Abstract
Background/Aims
Duodenal immune alterations have been reported in a subset of patients with functional dyspepsia (FD). The aim of this study was to investigate the effect of acute stress on immune cell counts and the expression of tight junction proteins in the duodenal mucosa.
Methods
Twenty-one male rats were divided into the following three experimental groups: 1) the nonstressed, control group, 2) the 2-hour-stressed group, and 3) the 4-hour-stressed group. Eosinophils, mast cells and CD4+ and CD8+ T lymphocytes in the duodenal mucosa were counted. The protein and mRNA expressions of occludin and zonula occludens-1 (ZO-1) were examined.
Results
Eosinophils, mast cells and CD8+ T lymphocyte counts did not differ between the stressed and control groups. The number of CD4+ T lymphocytes and the protein and mRNA expressions of occludin and ZO-1 were significantly lower in the 4-hour-stressed group compared with the control group. The plasma adrenocorticotrophic hormone and cortisol levels of the 4-hour-stressed group were significantly higher than those of the control group.
Conclusions
Acute stress reduces the number of CD4+ T lymphocytes and the expression of tight junction proteins in the duodenal mucosa, which might be associated with the duodenal immune alterations found in a subset of FD patients.
Keywords: Duodenum, Lymphocytes, Stress, Tight junction protein
INTRODUCTION
Functional dyspepsia (FD) is a heterogeneous disorder characterized by the presence of recurrent or persistent symptoms thought to originate in the gastroduodenal region without any organic, systemic or metabolic disease that is likely to explain the symptoms.1 The pathophysiology of FD is not completely understood yet. Although the stomach is traditionally believed to be mainly responsible for dyspeptic symptoms, recent studies have shown the possibility of duodenal involvement in the pathophysiology of FD.2 Abnormal motor and sensory responses to duodenal acid or lipids have been demonstrated in patients with FD.3-7 Alterations in the number of immune cells have been observed in the duodenal mucosa of patients with FD.8,9 Since cytokines, chemokines, and neuroactive chemicals released from immune cells may affect gastrointestinal motility and sensitivity,10-13 duodenal immune cell alterations may be implicated in the pathophysiology of FD. However, how these changes occur is unclear yet and remains to be explored.
FD is known to be related to stress.14,15 Experimental studies provide evidence that acute psychological stress exerts an inhibitory effect on the duodenal and gastric motility.16-18 In addition, stress is found to contribute to inflammation by altering epithelial permeability.19,20 Immunologic alterations found in the duodenal mucosa of FD patients might be associated with stress. So, we hypothesized that stress affect immune cells and epithelial tight junction (TJ) proteins in the duodenal mucosa.
Thus, the aim of the present study was to investigate the effect of acute stress on immune cell counts and the expression of TJ proteins in the duodenal mucosa of rats.
MATERIALS AND METHODS
1. Animals
Twenty-one adult male Sprague Dawley rats (250 to 300 g), aged 16 to 18 weeks were used for the present study. All rats were acclimated for 7 days before experimentation and allowed free access to food and water. Rats were kept on a 12-hour light:dark cycle and isolated from environmental stressors (e.g., noise) as much as possible. The animals were housed in pairs in cages and kept in a temperature-controlled room (21℃±1℃). The rats were handled daily for a week by the same examiner. All protocols were approved by the Institutional Animal Care and Use Committee at Ajou University School of Medicine (AMC-103).
2. Experimental protocols
Rats were divided into three experimental groups with seven rats per treatment group: 1) the nonstressed group, 2) the 2-hour-stressed group (rats were assigned to receive water-immersion restraint stress for 2 hours), and 3) the 4-hour-stressed group (rats were assigned to receive water-immersion restraint stress for 4 hours). Rats in those separate groups are identical in sex, weight and age. In all stress sessions, the total body of the animal from head to lower hind limbs was tightly placed in wire cages, and the entire body except the head was immersed vertically to the level of the xiphoid process in a water bath maintained at 19℃±1℃. The stress session was performed just one time. Control rats were placed in their home cages without exposure to any restraint stress. Immediately after completing the experiments according to the protocol, all rats were sacrificed by stunning and posterior exsanguination. Blood and tissues were collected immediately after sacrifice.
3. Histological evaluation
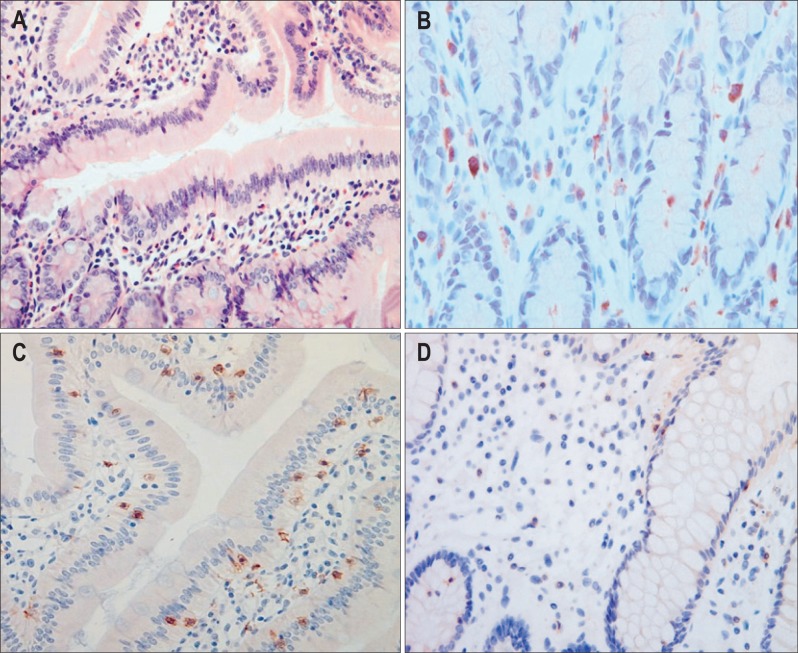
Mucosal tissues were obtained from the duodenum, immediately fixed in formalin and embedded in paraffin wax. Serial sections were stained with hematoxylin and eosin for routine histological evaluation under light microscopy. Eosinophils were counted in five nonoverlapping high power fields (HPF; final magnification, ×400) (Fig. 1A). Toluidine blue staining was performed to identify mast cells (Fig. 1B). Mast cells located in the mucosa were counted in five nonoverlapping HPF. CD4+ and CD8+ T lymphocytes were quantified using a standard immunoperoxidase technique (Fig. 1C). Briefly, sections were deparaffinized using xylene, rehydrated with a graded series of ethanol and immersed in distilled water. Endogenous peroxidase activity was blocked by incubation with Hydrogen Peroxide Block (Thermo Scientific, Fremont, CA, USA) for 5 minutes. After washing in distilled water and then in phosphate-buffered saline, the sections were incubated in a moist chamber for 30 minutes with the primary monoclonal antibodies; antimouse CD4 (NB100-64988; Novus Biologicals, Littleton, CO, USA) and antimouse CD8 (CBL1507; Millipore, Billerica, MA, USA). The samples were counterstained with Harris' hematoxylin, dehydrated and covered with synthetic resin. CD4+ and CD8+ cells in the mucosa were quantified within five consecutive nonoverlapping HPF. The mean numbers of eosinophils, mast cells or T lymphocytes were expressed per HPF. All histological sections were evaluated by an expert pathologist (Y.B. Kim), who was unaware of the experimental details.
Fig. 1.
Histological evaluation of eosinophils, mast cells and T lymphocytes in the duodenal mucosa (magnification, ×400). (A) Hematoxylin and eosin and (B) toluidine blue stains were performed to identify eosinophils and mast cells, respectively. (C) Immunostaining using antimouse CD4 for CD4+ cells and antimouse CD8 for CD8+ cells was performed. (D) The number of CD4+ immunostaining cells was reduced in the stressed group.
4. Expression of TJ proteins
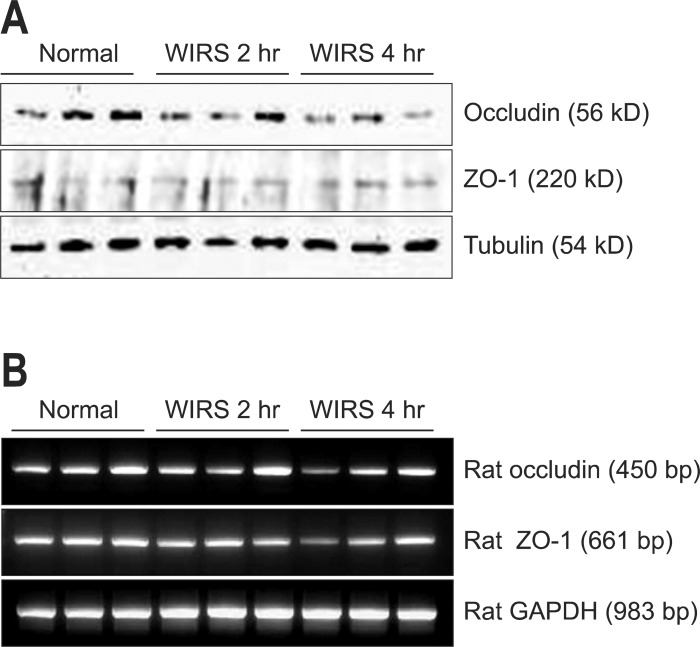
Protein and mRNA expressions of occludin and zonula occludens-1 (ZO-1) were examined, using the Western blot and reverse transcription-polymerase chain reaction (RT-PCR) analysis, respectively. The tissues were solubilized in lysis buffer containing 40 mM Tris, 7 M urea, 2 M thiourea, 4% CHAPS, 100 mM 1, 4-dithioerythritol and protease inhibitor cocktail. The lysate was sonicated thrice for approximately 10 seconds and then centrifuged at 100,000×g for 30 minutes. The supernatant proteins were electrophoresed on SDS-PAGE gels and then transferred to polyvinylidene difluoride membranes using a semidry transfer system (Hoeffer Pharmacia Biotech, San Francisco, CA, USA). The membrane was blocked with 5% nonfat dry milk in TBST (10 mM Tris-Cl, pH 8.0, 150 mM NaCl and 0.1%v/v Tween-20) at room temperature for 1 hour then incubated with specific primary antibodies for 2 hours. Primary antibodies (anti-ZO-1 at 1:3,000 overnight at 4℃, antioccludin 1:1,000 for 1 hour at room temperature) were applied followed by appropriate secondary antibodies (antigoat-horseradish peroxidase at 1:10,000 and antigoat-HRP at 1:5,000 for 1 hour at room temperature). Anti-ZO-1, antioccludin, and antigoat-HRP antibodies were purchased from Santa Cruz Biotechnology Inc. (sc-10804 and sc-133256 and sc-2005; Santa Cruz, CA, USA). Visualization was accomplished using an enhanced chemiluminescence detection kit (Amersham Pharmacia Biotech, Piscataway, NJ, USA). The expression level of each protein was analyzed with the image analysis software Image-Pro Plus 6.0 (Media Cybernetics, Silver Spring, MD, USA) and presented as band intensities (pixels) (Fig. 2A).
Fig. 2.
Expression of tight junction proteins. (A) Protein and (B) mRNA expression profiles for occludin and zonula occludens-1 (ZO-1) were examined using Western blot and reverse transcription-polymerase chain reaction, respectively.
WIRS, water immersion restraint stress; GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
Quantitative RT-PCR was employed for cognate mRNA detection and quantitation (Fig. 2B). Total RNA was extracted from the duodenal mucosa using the TRIzol reagent (Life Technologies Inc., Rockville, MD, USA) according to the manufacturer's instructions. The 2 µg of total RNA was reverse transcribed according to the manufacturer's instructions of M-MLV reverse transcriptase (Promega, Madison, WI, USA). The thermocycling conditions were as follows: initial activation at 95℃ for 5 minutes, 30 cycles of denaturation at 95℃, annealing at 60℃ for 1 minute, and final extension at 72℃ for 7 minutes. Primers were designed by a Primer-BLAST (NCBI, USA) according to the mRNA sequences (by GenBank) of occludin, ZO-1, and β-actin (as a control). The PCR products were run on a 1% agarose gel to confirm that products were of the expected size. Results were normalized against glyceraldehyde-3-phosphate dehydrogenase (GAPDH) expression. The sequences of primers were as follows: The forward primer of the GAPDH gene was 5'-TCCCTCAAGATTGTCAGCAA-3' and its reverse primer was 5'-AGATCCACAACGGATACATT-3'. The forward primer of the occludin gene was 5'-TGGAGTTGCGGGAGAGCGATC-3' and its reverse primer was 5'-GGGCAGTCGGGTTGACTCCCA-3'. The forward primer of the ZO-1 gene was 5'-GCTCCTCCCACCTCGCACGT-3' and its reverse primer was 5'-GACCTGCTGGAGCATAGGGCTG-3'.
5. Measurement of plasma adrenocorticotrophic hormone (ACTH) and cortisol levels
Blood samples from the central vein were collected in heparinized Eppendorf tubes immediately after sacrifice and centrifuged (10,000 rpm, 1 minute at 4℃) to obtain plasma, which was then stored at -70℃ until required for assay. ACTH was measured with a chemiluminescence enzyme-linked immunosorbent assay (AC562T-100; Calbiotech Inc., Spring Valley, CA, USA). Plasma cortisol levels were quantified using an ELISA kit (E90462Ra; USCNK Life Science Inc., Wuhan, China) according to the instruction manual.
6. Data analysis
All data are expressed as means±SEM for each group of rats. The numbers of eosinophils, mast cells and T lymphocytes, the expression intensity of TJ proteins, plasma ACTH levels, and plasma cortisol levels were compared between groups using ANOVA with Turkey post hoc tests. A p<0.05 was considered statistically significant. SPSS for Windows version 11.0 (SPSS Inc., Chicago, IL, USA) was used for all analyses.
RESULTS
1. Eosinophil, mast cell, and T lymphocyte counts
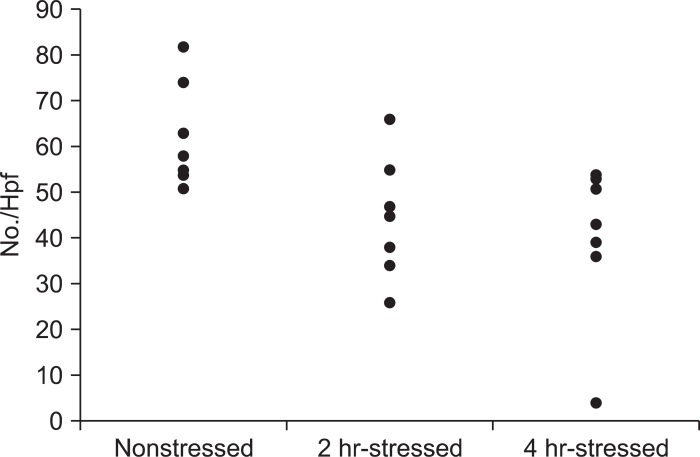
The number of eosinophils in the 2-hour and 4-hour-stressed groups was not significantly different from that in the nonstressed group. Mast cell counts in the 2-hour and 4-hour-stressed groups did not significantly differ, compared with the nonstressed group. The number of CD8+ cells in the 2-hour and 4-hour-stressed groups was not significantly different from that in the nonstressed group. The number of CD4+ cells tended to be lower in the 2-hour-stressed group (p=0.07) and was significantly lower in the 4-hour-stressed group, compared with the nonstressed group (Figs 1D and 3) (Table 1).
Fig. 3.
The number of CD4+ immunostaining cells. The number of CD4+ cells tended to be lower in the 2-hour-stressed group (p=0.07) and was significantly lower in the 4-hour-stressed group, compared with the nonstressed group.
Table 1.
Effect of Stress on the Number of Immune Cells in the Duodenal Mucosa
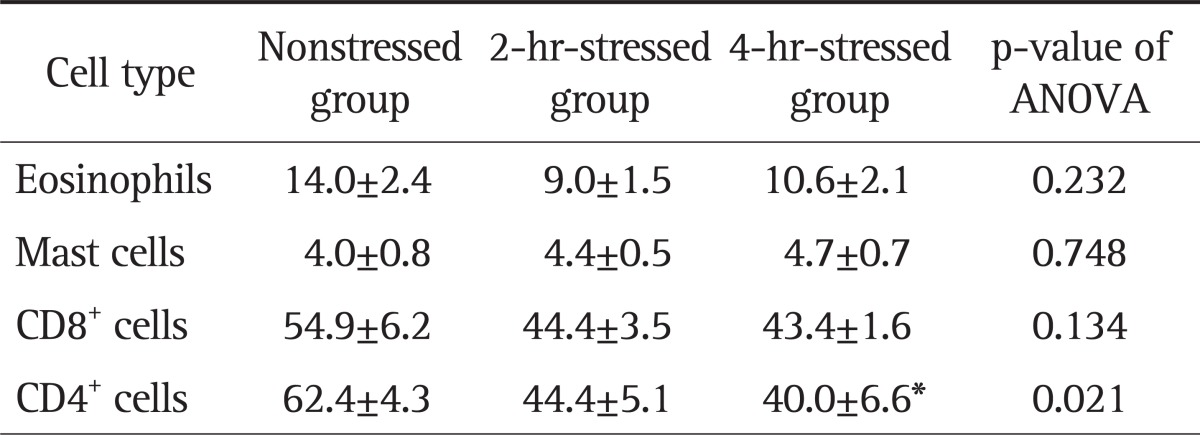
Data are presented as mean±SEM per high power field (magnification, ×400).
ANOVA, analysis of variance.
*p<0.05 vs the nonstressed group using ANOVA with Tukey post hoc tests.
2. Protein and mRNA expressions of TJ proteins
The expression level of occludin in the 4-hour-stressed group was significantly lower than in the nonstressed group, but that in the 2-hour-stressed group was not. There were no significant differences in the mRNA expression levels of occludin between groups. The expression levels of ZO-1 in the 2-hour and 4-hour-stressed groups were significantly lower, compared with the nonstressed group. Significantly lower mRNA expression of ZO-1 was observed in the 4-hour-stressed group, compared with the nonstressed group, but not in the 2-hour-stressed group (Table 2).
Table 2.
Effect of Stress on the Expression of Tight Junction Proteins in the Duodenal Mucosa
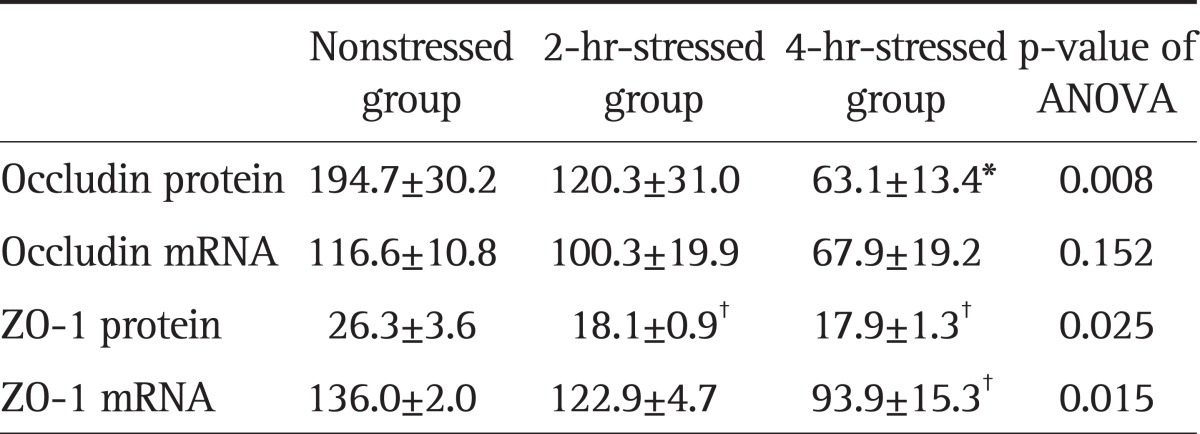
Data are presented as mean±SEM (pixels). The p<0.01 and p<0.05 versus the nonstressed group, using ANOVA with Tukey post hoc tests.
ANOVA, analysis of variance.
*p<0.01; †p<0.05.
3. Plasma ACTH and cortisol levels
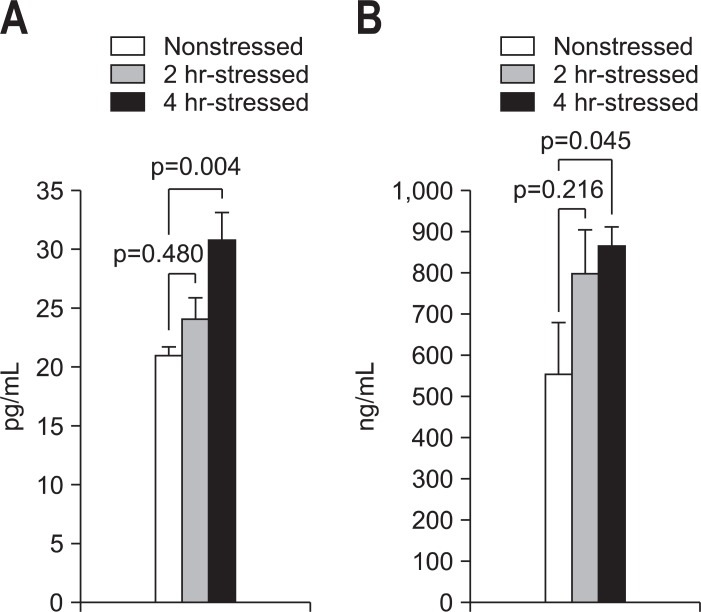
The plasma ACTH levels were significantly higher in the 4-hour-stressed group (30.7±2.4 pg/mL, p=0.004) than those of the nonstressed group (20.9±0.8 pg/mL), but not in the 2-hour-stressed group (24.0±1.9 pg/mL, p=0.480) (Fig. 4A). Significantly higher plasma cortisol levels were observed in the 4-hour-stressed group (864.5±47.9 ng/mL, p=0.045), compared with the nonstressed group (554.2±126.4 ng/mL), but not in the 2-hour-stressed group (799.3±106.1 ng/mL, p=0.216) (Fig. 4B).
Fig. 4.
Comparison of plasma adrenocorticotrophic hormone (ACTH) and cortisol levels. (A) The plasma ACTH level of the 4-hour-stressed group was significantly higher than that of the nonstressed group (p=0.004). (B) The plasma cortisol level of the 4-hour-stressed group was significantly higher than that of the nonstressed group (p=0.045).
DISCUSSION
In the present study, we demonstrated that CD4+ cells significantly reduced in the duodenal mucosa of the stressed group, compared with the nonstressed group. Whereas, the numbers of eosinophils, mast cells and CD8+ cells were not significantly altered in the stressed group. Protein and mRNA expressions of occludin and ZO-1 in the duodenal mucosa of stressed rats were significantly reduced, compared with nonstressed rats. Dysregulation of the mucosal immune system and weakening of epithelial barriers may lead to low grade inflammation or immune activation in the duodenal mucosa, which was found in a subset of patients with FD. Ongoing low grade inflammation and upregulation of cytokines, chemokines, or neuroactive chemicals may affect gastrointestinal motility and sensitivity. Actually, inflammation and immune activation in the gut are usually accompanied by gastrointestinal dysmotility and visceral hypersensitivity.10-13
Our results of the present study are in keeping with those of the previous report that the number of CD4+ cells is reduced in postinfectious FD.9 CD4+ cells are less frequently observed on duodenal biopsies in presumed post-infectious FD. Although focal aggregates of CD8+ cells were found in postinfectious FD, those are not observed in unspecified-onset FD patients.9 We did not find focal aggregates of CD8+ cells in the duodenum of stressed rats and the total number of CD8+ cells did not significantly differ between the nonstressed and stressed groups. CD4+ cells are known to play a crucial role in the immune response. Some subtypes of CD4+ cells have been shown to suppress antigen-specific immune responses and actively down-regulate a pathological immune response.21 Accordingly, decreased number of suppressive CD4+ cells may impair ability of the immune system to inhibit the inflammatory response to antigens, resulting in the persistence of a certain degree of inflammation. Actually, duodenal biopsy specimens from nonulcer dyspepsia patients have shown significantly higher inflammatory cell infiltration than control specimens.22 Several studies have shown that acute stress modifies the distribution of intestinal lymphocytes. Endocrine factors released during stress are suggested to modulate leukocyte trafficking and lead to the redistribution of leukocytes between the blood and other immune compartments.22 Thus, redistribution or apoptosis of lymphocytes might contribute to the decrease in the number of CD4+ T cells observed in stressed rats. Acute or short-term stress can enhance innate and adaptive immune responses.23 Our findings of the present study suggest the possibility that stress is involved in immune cell alterations or immune activation of the duodenal mucosa found in a subset of FD patients. The change in the number of T lymphocytes in the model of chronic stress warrants further investigation.
While duodenal eosinophilia in FD has been described, a significant increase in mast cells has not been observed in the duodenum of patients with FD.8,24 We investigated mast cell counts as well as eosinophil counts in the duodenal mucosa, because mucosal mast cells are known to be mediators of the stress responses in the gastrointestinal tract. We failed to find a significant difference in mast cell and eosinophil counts of the duodenum between the nonstressed and stressed rats. A previous study showed no significant difference in the number of mucosal mast cells in the colon of stressed animals compared with sham stress, but a significant increase in histamine content in mast cells of stressed animals compared with sham rats.25 Chronic stress might be associated with eosinophilia, which needs to be investigated.
Stress is believed to be a contributing factor in intestinal inflammatory diseases. Acute stressors may have profound effects on intestinal epithelial physiology, stimulating ion secretion, and reducing barrier function.26 It is presumed that acute stress induces barrier defect and allows uptake of immunogenic substances into the intestinal mucosa, initiating or exacerbating intestinal inflammation.27 Permeability across the epithelium is determined by the TJ. Actually, epithelial permeability can be estimated by TJ protein assay.28 The TJ is composed of a multiprotein complex that consists of the transmembrane proteins occludin and claudins that interact with ZO proteins.29 ZO-1 binds to the actin cytoskeleton and plays a crucial role in the final establishment of adherens junctions and the formation of TJs.30 The function of the TJs is dependent on levels and localization of junction proteins and also regulated by phosphorylation of the TJ proteins It has been reported that immobilization stress can induce an increase in intestinal permeability in rats, mainly due to its effect on the TJ proteins.20 Similarly, Ying et al.31 demonstrated that occludin and ZO-1 were reduced in intestinal mucosa both in mRNA and protein levels in the rat tail-suspension model. The stressed groups in the present study showed decreased expression of occludin and ZO-1, which may affect barrier function. Accordingly, acute stress appears to induce intestinal barrier dysfunction, leading to increased permeability. Intestinal permeability was not measured in the present study. However, since TJ proteins not only provide the epithelium with a barrier function but also change its permeability,28,32 duodenal permeability is likely to be increased in the stressed groups. A barrier defect induced by stress may initiate or exacerbate intestinal inflammation, which might be the plausible mechanism underlying subtle histopathological changes of the duodenum found in a subset of FD patients.
The model of water immersion restraint stress used in the present study produces physical stress in addition to psychological stress. This stress model is reported to increase plasma ACTH levels.33 Our results showed that water immersion restraint stress for 4 hours significantly increased plasma ACTH and cortisol levels, suggesting activation of the hypothalamic-pituitary-adrenal axis. It is conceivable that corticotrophin-releasing hormone (CRH) released by water immersion restraint stress plays a role in reducing the number of CD4+ cells and the expression of TJ proteins in the duodenal mucosa. Previous studies have shown that administration of CRH increases the intestinal permeability and CRH receptor antagonists prevents this stress-induced increase of mucosal permeability.29,34 Whether the pretreatment of CRH receptor antagonists can prevent an acute stress-induced decrease in the number of CD4+ cells and the expression of TJ proteins remains to be investigated. To our knowledge, this is the first study demonstrating the effect of acute stress on the number of CD4+ cells and the expression of TJ proteins in the duodenal mucosa. However, the present study has several limitations. First, acute stress, but not chronic stress, was used. Second, animal models may differ from human pathophysiology. Third, lymphocyte apoptosis was not measured and the location of TJ proteins was not determined. Fourth, mucosal permeability was not measured. Further studies using chronic stress or human models are warranted.
In conclusion, acute stress reduces the number of CD4+ T lymphocytes and the expression of TJ proteins in the duodenal mucosa of rats, which might be able to explain the duodenal immune alterations found in a subset of FD patients.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Tack J, Talley NJ, Camilleri M, et al. Functional gastroduodenal disorders. Gastroenterology. 2006;130:1466–1479. doi: 10.1053/j.gastro.2005.11.059. [DOI] [PubMed] [Google Scholar]
- 2.Lee KJ, Tack J. Duodenal implications in the pathophysiology of functional dyspepsia. J Neurogastroenterol Motil. 2010;16:251–257. doi: 10.5056/jnm.2010.16.3.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Samsom M, Verhagen MA, vanBerge Henegouwen GP, Smout AJ. Abnormal clearance of exogenous acid and increased acid sensitivity of the proximal duodenum in dyspeptic patients. Gastroenterology. 1999;116:515–520. doi: 10.1016/s0016-5085(99)70171-x. [DOI] [PubMed] [Google Scholar]
- 4.Lee KJ, Demarchi B, Demedts I, Sifrim D, Raeymaekers P, Tack J. A pilot study on duodenal acid exposure and its relationship to symptoms in functional dyspepsia with prominent nausea. Am J Gastroenterol. 2004;99:1765–1773. doi: 10.1111/j.1572-0241.2004.30822.x. [DOI] [PubMed] [Google Scholar]
- 5.Bratten J, Jones MP. Prolonged recording of duodenal acid exposure in patients with functional dyspepsia and controls using a radiotelemetry pH monitoring system. J Clin Gastroenterol. 2009;43:527–533. doi: 10.1097/MCG.0b013e31818e37ab. [DOI] [PubMed] [Google Scholar]
- 6.Fried M, Feinle C. The role of fat and cholecystokinin in functional dyspepsia. Gut. 2002;51(Suppl 1):i54–i57. doi: 10.1136/gut.51.suppl_1.i54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Björnsson E, Sjöberg J, Ringström G, Norström M, Simrén M, Abrahamsson H. Effects of duodenal lipids on gastric sensitivity and relaxation in patients with ulcer-like and dysmotility-like dyspepsia. Digestion. 2003;67:209–217. doi: 10.1159/000072059. [DOI] [PubMed] [Google Scholar]
- 8.Talley NJ, Walker MM, Aro P, et al. Non-ulcer dyspepsia and duodenal eosinophilia: an adult endoscopic population-based case-control study. Clin Gastroenterol Hepatol. 2007;5:1175–1183. doi: 10.1016/j.cgh.2007.05.015. [DOI] [PubMed] [Google Scholar]
- 9.Kindt S, Tertychnyy A, de Hertogh G, Geboes K, Tack J. Intestinal immune activation in presumed post-infectious functional dyspepsia. Neurogastroenterol Motil. 2009;21:832–e56. doi: 10.1111/j.1365-2982.2009.01299.x. [DOI] [PubMed] [Google Scholar]
- 10.Akiho H, Ihara E, Motomura Y, Nakamura K. Cytokine-induced alterations of gastrointestinal motility in gastrointestinal disorders. World J Gastrointest Pathophysiol. 2011;2:72–81. doi: 10.4291/wjgp.v2.i5.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liebregts T, Adam B, Bredack C, et al. Small bowel homing T cells are associated with symptoms and delayed gastric emptying in functional dyspepsia. Am J Gastroenterol. 2011;106:1089–1098. doi: 10.1038/ajg.2010.512. [DOI] [PubMed] [Google Scholar]
- 12.De Winter BY, De Man JG. Interplay between inflammation, immune system and neuronal pathways: effect on gastrointestinal motility. World J Gastroenterol. 2010;16:5523–5535. doi: 10.3748/wjg.v16.i44.5523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Humes DJ, Simpson J, Smith J, et al. Visceral hypersensitivity in symptomatic diverticular disease and the role of neuropeptides and low grade inflammation. Neurogastroenterol Motil. 2012;24:318–e163. doi: 10.1111/j.1365-2982.2011.01863.x. [DOI] [PubMed] [Google Scholar]
- 14.Haug TT, Svebak S, Wilhelmsen I, Berstad A, Ursin H. Psychological factors and somatic symptoms in functional dyspepsia. A comparison with duodenal ulcer and healthy controls. J Psychosom Res. 1994;38:281–291. doi: 10.1016/0022-3999(94)90033-7. [DOI] [PubMed] [Google Scholar]
- 15.Monnikes H, Tebbe JJ, Hildebrandt M, et al. Role of stress in functional gastrointestinal disorders. Evidence for stress-induced alterations in gastrointestinal motility and sensitivity. Dig Dis. 2001;19:201–211. doi: 10.1159/000050681. [DOI] [PubMed] [Google Scholar]
- 16.Mönnikes H, Schmidt BG, Raybould HE, Taché Y. CRF in the paraventricular nucleus mediates gastric and colonic motor response to restraint stress. Am J Physiol. 1992;262(1 Pt 1):G137–G143. doi: 10.1152/ajpgi.1992.262.1.G137. [DOI] [PubMed] [Google Scholar]
- 17.Gue M, Fioramonti J, Frexinos J, Alvinerie M, Bueno L. Influence of acoustic stress by noise on gastrointestinal motility in dogs. Dig Dis Sci. 1987;32:1411–1417. doi: 10.1007/BF01296668. [DOI] [PubMed] [Google Scholar]
- 18.Kellow JE, Langeluddecke PM, Eckersley GM, Jones MP, Tennant CC. Effects of acute psychologic stress on small-intestinal motility in health and the irritable bowel syndrome. Scand J Gastroenterol. 1992;27:53–58. doi: 10.3109/00365529209011167. [DOI] [PubMed] [Google Scholar]
- 19.Kiliaan AJ, Saunders PR, Bijlsma PB, et al. Stress stimulates transepithelial macromolecular uptake in rat jejunum. Am J Physiol. 1998;275:G1037–G1044. doi: 10.1152/ajpgi.1998.275.5.G1037. [DOI] [PubMed] [Google Scholar]
- 20.Mazzon E, Sturniolo GC, Puzzolo D, Frisina N, Fries W. Effect of stress on the paracellular barrier in the rat ileum. Gut. 2002;51:507–513. doi: 10.1136/gut.51.4.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Groux H, O'Garra A, Bigler M, et al. A CD4+ T-cell subset inhibits antigen-specific T-cell responses and prevents colitis. Nature. 1997;389:737–742. doi: 10.1038/39614. [DOI] [PubMed] [Google Scholar]
- 22.Dhabhar FS, Miller AH, McEwen BS, Spencer RL. Effects of stress on immune cell distribution. Dynamics and hormonal mechanisms. J Immunol. 1995;154:5511–5527. [PubMed] [Google Scholar]
- 23.Dhabhar FS. Enhancing versus suppressive effects of stress on immune function: implications for immunoprotection and immunopathology. Neuroimmunomodulation. 2009;16:300–317. doi: 10.1159/000216188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Walker MM, Talley NJ, Prabhakar M, et al. Duodenal mastocytosis, eosinophilia and intraepithelial lymphocytosis as possible disease markers in the irritable bowel syndrome and functional dyspepsia. Aliment Pharmacol Ther. 2009;29:765–773. doi: 10.1111/j.1365-2036.2009.03937.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Eutamene H, Theodorou V, Fioramonti J, Bueno L. Acute stress modulates the histamine content of mast cells in the gastrointestinal tract through interleukin-1 and corticotropin-releasing factor release in rats. J Physiol. 2003;553(Pt 3):959–966. doi: 10.1113/jphysiol.2003.052274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Saunders PR, Kosecka U, McKay DM, Perdue MH. Acute stressors stimulate ion secretion and increase epithelial permeability in rat intestine. Am J Physiol. 1994;267(5 Pt 1):G794–G799. doi: 10.1152/ajpgi.1994.267.5.G794. [DOI] [PubMed] [Google Scholar]
- 27.McCall IC, Betanzos A, Weber DA, Nava P, Miller GW, Parkos CA. Effects of phenol on barrier function of a human intestinal epithelial cell line correlate with altered tight junction protein localization. Toxicol Appl Pharmacol. 2009;241:61–70. doi: 10.1016/j.taap.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Saunders PR, Santos J, Hanssen NP, Yates D, Groot JA, Perdue MH. Physical and psychological stress in rats enhances colonic epithelial permeability via peripheral CRH. Dig Dis Sci. 2002;47:208–215. doi: 10.1023/a:1013204612762. [DOI] [PubMed] [Google Scholar]
- 29.Nusrat A, Turner JR, Madara JL. Molecular physiology and pathophysiology of tight junctions. IV. Regulation of tight junctions by extracellular stimuli: nutrients, cytokines, and immune cells. Am J Physiol Gastrointest Liver Physiol. 2000;279:G851–G857. doi: 10.1152/ajpgi.2000.279.5.G851. [DOI] [PubMed] [Google Scholar]
- 30.Tsukita S, Katsuno T, Yamazaki Y, Umeda K, Tamura A. Roles of ZO-1 and ZO-2 in establishment of the belt-like adherens and tight junctions with paracellular permselective barrier function. Ann N Y Acad Sci. 2009;1165:44–52. doi: 10.1111/j.1749-6632.2009.04056.x. [DOI] [PubMed] [Google Scholar]
- 31.Ying C, Chunmin Y, Qingsen L, et al. Effects of simulated weightlessness on tight junction protein occludin and zonula occluden-1 expression levels in the intestinal mucosa of rats. J Huazhong Univ Sci Technolog Med Sci. 2011;31:26–32. doi: 10.1007/s11596-011-0145-5. [DOI] [PubMed] [Google Scholar]
- 32.Koch S, Nusrat A. Dynamic regulation of epithelial cell fate and barrier function by intercellular junctions. Ann N Y Acad Sci. 2009;1165:220–227. doi: 10.1111/j.1749-6632.2009.04025.x. [DOI] [PubMed] [Google Scholar]
- 33.Nishioka T, Iyota K, Nakayama T, Suemaru S, Numata Y, Hashimoto K. Effects of ether-laparotomy and water immersion-restraint stress on CRH concentration in the hypothalamus, extrahypothalamic tissues and peripheral blood. Endocr J. 1993;40:213–220. doi: 10.1507/endocrj.40.213. [DOI] [PubMed] [Google Scholar]
- 34.Taché Y, Perdue MH. Role of peripheral CRF signalling pathways in stress-related alterations of gut motility and mucosal function. Neurogastroenterol Motil. 2004;16(Suppl 1):137–142. doi: 10.1111/j.1743-3150.2004.00490.x. [DOI] [PubMed] [Google Scholar]