Abstract
Background/Aims
We aimed to investigate the correlation between a disintegrin and metalloprotease with thrombospondin motif 2 (ADAMTS-2) and transforming growth factor-β1 (TGF-β1) in clinical human cirrhotic tissues.
Methods
The liver tissues of 24 patients (16 cases with cirrhotic portal hypertension as the cirrhosis group and eight cases with healthy livers as the normal group) were collected. Immunohistochemistry and Western blots were performed to evaluate the protein expression levels of ADAMTS-2 and TGF-β1. Western blots for other key mediators of cirrhotic progression, including SMAD2, SMAD3, TGF-β receptor II (TGFβRII), matrix metalloproteinases 2 (MMP2), and tissue inhibitor of matrix metalloproteinases 2 (TIMP2), were also performed.
Results
Cirrhotic tissues showed higher percentages of collagen. The protein expression levels of ADAMTS-2 and TGF-β1 were significantly higher in the cirrhotic group as compared to the matched normal group (p<0.05), and there was a positive correlation between these two proteins (r=0.862, p<0.01). The protein expressions of MMP2, TIMP2, and TGFβRII, as well as the phosphorylated forms of SMAD2 and SMAD3, were significant higher in the cirrhotic group (p<0.01 or p<0.05).
Conclusions
These findings suggested that ADAMTS-2 and TGF-β1 may play important roles in the pathogenesis of human cirrhosis; specifically, TGF-β1 may induce the expression of ADAMTS-2 through the TGFβ/SMAD pathway.
Keywords: A disintegrin and metalloprotease with thrombospondin motif-2, Transforming growth factor beta 1, Cirrhosis, Humans
INTRODUCTION
Liver disease is a kind of frequently encountered disease which is seriously harmful to human health worldwide. As an end-stage of liver disease, cirrhosis is due to a large number of extracellular matrix (ECM) synthesis through the activation of hepatic stellate cells (HSCs).1
In human, transforming growth factor-β1 (TGF-β1) shows a variety of pleiotropic functions, including inhibition of epithelial, induction of ECM synthesis and deposition, regulation of pericellular proteolytic activity, stimulation of cytokine cascade, chemoattraction, angiogenesis, and suppression of immunity.1,2 In the pathogenesis of hepatic fibrosis and cirrhosis, TGF-β1 is considered as one of the central regulators since it regulates the gene transcription of type I collagen, a common hallmark of fibrosis. Through binding with TGF-β receptor II (TGFβRII), TGF-β1 induces the signal transduction of SMAD family members to accelerate the activation of HSCs, leading to fibrosis and even cirrhosis.3 Moreover, TGF-β1 is capable to induce the expression of matrix metalloproteinases (MMPs) and tissue inhibitor of matrix metalloproteinases (TIMPs). MMPs are zinc-dependent endopeptidases which degrade all kinds of ECM proteins while TIMPs inhibit the degradation of collagens. The balance between MMPs and TIMPs determines the progression and regression of ECM accumulation in the liver, although the expression of both MMPs and TIMPs are increased in the fibrotic liver.4
A disintegrin and metalloprotease with thrombospondin motif (ADAMTS) is a class of disintegrin-like metalloproteinases containing I-thrombospondin (TSP-1) motif, which is widely presented in mammals and invertebrates. ADAMTS-2 has been regarded as a key regulator of procollagen maturation and collagen fibril formation.5 It has a characteristic structure contains four TSP-1 repeats, which has already been proven to react with TGF-β1 intimately. It was observed that the inclusion of TSP-1 blocking peptide to cells which were stimulated by elevated glucose concentration abrogated the activation of TGF-β1 and TGF-β1 in turn modulated the synthesis of TSP-1 synthesis, indicating an autocrine loop between TSP-1 and TGF-β1.6 However, to date, the relationship and mechanism between the expression between TGF-β1 and ADAMTS-2 are poorly understood clinically. Based on the previous findings in animal studies, we hypothesized that ADAMTS-2 may be involved in the progression of human cirrhosis with biological relations to TGF-β1. Thus, in this study, the protein expression of ADAMTS-2 and TGF-β1 in clinical cirrhotic cases was investigated in comparison with healthy cases. Key mediators related to TGF-β1 signaling, including TGFβRII, SMAD 2, SMAD3, MMP2, and TIMP2 were also characterized.
MATERIALS AND METHODS
1. Collection of patient samples and patient grouping
Liver tissues were obtained from 16 patients with portal hypertension and eight people of normal liver tissue in Xiangya Hospital, Central South University Xiangya School of Medicine from March to June 2011. All liver specimens were collected in patients with signed informed consent. For cirrhotic patients with portal hypertension, the liver specimens were collected during the portosystemic shunt and were originally taken for the postoperative pathological diagnostic use. For healthy people, the liver specimens were collected during the surgical mending of the liver damaged in accidents. All specimens were only used for research purposes. Patients and their family members were informed about the detail procedures and research purposes of specimen collection before operations. The entire procedure of specimen collection was in conformity with the ethical principles of medicine. The mean age of the patients was 57 years (range, 27 to 74 years). Clinical baseline characteristics of 24 patients (including age, gender, etiology of cirrhosis, Child-Pugh score, ascites, hepatic encephalopathy, and megalosplenia) were concluded in Table 1. A 1×1 cm liver tissue was collected from the edge of the left lateral lobe of each person and then diagnosed by two independent pathologists in the same hospital. Specimen of each patient was divided into two parts, one was immediately placed in -70℃ freezer for further Western blot assay; the other was prepared for routine pathological observation. Liver tissues with portal hypertension (16 cases) were classified as the cirrhosis group and eight cases of normal liver tissues were set as the control group. The protocol was approved by Medical Ethic Committee of Xiangya Hospital, Central South University Xiangya School of Medicine, Changsha, China.
Table 1.
Clinical Characteristics of 24 Patients
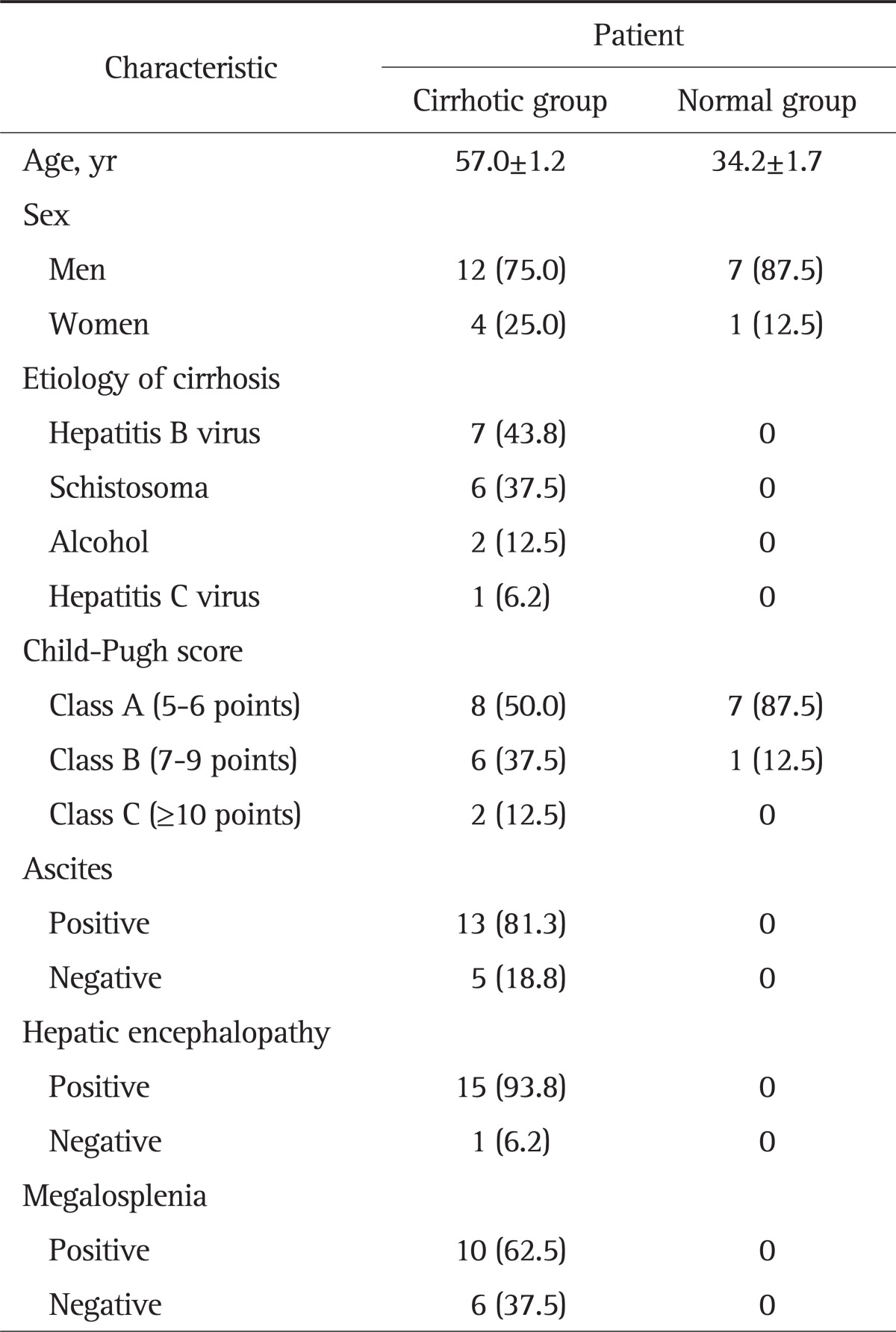
Data are presented as mean±SD or number (%).
2. Reagents
ADAMTS-2 and TGF-β1 antibodies were purchased from Abcam (Abcam, Cambridge, UK). Antibodies for TGFβRII, MMP2, TIMP2, phosphorylated SMAD2 at Ser465/467, total SMAD2, phosphorylated SMAD3 at Ser423/425, and total SMAD3 were bought from Cell Signaling (Cell Signaling Technology, Beverly, MA, USA). The two-step method antirabbit/mouse universal immunohistochemistry kit (diaminobenzidine, DAB method) was bought from DAKO (DAKO, Glostrup, Denmark) and used according to the manual. Enhanced chemiluminescence (ECL) reagent was from GE Healthcare, Little Chalfont, UK.
3. Masson's trichrome staining
The percentage of collagen in each liver tissue section was determined by using Masson's trichrome staining. After deparaffinization, sections were refixed in Bouin's solution to improve staining quality. Then sections were stained in Weigert's iron hematoxylin, Biebrich scarlet-acid fuchsin solution, and differentiate in phosphomolybdic-phosphotungstic acid solution in order. The final staining was aniline blue solution for collagen presentation. After staining, collagen was quantified by using Motic Fluo 1.0 software (Xinzhen Inc., Shanghai, China).
4. Immunohistochemistry of ADAMTS-2 and TGF-β1
ADAMTS-2 and TGF-β1 immunohistochemistry was conducted using the standard method. Antigen retrieval was carried out in citrate buffer (pH 6.0) for 45 minutes in an atmospheric pressure steamer. Slides were then stained using antibodies against ADAMTS-2 or TGF-β1 at 1:200 dilution in Cyto-Q reagent (Innovex Biosciences, Richmond, CA, USA) overnight at 4℃. Primary antibody detection was achieved with Mach 4 HRP polymer (Biocare Medical, Concord, CA, USA) for 20 minutes at room temperature, followed by 3,3'-DAB incubation.
Criteria of staining, we set known positive slide as the positive control, while PBS instead of primary antibody-stained slide as the negative control. The cell with nucleus or cytoplasm showing brown color was considered as positive cell. The nuclei of cells in negative control group showed blue instead of brown staining. Quantitative analyses of ADAMTS-2 and TGF-β1 in both cirrhotic and normal groups were conducted through the HPIAS-1000-type analysis system (Tongji Medical University, Wuhan, China, the company thousands of screen image) from 10 randomly selected slide.
5. Western blot analysis
Protein samples were quantified by Bradford method after extraction with lysis buffer (NaCl, 150 mM; 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid, 10 mM; pH 7.9; ethylenediaminetetraacetic acid, 1 mM; NP-40, 0.6%; phenylmethylsulfonyl fluoride, 0.5 mM; leupeptin, 1 µg/mL; aprotonin, 1 µg/mL; and trypsin inhibitor, 10 µg/mL). Fifty micrograms protein from each sample was subjected to Western blot assay. Samples were separated in a denaturing 10% polyacrylimide gel and transferred to a 0.1 µm pore nitrocellulose membrane. Nonspecific binding sites were blocked with Tris-buffered saline (TBS; Tris 40 mM, pH 7.6, NaCl 300 mM) containing 5% nonfat dry milk for 1 hour at room temperature. Membranes were then incubated with appropriate primary antibodies in TBS with 0.1% tween 20. Membranes were washed and incubated with secondary antibodies conjugated to horseradish peroxidase to show the result bands with ECL buffer. Parallel blotting of β-actin was used as internal control.
6. Data analysis
Data were shown in the form of mean±SEM deviation. The mean optical density and mean gray values of the particles from positive immunohistochemical reaction were analyzed by SPSS version 13.0 (SPSS Inc., Chicago, IL, USA) statistical package by using two independent sample t-test. For the correlation between ADAMTS-2 and TGF-β1 expression in cirrhotic group, bivariate correlation analysis has been applied. All statistical method have two-sided test and set p<0.05 as significant statistical difference.
RESULTS
1. Collagen formation was higher in cirrhotic patients
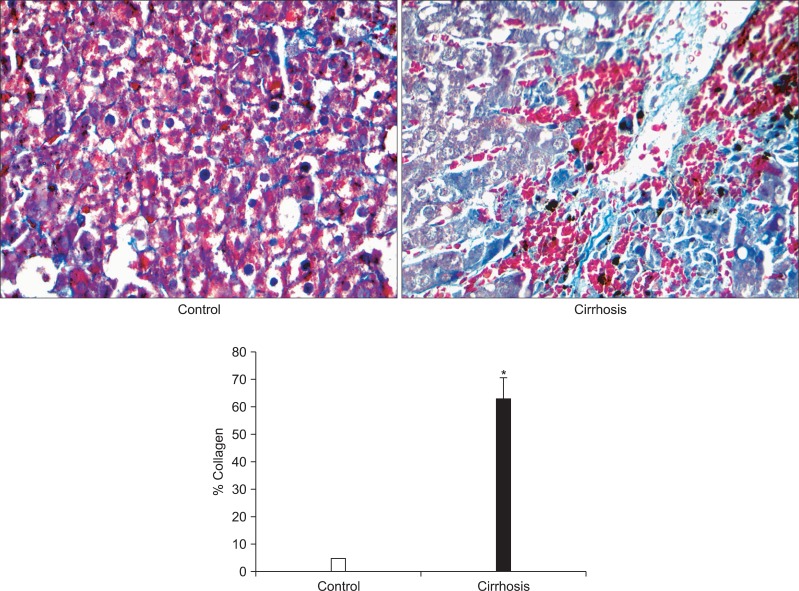
In liver sections from cirrhotic patients, the percentage of collagen was significant higher (up to 62%) than that in the control group (up to 6%, p<0.001), shown by Masson's trichrome staining, indicating the development of cirrhosis in the patients (Fig. 1).
Fig. 1.
Change in the percentage of collagen in liver sections between normal and cirrhotic samples. Representative results of Manson's trichrome stain were conducted in both samples (×400). The quantitative results of the section staining were analyzed using Motic Fluo 1.0 software (Motic Instruments Inc.). Data are presented as mean±SEM deviation and analyzed with the SPSS version 13.0 statistical package (SPSS Inc.) using the two independent sample t-test. *p<0.001 vs control.
2. Immunohistochemistry of ADAMTS-2 and TGF-β1
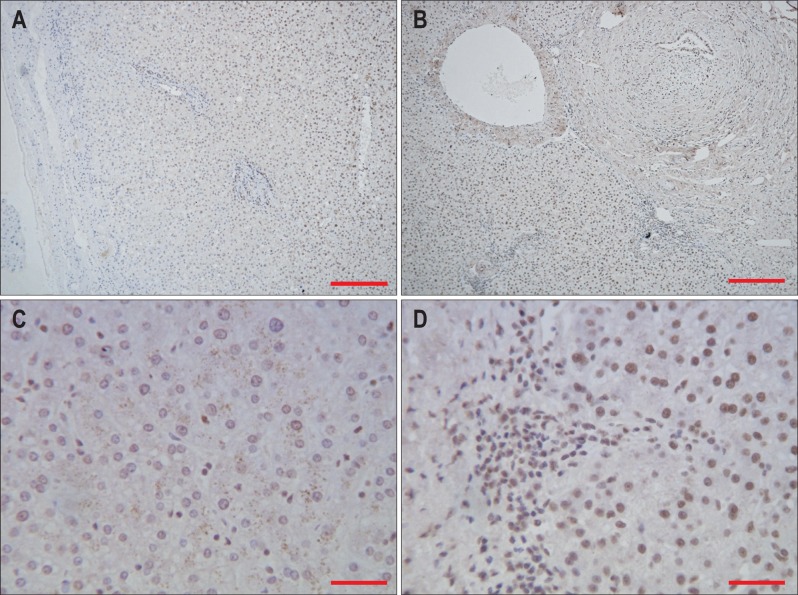
In the control group, there was a small amount of positive ADAMTS-2 staining of liver cells, mainly in the nucleus and marginally around the vessels and in some biliary epithelial cells (Fig. 2A and C). In the cirrhotic group, there were obvious pseudolobule formations, disordered arrangements of hepatocyte cords, and strong liver cell nuclei positive ADAMTS-2 staining in the vascular wall and bile duct epithelial cells (Fig. 2B and D).
Fig. 2.
Expression of a disintegrin and metalloprotease with thrombospondin motif 2 (ADAMTS-2) in normal and cirrhotic liver tissue. (A) Expression of ADAMTS-2 in normal liver tissue (DAB stain, ×100; scale bar, 100 µm). (B) Expression of ADAMTS-2 in cirrhotic liver tissue (DAB stain, ×100; scale bar, 100 µm). (C) Expression of ADAMTS-2 in normal liver tissue (DAB stain, ×400; scale bar, 25 µm). (D) Expression of ADAMTS-2 in cirrhotic liver tissue (DAB stain, ×400; scale bar, 25 µm).
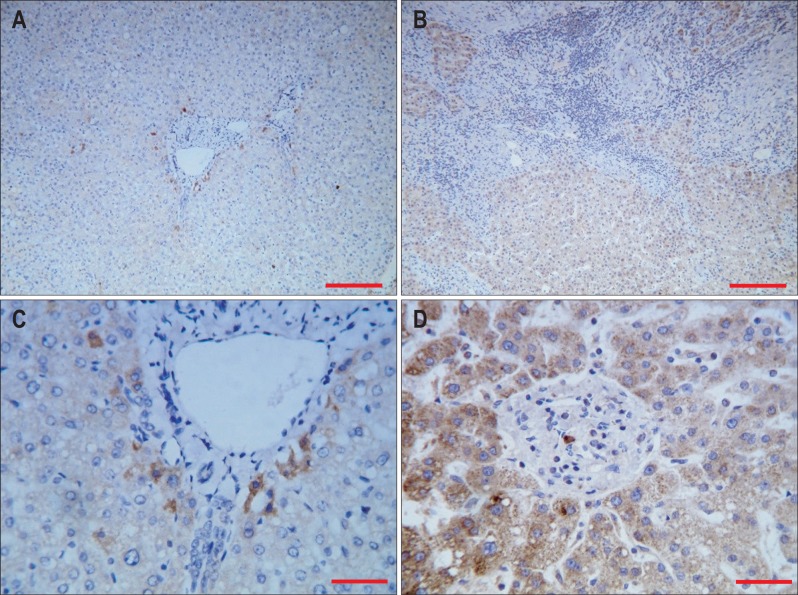
In the control group, signals of TGF-β1 were mainly found in the liver cytoplasm but not in the nucleus. Portal area and bile duct epithelial cells also showed positive staining of TGF-β1 (Fig. 3A and C). Cirrhotic group showed structural disruption of the hepatic lobule, as well as strong staining signals in hepatic cytoplasm, portal area and bile duct epithelial cells. Small amount of staining were also found in the fibrous tissue (Fig. 3B and D).
Fig. 3.
Expression of transforming growth factor-β1 (TGF-β1) in normal and cirrhotic liver tissue. (A) Expression of TGF-β1 in normal liver tissue (DAB stain, ×100; scale bar, 100 µm). (B) Expression of TGF-β1 in cirrhotic liver tissue (DAB stain, ×100; scale bar, 100 µm). (C) Expression of TGF-β1 in normal liver tissue (DAB stain, ×400; scale bar, 25 µm). (D) Expression of TGF-β1 in cirrhotic liver tissue (DAB stain, ×400; scale bar, 25 µm).
3. Analysis of ADAMTS-2 and TGF-β1 expressions in normal and cirrhotic tissues
Image analysis showed that the mean optical density of ADAMTS-2 protein expression in the control group was 0.145±0.025 and the mean gray value was 156.760±8.189; in the cirrhotic group, the mean optical density of ADAMTS-2 protein expression was 0.207±0.032 and the man gray value was 135.878±10.021. The difference between control and cirrhotic groups was significant (t=14.965 and 15.868, respectively, p<0.05) (Table 2). Similarly, the average optical density of TGF-β1 protein expression in normal control group was 0.142±0.023 and the average gray value was 157.562±7.493; the average optical density of TGF-β1 protein expression in cirrhotic group was 0.211±0.041 and the average gray value was 135.030±12.641. The difference was statistically significant (t-values were 14.475 and 15.431, p<0.05) (Table 3).
Table 2.
Expression of a Disintegrin and Metalloprotease with Thrombospondin Motif 2 in the Two Groups
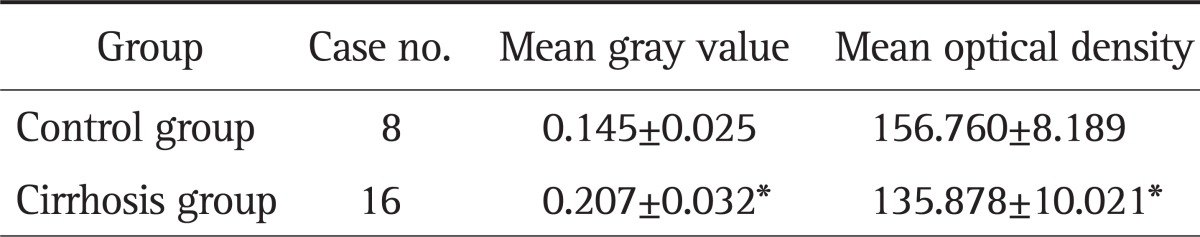
*p<0.05 as compared to the control group.
Table 3.
Expression of Transforming Growth Factor-β1 in the Cirrhotic and Normal Groups
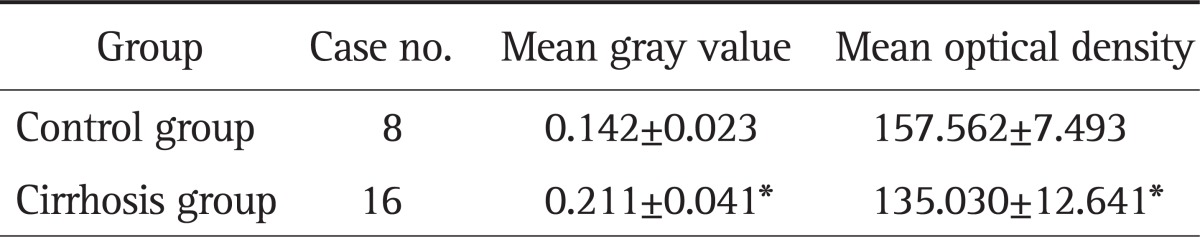
*p<0.05 as compared to the control group.
4. Western blot analysis of ADAMTS-2 and TGF-β1
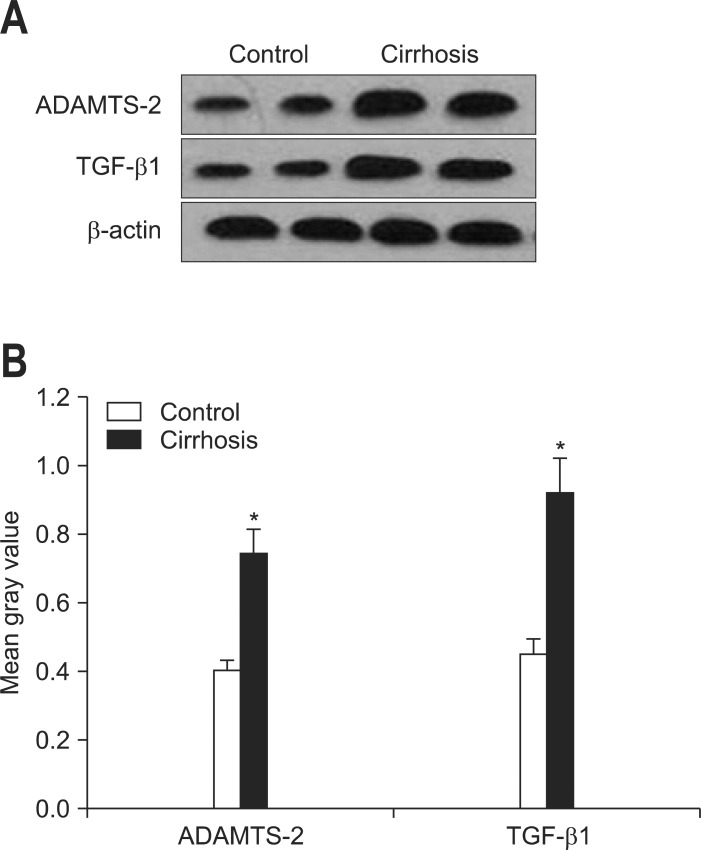
In Western blot assay, the molecular weight of ADAMTS-2, TGF-β1, and β-actin was 100, 44, and 43 kDa, respectively (Fig. 4). It was very clear that in the cirrhotic group, the protein expressions of both ADAMTS-2 and TGF-β1 were highly elevated when compared with the control group. Quantitative data of the Western blot bands further confirmed the results (p<0.05).
Fig. 4.
(A) Western blot results for a disintegrin and metalloprotease with thrombospondin motif 2 (ADAMTS-2), transforming growth factor-β1 (TGF-β1), β-actin protein expression. (B) Quantitative contrast of the protein expression levels of ADAMTS-2 and TGF-β1 in normal (n=8) and cirrhotic (n=16) liver tissues. Data are presented as mean±SEM deviation and analyzed with the SPSS version 13.0 statistical package (SPSS Inc.) by using two independent sample t-tests
*p<0.05 vs control.
5. Correlation analysis of ADAMTS-2 and TGF-β1 expression in cirrhotic tissue
From the results of Western blot assay, it could be seen that in the cirrhotic group, there was a significant positive correlation between ADAMTS-2 and TGF-β1 protein expression (Table 4), with statistical significance (r=0.862, p<0.01).
Table 4.
Correlation Analysis of ADAMTS-2 and TGF-β1 Expression in Gray-Scale as Detected by Western Blot (r=0.862, p<0.01)
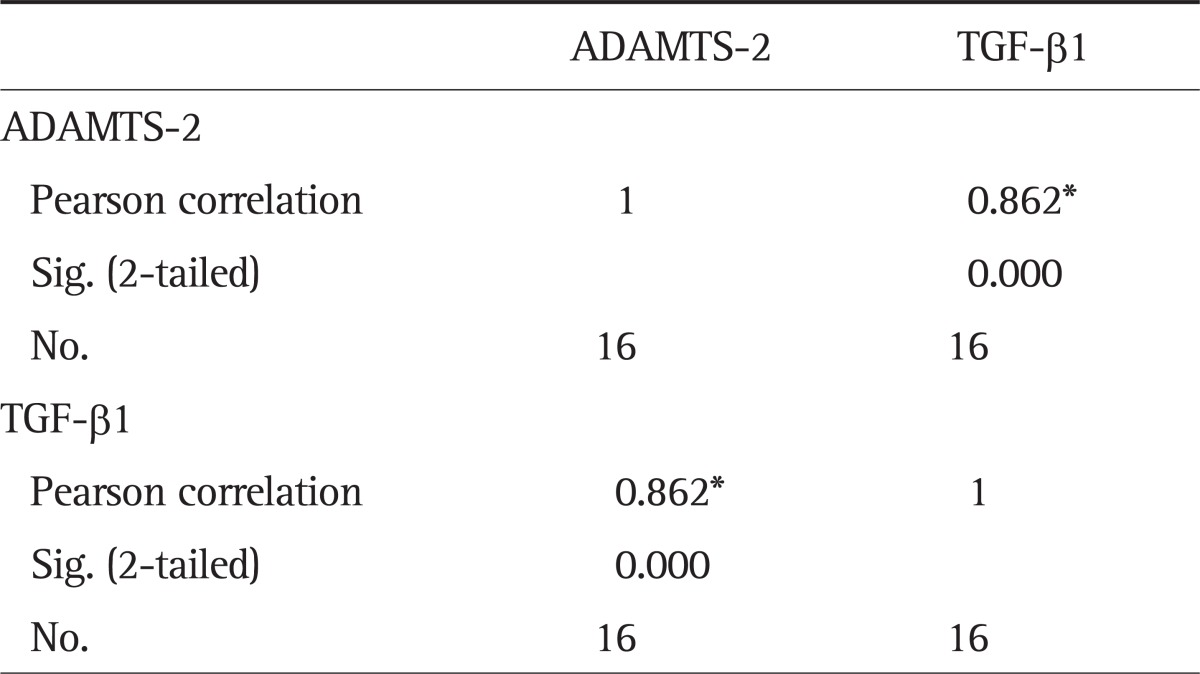
ADAMTS-2, a disintegrin and metalloprotease with thrombospondin motif 2; TGF-β1, transforming growth factor-β1.
*Correlation is significant at the 0.01 level (2-tailed).
However, correlation analysis of immunohistochemical assay (mean optical density of ADAMTS-2 and TGF-β1 in the cirrhotic tissue) showed no significant difference (r=0.098, p=0.307) (Table 5).
Table 5.
Correlations Analysis of the Average Optical Density Analysis of ADAMTS-2 and TGF-β1 as Detected by Immunohistochemistry (r=0.098, p=0.307)
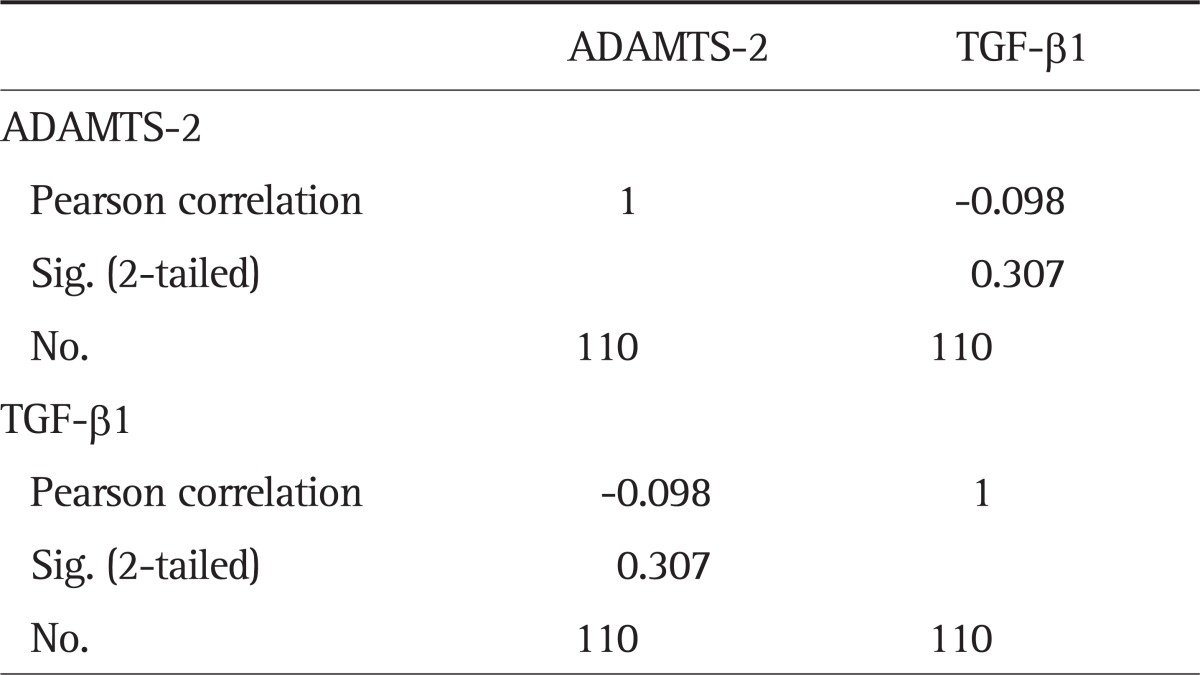
ADAMTS-2, a disintegrin and metalloprotease with thrombospondin motif 2; TGF-β1, transforming growth factor-β1.
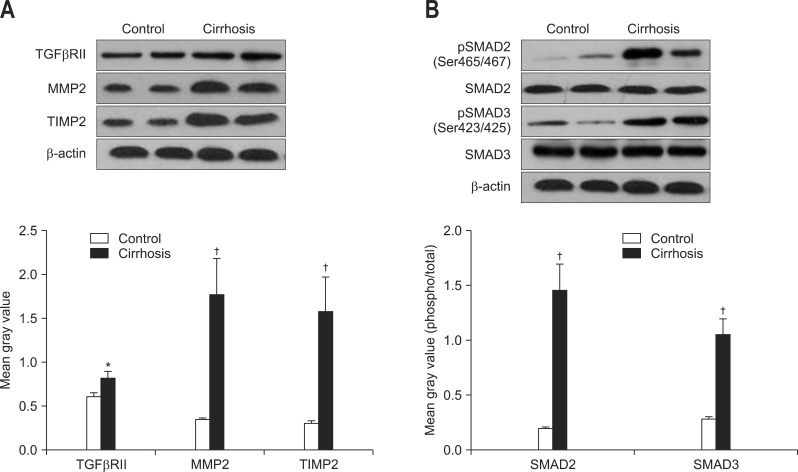
6. Western blot of TGFβRII, SMAD2, SMAD3, MMP2, and TIMP2 in cirrhotic and normal livers
To further investigate the relationship between TGF-β1 and ADAMTS-2, we analyzed the protein expressions of key mediators in TGF-β signaling and hepatic fibrosis. In cirrhotic tissues, the expressions of TGFβRII, MMP2, and TIMP2 were significantly up-regulated when compared with the expressions in control tissues (p<0.01 or p<0.05) (Fig. 5A). Development of cirrhosis also increased the phosphorylation of SMAD2 and SMAD3 without disturbing their basal protein levels (Fig. 5B).
Fig. 5.
Representative Western blots and quantitative results of (A) transforming growth factor-β receptor II (TGFβRII), matrix metalloproteinase 2 (MMP2), tissue inhibitor of matrix metalloproteinase 2 (TIMP2), and (B) SMAD2/3 expression in normal (n=8) and cirrhotic (n=16) liver tissues. Data are presented as mean±SEM deviation and analyzed with the SPSS version 13.0 statistical package (SPSS Inc.) by using two independent sample t-tests. *p<0.05 vs control; †p<0.01 vs control.
DISCUSSION
As one of the end-stage events of liver disease, cirrhosis has been the focus of hepatologist for a long time. However, there is no available specific therapy for cirrhosis. Theoretically, two approaches targeted at the reduction of ECM formation should be possible in clinical therapy. The first one is to accelerate the degradation of excessive ECM in the liver (e.g., promoting the activity of TIMPs). The second one is to reduce the synthesis of the fibrous extracellular molecules. Since collagen formation is a well-documented event in cirrhotic liver development, it might be a good target for therapeutic intervention.7
In ADAMTS family, ADAMTS-2 is responsible for the final step of procollagen post-translational processing, which is the key step for fibrillar collagens accumulation in the liver.8 Both TNF-α and TGF-β1 are able to promote the secretion of ADAMTS-2,9,10 which indicated that the activation of ADAMTS-2 is probably a downstream event in cirrhosis. Moreover, previous study found that in human cirrhotic tissue, the mRNA expression level of ADAMTS-2 was elevated in HSCs. ADAMTS-2 knocked-out mice exhibited attenuated liver fibrosis after chronic treatment by carbon tetrachloride treatment.8,11 Therefore, activation of ADAMTS-2 is probably an indispensable step for cirrhosis development. To date, there is no relevant report regarding ADAMTS-2 expression in human cirrhotic tissue although there are some in animal studies.8,12,13 In this study we found that positive ADAMTS-2 staining was mainly found in the nucleus, the vascular wall and part of the bile duct epithelial cells in the human liver with cirrhosis. In addition, the protein expression of ADAMTS-2 in the cirrhotic tissue was significantly increased comparing to normal liver tissues, suggesting that ADAMTS-2 might participated in the development of cirrhosis clinically. Regarding that the variation of ADAMTS-2 protein expression within the cirrhotic group is relatively low, the relationship between ADAMTS-2 and clinical baseline characteristics of cirrhotic patients should not be significant (data not shown).
Many cytokines has been proven to participate in the progress of either liver fibrosis or cirrhosis. TGF-β1 and its signaling pathway are of the highest importance both experimentally and clinically. Through the binding of TGFβRII on the cell surface, TGF-β1 activates the SMAD pathway to initiate the transcription of collagen genes, leading to the formation of collagens, activation of HSCs, and promotion of ECM synthesis in hepatic development of fibrosis.14,15 In consistent with those facts, this current study confirmed that the protein expressions of TGF-β1 and key components of TGF-β1 pathway (i.e., TGFβRII, activated SMAD2, and SMAD3) were significantly higher in the human cirrhosis tissue than normal liver. This result confirmed the closely relationship between TGF-β1 and the progression of human cirrhosis and their clinical significance. In addition, key enzymes responsible for collagen and ECM formation in the liver, MMP-2 and TIMP2, also showed up-regulated protein expressions in the cirrhotic livers, indicating a provoked balance between ECM degradation and formation.
In human cirrhosis, TGF-β1 must be activated before exerting its biological functions. TSP-1 is known to be the strongest activator to TGF-β1. Previous study also demonstrated that there was a mutual promotion between TGF-β1 and TSP-1.16 However, due to their wild range of biological functions, neither TGF-β1 nor TSP-1 is the ideal target for the gene therapy of cirrhosis. In addition, TGF-β1 can promote the activation of TSP-1 at both transcriptional and translational level in HSCs. Since ADAMTS is a class of disintegrin-like metalloproteinase which contains TSP-1 motif and widely presents in mammals and invertebrates in vivo, it is reasonable to speculate the possible induction of TGF-β1 by ADAMTS-2. However, although TGF-β1 was shown to induce the production and secretion of ADAMTS-2,10 there is no study to demonstrate the opposite induction. Moreover, there are some studies investigated the relationship between ADAMTS-2 and TGF-β in animal studies, but there is no relevant report regarding ADAMTS-2 expression in human cirrhotic tissue, as well as its underlying mechanisms. In this study, we demonstrated a positive connection between ADAMTS-2 and TGF-β1, via the TGF-β/SMAD pathway, by immunohistochemistry and Western blot. Based on the two facts that ADAMTS-2 has four TSP-1 repeats and TSP-1 has an intimated interaction with TGF-β1, we hypothesized that human ADAMTS-2 may also be regulated by TGF-β1. Further investigations should be conducted to evaluate the knock-down effects of ADAMTS-2, which may reveal the therapy role of this gene in human cirrhosis. Additionally, inhibition studies, which demonstrate the inhibition of TGFβ/SMAD pathway leads to ADAMTS-2 down-regulation and less fibrosis/cirrhosis, are also necessary. In conclusion, we verified the possible role of both TGF-β1 and ADAMTS-2 in the development of human cirrhosis. Moreover, ADAMTS-2 expression was positively correlated with TGF-β1, which indicates that ADAMTS-2 may be a novel target for the treatment of liver cirrhosis.
ACKNOWLEDGEMENTS
This work is supported by Key Project of Hunan Provincial Natural Science Foundation of China (No. 09JJ3080).
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Kirmaz C, Terzioglu E, Topalak O, et al. Serum transforming growth factor-beta1 (TGF-beta1) in patients with cirrhosis, chronic hepatitis B and chronic hepatitis C [corrected] Eur Cytokine Netw. 2004;15:112–116. [PubMed] [Google Scholar]
- 2.Li HJ, Dong C, Chang S. Effect of transforming growth factor-β1 on thrombospondin-1 in hepatic fibrosis. Chin J Exp Surg. 2010;27:1674–1676. [Google Scholar]
- 3.Inagaki Y, Okazaki I. Emerging insights into transforming growth factor beta Smad signal in hepatic fibrogenesis. Gut. 2007;56:284–292. doi: 10.1136/gut.2005.088690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Iredale JP. Hepatic stellate cell behavior during resolution of liver injury. Semin Liver Dis. 2001;21:427–436. doi: 10.1055/s-2001-17557. [DOI] [PubMed] [Google Scholar]
- 5.Colige A, Ruggiero F, Vandenberghe I, et al. Domains and maturation processes that regulate the activity of ADAMTS-2, a metalloproteinase cleaving the aminopropeptide of fibrillar procollagens types I-III and V. J Biol Chem. 2005;280:34397–34408. doi: 10.1074/jbc.M506458200. [DOI] [PubMed] [Google Scholar]
- 6.Yung S, Lee CY, Zhang Q, Lau SK, Tsang RC, Chan TM. Elevated glucose induction of thrombospondin-1 up-regulates fibronectin synthesis in proximal renal tubular epithelial cells through TGF-beta1 dependent and TGF-beta1 independent pathways. Nephrol Dial Transplant. 2006;21:1504–1513. doi: 10.1093/ndt/gfl017. [DOI] [PubMed] [Google Scholar]
- 7.Brenner DA, Westwick J, Breindl M. Type I collagen gene regulation and the molecular pathogenesis of cirrhosis. Am J Physiol. 1993;264(4 Pt 1):G589–G595. doi: 10.1152/ajpgi.1993.264.4.G589. [DOI] [PubMed] [Google Scholar]
- 8.Kesteloot F, Desmoulière A, Leclercq I, et al. ADAM metallopeptidase with thrombospondin type 1 motif 2 inactivation reduces the extent and stability of carbon tetrachloride-induced hepatic fibrosis in mice. Hepatology. 2007;46:1620–1631. doi: 10.1002/hep.21868. [DOI] [PubMed] [Google Scholar]
- 9.Nicholson AC, Malik SB, Logsdon JM, Jr, Van Meir EG. Functional evolution of ADAMTS genes: evidence from analyses of phylogeny and gene organization. BMC Evol Biol. 2005;5:11. doi: 10.1186/1471-2148-5-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang WM, Lee S, Steiglitz BM, et al. Transforming growth factor-beta induces secretion of activated ADAMTS-2. A procollagen III N-proteinase. J Biol Chem. 2003;278:19549–19557. doi: 10.1074/jbc.M300767200. [DOI] [PubMed] [Google Scholar]
- 11.Schwettmann L, Wehmeier M, Jokovic D, et al. Hepatic expression of A disintegrin and metalloproteinase (ADAM) and ADAMs with thrombospondin motives (ADAM-TS) enzymes in patients with chronic liver diseases. J Hepatol. 2008;49:243–250. doi: 10.1016/j.jhep.2008.03.020. [DOI] [PubMed] [Google Scholar]
- 12.Moss ML, Bartsch JW. Therapeutic benefits from targeting of ADAM family members. Biochemistry. 2004;43:7227–7235. doi: 10.1021/bi049677f. [DOI] [PubMed] [Google Scholar]
- 13.Le Goff C, Cormier-Daire V. The ADAMTS(L) family and human genetic disorders. Hum Mol Genet. 2011;20(R2):R163–R167. doi: 10.1093/hmg/ddr361. [DOI] [PubMed] [Google Scholar]
- 14.Kato J, Ido A, Hasuike S, et al. Transforming growth factor-beta-induced stimulation of formation of collagen fiber network and anti-fibrotic effect of taurine in an in vitro model of hepatic fibrosis. Hepatol Res. 2004;30:34–41. doi: 10.1016/j.hepres.2004.04.006. [DOI] [PubMed] [Google Scholar]
- 15.Nakano S, Nagasawa T, Ijiro T, et al. Bezafibrate prevents hepatic stellate cell activation and fibrogenesis in a murine steatohepatitis model, and suppresses fibrogenic response induced by transforming growth factor-beta1 in a cultured stellate cell line. Hepatol Res. 2008;38:1026–1039. doi: 10.1111/j.1872-034X.2008.00363.x. [DOI] [PubMed] [Google Scholar]
- 16.Kondou H, Mushiake S, Etani Y, Miyoshi Y, Michigami T, Ozono K. A blocking peptide for transforming growth factor-beta1 activation prevents hepatic fibrosis in vivo. J Hepatol. 2003;39:742–748. doi: 10.1016/s0168-8278(03)00377-5. [DOI] [PubMed] [Google Scholar]