Abstract
Background/Aims
This study was performed to investigate the cost effectiveness of Helicobacter pylori screening/eradication in South Korean patients treated with nonsteroidal anti-inflammatory drugs (NSAIDs) and/or aspirin.
Methods
A decision Markov model was used to estimate the effectiveness and economic impact of an H. pylori screening/eradication strategy compared to a no-screening strategy among patients who were included in the model at the age of 40 years. Utility weights were applied to four of the health status groups to reflect quality-adjusted life years (QALY). The costs of screening, H. pylori eradication, and managing peptic ulcer and ulcer complications were obtained from South Korea-specific data.
Results
The total costs per patient were US $2,454 for the H. pylori screening/eradication and US $3,182 for the no-screening strategy. The QALYs for the two strategies were 16.05 and 15.73, respectively. The results were robust for the analyses of all different cohort groups who entered the model at the age of 30, 50, or 60 years and for NSAIDs-naïve patients. Through the probabilistic sensitivity analysis, the robustness of our study's results was also determined.
Conclusions
The H. pylori screening/eradication strategy was found to be less expensive and more effective compared to the no-screening strategy among South Korean patients taking NSAIDs and/or aspirin.
Keywords: Cost-benefit analysis, Helicobacter pylori, Mass screening
INTRODUCTION
The prevalence of Helicobacter pylori infection was reported to be higher in developing countries than developed countries.1 With economic developments, the prevalence of H. pylori in South Korea has decreased but still remains higher than that of Western societies. In 1998, 66.8% of individuals without a history of H. pylori eradication had a positive serology test results; this declined to 59.6% in 2005.2
A recent systematic review reported that the annual incidence of peptic ulcer disease (PUD) in Western countries is 0.10% to 0.19% for physician-diagnosed PUD cases and 0.03% to 0.17% based on hospitalization.3 Over time, the incidence or prevalence of PUD has slightly decreased, which may have been caused by decreased rates of H. pylori infection.3 There have been few data for the prevalence of PUD among Asians, which might be different among Asians. In South Korea, the prevalence of PUD has not decreased although the prevalence of H. pylori infection has declined.4 This may have been caused by the increasing use of nonsteroidal anti-inflammatory drugs (NSAIDs) and low-dose aspirin due to the rapidly increased elderly population. In particular, patients treated with NSAIDs and aspirin have a higher risk of developing severe ulcer complications without any symptomatic signs due to the analgesic effects of these drugs.
Eradication of H. pylori is generally regarded as a cost-effective method for preventing PUD associated with NSAIDs treatments in Western countries. National Institute for Health and Clinical Excellence (NICE) compared several strategies (i.e., do nothing, H. pylori eradication alone, treatment with proton pump inhibitor [PPI], administration of misoprostol, H. pylori eradication followed by misoprostol treatment, or H. pylori eradication followed by PPI treatment) as a prophylaxis against peptic ulcer bleeding in NSAIDs users.5 This group concluded that two strategies including 'H. pylori eradication alone' and 'H. pylori eradication followed by misoprostol if not tolerated, otherwise switching to PPI' were the most cost-effective.5
There are other reasons for the preference of H. pylori eradication over antisecretory treatment. First, antisecretory treatments require long-term compliance. Second, the cost of long-term treatment with antisecretory agents is overpriced. Guidelines by American College of Gastroenterology recommended H. pylori screening and treatment with H. pylori eradication therapy for patients who need long-term NSAIDs therapy regardless of the associated risk factors.6,7 However, in Asian countries like South Korea, which have a higher prevalence of H. pylori infection, there is controversy over the universal application for the screening/eradication of H. pylori infection. The guidelines by Korean Association of Gastroenterology have mentioned long-term NSAIDs users as one of possible indications for the screening/eradication of H. pylori.8 However, they are not covered by national insurance.9
Based on this background, the aim of the present study was to evaluate the cost-effectiveness of H. pylori screening/eradication for NSAIDs or aspirin users in South Korea, which has a high prevalence of H. pylori.
MATERIALS AND METHODS
1. Decision analytic model overview
A combined decision tree and Markov process model were used to estimate the effectiveness and economic impact of a H. pylori screening/eradication strategy compared to a no-screening strategy among patients who required treatment with NSAIDs or aspirin. This model assumed that all patients were screened and H. pylori infection was eradicated if they were positive for H. pylori in H. pylori screening/eradication strategy. Patients treated by the no-screening strategy were neither screened nor treated for H. pylori infection.
For the base model, patients entered the model at the age of 40 years old. The 3-month long cycle reflected disease processes such as treatment or exacerbation. Patients in this model died based on the death transition probability due to ulcer complication or natural causes depending on age-stratified life expectancy. It was assumed that all of these individuals died before the age of 100 years.
There were five possible health statuses into which a patient could transit: well, ulcer, ulcer complication (bleeding), death due to bleeding, and death due to other cause.
The definitions of ulcer, ulcer complication, death due to bleeding, death due to other cause were as follows: Well is no gastric intestinal symptoms in addition to no endoscopic lesion required treatments; Ulcer is defined endoscopical ulcer of which diameter at least 5 mm which needs treatments; Ulcer complication is defined as perforated and bleeding peptic ulcers; Death due to bleeding is mortality due to ulcer complication; Death due to other cause means natural death due to other causes than ulcer complication.
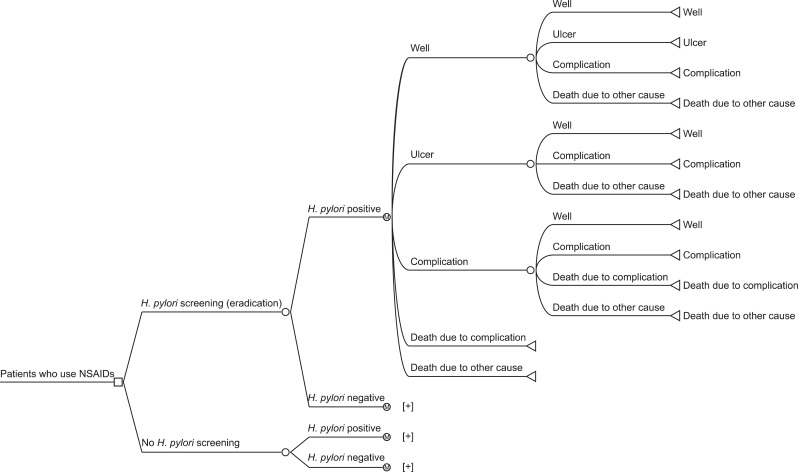
The probability of disease transition for each health status was obtained from the literature.10-12 For the present study, values for all causes of mortality rate were obtained through the Complete Life Table published in 2009 by the South Korean Statistical Office,13 and values for death rate due to complication were derived from published data sources.11 Transition into the next health status depended on the current health status. This model was established after several meetings with clinicians and methodologists to reflect real-world situations. The basic model is described in Fig. 1. The conceptual framework for this Markov process model was similar to that of a previous study.5
Fig. 1.
Decision analytic model for Helicobacter pylori screening/eradication versus no-H. pylori screening.
NSAIDs, nonsteroidal anti-inflammatory drugs.
2. Epidemiology data
We used South Korean-specific H. pylori prevalence data.2 This data was collected from a total of 7,792 adults (≥30 years old) who visited hospitals located in all parts of South Korea for medical check-ups and had no history of H. pylori eradication.2 Overall seropositivity rates for H. pylori were within a range of 50% to 65% depending on patient age. As shown in Table 1, the per-protocol eradication rate of PPI-based triple therapy was assumed to be 80% in South Korea.14
Table 1.
Model Input Parameters
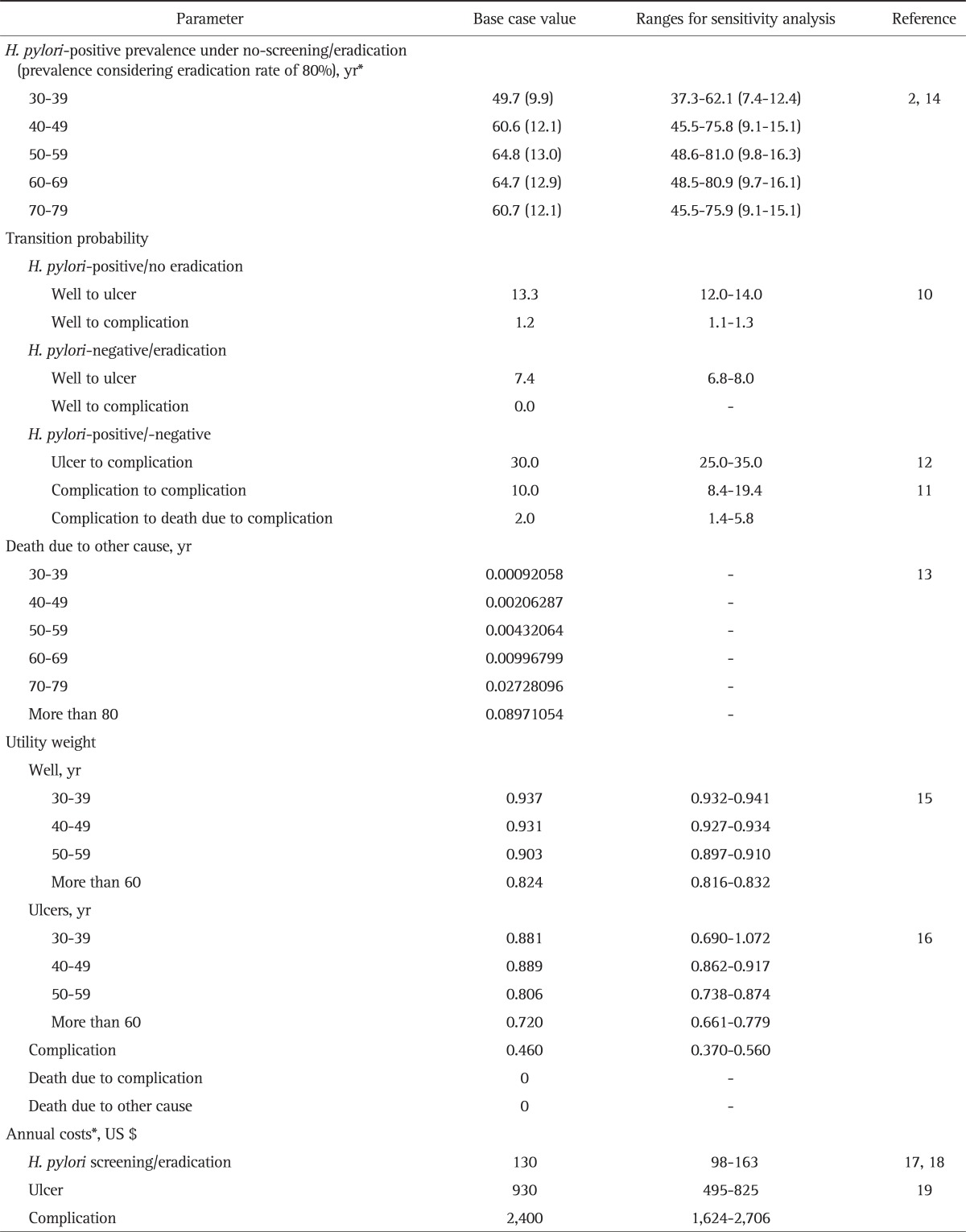
Data are presented as percentage or transition probabilities.
*SD was calculated using the mean value of ±25%.
3. Effectiveness of H. pylori eradication to prevent development of PUD
Since South Korean data for the transition probability for PUD development and complications when comparing H. pylori eradication to none among NSAIDs or aspirin users were not available we obtained from a literature review. Based on the Vergara et al.'s meta-analysis regarding the probability of transition to ulcers and complications among individuals taking NSAIDs were used.10 The development rate of PUD was 7.4% and 13.3% for the H. pylori eradication and noneradication groups, respectively, among NSAIDs users. If the data were restricted to the NSAIDs-naïve patients, the difference for PUD development was significantly greater. That is, 13.7% of the patients in the noneradicated group had PUD while 3.8% of the patients in the eradicated group developed this disease. This meta-analysis result was almost similar to the NICE health technology assessments published in 2007.5
4. Quality of life
Utility weights were applied to five health statuses (i.e., well, ulcer, complication, death due to complication, and death due to other cause) in this model to reflect quality adjusted life year (QALY). Since utility weight worsens with aging, participants designated with "well" and "ulcer" status had the utility weights depending on their age. Specific utility weights for applicable ages were obtained using the 2007 to 2009 Korean National Health and Nutritional Examination Survey Data, which was a national survey representative of the South Korean population.15 For example, the utility weights for the "well" and "ulcer" status at 40 years of age were 0.931 and 0.889, respectively, using the same database.15 The utility weights of complication during the initial 3 months of "bleeding" status was 0.460 based on a previous report.16 Death was valued as zero regardless of the cause (Table 1).
5. Medical costs
The screening/eradication strategy was associated with screening and eradication costs; the no-screening strategy was not. Costs for the screening strategy were determined by multiplying each health examinations by unit costs. Screening costs included costs for physician visits, endoscopic examination, and biopsy for the H. pylori test, and Campylobacter like organism tests. Eradication costs included ones for medical therapy with PPI-based triple therapy.17 This regimen included the standard therapy such as b.i.d. for PPI, 1 g b.i.d. amoxocillin, and 0.5 g b.i.d. clarythromycin for 1 week. The costs for these medications were calculated using weighted average costs.18 Eradication therapy was assumed to be administered only once when patients had positive H. pylori screening results. The positive rate for H. pylori screening was assumed to be 60% based on South Korean-specific H. pylori prevalence data.2
Patients with a "well" status had no associated costs because they did not have any clinical symptoms. Costs for treating ulcers with bleeding or without bleeding were drawn from a previous report,19 In this South Korean report, the annual cost for treating ulcers without bleeding was around US $930 (benign gastric ulcers, US $959.6; duodenal ulcer, US $901.4). When bleeding occurred due to PUD then the cost increased 2.6 times compared to ulcers without bleeding (benign gastric ulcers, US $2,553.1; duodenal ulcers, US $2,316.4).19 These costs were divided by four before being entered into the model to account for the periods for each cycle. Costs for death were entered as zero (Table 1). All costs are adjusted for 2011 currency values.
6. Statistical analysis
In the base model, the cost-effectiveness of the H. pylori screening/eradication strategy was compared to the no-screening strategy based on the outcomes (i.e., incurred costs and gained QALY) observed when the cohort patients were 40 years old. Aside from the base model analysis using data which was collected starting at the age of 40 years old, a different cohort group (i.e., patients who entered the model at the age of 30, 50, or 60 years) was analyzed. In addition, a cohort of patients who were NSAIDs-naïve and 40 years old was analyzed.
To evaluate the impact of the uncertainty of input data, probabilistic sensitivity analysis with 10,000 simulations was carried out. Costs and disease transition probabilities were assumed to have a gamma distribution while utility weights were assumed to have a triangular distribution. From the applicable distribution for cost, transition probability, and utility weight, one random value was drawn, and then incremental cost and QALY were calculated. If the process is repeated 10,000 times, 10,000 plot values are drawn in the plane of the incremental cost and QALY. Using this scatter plot, we could determine whether the study results were robust or not. A discount rate of 3% was applied to both the effectiveness and cost. TreeAge 2009 (TreeAge Software Inc., Williamstown, MA, USA) and Microsoft Excel 2007 (Microsoft Corp., Redmond, CA, USA) were used for these analyses.
RESULTS
1. Base case analysis
Total costs per patient for H. pylori screening/eradication was US $2,454 and that for the no-screening strategy was and US $3,182. QALYs for the two strategies were 16.05 and 15.73, respectively. The H. pylori screening/eradication strategy was less expensive (US $728 in cost saving) and more effective (QALY gain of 0.32) compared to the no-screening strategy (Table 2).
Table 2.
Base Case Analysis and Sensitivity Analysis According to Cohort Starting Age and NSAIDs Naïve-Patients
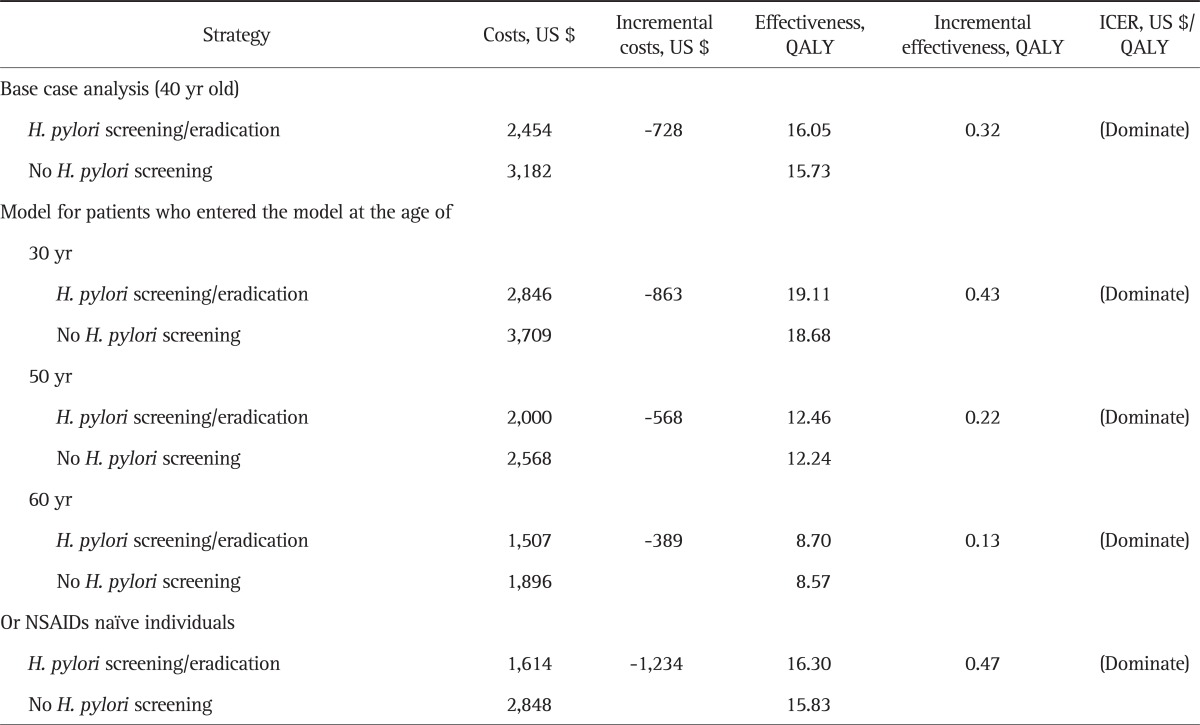
NSAIDs, nonsteroidal anti-inflammatory drugs; QALY, quality-adjusted life years; ICER, incremental cost-effectiveness ratio.
Results of different cohort group analysis for participants aged 30, 50, and 60 years old were similar to those of base analysis. The H. pylori screening/eradication strategy was dominant for all cohorts. In addition, the H. pylori screening/eradication strategy was more cost effective among the younger cohorts. For the cohorts who entered the model at the ages of 30, 40 (base case), 50, and 60 years, QALY gains were 0.43, 0.32, 0.22, and 0.13, respectively, and the cost savings were US $863, US $728, US $568, and US $389, respectively. Furthermore, the subgroup of NASIDs-naïve patients had the greater QALY gains (0.47) and cost savings (US $1,234), when compared to the base case or other subgroups.
2. Sensitivity analysis
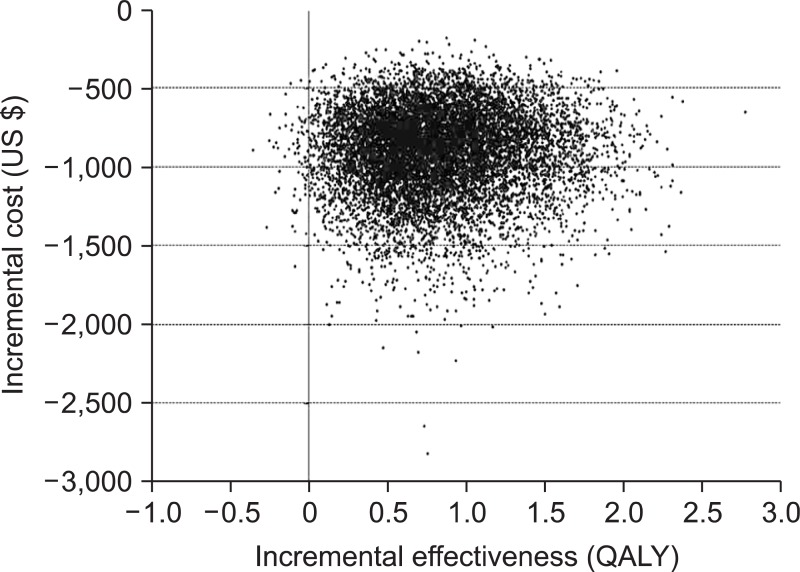
The probabilistic sensitivity analysis with 10,000 simulations showed that incremental QALYs (i.e., QALYs for the H. pylori screening/eradication strategy minus QALYs for the no-screening strategy) were positive for 95% or more cases, and incremental cost values of 95% or more cases were negative. In addition, a scatterplot was drawn (Fig. 2). In this plot, most dots were located in the area representing positive value for incremental effectiveness (i.e., higher QALY) and negative value for incremental cost (i.e., lower costs) compared to no-screening strategy. These findings indicate the robustness of our study results.
Fig. 2.
Incremental cost effectiveness scatterplot for the base model. For probabilistic sensitivity analysis with 10,000 simulations, a scatterplot was created. In this scatter plot, most dots were located in the area representing positive values of the incremental effectiveness (i.e., quality adjusted life years [QALY] gain) and negative values of incremental cost (i.e., cost saving). Incremental effectiveness was calculated by subtracting the QALY of the no-Helicobacter pylori screening from the QALY of the H. pylori screening/eradication. In addition, incremental cost was calculated by subtracting the costs of the no-H. pylori screening from the costs of the H. pylori screening/eradication.
DISCUSSION
In the present study, a H. pylori screening/eradication strategy was found to be less expensive and more effective compared to a no-screening strategy in South Korea, an area where H. pylori is endemic. Based on the different cohort group analysis results (i.e., for patients who entered the model at the age of 30, 40, 50, or 60 years), the H. pylori screening/eradication strategy was more cost-effective for the younger cohort than the older cohort. This result was consistent with those of previous economic analyses for H. pylori screening/eradication in patients treated with NSAIDs in Western countries.5 Health technology assessments by NICE also showed that H. pylori screening/eradication is the most cost-effective strategy.5 This report found that, H. pylori screening/eradication strategy has a QALY was more than 0.18 QALY and incremental costs was £235 less, compared to the do nothing.5 Interestingly, these numbers are similar to those from our study (i.e., a QALY gain of 0.32 and cost saving of US $728).
In a randomized study performed in China with a sample size of 100 patients, showed that, H. pylori screening/eradication prior to initiation of NSAIDs treatments reduced ulcer formation rates and mean direct medical costs compared to a noeradication strategy.20 However, one article from the United States reported substantial incremental costs of US $16,805 and US $31,842 to prevent symptomatic ulcer and complicated ulcer respectively, with a H. pylori screening/eradication strategy compared to a no-screening strategy.21 This study demonstrated the benefit of H. pylori screening/eradication for the prevention of ulcers similar to our study. However, H. pylori screening/eradication was not beneficial in terms of cost, which is different from the conclusions based on the present study. That is, this study raised question regarding whether effectiveness outweighs costs.21 The authors also suggested that H. pylori screening/eradication may be cost-effective if the risk of ulcers due to H. pylori infection was assumed to be higher. Several reasons might explain the difference between these two studies. First, the assumed ulcer development rates were different. This study applied 2.1% versus 1.4% ulcer development rates among NSAIDs users depending on the status of H. pylori positive and negative,21 but our study used 13.3% versus 7.4% ulcer development rates, respectively. The difference of ulcer development rates between two studies may be due to ethnicity and regional difference as indicated in previous studies.22-24 Generally, Asian countries have higher PUD prevalence than Western countries, which is seemed to be contributed by the differences of the prevalence of H. pylori between two areas. Higher prevalence of H. pylori in Asian countries is seemed to be caused by the environmental sanitization, especially clean water. Cultural practice may be another reason. In Asian countries, close maternal contact to children such as sharing the same bed and eating with sharing the bowel would increase mother-to-child transmission of H. pylori and passing the grass around when people drink together may also increase the infection of H. pylori. In addition, differences on diet and genetics seem to be the risk factors for greater prevalence of H. pylori infection in Asians.22
Second, the H. pylori infection prevalence and medical costs reported by the two studies were different. It is already known that cost-effectiveness analysis is affected by disease epidemiology, availability of healthcare resources, variation in clinical practice, and relative price or costs, which is challengeable to generalize study results across countries.25 Furthermore, in the present study the QALY gains were 0.47 versus 0.32 (base case) and cost savings were US $1,234 versus US $728 (base case) for the subgroup of NSAIDs naïve patients and all patients respectively. These results indicate that when H. pylori screening/eradication conducted among NSAIDs-naïve patients who required long-term NSAIDs treatment would effectively prevent PUD.26-28
H. pylori infection is known to be closely associated with a number of diverse gastrointestinal diseases such as gastric mucosa-associated lymphoid tissue lymphoma, and gastric cancers,29 and peptic ulcers.30 In addition to H. pylori, the use of acetyl salicylic acid (i.e., aspirin) and NSAIDs, and smoking are also known to be risk factors for PUD. General population attributable risk for H. pylori was highest (48%) followed by the use of NSAIDs (24%) and smoking (23%).31 The risk of peptic ulcer might be much higher for both H. pylori infection and NSAIDs administration in subjects with either H. pylori infection or NSAIDs-associated damage to the mucosa, because the mechanism of action is different. While NSAIDs induce prostanoid inhibition and nitric oxide mediated neutrophil recruitments into the microvasculature through topical and systemic effects, H. pylori infection induces an inflammatory response in the mucosa.32 Synergistic and additive effects of H. pylori infection and NSAIDs on the mucosa are possible. However, H. pylori screening/eradication in the chronic users of NSAIDs/aspirin are still optional instead of mandatory and are not covered by medical insurance provided by the South Korean government.9 Thus, H. pylori screening/eradication is not routinely performed in clinical practice in South Korea. Furthermore, there are some drawbacks for that. Side-effects such as diarrhea, vomiting, nausea, abdominal pain, and abdominal bloating frequently develop during H. pylori eradication, resulting in poor patient compliance, eradication failure, and antimicrobial resistance.33-35 However, the present study clearly demonstrated the cost saving associated with H. pylori screening/eradication, for the first time in a South Korean population.
This study had several limitations. First, we used meta-analysis results to determine the rates of ulcers and ulcer-related complications among NSAIDs or aspirin users depending on H. pylori eradication. We tried to find additional journals which deal with transition probability for PUD development under similar condition of our study design, but we could not find additional article after this meta-analysis result. Nonetheless, this meta-analysis included two China studies among five studies,26,27 which are very higher H. pylori infection rate like our country. In addition, these reports included the case of endoscopic ulcer instead of clinical ulcers. Actually clinical ulcers are the ones that ultimately will likely transition to ulcer complications and the risks of bleeding and perforation. However, the differentiation of clinical ulcers from endoscopic ulcers looks like very difficult especially when transition probability for PUD development is under two conditions with or without long-term use of NSAIDs and/or aspirin. Second, the present study was based on the annual medical costs for ulcers and ulcer-related complications, around US $900 and US $2,400, respectively,19 which is much lower than those in Western countries. The annual medical cost for treating PUD in the United States is US $24,000.36 If the medical cost is very low to treat disease as like the situation of South Korea, universal prevention strategies may not be cost-effective from an economic perspective. However, our study clearly demonstrated the cost-effectiveness associated with H. pylori screening/eradication, for individuals taking NSAIDs or aspirin. Finally, we did not account for side effects of H. pylori eradication, such as gastrointestinal disorders, in the cost-effectiveness analysis. Since these symptoms are mild and can be reduced with supplementary probiotic therapies,35 we assumed that they did not influence QALY.
In conclusion, the present study found that the H. pylori screening/eradication strategy was less expensive and more effective compared to the no-screening strategy. This was more apparent in younger individuals. Our results may help establish strategies for H. pylori screening in patients who required treatment with NSAIDs or aspirin in areas of endemic of H. pylori infection such as South Korea.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Graham DY, Adam E, Reddy GT, et al. Seroepidemiology of Helicobacter pylori infection in India. Comparison of developing and developed countries. Dig Dis Sci. 1991;36:1084–1088. doi: 10.1007/BF01297451. [DOI] [PubMed] [Google Scholar]
- 2.Yim JY, Kim N, Choi SH, et al. Seroprevalence of Helicobacter pylori in South Korea. Helicobacter. 2007;12:333–340. doi: 10.1111/j.1523-5378.2007.00504.x. [DOI] [PubMed] [Google Scholar]
- 3.Sung JJ, Kuipers EJ, El-Serag HB. Systematic review: the global incidence and prevalence of peptic ulcer disease. Aliment Pharmacol Ther. 2009;29:938–946. doi: 10.1111/j.1365-2036.2009.03960.x. [DOI] [PubMed] [Google Scholar]
- 4.Jang HJ, Choi MH, Shin WG, et al. Has peptic ulcer disease changed during the past ten years in Korea? A prospective multi-center study. Dig Dis Sci. 2008;53:1527–1531. doi: 10.1007/s10620-007-0028-6. [DOI] [PubMed] [Google Scholar]
- 5.Leontiadis GI, Sreedharan A, Dorward S, et al. Systematic reviews of the clinical effectiveness and cost-effectiveness of proton pump inhibitors in acute upper gastrointestinal bleeding. Health Technol Assess. 2007;11:iii–iiv. 1–164. doi: 10.3310/hta11510. [DOI] [PubMed] [Google Scholar]
- 6.Lanza FL, Chan FK, Quigley EM Practice Parameters Committee of the American College of Gastroenterology. Guidelines for prevention of NSAID-related ulcer complications. Am J Gastroenterol. 2009;104:728–738. doi: 10.1038/ajg.2009.115. [DOI] [PubMed] [Google Scholar]
- 7.Graham DY, Chan FK. NSAIDs, risks, and gastroprotective strategies: current status and future. Gastroenterology. 2008;134:1240–1246. doi: 10.1053/j.gastro.2008.02.007. [DOI] [PubMed] [Google Scholar]
- 8.Lee JH, Lee YC, Jeon SW, et al. Guidelines of prevention and treatment for NSAID-related peptic ulcers. Korean J Gastroenterol. 2009;54:309–317. doi: 10.4166/kjg.2009.54.5.309. [DOI] [PubMed] [Google Scholar]
- 9.Lee SW. Current status of health insurance in the treatment of Helicobacter pylori infection. Hanyang Med Rev. 2007;27:103–106. [Google Scholar]
- 10.Vergara M, Catalán M, Gisbert JP, Calvet X. Meta-analysis: role of Helicobacter pylori eradication in the prevention of peptic ulcer in NSAID users. Aliment Pharmacol Ther. 2005;21:1411–1418. doi: 10.1111/j.1365-2036.2005.02444.x. [DOI] [PubMed] [Google Scholar]
- 11.Lau JY, Sung J, Hill C, Henderson C, Howden CW, Metz DC. Systematic review of the epidemiology of complicated peptic ulcer disease: incidence, recurrence, risk factors and mortality. Digestion. 2011;84:102–113. doi: 10.1159/000323958. [DOI] [PubMed] [Google Scholar]
- 12.Kim JJ, Kim N, Park HK, et al. Clinical characteristics of patients diagnosed as peptic ulcer disease in the third referral center in 2007. Korean J Gastroenterol. 2012;59:338–346. doi: 10.4166/kjg.2012.59.5.338. [DOI] [PubMed] [Google Scholar]
- 13.Statistics Korea. Complete Life Table 2009 [Internet] Daejeon: Statistics Korea; c1996. [cited 2010 Nov 21]. Available from: http://kostat.go.kr. [Google Scholar]
- 14.Kim N, Kim JJ, Choe YH, et al. Diagnosis and treatment guidelines for Helicobacter pylori infection in Korea. Korean J Gastroenterol. 2009;54:269–278. doi: 10.4166/kjg.2009.54.5.269. [DOI] [PubMed] [Google Scholar]
- 15.Korean Ministry of Health and Welfare Affairs. Korean national health and nutritional examination survey (2007-2009) Seoul: Korean Ministry of Health and Welfare Affairs; 2010. [Google Scholar]
- 16.Latimer N, Lord J, Grant RL, et al. Cost effectiveness of COX 2 selective inhibitors and traditional NSAIDs alone or in combination with a proton pump inhibitor for people with osteoarthritis. BMJ. 2009;339:b2538. doi: 10.1136/bmj.b2538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Health Insurance Review and Assessment. Medical/dental service fee. Seoul: Health Insurance Review and Assessment; 2011. [Google Scholar]
- 18.Health Insurance Review and Assessment. Weighted average price by active ingredient of pharmaceutical product. Seoul: Health Insurance Review and Assessment; 2010. [Google Scholar]
- 19.Kim N, Lee MK, Park JH, Nam RH, Choi YA, Min SJ. The incidence and direct medical costs of peptic ulcer disease in Korean tertiary medical center. Seoul: Health Insurance Review and Assessment; 2009. [Google Scholar]
- 20.You JH, Lau W, Lee IY, et al. Helicobacter pylori eradication prior to initiation of long-term non-steroidal anti-inflammatory drug therapy in Chinese patients-a cost-effectiveness analysis. Int J Clin Pharmacol Ther. 2006;44:149–153. doi: 10.5414/cpp44149. [DOI] [PubMed] [Google Scholar]
- 21.Scheiman JM, Bandekar RR, Chernew ME, Fendrick AM. Helicobacter pylori screening for individuals requiring chronic NSAID therapy: a decision analysis. Aliment Pharmacol Ther. 2001;15:63–71. doi: 10.1046/j.1365-2036.2001.00874.x. [DOI] [PubMed] [Google Scholar]
- 22.Leong RW. Differences in peptic ulcer between the East and the West. Gastroenterol Clin North Am. 2009;38:363–379. doi: 10.1016/j.gtc.2009.03.010. [DOI] [PubMed] [Google Scholar]
- 23.Feinstein LB, Holman RC, Yorita Christensen KL, Steiner CA, Swerdlow DL. Trends in hospitalizations for peptic ulcer disease, United States, 1998-2005. Emerg Infect Dis. 2010;16:1410–1418. doi: 10.3201/eid1609.091126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dong WG, Cheng CS, Liu SP, Yu JP. Epidemiology of peptic ulcer disease in Wuhan area of China from 1997 to 2002. World J Gastroenterol. 2004;10:3377–3379. doi: 10.3748/wjg.v10.i22.3377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Drummond MF. Comparing cost-effectiveness across countries: the model of acid-related disease. Pharmacoeconomics. 1994;5:60–67. [Google Scholar]
- 26.Chan FK, Sung JJ, Chung SC, et al. Randomised trial of eradication of Helicobacter pylori before non-steroidal anti-inflammatory drug therapy to prevent peptic ulcers. Lancet. 1997;350:975–979. doi: 10.1016/s0140-6736(97)04523-6. [DOI] [PubMed] [Google Scholar]
- 27.Chan FK, To KF, Wu JC, et al. Eradication of Helicobacter pylori and risk of peptic ulcers in patients starting long-term treatment with non-steroidal anti-inflammatory drugs: a randomised trial. Lancet. 2002;359:9–13. doi: 10.1016/s0140-6736(02)07272-0. [DOI] [PubMed] [Google Scholar]
- 28.Labenz J, Blum AL, Bolten WW, et al. Primary prevention of diclofenac associated ulcers and dyspepsia by omeprazole or triple therapy in Helicobacter pylori positive patients: a randomised, double blind, placebo controlled, clinical trial. Gut. 2002;51:329–335. doi: 10.1136/gut.51.3.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Go MF. Review article: natural history and epidemiology of Helicobacter pylori infection. Aliment Pharmacol Ther. 2002;16(Suppl 1):3–15. doi: 10.1046/j.1365-2036.2002.0160s1003.x. [DOI] [PubMed] [Google Scholar]
- 30.NIH Consensus conference. Helicobacter pylori in peptic ulcer disease. NIH consensus development panel on Helicobacter pylori in peptic ulcer disease. JAMA. 1994;272:65–69. [PubMed] [Google Scholar]
- 31.Kurata JH, Nogawa AN. Meta-analysis of risk factors for peptic ulcer. Nonsteroidal antiinflammatory drugs, Helicobacter pylori, and smoking. J Clin Gastroenterol. 1997;24:2–17. doi: 10.1097/00004836-199701000-00002. [DOI] [PubMed] [Google Scholar]
- 32.Hunt RH, Bazzoli F. Review article: should NSAID/low-dose aspirin takers be tested routinely for H. pylori infection and treated if positive? Implications for primary risk of ulcer and ulcer relapse after initial healing. Aliment Pharmacol Ther. 2004;19(Suppl 1):9–16. doi: 10.1111/j.0953-0673.2004.01830.x. [DOI] [PubMed] [Google Scholar]
- 33.Adamsson I, Nord CE, Lundquist P, Sjöstedt S, Edlund C. Comparative effects of omeprazole, amoxycillin plus metronidazole versus omeprazole, clarithromycin plus metronidazole on the oral, gastric and intestinal microflora in Helicobacter pylori-infected patients. J Antimicrob Chemother. 1999;44:629–640. doi: 10.1093/jac/44.5.629. [DOI] [PubMed] [Google Scholar]
- 34.de Boer WA, Tytgat GN. The best therapy for Helicobacter pylori infection: should efficacy or side-effect profile determine our choice? Scand J Gastroenterol. 1995;30:401–407. doi: 10.3109/00365529509093298. [DOI] [PubMed] [Google Scholar]
- 35.Tong JL, Ran ZH, Shen J, Zhang CX, Xiao SD. Meta-analysis: the effect of supplementation with probiotics on eradication rates and adverse events during Helicobacter pylori eradication therapy. Aliment Pharmacol Ther. 2007;25:155–168. doi: 10.1111/j.1365-2036.2006.03179.x. [DOI] [PubMed] [Google Scholar]
- 36.Joish VN, Donaldson G, Stockdale W, et al. The economic impact of GERD and PUD: examination of direct and indirect costs using a large integrated employer claims database. Curr Med Res Opin. 2005;21:535–544. doi: 10.1185/030079905X38240. [DOI] [PubMed] [Google Scholar]