Abstract
AIM: To investigate the attenuation patterns and detectability of common bile duct (CBD) stones by multidetector computed tomography (MDCT).
METHODS: Between March 2010 and February 2012, 191 patients with suspicion of CBD stones undergoing both MDCT and endoscopic retrograde cholangiopancreatography (ERCP) were enrolled and reviewed retrospectively. The attenuation patterns of CBD stones on MDCT were classified as heavily calcified, radiopaque, less radiopaque, or undetectable. The association between the attenuation patterns of CBD stones on MDCT and stone type consisting of pure cholesterol, mixed cholesterol, brown pigment, and black pigment and the factors related to the detectability of CBD stones by MDCT were evaluated.
RESULTS: MDCT showed CBD stones in 111 of 130 patients in whom the CBD stones were demonstrated by ERCP with 85.4% sensitivity. The attenuation patterns of CBD stones on MDCT were heavily calcified 34 (26%), radiopaque 31 (24%), less radiopaque 46 (35%), and undetectable 19 (15%). The radiopacity of CBD stones differed significantly according to stone type (P < 0.001). From the receiver operating characteristic curve, stone size was useful for the determination of CBD stone by MDCT (area under curve 0.779, P < 0.001) and appropriate cut-off stone size on MDCT was 5 mm. The factors related to detectability of CBD stones on MDCT were age, stone type, and stone size on multivariate analysis (P < 0.05).
CONCLUSION: The radiopacity of CBD stones on MDCT differed according to stone type. Stone type and stone size were related to the detectability by MDCT, and appropriate cut-off stone size was 5 mm.
Keywords: Common bile duct gallstones, Gallstones, Multidetector computed tomography, Endoscopic retrograde cholangiography
INTRODUCTION
Traditionally, endoscopic retrograde cholangiopancreatography (ERCP) was the gold standard for investigation of bile duct diseases, but its role has been limited to therapeutic use due to the invasiveness for the procedure[1-4]. Therefore, multiple imaging tests have been used to diagnose stones in the common bile duct (CBD) instead of ERCP. Commonly performed imaging tests are abdominal sonography, computed tomography (CT), magnetic retrograde cholangiopancreatography (MRCP), and endoscopic ultrasonography (EUS). Abdominal sonography is easy to perform, but it cannot evaluate the overall CBD. MRCP and EUS have been reported to be accurate for the diagnosis of choledocholithiasis[5-8]. However, they both have some limitations that prevent active use on patients; metal in the body, claustrophobia, and lack of rapidity for MRCP; operator dependency for EUS; high cost and the differences of the facilities among different centers for MRCP and EUS.
CT scans are being used more frequently and performed as the initial imaging technique in patients with abnormal liver function test results or possible symptoms related to the biliary tract because it can be performed rapidly, is equipped in most centers, has relatively low cost, and gives extensive information on pancreaticobiliary structures including CBD stones. However, CT has been reported to have lower accuracy for the diagnosis of CBD stones than MRCP or EUS[9-11], which does not make CT the imaging technique of choice for patients with clinical suspicion of choledocholithiasis. The development of multi-detector computed tomography (MDCT) has shown promise in increasing the accuracy of CT in the diagnosis of pancreaticobiliary diseases. With an almost universal use of MDCT, acquisition of images with high spatial resolution is now routine and the advent of more recent MDCT technology may further advance the use of CT for these diagnoses given its ability to acquire an isotropic data set with minimal motion artifacts[12]. A recent study showed that MDCT was comparable with MRCP or EUS for the detection of CBD stone with 87% sensitivity, 85% specificity, and 86% accuracy[13].
Gallstones are classified into cholesterol stones (pure cholesterol, combination, or mixed) and pigment stones (black or brown) according to the National Institutes of Health (NIH)-International Workshop on Pigment Gallstone Disease[14] and the Gallstone Research Committee form the Japanese Society of Gastroenterology[15]. CT can reveal the heterogeneous nature of these biliary stones in attenuation patterns ranging from being heavily calcified and radiopaque, to being slightly less radiopaque than bile due to cholesterol, to having gas attenuation due to locules of nitrogen gas[16]. The association between the attenuation patterns of CBD stones on MDCT and the gallstone type has not been clarified. It is also not fully elucidated how the types and size of stones affect the detectability of MDCT. Therefore, we investigated the attenuation patterns and the detectability of CBD stones by MDCT according to stone type and stone size, and intended to find the factors related to the detectability of CBD stones by MDCT.
MATERIALS AND METHODS
Patients
We consecutively enrolled patients with suspicion of choledocholithiasis undergoing both MDCT and ERCP during the period from March 2010 and February 2012 at a single institution. Hematologic and biochemical tests were performed at the time of admission. Suspected choledocholithiasis was defined as follows: recent abdominal pain, leukocytosis, and/or abnormal blood chemistry findings including total bilirubin, alanine aminotransaminase, alkaline phosphatase, or γ-glutamyl transferase levels. Exclusion criteria were as follows: CT from other institution, more than one month duration between MDCT and ERCP, failure in removing CBD stones by ERCP, and patients suspected of cystic duct stones. After MDCT, MRCP or EUS was performed for further determination of CBD stones if possible. ERCP was performed when the imaging study showed CBD stones or CBD stones were highly suspected despite negative imaging studies. Patient anonymity was preserved and the Institutional Review Board of our hospital approved this study (HC12RISI0038). This study protocol was in complete compliance with the Declaration of Helsinki, as revised in Seoul in 2008.
Multidetector computed tomography
CT studies were performed either a 64-slice MDCT scanner (Somatom Sensation 64; Siemens, Erlangen, Germany) with a detector collimation of 24 mm × 1.2 mm, a table feed of 28.8 mm per rotation, a rotation time of 1 s, a tube current of 200 effective mAs, and a tube voltage of 120 kV or a 16-slice MDCT scanner (Somatom Sensation 16; Siemens, Erlangen, Germany) with a detector collimation of 16 mm × 1.5 mm, a table feed of 24 mm per rotation, a rotation time of 1 s, a tube current of 200 effective mAs, and a tube voltage of 120 kV. Precontrast scan ranged from the diaphragm to the iliac crest and postcontrast scan ranged from the diaphragm to the symphysis pubis. Contrast material (Ultravist 300, Bayer, Berlin, Germany) with a volume of 120 mL and an injection rate of 2 mL/s was injected into the antecubital vein. Image acquisition was initiated after 90 s of contrast injection. Pre and post contrast axial images were reconstructed by 5 mm without overlap and postconstrast coronal images by 3 mm without overlap.
Endoscopic retrograde cholangiopancreatography
ERCP was performed with a duodenoscope (JF 240; Olympus Optical Co., Ltd., Tokyo, Japan). Two experienced gastroenterologists who had performed > 1000 ERCPs conducted the procedures. Conscious sedation was achieved with midazolam and pethidine hydrochloride. Contrast media (iopromide; 1:1 dilution with saline) was injected to obtain a cholangiogram after cannulation of the common bile duct without papillotomy. If the cholangiogram was interpreted as positive for CBD stones, endoscopic sphincterotomy was performed and the bile duct was swept with a Dormia basket and a retrieval balloon catheter to remove the calculi. Following this, the calculi were taken out for the evaluation of stone type. If the cholangiogram was considered as negative for CBD stones, the absence of stones were confirmed again by a basket and a retrieval balloon catheter.
Analysis of images on multidetector computed tomography
CT scan data sets were transferred to picture archiving and communication system (PACS) workstations for analysis, and CT images were interpreted by one radiologist who had more than ten years of experience interpreting gastroenterological images. The radiologist received no clinical information or results of ERCP and used pre-contrast and portal venous phase images. Pre-contrast images were used for the initial detection of CBD stones. If the stones could not be discriminated in pre-contrast images, portal venous phase images served as references. The radiologist was free to use the window settings he preferred, which included narrow settings if a common duct stone was not initially identified on soft tissue window settings. The mean Hounsfield units of the stones were measured on PACS workstations. The attenuation patterns of CBD stones were classified as follows (Figure 1): (1) heavily calcified as having very high attenuation (mean Hounsfield unit > 150); (2) radiopaque as having distinctly higher attenuation than surrounding structures (150 ≥ mean Hounsfield unit > 80); (3) less radiopaque as having slightly higher attenuation than surroundings (mean Hounsfield unit ≤ 80); and (4) gas attenuation as having a gas in or around the stones[16]. Common bile duct caliber was measured using the axial image of CT.
Figure 1.
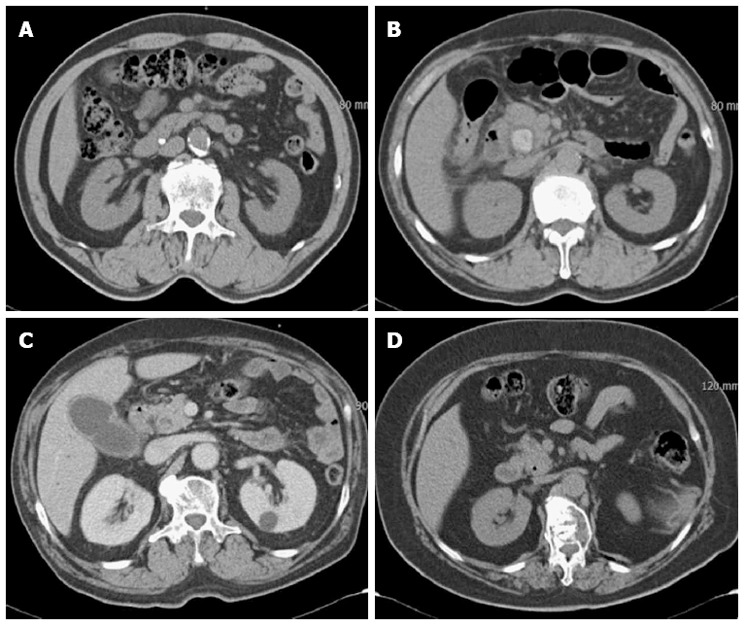
Attenuation patterns of common bile duct stones on multidetector computed tomography. A: Heavily calcified; B: Radiopaque; C: Less radiopaque; D: Gas attenuation in the stone.
Type and size of common bile duct stones
Types of stones were classified by morphology as black pigment, brown pigment, and cholesterol stones according to the NIH-International Workshop[14]. The color and shape on the external appearance and cross sectional shape on the internal structure were used as indexes. Cholesterol stones were subclassified into pure cholesterol stones, mixed stones, and combination stones according to the classification of the Japanese Society of Gastroenterology[15]. To reduce the confusion in discrimination between mixed and combination stones, they both were classified as mixed stone. For measuring the stone size and number, MDCT (axial and coronal), MRCP, EUS, and/or ERCP were utilized together. The largest diameter of the stone, as the representative of size, was measured using electronic calipers on the workstation. If there were more than two stones, the largest stone was selected for the evaluation of stone type and stone size.
Statistical analysis
A Pearson’s χ2 test or Fisher’s exact test was used to compare categorical data and Student’s t-test or Mann-Whitney U-test was used for comparisons of continuous data to analyze the attenuation patterns and the detectability of CBD stones. A linear by linear association was used to analyze the trend between the attenuation patterns and the stone type. Receiver operating characteristic curve for CT detectability of CBD stone according to stone size was plotted. The area under the curve and the optimal cut-off value of stone size for CT detectability were calculated. Multivariate analysis for the related factors to the detectability of CBD stones by MDCT was performed with the significant factors identified from univariate analysis using binary logistic regression analysis (enter method). Statistical analyses were performed with SPSS, version 14 (SPSS, Inc., Chicago, IL, United States). P-values < 0.05 were considered significant.
RESULTS
Patients
One hundred ninety-one patients with suspicion of CBD stones undergoing MDCT and ERCP were consecutively enrolled from March 2010 and February 2012 (Figure 2). Twenty-four patients were excluded because they did not undergo CT within 1 mo (3), received CT from other institution (7), and failed to remove CBD stones by ERCP (14). This resulted in a study population of 167 patients consisting of 86 males and 81 females with a mean age of 65.7 years (SD, ± 15.9 years). The mean time between MDCT and ERCP was 2.4 d (SD, ± 4.5 d). MDCT was performed with 64-channel or 16-channel in 76% and 23%, respectively. CBD stones were demonstrated by ERCP in 130 patients. Of them, 54 (42%) patients had accompanying gallbladder stones and 31 (24%) previously received cholecystectomy. Thirteen patients (10%) underwent endoscopic sphincterotomy previously. The mean number of CBD stones was 2.0 (SD, ± 1.6). The types of stones revealed by ERCP was as follows: 23 (18%) black pigment stones, 38 (29%) mixed cholesterol stones, 60 (46%) brown pigment stones, and 9 (7%) pure cholesterol stones. The mean size of stones were 10.1 mm (SD, ± 5.4 mm), 8.0 mm (SD, ± 3.9 mm), 5.8 mm (SD, ± 3.2 mm), and 5.7 mm (SD, ± 3.8 mm) in brown pigment, mixed cholesterol, black pigment, and pure cholesterol stones, respectively. The brown pigment stones were significantly larger than other types of stones (P < 0.05).
Figure 2.
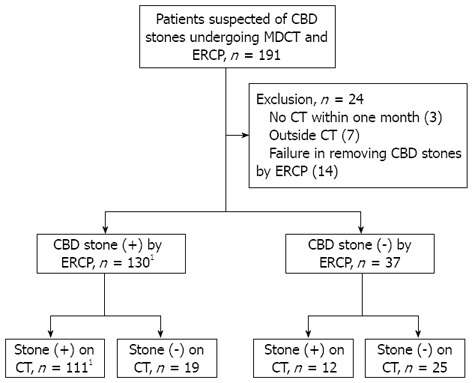
Flow chart of patients with suspicion of common bile duct stones undergoing multidetector computed tomography and endoscopic retrograde cholangiopancreatography. The sensitivity of multidetector computed tomography (CT) for common bile duct stones was 85.4%. 1Two patients had recurrent common bile duct stones during the study period. CBD: Common bile duct; ERCP: Endoscopic retrograde cholangiopancreatography.
Attenuation patterns of common bile duct stones
The attenuation patterns of CBD stones consisted of heavily calcified 34 (31%), radiopaque 31 (28%), and less radiopaque (41%, Table 1). The mean Hounsfield units were 421 (range, 156-1552), 104 (range, 81-141), and 59 (range, 11-80) in heavily calcified, radiopaque, and less radiopaque patterns, respectively. The radiopacity of stones differed significantly according to stone type (P < 0.001). An increasing trend in the radiopacity was observed among brown pigment, mixed cholesterol, and black pigment stones by analysis with a linear-by-linear association (P < 0.001). The mean size of heavily calcified stones, radiopaque stones, and less radiopaque stones differed significantly (P < 0.05). The order of mean stone size according to the radiopacity was radiopaque stones, less radiopaque stones, and heavily calcified stones. Heavily calcified and radiopaque stones were well discriminated on the pre-contrast images of MDCT. For less radiopaque stones, the images of portal venous phase were helpful in discriminating CBD stones because bile duct was well shown in that phase. The coronal reconstructed CT scan was useful in one patient. One mixed cholesterol stone with less radiopacity was ambiguous on portal venous-phase images, but the coronal reconstructed CT scan showed less radiopaque stone near the major ampulla (Figure 3). Of 46 less radiopaque stones, six stones had a pattern of gas attenuation in or around the stones which were less radiopaque or not well discriminated. All patients with gas attenuated stones had previously undergone endoscopic sphincterotomy.
Table 1.
Correlation between the attenuation patterns of common bile duct stones on multidetector computed tomography and stone type (n = 111)
|
Stone type,
n
(size, mean ± SD, mm) |
|||||
| Attenuation patterns | Black pigment | Mixed cholesterol | Brown pigment | Pure cholesterol | Overall |
| Heavily calcified | 15 | 14 | 5 | 0 | 34 |
| (6.1 ± 3.4) | (7.0 ± 3.2) | (9.2 ± 4.1) | (6.9 ± 3.5) | ||
| Radiopaque | 3 | 12 | 16 | 0 | 31 |
| (7.0 ± 1.0) | (10.2 ± 4.3) | (13.6 ± 4.8) | (11.6 ± 4.8) | ||
| Less radiopaque1 | 3 | 10 | 33 | 0 | 46 |
| (5.7 ± 4.0) | (7.6 ± 3.5) | (9.9 ± 5.1) | (9.1 ± 4.8) | ||
| Overall | 21 | 36 | 54 | 0 | |
| (6.2 ± 3.1) | (8.2 ± 3.6) | (10.9 ± 5.1) | |||
Including six less radiopaque stones with gas attenuation. P < 0.001.
Figure 3.
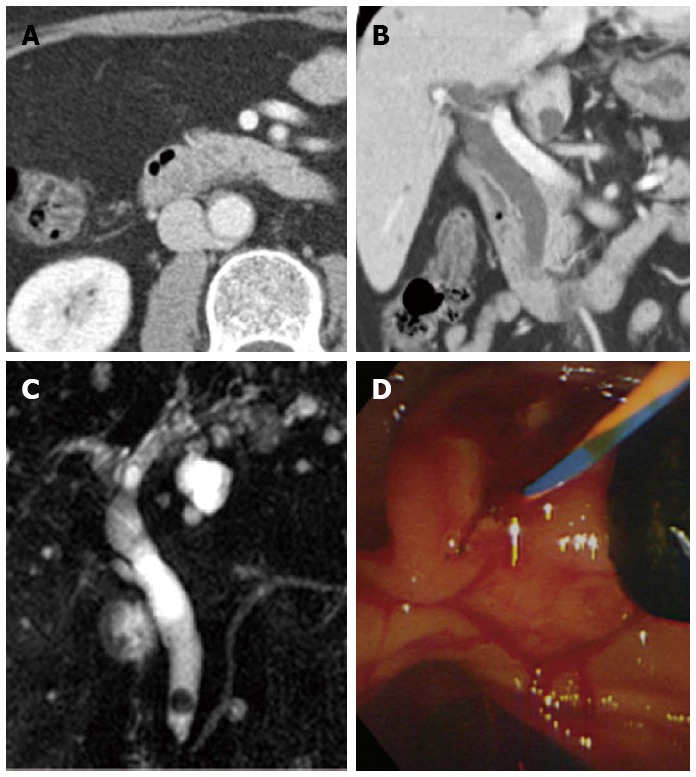
A case of difficult discrimination of common bile duct stone on the axial image of multidetector computed tomography. The coronal reconstructed image was helpful. A: The stone was ambiguous on the portal venous-phase axial computed tomography (CT) scan; B: The coronal reconstructed CT scan showed a less radiopaque stone near the major ampulla; C, D: Magnetic retrograde cholangiopancreatography and endoscopic retrograde cholangiopancreatography showed a six mm, mixed common bile duct stone.
Detectability of common bile duct stones
The detectability of CBD stones by MDCT increased according to stone size (Table 2). 96% of stones larger than 5 mm were detectable by MDCT, but 67% of stones smaller than 5 mm were detectable (P < 0.001). Three stones were not detected by MDCT despite having a size of more than 5 mm and they all were determined to be pure cholesterol stones. The detection rate of CBD stones less than 3 mm was 44% (4/9). Although this rate was less than the detection rate of CBD stones between 3-5 mm (75%, 29/40), it was not statistically significant. From the receiver operating characteristic curve, stone size was useful for the determination of CBD stone by MDCT (area under curve 0.779, P < 0.001, Figure 4). Appropriate cut-off stone size considering sensitivity and specificity was 5 mm. The detection rate of black pigment or mixed cholesterol stones (93%) was higher than that of brown pigment or pure cholesterol stones (78%, P = 0.023). However, the detection rate did not differ among black pigment, mixed cholesterol, and brown pigment stones. Nineteen undetected CBD stones by MDCT consisted of nine pure cholesterol stones, six brown pigment stones, two black pigment stones, and two mixed cholesterol stones. Of them, the sizes of all stones except for the pure cholesterol stones were less than 5 mm. The size of two black pigment stones which were undetected by MDCT were as small as 1 and 3 mm.
Table 2.
Detectability of common bile duct stones according to stone size and stone type n (%)
| n | Detectable (n = 111) | Undetectable (n = 19) | P value | |
| Size (mm) | ||||
| < 3 | 9 | 4 (44) | 5 (56) | < 0.001 |
| 3–5 | 40 | 29 (74) | 11 (26) | |
| 6–10 | 45 | 43 (96) | 2 (4) | |
| 11–15 | 26 | 25 (96) | 1 (4) | |
| > 15 | 10 | 10 (100) | 0 (0) | |
| ≤ 5 | 49 | 33 (67) | 16 (33) | < 0.001 |
| > 5 | 81 | 78 (96) | 3 (4) | |
| Type | ||||
| Black pigment | 23 | 21 (91) | 2 (9) | < 0.001 |
| Mixed cholesterol | 38 | 36 (95) | 2 (5) | |
| Brown pigment | 60 | 54 (90) | 6 (10) | |
| Pure cholesterol | 9 | 0 (0) | 9 (100) | |
| Black or mixed | 61 | 57 (93) | 4 (7) | 0.023 |
| Brown or pure cholesterol | 69 | 54 (78) | 15 (22) |
Figure 4.
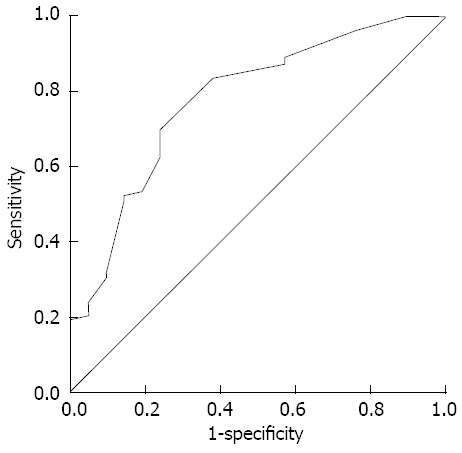
Receiver operating characteristic curve of the detectability of multidetector computed tomography for common bile duct stones according to stone size. The area under the curve was 0.779 and the optimal cut-off value of stone size was 5 mm.
The related factors to the detectability of CBD stones were age, stone type, stone size, and CBD diameter in univariate analysis (P < 0.01, Table 3). In multivariate analysis, age, stone size, and stone type were significant independent factors for CBD stone detectability by MDCT (P < 0.05, Table 4). Of them, stone size and stone type had large odds ratios of 8.851 (95%CI: 2.249−34.84) and 1.437 (95%CI: 1.153−1.791), respectively.
Table 3.
Univariate analysis of the factors related to the detectability of common bile duct stones by multidetector computed tomography n (%)
| Factors | Detectable (n = 111) | Undetectable (n = 19) | P value |
| Age (yr) | 68.3 ± 14.2 | 58.0 ± 16.2 | 0.009 |
| Male sex | 58 (52) | 9 (47) | 0.966 |
| Accompanying GB stones | 43 (39) | 11 (58) | 0.117 |
| Previous cholecystectomy | 28 (25) | 3 (16) | 0.561 |
| Previous sphincterotomy | 13 (12) | 0 (0) | 0.213 |
| Black or mixed stone | 57 (51) | 4 (21) | 0.009 |
| Stone size (mm) | 9.1 ± 4.8 | 4.3 ± 2.9 | < 0.001 |
| CBD diameter (mm) | 12.4 ± 4.6 | 9.2 ± 3.5 | 0.005 |
| WBC (× 109/L) | 10.9 ± 6.01 | 9.86 ± 3.07 | 0.251 |
| Alanine aminotransaminase (IU/L) | 210 ± 250 | 250 ± 208 | 0.513 |
| Total bilirubin (mg/dL) | 3.8 ± 3.9 | 3.3 ± 2.8 | 0.565 |
| Alkaline phosphatase (IU/L) | 215 ± 153 | 209 ± 114 | 0.862 |
| γ-glutamyl transferase (IU/L) | 412 ± 342 | 500 ± 465 | 0.327 |
CBD: Common bile duct; WBC: White blood cell.
Table 4.
Multivariate analysis of the factors related to the detectability of common bile duct stones by multidetector computed tomography
| Factors | P value | Odds ratio (95%CI) |
| Age | 0.032 | 1.049 (1.004-1.095) |
| Stone type (black or mixed vs brown or pure cholesterol) | 0.002 | 8.851 (2.249-34.84) |
| Stone size | 0.001 | 1.437 (1.153-1.791) |
| CBD diameter | 0.538 |
CBD: Common bile duct.
DISCUSSION
Abdominal CT has been a very popular procedure in routine clinical practice. Rapidity, relatively low cost, extensive information about the abdomen, and wide availability facilitate the use of CT. Especially in the emergency department, the utilization of CT has increased for the rapid detection of pancreato-biliary diseases. CT has been developed from conventional to helical CT. Recently, multi-detector CT was introduced and has been used in many institutions. Helical CT using MDCT technology can use thin slice images in a single breath-hold and reconstruct those slices retrospectively using a variable overlap. It reduces much of the image degradation previously experienced from motion artifacts and volume averaging[13]. As CT technology continues to improve with the increasing use of 16-, 32-, 64-, and 256-MDCT, it is likely that the accuracy for the detection of choledocholithiasis in patients undergoing routine abdominal CT will improve[12]. MDCT showed better results than previous studies that showed various sensitivities for the detection of choledocholithiasis by CT range from 71% to 93%[17-21], with a mean sensitivity of approximately 80%. In the present study, MDCT displayed a sensitivity of 85.4% for the detection of CBD stones. Although MDCT has a better resolution and can detect greater number of small stones, there is a theoretic limitation for the detectability of choledocholithiasis by CT due to the iso or slightly hypoattenuating nature of pure cholesterol stones relative to bile, making them difficult to detect[13,22-24].
The detectability of CBD stones by MDCT depends on several factors. The present study showed that the factors related to the detectability of CBD stone were age, stone size, and stone type. Although the optimal size of CBD stones for MDCT detection was determined as 5 mm, 67% of CBD stones smaller than 5 mm were detectable by MDCT. This showed that MDCT had some role in the initial screening even for small CBD stones less than 5 mm. The radiopacity of stones differed significantly according to stone type. Black pigment and mixed cholesterol stones were more detectable than brown and pure cholesterol stones. However, attenuation patterns could not discriminate between specific stone types because of the overlap in CT attenuation. A previous study showed that the mean CT attenuation of cholesterol stones was lower than that of pigment gallstones, and CT attenuation measurement was not useful for the determination of gallstone composition due to the overlap of CT attenuation values[25]. Besides the type and size of stones, the position of stones can influence the detectability. It is recognized that small stones impacted at the ampulla are difficult to identify, particularly in non-dilated biliary ducts. One patient in the present study has a CBD stone near the ampulla, and it was detected by a coronal image rather than an axial image. Unfortunately, a previous study revealed that CT coronal images did not show significant improvement of diagnosis for CBD stones[11]. We consider that coronal images do not increase the general detectability of CBD stones, but it may become helpful in special situations such as stones near the ampulla. The phase of CT image is another factor concerning the detectability of CBD stones. It was reported that portal venous phase CT images are specific and sensitive for the detection of biliary duct narrowing and choledocholithiasis[26]. Radiopaque stones are well demarcated on a precontrast image, but less radiopaque stones are not easily discriminated on a precontrast image. Portal venous phase images show common bile duct clearly, so it helps discriminate CBD stones especially for less radiopaque stones. Peak voltage setting also affects the detection of gallstones. In a study to evaluate the effect of four peak voltage settings (80, 100, 120, and 140 kV) on the in vitro conspicuity of gallstones in an anthropomorphic phantom by CT, the sensitivity for gallstone detection was significantly higher at 140 kV[25]. We used 120 kV for the peak voltage setting on MDCT. 140 kV would increase the sensitivity of MDCT according to a previous study. However, the danger of increasing radiation hazard cannot be neglected. In the present study, the diameter of the bile duct affected the CT detectability of CBD stones in univariate analysis, but not in multivariate analysis. This was probably because the diameter of the bile duct is related to stone size. Although age was a significant factor related to the detectability, the odds ratio of age was as small as 1.049 and the lower limit of 95% confidence interval was close to 1 (1.004). Therefore, it can be stated that age showed little clinical significance. With regard to age, it could be considered that younger patients had a tendency of small stones and cholesterol stones. However, this needs the further investigation.
Formation of pigment stones in the bile duct is a late complication of endoscopic sphincterotomy[27]. Sphincterotomy permits chronic bacterial colonization of the bile duct that results in deconjugation of bilirubin and precipitation of pigment stones. In the present study, all six patients with gas attenuated stones had previously undergone endoscopic sphincterotomy. According to our results, if CBD stones were indistinct by CT and air attenuations were found in the CBD in patients previously undergoing endoscopic sphincterotomy, CBD stones would be strongly suspected and further studies should be performed for the confirmation of CBD stones.
Gallstones are extremely common in Western countries, where the prevalence of bile-duct stones is relatively low. In contrast, primary choledocholithiasis and hepatolithiasis appear to be more frequent in East Asian countries than in Western societies[28,29]. Primary bile duct stones are predominantly composed of calcium bilirubinate, namely brown pigment stones. The pathogenesis of primary bile duct stones is based on bile stasis and infection, which are associated with bile duct strictures, extrahepatic anomalies, and biliary parasites. In contrast, secondary stones are considered to originate from gallbladder stones, and are commonly composed of cholesterol. In the present study, the proportion of cholesterol stones, black pigment stones, and brown pigment stones were 36%, 18%, and 46%, respectively. Tazuma[28] reported that the proportion of cholesterol stones, black pigment stones, and brown pigment stones in the CBD were 31%, 12%, and 54%, respectively in Japan. This was similar to our study. In a Korean population study of bile duct stones 14 years ago, the majority were brown pigment stones (76%), and the remaining were cholesterol stones (18%) and black pigment stones (4%)[30]. The discrimination of stone type is sometimes difficult. Atypical stones with a black or brown colored surface, but with a radial fashioned surface and a high cholesterol content are not rare[15,31,32]. These stones are frequently confused by their external appearance with black pigment stones originating from the gallbladder. We used color and shape on the external appearance and cross sectional shape on the internal structure as the indexes to reduce such confusion. Mixed and combination stones were classified as mixed stones to make a clear classification. Some investigators classify mixed or combination stone as stones of the intermediate group which are difficult to classify into either cholesterol stones or pigment stones[15].
There were some limitations in the present study. First, this was a retrospective study. Some disparity in the interpretation of CBD stones between the original time of the examination and the time of the study can exist. The interpreter in the present study who was primed for the detection of choledocholithiasis likely led to a little improved sensitivity for detection for bile duct stones compared with real-time interpretations at the time of the examination. Second, stone composition analysis was not performed. Analysis using spectroscopy would help determine stone type more precisely. However, we exerted the greatest effort to determine stone type by using the color and shape on the external appearance and cross sectional shape on the internal structure. Third, reformation of MDCT images was not performed. Reformation process such as multiplanar reformation or minimum intensity reformation helps with the detection of gallstones[33,34]. However, reformation needs an additional process which requires much time and effort. Most hospitals do not perform reformation as a routine procedure due to this restraint.
In conclusion, MDCT showed a moderately high sensitivity for the detection of CBD stones, and the radiopacity of CBD stones by MDCT differed according to stone type. Type and size of stones were significant factors related to the detectability of CBD stones. We expect further prospective studies with a larger cohort of patients to demonstrate the characteristics of CBD stones by MDCT.
COMMENTS
Background
Multidetector computed tomography (MDCT) can reveal the heterogeneous nature of the biliary stones in attenuation patterns ranging from being heavily calcified and radiopaque, to being slightly less radiopaque than bile due to cholesterol, to having gas attenuation due to locules of nitrogen gas. The association between the attenuation patterns of common bile duct (CBD) stones on MDCT and the gallstone type has not been clarified. It is also not fully elucidated how the types and size of stones affect the detectability of MDCT.
Research frontiers
This study demonstrated that the radiopacity of CBD stones differed significantly according to stone type. Stone size was important for the determination of CBD stone by MDCT, and appropriate cut-off stone size was 5 mm. The factors related to detectability of CBD stones on MDCT were age, stone type, and stone size.
Innovations and breakthroughs
This is the first report the radiopacity of CBD stones according to stone type. MDCT showed moderately high sensitivity for the detection of CBD stones. Although magnetic retrograde cholangiopancreatography or endoscopic ultrasonography is considered more accurate method for the detection of CBD stones, MDCT also had good sensitivity and can give information about stone type.
Applications
MDCT is useful mothod for dectection of CBD stones, especially when CBD stones are more than 5 mm. Stone type can be estimated by the radiopacity of CBD stones on MDCT
Peer review
The author evaluated the role of MDCT in detection of CBD stones, and found a moderately high sensitivity for the detection of CBD stones, and the radiopacity of CBD stones by MDCT differed according to stone type. The article is of great significance in clinical evaluation of CBD stones by this approach.
Footnotes
P- Reviewer Zhu JF S- Editor Wen LL L- Editor A E- Editor Zhang DN
References
- 1.NIH state-of-the-science statement on endoscopic retrograde cholangiopancreatography (ERCP) for diagnosis and therapy. NIH Consens State Sci Statements. 2002;19:1–26. [PubMed] [Google Scholar]
- 2.Loperfido S, Angelini G, Benedetti G, Chilovi F, Costan F, De Berardinis F, De Bernardin M, Ederle A, Fina P, Fratton A. Major early complications from diagnostic and therapeutic ERCP: a prospective multicenter study. Gastrointest Endosc. 1998;48:1–10. doi: 10.1016/s0016-5107(98)70121-x. [DOI] [PubMed] [Google Scholar]
- 3.Freeman ML, Nelson DB, Sherman S, Haber GB, Herman ME, Dorsher PJ, Moore JP, Fennerty MB, Ryan ME, Shaw MJ, et al. Complications of endoscopic biliary sphincterotomy. N Engl J Med. 1996;335:909–918. doi: 10.1056/NEJM199609263351301. [DOI] [PubMed] [Google Scholar]
- 4.Halme L, Doepel M, von Numers H, Edgren J, Ahonen J. Complications of diagnostic and therapeutic ERCP. Ann Chir Gynaecol. 1999;88:127–131. [PubMed] [Google Scholar]
- 5.Canto MI, Chak A, Stellato T, Sivak MV. Endoscopic ultrasonography versus cholangiography for the diagnosis of choledocholithiasis. Gastrointest Endosc. 1998;47:439–448. doi: 10.1016/s0016-5107(98)70242-1. [DOI] [PubMed] [Google Scholar]
- 6.Verma D, Kapadia A, Eisen GM, Adler DG. EUS vs MRCP for detection of choledocholithiasis. Gastrointest Endosc. 2006;64:248–254. doi: 10.1016/j.gie.2005.12.038. [DOI] [PubMed] [Google Scholar]
- 7.Fernández-Esparrach G, Ginès A, Sánchez M, Pagés M, Pellisé M, Fernández-Cruz L, López-Boado MA, Quintó L, Navarro S, Sendino O, et al. Comparison of endoscopic ultrasonography and magnetic resonance cholangiopancreatography in the diagnosis of pancreatobiliary diseases: a prospective study. Am J Gastroenterol. 2007;102:1632–1639. doi: 10.1111/j.1572-0241.2007.01333.x. [DOI] [PubMed] [Google Scholar]
- 8.Ainsworth AP, Rafaelsen SR, Wamberg PA, Durup J, Pless TK, Mortensen MB. Is there a difference in diagnostic accuracy and clinical impact between endoscopic ultrasonography and magnetic resonance cholangiopancreatography? Endoscopy. 2003;35:1029–1032. doi: 10.1055/s-2003-44603. [DOI] [PubMed] [Google Scholar]
- 9.Moon JH, Cho YD, Cha SW, Cheon YK, Ahn HC, Kim YS, Kim YS, Lee JS, Lee MS, Lee HK, et al. The detection of bile duct stones in suspected biliary pancreatitis: comparison of MRCP, ERCP, and intraductal US. Am J Gastroenterol. 2005;100:1051–1057. doi: 10.1111/j.1572-0241.2005.41057.x. [DOI] [PubMed] [Google Scholar]
- 10.Lee JK, Kim TK, Byun JH, Kim AY, Ha HK, Kim PN, Lee MG. Diagnosis of intrahepatic and common duct stones: combined unenhanced and contrast-enhanced helical CT in 1090 patients. Abdom Imaging. 2006;31:425–432. doi: 10.1007/s00261-006-9076-1. [DOI] [PubMed] [Google Scholar]
- 11.Tseng CW, Chen CC, Chen TS, Chang FY, Lin HC, Lee SD. Can computed tomography with coronal reconstruction improve the diagnosis of choledocholithiasis? J Gastroenterol Hepatol. 2008;23:1586–1589. doi: 10.1111/j.1440-1746.2008.05547.x. [DOI] [PubMed] [Google Scholar]
- 12.Anderson SW, Zajick D, Lucey BC, Soto JA. 64-detector row computed tomography: an improved tool for evaluating the biliary and pancreatic ducts? Curr Probl Diagn Radiol. 2007;36:258–271. doi: 10.1067/j.cpradiol.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 13.Anderson SW, Lucey BC, Varghese JC, Soto JA. Accuracy of MDCT in the diagnosis of choledocholithiasis. AJR Am J Roentgenol. 2006;187:174–180. doi: 10.2214/AJR.05.0459. [DOI] [PubMed] [Google Scholar]
- 14.Trotman BW, Soloway RD. Pigment gallstone disease: Summary of the National Institutes of Health--international workshop. Hepatology. 1982;2:879–884. doi: 10.1002/hep.1840020624. [DOI] [PubMed] [Google Scholar]
- 15.Kim IS, Myung SJ, Lee SS, Lee SK, Kim MH. Classification and nomenclature of gallstones revisited. Yonsei Med J. 2003;44:561–570. doi: 10.3349/ymj.2003.44.4.561. [DOI] [PubMed] [Google Scholar]
- 16.Yeh BM, Liu PS, Soto JA, Corvera CA, Hussain HK. MR imaging and CT of the biliary tract. Radiographics. 2009;29:1669–1688. doi: 10.1148/rg.296095514. [DOI] [PubMed] [Google Scholar]
- 17.Neitlich JD, Topazian M, Smith RC, Gupta A, Burrell MI, Rosenfield AT. Detection of choledocholithiasis: comparison of unenhanced helical CT and endoscopic retrograde cholangiopancreatography. Radiology. 1997;203:753–757. doi: 10.1148/radiology.203.3.9169700. [DOI] [PubMed] [Google Scholar]
- 18.Jiménez Cuenca I, del Olmo Martínez L, Pérez Homs M. Helical CT without contrast in choledocholithiasis diagnosis. Eur Radiol. 2001;11:197–201. doi: 10.1007/s003300000609. [DOI] [PubMed] [Google Scholar]
- 19.Soto JA, Velez SM, Guzmán J. Choledocholithiasis: diagnosis with oral-contrast-enhanced CT cholangiography. AJR Am J Roentgenol. 1999;172:943–948. doi: 10.2214/ajr.172.4.10587126. [DOI] [PubMed] [Google Scholar]
- 20.Sugiyama M, Atomi Y. Endoscopic ultrasonography for diagnosing choledocholithiasis: a prospective comparative study with ultrasonography and computed tomography. Gastrointest Endosc. 1997;45:143–146. doi: 10.1016/s0016-5107(97)70237-2. [DOI] [PubMed] [Google Scholar]
- 21.Mitchell SE, Clark RA. A comparison of computed tomography and sonography in choledocholithiasis. AJR Am J Roentgenol. 1984;142:729–733. doi: 10.2214/ajr.142.4.729. [DOI] [PubMed] [Google Scholar]
- 22.Baron RL, Rohrmann CA, Lee SP, Shuman WP, Teefey SA. CT evaluation of gallstones in vitro: correlation with chemical analysis. AJR Am J Roentgenol. 1988;151:1123–1128. doi: 10.2214/ajr.151.6.1123. [DOI] [PubMed] [Google Scholar]
- 23.Brakel K, Laméris JS, Nijs HG, Terpstra OT, Steen G, Blijenberg BC. Predicting gallstone composition with CT: in vivo and in vitro analysis. Radiology. 1990;174:337–341. doi: 10.1148/radiology.174.2.2296642. [DOI] [PubMed] [Google Scholar]
- 24.Baron RL. Diagnosing choledocholithiasis: how far can we push helical CT? Radiology. 1997;203:601–603. doi: 10.1148/radiology.203.3.9169674. [DOI] [PubMed] [Google Scholar]
- 25.Chan WC, Joe BN, Coakley FV, Prien EL, Gould RG, Prevrhal S, Barber WC, Kirkwood KS, Qayyum A, Yeh BM. Gallstone detection at CT in vitro: effect of peak voltage setting. Radiology. 2006;241:546–553. doi: 10.1148/radiol.2412050947. [DOI] [PubMed] [Google Scholar]
- 26.Anderson SW, Rho E, Soto JA. Detection of biliary duct narrowing and choledocholithiasis: accuracy of portal venous phase multidetector CT. Radiology. 2008;247:418–427. doi: 10.1148/radiol.2472070473. [DOI] [PubMed] [Google Scholar]
- 27.Tanaka M, Takahata S, Konomi H, Matsunaga H, Yokohata K, Takeda T, Utsunomiya N, Ikeda S. Long-term consequence of endoscopic sphincterotomy for bile duct stones. Gastrointest Endosc. 1998;48:465–469. doi: 10.1016/s0016-5107(98)70086-0. [DOI] [PubMed] [Google Scholar]
- 28.Tazuma S. Gallstone disease: Epidemiology, pathogenesis, and classification of biliary stones (common bile duct and intrahepatic) Best Pract Res Clin Gastroenterol. 2006;20:1075–1083. doi: 10.1016/j.bpg.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 29.Ko CW, Lee SP. Epidemiology and natural history of common bile duct stones and prediction of disease. Gastrointest Endosc. 2002;56:S165–S169. doi: 10.1067/mge.2002.129005. [DOI] [PubMed] [Google Scholar]
- 30.Shim CS. The color of gallstones: yellow, brown or black. Present features of Korean gallstone disease. Gastrointest Endosc. 2000;52:144–145. doi: 10.1016/s0016-5107(00)70266-5. [DOI] [PubMed] [Google Scholar]
- 31.Kim MH, Sekijima J, Park HZ, Lee SP. Structure and composition of primary intrahepatic stones in Korean patients. Dig Dis Sci. 1995;40:2143–2151. doi: 10.1007/BF02208998. [DOI] [PubMed] [Google Scholar]
- 32.Malet PF. Nomenclature for pigment gallstones. Hepatology. 1987;7:988. doi: 10.1002/hep.1840070544. [DOI] [PubMed] [Google Scholar]
- 33.Kim HJ, Park DI, Park JH, Cho YK, Sohn CI, Jeon WK, Kim BI, Kim SK. Multidetector computed tomography cholangiography with multiplanar reformation for the assessment of patients with biliary obstruction. J Gastroenterol Hepatol. 2007;22:400–405. doi: 10.1111/j.1440-1746.2006.04503.x. [DOI] [PubMed] [Google Scholar]
- 34.Denecke T, Degutyte E, Stelter L, Lehmkuhl L, Valencia R, Lopez-Hänninen E, Felix R, Stroszczynski C. Minimum intensity projections of the biliary system using 16-channel multidetector computed tomography in patients with biliary obstruction: comparison with MRCP. Eur Radiol. 2006;16:1719–1726. doi: 10.1007/s00330-006-0172-y. [DOI] [PubMed] [Google Scholar]


