Abstract
Background/Aims
Endoscopic ultrasound-guided fine needle aspiration (EUS-FNA) and Trucut biopsy (TCB) are sensitive techniques for diagnosing mediastinal lesions, but it is unclear how either one or both should be used to obtain a pathologic diagnosis. The objective of our study was to evaluate whether EUS-TCB impacts the diagnosis of mediastinal lesions after the initial on-site review of EUS-FNA specimen suggests a suboptimal result.
Methods
We enrolled consecutive patients with mediastinal lesions who underwent EUS-TCB during the same procedure if the initial EUS-FNA demonstrated an inadequate FNA sample or suggested that histopathology was required for diagnosis. Diagnostic accuracies between procedures were compared as the main outcome.
Results
Twenty-seven patients (14 men; median age, 56 years; range, 19 to 82 years) underwent EUS-FNA and EUS-TCB to evaluate a mediastinal lymphadenopathy or mass (n=17), to determine the cancer stage (n=3) or to exclude tumor recurrence or metastasis (n=7). The overall diagnostic accuracies of EUS-FNA and EUS-TCB were 78% and 67%, respectively (p=0.375). The combined diagnostic accuracy of EUS-FNA plus EUS-TCB was 82%. In six patients with nondiagnostic EUS-FNA, EUS-TCB provided a final diagnosis in one patient (17%).
Conclusions
In the current series of patients with mediastinal masses or adenopathy, the administration of EUS-TCB following suboptimal results for the on-site cytology review did not increase the diagnostic yield.
Keywords: Endoscopic ultrasound, Endoscopic ultrasound-guided fine needle aspiration, Endoscopic ultrasound-guided Trucut biopsy, Mediastinum
INTRODUCTION
Tissue acquisition from benign and malignant posterior mediastinal lymph nodes or masses is necessary to guide management. The traditional approaches to sampling of mediastinal lesions include: computed tomography (CT)-guided percutaneous biopsy, transbronchial fine needle aspiration (FNA), mediastinoscopy, mediastinotomy, and thoracoscopy. However, these approaches have various limitations of poor tissue yield, adverse safety profile and increased costs.1 Endoscopic ultrasound (EUS)-FNA is a sensitive test for the diagnosis of mediastinal lesions such as lymphadenopathy of unknown causes, staging of nonsmall cell lung cancer or esophageal cancer, and evaluation of benign conditions such as sarcoidosis.2-4 Although the diagnostic accuracy of EUS-FNA is generally high, it has several limitations such as the variable availability of on-site cytology assessment and the acquisition of only a cellular specimen for evaluation.5,6 Interpretation of cytologic specimens may be difficult due to obscuring blood, necrotic material, and inflammatory cells. Furthermore, inadequate cytologic specimens may preclude performance of immunostains required to classify tumors such as lymphomas and mesenchymal tumors.7
Since its introduction in 2002, EUS-guided Trucut biopsy (EUS-TCB) has attempted to overcome the limitations of EUS-FNA by acquisition of histology specimens which permit evaluation of tissue architecture and use of immunohistochemistry (IHC) when necessary. Nevertheless, the role of EUS-TCB for sampling of various lesions remains debatable. Criticisms of EUS-TCB include difficult maneuverability due to needle rigidity and spring-loaded mechanism, increased cost, uncertain safety, and paucity of clinical data to develop practice guidelines.
Studies comparing the diagnostic yield of EUS-FNA to EUS-TCB have shown that TCB is at least as accurate as FNA and the supplementary value of EUS-TCB to EUS-FNA remains controversial.8-17 There are little data evaluating the impact of additional histology obtained with TCB following an inconclusive, unknown or unevaluated FNA result. One study evaluating the additional value of EUS-TCB to EUS-FNA in patients with mediastinal lesions demonstrated that the diagnostic yield of both procedures was comparable and authors recommended limiting the use of EUS-TCB to specific cases in which EUS-FNA is not conclusive.12 However, there are no data on the impact of rescue EUS-TCB for mediastinal lesions after the results of on-site cytology review of EUS-FNA are inconclusive. In the current study, we aim to evaluate the diagnostic impact of EUS-TCB in mediastinal lesions after initial on-site review of EUS-FNA suggests a suboptimal cytology specimen.
MATERIALS AND METHODS
1. Study population and design
This study was approved by the Institutional Review Board at Indiana University Medical Center. Utilizing a prospectively updated EUS database, we identified all patients from November 2005 through July 2010 who underwent EUS-TCB of any mediastinal lymph node or mass. EUS-TCB was considered in a lesion measuring at least 18 mm in maximal diameter (size of histology tray on the needle) when 1) on-site cytopathology review of initial EUS-FNA was nondiagnostic or hypocellular; or 2) histopathology was deemed to be helpful by the cytopathologist or endoscopist (i.e., suspected lymphoma, well differentiated carcinoma, mesenchymal tumors, or lesions requiring immunostaining) to confirm the final diagnosis. The anatomical position of the lesion was based on the American Thoracic Society mediastinal station system18 using a combination of prior imaging including CT or magnetic resonance imaging and the final results of EUS.
The following data were abstracted from medical records and our prospectively updated EUS database: prior radiographic data, subject demographics, EUS examination results (including sites biopsied by EUS-TCB or EUS-FNA, number of needle passes, results of cytology and histology, and immunochemistry if performed), procedural complications, requirement for any other diagnostic procedures after EUS, and postprocedure follow-up data. The EUS characteristics of the lesion biopsied included: long axis diameter, shape, homogeneity, echogenicity, margins, and presence of invasion were also recorded. When surgery was performed, the type of surgery, surgical margins, final histopathology and immunostain (if available) of the primary tumor, and lymph nodes or metastatic sites were noted. When available, surgical histopathology was considered the final diagnosis for each patient. In the absence of surgery, results of EUS-FNA or EUS-TCB and clinical follow-up were considered the reference standard for diagnosis.
2. EUS procedures and specimen processing
After written informed consent was obtained, the study participants received conscious sedation with various combinations of intravenous midazolam, meperidine, fentanyl, or propofol under appropriate cardiorespiratory monitoring. All procedures were performed by one among five experienced endosonographers, each of whom had performed more than 2,000 procedures before the commencement of this study.
In some patients, EUS was initially performed with a radial echoendoscope (GFUM-130, GF-UM160, or GF-UE160; Olympus America Inc., Center Valley, PA, USA). EUS-FNA was performed in the standard fashion using a curvilinear array echoendoscope (GF-UC140P-AL5; Olympus America Inc.). Under EUS guidance, each lesion was aspirated by a 22-gauge EUS-FNA needle (EchoTip; Cook Medical Inc., Winston-Salem, NC, USA). Doppler color ultrasonography was used to ensure the absence of intervening vascular structures along the anticipated needle path. The FNA needle was passed through the largest diameter possible in each lesion to maximize tissue sampling. Manual suction was applied to the FNA needle with a 10 mL syringe for the first pass. If a bloody specimen was encountered with the first pass, suction pressure was reduced or eliminated in some cases at the discretion of the pathologist and endoscopist. Each aspirate was placed on glass slides, and both air-dried and alcohol-fixed smears were prepared. The remaining material was then flushed from the needle and collected for subsequent preparation of a cell block. Air-dried smears were stained with Diff-Quik method for on-site review and to assess for the need for additional passes for review or immunostaining. An attending cytopathologist was present on-site for immediate slide review and to determine the adequacy of the specimens and provide a preliminary diagnosis when possible. Alcohol-fixed smears were later stained by Papanicolaou method and reviewed by one or more cytopathologists. Immunocytochemistry from cytology specimens was later performed selectively at the discretion of the pathology department based on results from a combination of initial on-site cytology review, cellularity of obtained cell block and results and adequacy of histology from core biopsy. Depending on the discretion of on-site cytopathologist, 1 to 2 additional passes were made and placed into a cell block preparation. In patients with suspected lymphoma based on clinical information, material was obtained for flow cytometry.
If on-site review indicated a nondiagnostic (no viable tumor cells), hypocellular (tumor cells present but of insufficient quality for definitive diagnosis) or bloody specimen or when tissue architecture was sought, then EUS-TCB was performed. Patients in whom EUS-TCB was performed comprised the study population. At least two EUS-FNA passes were made prior to consideration of EUS-TCB.
Core biopsy was obtained using a 19-gauge EUS-TCB needle (Quick-Core; Cook Medical Inc.). The biopsy device was initially placed in the firing position by the endoscopy assistant and the needle assembly was advanced through the accessory channel of the endoscope. Before advancing the Trucut needle into the lesion, the projected path was interrogated with power Doppler to ensure the absence of intervening blood vessels. After the needle was inserted into the tumor, the instrument tip was straightened; the tissue tray was fired into the tumor completely, so that the longest possible fragment was obtained.
EUS-TCB was repeated until one or more visible cores were obtained. Immediate touch preps were not performed. The core biopsy specimen was placed into formalin and processed later with hematoxylin and eosin (H&E) stain for histopathologic analysis. IHC was attempted whenever this aided the diagnosis.
Per department policy, all patients were called 24 to 72 hours after EUS procedure to assess short-term complications. At each call, each subject was asked specially about any change in baseline abdominal pain, symptoms of GI bleeding, sore throat, or visits to other health care facilities for any treatment related to the endoscopy.
3. Cytological and histological analysis
Histologic and cytologic samples were reviewed by various attending pathologists for adequacy and diagnosis. Inadequate specimens were defined as tissue samples that contained no representative or useful tissue to render a pathologic diagnosis. The gold standard for the final diagnosis included surgical resection when available or the results of patients' history, FNA+TCB, and a clinical follow-up for at least 6 months with repeated imaging to assess for disease progression. In the absence of surgical resection, a diagnostic biopsy from either TCB or FNA for a specific malignancy (i.e., lymphoma) or benign disease (i.e., sarcoidosis) was utilized as a gold standard for comparison to the other biopsy technique. Patients with a diagnosis of benign lymphadenopathy were confirmed by clinical follow-up and imaging tests to ensure that no malignancy developed. In patients with final diagnosis of lymphoma, the diagnosis was accepted if monoclonal proliferation of lymphoid cells was proved by flow cytometry/immunostaining or histological core biopsy on EUS. Atypical or abnormal cells were considered a nondiagnostic result. Other results were considered satisfactory for nonoperative diagnosis as follows: diagnostic morphology or immunostains when required for mesenchymal and solid tumors; noncaseating granulomas for sarcoidosis; identification of organism for infection; epithelial lining for benign cyst. For calculation of diagnostic yield (final diagnosis established by EUS-FNA, EUS-TCB, or combination of the techniques), inadequate biopsy specimens were considered as nondiagnostic.
4. Statistical analysis
For analysis, continuous variables were expressed as means with standard deviations or medians with ranges, and dichotomous or categorical variables were expressed as simple proportions with or without 95% confidence limits. Student's t-test and chi-square or Fisher's exact tests were used to test for differences in comparisons between continuous and dichotomous variables, respectively. The comparison of the diagnostic yields between the two techniques according to each other in which they were performed was assessed using the McNemar test. The mean number of needle passes between the two techniques was compared using the paired t-test. Statistical analyses were performed by using SPSS version 12.0 (SPSS Inc., Chicago, IL, USA). A p-value of 0.05 or less was considered as indicating statistical significance.
RESULTS
1. Study population
Between November 2005 and July 2010, a total of 30 patients (14 men; median age, 56 years; range, 19 to 82 years) underwent both EUS-FNA and EUS-TCB for 31 mediastinal lesions. One lesion was excluded due to loss of TCB sampling tissue during pathologic processing. On-site cytology evaluation for FNA specimen was not performed in three cases and these were excluded from data analysis. The indications for the 27 procedures were to evaluate mediastinal lymphadenopathy or mass in 17 (63%), to determine cancer stage in three (11%), and to exclude tumor recurrence or metastasis in seven (26%). Biopsy specimens were taken from lymph nodes or masses in the subcarinal region in 18, paraesophageal lesion in two, and one each in retrotracheal region, lower paratracheal region, aortopulmonary window, middle mediastinum and distal esophageal wall. In two with lymphadenopathy, the site of biopsy was not specified.
The mean size of lesions biopsied was 40±19 mm. The median number of needle passes performed was greater with EUS-FNA (four passes; range, 2 to 8) compared to EUS-TCB (three passes; range, 1 to 10) (p=0.046). Grossly visible core tissue by EUS-TCB was obtained in 23 sites (85%). No endoscopic complications were noted.
2. Diagnosis by EUS-FNA and EUS-TCB
Among 27 patients who underwent EUS-guided biopsy, the final clinical diagnosis (malignant in 13 and benign in 14) (Table 1) was established by surgery in four, EUS-guided biopsies +/- flow cytometry or immunostaining in 16, EUS imaging in one, and clinical and imaging follow-up in six. EUS-FNA +/- EUS-TCB established the diagnosis in 22 (82%). In the remaining five, the final diagnosis was confirmed by EUS image in one (bronchogenic cyst), serologic test and clinical follow-up in one (histoplasmosis) and other invasive procedures including cervical lymph node excision, mediastinoscopy, and thoracotomy in three (lymphoma).
Table 1.
Results of EUS-FNA and EUS-TCB by Final Diagnosis in 27 Patients

EUS-FNA, endoscopic ultrasound-guided fine needle aspiration; EUS-TCB, endoscopic ultrasound-guided Trucut biopsy; SCLC, small cell lung cancer; NSCLC, nonsmall cell lung cancer; RCC, renal cell carcinoma; GIST, gastrointestinal stromal tumor; AP, aortopulmonary.
EUS-FNA was technically feasible in all sites. The diagnostic yield of cytology from EUS-FNA was 78% (21/27). Among diagnostic FNA specimens, immunocytochemical studies were performed for further characterization of the primary tumor in four and flow cytometry for evaluation of possible lymphoma were also done in four. Of these, immunocytochemistry contributed to the final diagnosis of gastrointestinal stromal tumor (GIST) in one patient and lymphoma in another. EUS-TCB was technically feasible in all attempted biopsies. Overall, diagnostic histology was obtained for 18 (67%) patients which was similar to the yield of diagnostic cytology from EUS-FNA (p=0.375). Among specimens with a visible core of tissue (median length, 4 mm; range, 1 to 15 mm), the diagnostic accuracy was 74% (17/23). Two of seven histology specimens (one each of a GIST and malignant glomus tumor) sent for immunohistochemical study contributed to the final diagnosis. A visible core tissue was obtained in six of nine nondiagnostic EUS-TCB specimens. However, these six did not contribute to obtaining a final diagnosis because of insufficient specimen for immunohistochemical study in three and H&E stain in one, acquisition of normal gut wall in one, and necrotic tissue only in one, Combined diagnostic accuracy of EUS-FNA and EUS-TCB was 82% (22/27), which is similar to EUS-FNA alone (p=1.0) but superior to EUS-TCB alone (p=0.125), that is statistically not significant.
Comparison of the number of needle passes for each of the four subgroups by diagnostic contribution (Table 2) showed that the subgroup with diagnostic FNA and TCB had fewer needle passes performed with EUS-TCB (three passes; range, 1 to 8) compared to EUS-FNA (four passes; range, 2 to 8; p=0.046).
Table 2.
Comparison of Needle Passes According to the Diagnostic Contribution of FNA and TCB

Data are presented as median (range).
FNA, fine needle aspiration; TCB, Trucut biopsy; NA, not available.
DISCUSSION
EUS-FNA is a well-established technique in diagnosing lymph nodes and masses of the posterior mediastinum, obtaining samples for cytological examinations either as a primary diagnostic procedure or in cases where other biopsy techniques have failed.19 It can be safely performed with an overall diagnostic accuracy of 60% to 90%, depending on the site and availability of on-site cytopathologist.17,20-22
Certain neoplasms such as lymphomas, well-differentiated cancers and stromal tumors are often difficult to diagnose by cytological assessment alone. TCB can overcome the limitations of EUS-FNA by providing histologic samples. Since the introduction of EUS-TCB in 2002, there has been continuous debate as to the need and role of EUS-TCB and its use has varied considerably among institutions.6
Our study shows that the diagnostic accuracy of EUS-TCB for mediastinal lesions is equivalent to an initially suspected suboptimal EUS-FNA specimen of the same lesion. These findings are similar to other studies (Table 3).8-17 This relatively high cytology yield in our study was somewhat surprising given the requirement for preliminary nondiagnostic or suboptimal cytology review. This highlights the limitations of on-site cytology review of air-dried specimens for determination of specimen adequacy. Previous studies have suggested that the combined techniques are superior to either one alone.13,15 However, Berger et al.12 showed no significant difference in the diagnostic accuracy among EUS-FNA, EUS-TCB, and the combination. We found that the combined diagnostic accuracy of EUS-FNA and EUS-TCB was equal to EUS-FNA alone but higher than EUS-TCB alone. For malignant intra-abdominal lesions, EUS-FNA has a slightly higher accuracy than TCB and a combined approach may be helpful.
Table 3.
Published Results for the Comparison of Diagnostic Accuracy between EUS-FNA and EUS-TCB
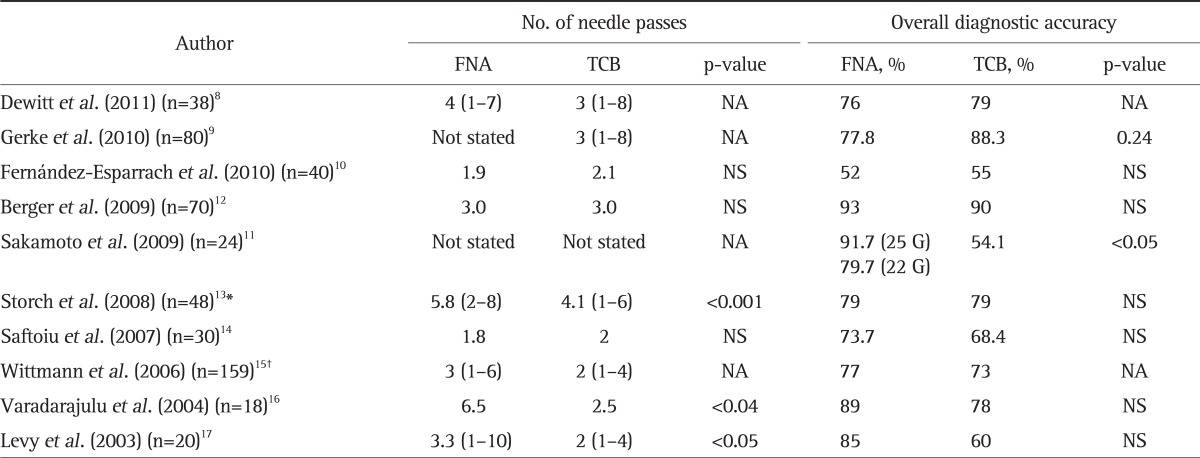
EUS-FNA, endoscopic ultrasound-guided fine needle aspiration; EUS-TCB, endoscopic ultrasound-guided Trucut biopsy; NA, not available; NS, not significant.
*The combined accuracy of both FNA and TCB was 98% (p=0.007 vs EUS-TCB); †91% (p=0.008 vs EUS-TCB).
One of the factors limiting the performance of TCB is a technical one related to the difficulty in inserting and firing the needle when the endoscope is in an angulated position (e.g., in the duodenum and gastric fundus). This is rarely a problem in the esophagus as the scope is in a straight position and in the current study TCB was successfully carried out in all patients. Nevertheless, a visible core biopsy sample was not procured in four. In our study the diagnostic accuracy of EUS-FNA was below 90%. The reason for a lower EUS-FNA diagnostic accuracy (78%) than previously reported in the literature (over 90%) was likely due to several factors, including a significant percentage of patients with benign diseases (52%) where cytology is frequently limited, and our definition of diagnostic accuracy. Therefore, until more data is available, we recommend the use of EUS-FNA with on-site cytopathology. Rescue EUS-TCB for mediastinal lesions may then be considered with a small incremental diagnostic yield.
An alternative to the current approach of performing FNA first followed by TCB has been performed in a prospective study with 30 patients.17 In this trial, FNA was used as a rescue procedure to TCB if touch prep in the room revealed benign disease (nondiagnostic) or if Trucut failed to obtain a core specimen. Although this approach is novel, it still requires on-site review of a touch prep specimen similar as performed during on-site cytology review and would not avoid occasional failures to obtain a tissue core. A recent prospective cohort study using a newly developed 19-gauge EUS-guided core biopsy device (ProCore; Cook Medical Inc.) reported that 102 (89%) of the 114 intramural or extramural lesions biopsied were able to be assessed histologically with a sensitivity and specificity of 90% and 100%, respectively.23 Further studies of this needle are needed to confirm these promising findings.
The current study represents the first description of the impact of rescue EUS-TCB for mediastinal lesions after inconclusive on-site cytology review of EUS-FNA specimens. Nevertheless, there are several limitations that merit discussion. First, a variety of mediastinal sites and lesions were sampled for this study which limits potential application of our results to any particular anatomical station or region. Second, the use of EUS-TCB only in patients with suspected nondiagnostic/hypocellular specimens of on-site review limits its utility to these patients. Finally, our retrospective design and the lack of matched subgroup cohorts (with respect to lesion types and number of patients included in each of the four subgroups in Table 1) raise potential bias.
In conclusion, our findings show no additional impact of EUS-TCB on the accuracy of EUS-FNA for the diagnosis of mediastinal lesions. The combined diagnostic accuracy of EUS-FNA plus EUS-TCB is numerically but not statistically higher compared to FNA alone or TCB alone. Thus, EUS-FNA with on-site cytology review should be considered the primary method for obtaining tissue from mediastinal lesions. EUS-TCB may supplement a suboptimal result of EUS-FNA for these lesions.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Detterbeck FC, Jantz MA, Wallace M, Vansteenkiste J, Silvestri GA American College of Chest Physicians. Invasive mediastinal staging of lung cancer: ACCP evidence-based clinical practice guidelines (2nd edition) Chest. 2007;132(3 Suppl):202S–220S. doi: 10.1378/chest.07-1362. [DOI] [PubMed] [Google Scholar]
- 2.Fritscher-Ravens A, Sriram PV, Bobrowski C, et al. Mediastinal lymphadenopathy in patients with or without previous malignancy: EUS-FNA-based differential cytodiagnosis in 153 patients. Am J Gastroenterol. 2000;95:2278–2284. doi: 10.1111/j.1572-0241.2000.02243.x. [DOI] [PubMed] [Google Scholar]
- 3.Wildi SM, Judson MA, Fraig M, et al. Is endosonography guided fine needle aspiration (EUS-FNA) for sarcoidosis as good as we think? Thorax. 2004;59:794–799. doi: 10.1136/thx.2003.009472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Annema JT, Veseliç M, Rabe KF. Endoscopic ultrasound-guided fine-needle aspiration for the diagnosis of sarcoidosis. Eur Respir J. 2005;25:405–409. doi: 10.1183/09031936.05.00098404. [DOI] [PubMed] [Google Scholar]
- 5.Klapman JB, Logrono R, Dye CE, Waxman I. Clinical impact of on-site cytopathology interpretation on endoscopic ultrasound-guided fine needle aspiration. Am J Gastroenterol. 2003;98:1289–1294. doi: 10.1111/j.1572-0241.2003.07472.x. [DOI] [PubMed] [Google Scholar]
- 6.Levy MJ. Endoscopic ultrasound-guided trucut biopsy of the pancreas: prospects and problems. Pancreatology. 2007;7:163–166. doi: 10.1159/000104240. [DOI] [PubMed] [Google Scholar]
- 7.Jhala NC, Jhala DN, Chhieng DC, Eloubeidi MA, Eltoum IA. Endoscopic ultrasound-guided fine-needle aspiration. A cytopathologist's perspective. Am J Clin Pathol. 2003;120:351–367. doi: 10.1309/MFRF-J0XY-JLN8-NVDP. [DOI] [PubMed] [Google Scholar]
- 8.DeWitt J, Emerson RE, Sherman S, et al. Endoscopic ultrasound-guided Trucut biopsy of gastrointestinal mesenchymal tumor. Surg Endosc. 2011;25:2192–2202. doi: 10.1007/s00464-010-1522-z. [DOI] [PubMed] [Google Scholar]
- 9.Gerke H, Rizk MK, Vanderheyden AD, Jensen CS. Randomized study comparing endoscopic ultrasound-guided Trucut biopsy and fine needle aspiration with high suction. Cytopathology. 2010;21:44–51. doi: 10.1111/j.1365-2303.2009.00656.x. [DOI] [PubMed] [Google Scholar]
- 10.Fernández-Esparrach G, Sendino O, Solé M, et al. Endoscopic ultrasound-guided fine-needle aspiration and trucut biopsy in the diagnosis of gastric stromal tumors: a randomized crossover study. Endoscopy. 2010;42:292–299. doi: 10.1055/s-0029-1244074. [DOI] [PubMed] [Google Scholar]
- 11.Sakamoto H, Kitano M, Komaki T, et al. Prospective comparative study of the EUS guided 25-gauge FNA needle with the 19-gauge Trucut needle and 22-gauge FNA needle in patients with solid pancreatic masses. J Gastroenterol Hepatol. 2009;24:384–390. doi: 10.1111/j.1440-1746.2008.05636.x. [DOI] [PubMed] [Google Scholar]
- 12.Berger LP, Scheffer RC, Weusten BL, et al. The additional value of EUS-guided Tru-cut biopsy to EUS-guided FNA in patients with mediastinal lesions. Gastrointest Endosc. 2009;69:1045–1051. doi: 10.1016/j.gie.2008.09.034. [DOI] [PubMed] [Google Scholar]
- 13.Storch I, Shah M, Thurer R, Donna E, Ribeiro A. Endoscopic ultrasound-guided fine-needle aspiration and Trucut biopsy in thoracic lesions: when tissue is the issue. Surg Endosc. 2008;22:86–90. doi: 10.1007/s00464-007-9374-x. [DOI] [PubMed] [Google Scholar]
- 14.Saftoiu A, Vilmann P, Guldhammer Skov B, Georgescu CV. Endoscopic ultrasound (EUS)-guided Trucut biopsy adds significant information to EUS-guided fine-needle aspiration in selected patients: a prospective study. Scand J Gastroenterol. 2007;42:117–125. doi: 10.1080/00365520600789800. [DOI] [PubMed] [Google Scholar]
- 15.Wittmann J, Kocjan G, Sgouros SN, Deheragoda M, Pereira SP. Endoscopic ultrasound-guided tissue sampling by combined fine needle aspiration and trucut needle biopsy: a prospective study. Cytopathology. 2006;17:27–33. doi: 10.1111/j.1365-2303.2006.00313.x. [DOI] [PubMed] [Google Scholar]
- 16.Varadarajulu S, Fraig M, Schmulewitz N, et al. Comparison of EUS-guided 19-gauge Trucut needle biopsy with EUS-guided fine-needle aspiration. Endoscopy. 2004;36:397–401. doi: 10.1055/s-2004-814316. [DOI] [PubMed] [Google Scholar]
- 17.Levy MJ, Jondal ML, Clain J, Wiersema MJ. Preliminary experience with an EUS-guided trucut biopsy needle compared with EUS-guided FNA. Gastrointest Endosc. 2003;57:101–106. doi: 10.1067/mge.2003.49. [DOI] [PubMed] [Google Scholar]
- 18.Murray JG, Breatnach E. The American Thoracic Society lymph node map: a CT demonstration. Eur J Radiol. 1993;17:61–68. doi: 10.1016/0720-048x(93)90037-n. [DOI] [PubMed] [Google Scholar]
- 19.Tio TL, Tytgat GN. Endoscopic ultrasonography in analysing peri-intestinal lymph node abnormality. Preliminary results of studies in vitro and in vivo. Scand J Gastroenterol Suppl. 1986;123:158–163. doi: 10.3109/00365528609091878. [DOI] [PubMed] [Google Scholar]
- 20.Catalano MF, Rosenblatt ML, Chak A, Sivak MV, Jr, Scheiman J, Gress F. Endoscopic ultrasound-guided fine needle aspiration in the diagnosis of mediastinal masses of unknown origin. Am J Gastroenterol. 2002;97:2559–2565. doi: 10.1111/j.1572-0241.2002.06023.x. [DOI] [PubMed] [Google Scholar]
- 21.Itoi T, Itokawa F, Sofuni A, et al. Puncture of solid pancreatic tumors guided by endoscopic ultrasonography: a pilot study series comparing Trucut and 19-gauge and 22-gauge aspiration needles. Endoscopy. 2005;37:362–366. doi: 10.1055/s-2004-826156. [DOI] [PubMed] [Google Scholar]
- 22.Fernandez-Esparrach G, Pellisé M, Solé M, et al. Usefulness of endoscopic ultrasound-guided fine needle aspiration in the diagnosis of mediastinal lesions. Arch Bronconeumol. 2007;43:219–224. doi: 10.1016/s1579-2129(07)60054-8. [DOI] [PubMed] [Google Scholar]
- 23.Iglesias-Garcia J, Poley JW, Larghi A, et al. Feasibility and yield of a new EUS histology needle: results from a multicenter, pooled, cohort study. Gastrointest Endosc. 2011;73:1189–1196. doi: 10.1016/j.gie.2011.01.053. [DOI] [PubMed] [Google Scholar]


