathophysiology of portal hypertension. Ann Hepatol. 2007;6:28–36. [PubMed] [Google Scholar]
- 99.Martin PY, Ginès P, Schrier RW. Nitric oxide as a mediator of hemodynamic abnormalities and sodium and water retention in cirrhosis. N Engl J Med. 1998;339:533–541. doi: 10.1056/NEJM199808203390807. [DOI] [PubMed] [Google Scholar]
- 100.Thiesson HC, Skøtt O, Jespersen B, Schaffalitzky de Muckadell OB. Nitric oxide synthase inhibition does not improve renal function in cirrhotic patients with ascites. Am J Gastroenterol. 2003;98:180–186. doi: 10.1111/j.1572-0241.2003.07174.x. [DOI] [PubMed] [Google Scholar]
- 101.Wiest R, Shah V, Sessa WC, Groszmann RJ. NO overproduction by eNOS precedes hyperdynamic splanchnic circulation in portal hypertensive rats. Am J Physiol. 1999;276:G1043–G1051. doi: 10.1152/ajpgi.1999.276.4.G1043. [DOI] [PubMed] [Google Scholar]
- 102.Theodorakis NG, Wang YN, Wu JM, Maluccio MA, Sitzmann JV, Skill NJ. Role of endothelial nitric oxide synthase in the development of portal hypertension in the carbon tetrachloride-induced liver fibrosis model. Am J Physiol Gastrointest Liver Physiol. 2009;297:G792–G799. doi: 10.1152/ajpgi.00229.2009. [DOI] [PubMed] [Google Scholar]
- 103.Theodorakis NG, Wang YN, Skill NJ, Metz MA, Cahill PA, Redmond EM, Sitzmann JV. The role of nitric oxide synthase isoforms in extrahepatic portal hypertension: studies in gene-knockout mice. Gastroenterology. 2003;124:1500–1508. doi: 10.1016/s0016-5085(03)00280-4. [DOI] [PubMed] [Google Scholar]
- 104.Wei CL, Hon WM, Lee KH, Khoo HE. Chronic administration of aminoguanidine reduces vascular nitric oxide production and attenuates liver damage in bile duct-ligated rats. Liver Int. 2005;25:647–656. doi: 10.1111/j.1478-3231.2005.01063.x. [DOI] [PubMed] [Google Scholar]
- 105.Bauer TM, Schwacha H, Steinbrückner B, Brinkmann FE, Ditzen AK, Aponte JJ, Pelz K, Berger D, Kist M, Blum HE. Small intestinal bacterial overgrowth in human cirrhosis is associated with systemic endotoxemia. Am J Gastroenterol. 2002;97:2364–2370. doi: 10.1111/j.1572-0241.2002.05791.x. [DOI] [PubMed] [Google Scholar]
- 106.Guarner C, Soriano G, Tomas A, Bulbena O, Novella MT, Balanzo J, Vilardell F, Mourelle M, Moncada S. Increased serum nitrite and nitrate levels in patients with cirrhosis: relationship to endotoxemia. Hepatology. 1993;18:1139–1143. [PubMed] [Google Scholar]
- 107.Albillos A, de la Hera A, González M, Moya JL, Calleja JL, Monserrat J, Ruiz-del-Arbol L, Alvarez-Mon M. Increased lipopolysaccharide binding protein in cirrhotic patients with marked immune and hemodynamic derangement. Hepatology. 2003;37:208–217. doi: 10.1053/jhep.2003.50038. [DOI] [PubMed] [Google Scholar]
- 108.Morales-Ruiz M, Jiménez W, Pérez-Sala D, Ros J, Leivas A, Lamas S, Rivera F, Arroyo V. Increased nitric oxide synthase expression in arterial vessels of cirrhotic rats with ascites. Hepatology. 1996;24:1481–1486. doi: 10.1053/jhep.1996.v24.pm0008938184. [DOI] [PubMed] [Google Scholar]
- 109.Bhimani EK, Serracino-Inglott F, Sarela AI, Batten JJ, Mathie RT. Hepatic and mesenteric nitric oxide synthase expression in a rat model of CCl(4)-induced cirrhosis. J Surg Res. 2003;113:172–178. doi: 10.1016/s0022-4804(03)00163-x. [DOI] [PubMed] [Google Scholar]
- 110.Kwon SY, Groszmann RJ, Iwakiri Y. Increased neuronal nitric oxide synthase interaction with soluble guanylate cyclase contributes to the splanchnic arterial vasodilation in portal hypertensive rats. Hepatol Res. 2007;37:58–67. doi: 10.1111/j.1872-034X.2007.00005.x. [DOI] [PubMed] [Google Scholar]
- 111.Lores-Arnaiz S, Perazzo JC, Prestifilippo JP, Lago N, D'Amico G, Czerniczyniec A, Bustamante J, Boveris A, Lemberg A. Hippocampal mitochondrial dysfunction with decreased mtNOS activity in prehepatic portal hypertensive rats. Neurochem Int. 2005;47:362–368. doi: 10.1016/j.neuint.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 112.Fernández M, Semela D, Bruix J, Colle I, Pinzani M, Bosch J. Angiogenesis in liver disease. J Hepatol. 2009;50:604–620. doi: 10.1016/j.jhep.2008.12.011. [DOI] [PubMed] [Google Scholar]
- 113.Bosch J, Abraldes JG, Fernández M, García-Pagán JC. Hepatic endothelial dysfunction and abnormal angiogenesis: new targets in the treatment of portal hypertension. J Hepatol. 2010;53:558–567. doi: 10.1016/j.jhep.2010.03.021. [DOI] [PubMed] [Google Scholar]
- 114.Fernandez M, Vizzutti F, Garcia-Pagan JC, Rodes J, Bosch J. Anti-VEGF receptor-2 monoclonal antibody prevents portal-systemic collateral vessel formation in portal hypertensive mice. Gastroenterology. 2004;126:886–894. doi: 10.1053/j.gastro.2003.12.012. [DOI] [PubMed] [Google Scholar]
- 115.Mejias M, Garcia-Pras E, Tiani C, Miquel R, Bosch J, Fernandez M. Beneficial effects of sorafenib on splanchnic, intrahepatic, and portocollateral circulations in portal hypertensive and cirrhotic rats. Hepatology. 2009;49:1245–1256. doi: 10.1002/hep.22758. [DOI] [PubMed] [Google Scholar]
- 116.Zhao Y, Wang Y, Wang Q, Liu Z, Liu Q, Deng X. Hepatic stellate cells produce vascular endothelial growth factor via phospho-p44/42 mitogen-activated protein kinase/cyclooxygenase-2 pathway. Mol Cell Biochem. 2012;359:217–223. doi: 10.1007/s11010-011-1016-x. [DOI] [PubMed] [Google Scholar]
- 117.Medina J, Arroyo AG, Sánchez-Madrid F, Moreno-Otero R. Angiogenesis in chronic inflammatory liver disease. Hepatology. 2004;39:1185–1195. doi: 10.1002/hep.20193. [DOI] [PubMed] [Google Scholar]
- 118.Sieber CC, Sumanovski LT, Stumm M, van der Kooij M, Battegay E. In vivo angiogenesis in normal and portal hypertensive rats: role of basic fibroblast growth factor and nitric oxide. J Hepatol. 2001;34:644–650. doi: 10.1016/s0168-8278(00)00064-7. [DOI] [PubMed] [Google Scholar]
- 119.Sumanovski LT, Battegay E, Stumm M, van der Kooij M, Sieber CC. Increased angiogenesis in portal hypertensive rats: role of nitric oxide. Hepatology. 1999;29:1044–1049. doi: 10.1002/hep.510290436. [DOI] [PubMed] [Google Scholar]
- 120.Ahmad S, Hewett PW, Wang P, Al-Ani B, Cudmore M, Fujisawa T, Haigh JJ, le Noble F, Wang L, Mukhopadhyay D, et al. Direct evidence for endothelial vascular endothelial growth factor receptor-1 function in nitric oxide-mediated angiogenesis. Circ Res. 2006;99:715–722. doi: 10.1161/01.RES.0000243989.46006.b9. [DOI] [PubMed] [Google Scholar]
- 121.Huang HC, Wang SS, Hsin IF, Chang CC, Lee FY, Lin HC, Chuang CL, Lee JY, Hsieh HG, Lee SD. Cannabinoid receptor 2 agonist ameliorates mesenteric angiogenesis and portosystemic collaterals in cirrhotic rats. Hepatology. 2012;56:248–258. doi: 10.1002/hep.25625. [DOI] [PubMed] [Google Scholar]
- 122.Huang HC, Chu CJ, Lee FY, Chang FY, Wang SS, Lin HC, Hou MC, Chan CC, Wu SL, Chen CT, et al. Chronic inhibition of nitric oxide ameliorates splanchnic hyposensitivity to glypressin in a hemorrhage-transfused rat model of portal hypertension. Scand J Gastroenterol. 2000;35:1308–1313. doi: 10.1080/003655200453674. [DOI] [PubMed] [Google Scholar]
- 123.Pizcueta P, Piqué JM, Fernández M, Bosch J, Rodés J, Whittle BJ, Moncada S. Modulation of the hyperdynamic circulation of cirrhotic rats by nitric oxide inhibition. Gastroenterology. 1992;103:1909–1915. doi: 10.1016/0016-5085(92)91451-9. [DOI] [PubMed] [Google Scholar]
- 124.Forrest EH, Jones AL, Dillon JF, Walker J, Hayes PC. The effect of nitric oxide synthase inhibition on portal pressure and azygos blood flow in patients with cirrhosis. J Hepatol. 1995;23:254–258. doi: 10.1016/0168-8278(95)80468-4. [DOI] [PubMed] [Google Scholar]
- 125.González-Abraldes J, García-Pagán JC, Bosch J. Nitric oxide and portal hypertension. Metab Brain Dis. 2002;17:311–324. doi: 10.1023/a:1021957818240. [DOI] [PubMed] [Google Scholar]
- 126.Groszmann RJ. Beta-adrenergic blockers and nitrovasodilators for the treatment of portal hypertension: the good, the bad, the ugly. Gastroenterology. 1997;113:1794–1797. doi: 10.1053/gast.1997.v113.agast971131794. [DOI] [PubMed] [Google Scholar]
- 127.Dudenhoefer AA, Loureiro-Silva MR, Cadelina GW, Gupta T, Groszmann RJ. Bioactivation of nitroglycerin and vasomotor response to nitric oxide are impaired in cirrhotic rat livers. Hepatology. 2002;36:381–385. doi: 10.1053/jhep.2002.34739. [DOI] [PubMed] [Google Scholar]
- 128.Bosch J, Abraldes JG, Groszmann R. Current management of portal hypertension. J Hepatol. 2003;38 Suppl 1:S54–S68. doi: 10.1016/s0168-8278(02)00430-0. [DOI] [PubMed] [Google Scholar]
- 129.Fiorucci S, Antonelli E, Morelli O, Mencarelli A, Casini A, Mello T, Palazzetti B, Tallet D, del Soldato P, Morelli A. NCX-1000, a NO-releasing derivative of ursodeoxycholic acid, selectively delivers NO to the liver and protects against development of portal hypertension. Proc Natl Acad Sci USA. 2001;98:8897–8902. doi: 10.1073/pnas.151136298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Fiorucci S, Antonelli E, Brancaleone V, Sanpaolo L, Orlandi S, Distrutti E, Acuto G, Clerici C, Baldoni M, Del Soldato P, et al. NCX-1000, a nitric oxide-releasing derivative of ursodeoxycholic acid, ameliorates portal hypertension and lowers norepinephrine-induced intrahepatic resistance in the isolated and perfused rat liver. J Hepatol. 2003;39:932–939. doi: 10.1016/s0168-8278(03)00393-3. [DOI] [PubMed] [Google Scholar]
- 131.Loureiro-Silva MR, Cadelina GW, Iwakiri Y, Groszmann RJ. A liver-specific nitric oxide donor improves the intra-hepatic vascular response to both portal blood flow increase and methoxamine in cirrhotic rats. J Hepatol. 2003;39:940–946. doi: 10.1016/j.jhep.2003.09.018. [DOI] [PubMed] [Google Scholar]
- 132.Berzigotti A, Bellot P, De Gottardi A, Garcia-Pagan JC, Gagnon C, Spénard J, Bosch J. NCX-1000, a nitric oxide-releasing derivative of UDCA, does not decrease portal pressure in patients with cirrhosis: results of a randomized, double-blind, dose-escalating study. Am J Gastroenterol. 2010;105:1094–1101. doi: 10.1038/ajg.2009.661. [DOI] [PubMed] [Google Scholar]
- 133.Saavedra JE, Billiar TR, Williams DL, Kim YM, Watkins SC, Keefer LK. Targeting nitric oxide (NO) delivery in vivo. Design of a liver-selective NO donor prodrug that blocks tumor necrosis factor-alpha-induced apoptosis and toxicity in the liver. J Med Chem. 1997;40:1947–1954. doi: 10.1021/jm9701031. [DOI] [PubMed] [Google Scholar]
- 134.Qu W, Liu J, Fuquay R, Saavedra JE, Keefer LK, Waalkes MP. The nitric oxide prodrug, V-PYRRO/NO, mitigates arsenic-induced liver cell toxicity and apoptosis. Cancer Lett. 2007;256:238–245. doi: 10.1016/j.canlet.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.González R, Cruz A, Ferrín G, López-Cillero P, Fernández-Rodríguez R, Briceño J, Gómez MA, Rufián S, Mata Mde L, Martínez-Ruiz A, et al. Nitric oxide mimics transcriptional and post-translational regulation during α-tocopherol cytoprotection against glycochenodeoxycholate-induced cell death in hepatocytes. J Hepatol. 2011;55:133–144. doi: 10.1016/j.jhep.2010.10.022. [DOI] [PubMed] [Google Scholar]
- 136.DeLeve LD, Wang X, Kanel GC, Ito Y, Bethea NW, McCuskey MK, Tokes ZA, Tsai J, McCuskey RS. Decreased hepatic nitric oxide production contributes to the development of rat sinusoidal obstruction syndrome. Hepatology. 2003;38:900–908. doi: 10.1053/jhep.2003.50383. [DOI] [PubMed] [Google Scholar]
- 137.Moal F, Veal N, Vuillemin E, Barrière E, Wang J, Fizanne L, Oberti F, Douay O, Gallois Y, Bonnefont-Rousselot D, et al. Hemodynamic and antifibrotic effects of a selective liver nitric oxide donor V-PYRRO/NO in bile duct ligated rats. World J Gastroenterol. 2006;12:6639–6645. doi: 10.3748/wjg.v12.i41.6639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Edwards C, Feng HQ, Reynolds C, Mao L, Rockey DC. Effect of the nitric oxide donor V-PYRRO/NO on portal pressure and sinusoidal dynamics in normal and cirrhotic mice. Am J Physiol Gastrointest Liver Physiol. 2008;294:G1311–G1317. doi: 10.1152/ajpgi.00368.2007. [DOI] [PubMed] [Google Scholar]
- 139.Biecker E, Trebicka J, Kang A, Hennenberg M, Sauerbruch T, Heller J. Treatment of bile duct-ligated rats with the nitric oxide synthase transcription enhancer AVE 9488 ameliorates portal hypertension. Liver Int. 2008;28:331–338. doi: 10.1111/j.1478-3231.2008.01664.x. [DOI] [PubMed] [Google Scholar]
- 140.Das A, Mukherjee P, Singla SK, Guturu P, Frost MC, Mukhopadhyay D, Shah VH, Patra CR. Fabrication and characterization of an inorganic gold and silica nanoparticle mediated drug delivery system for nitric oxide. Nanotechnology. 2010;21:305102. doi: 10.1088/0957-4484/21/30/305102. [DOI] [PMC free article] [PubMed] [Google Scholar]


