Abstract
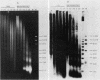
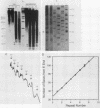
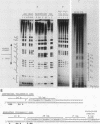
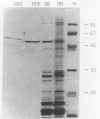
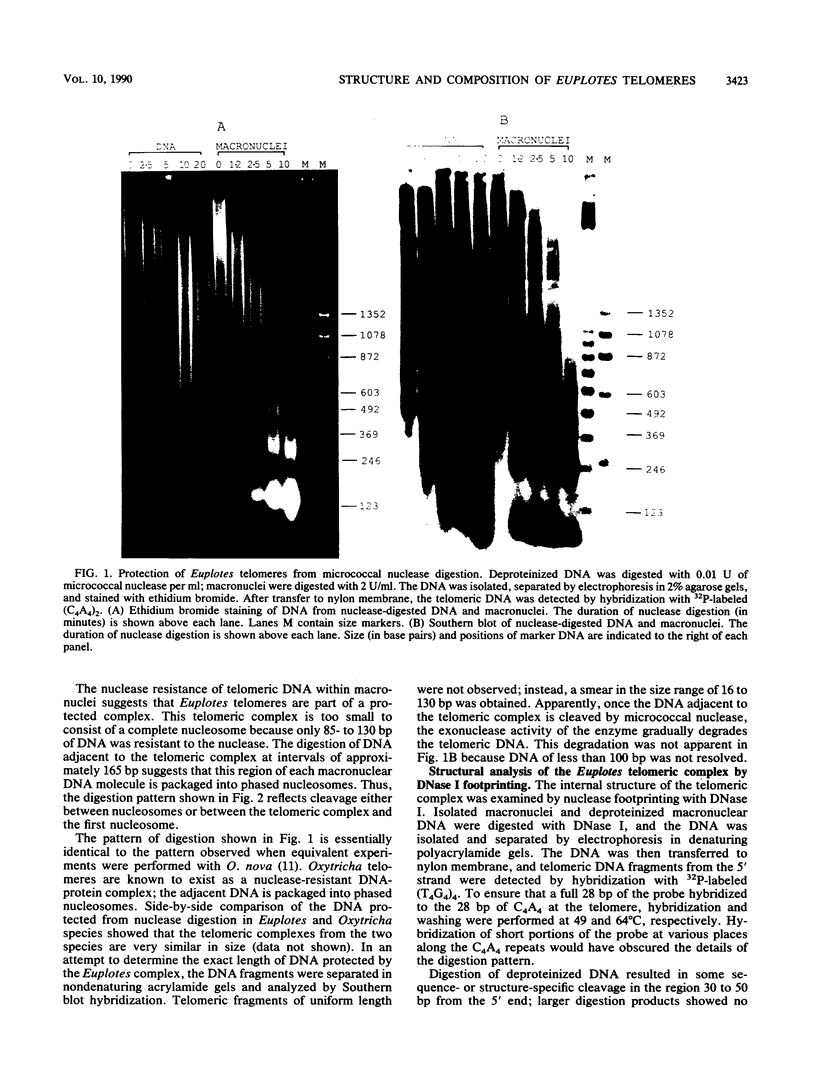
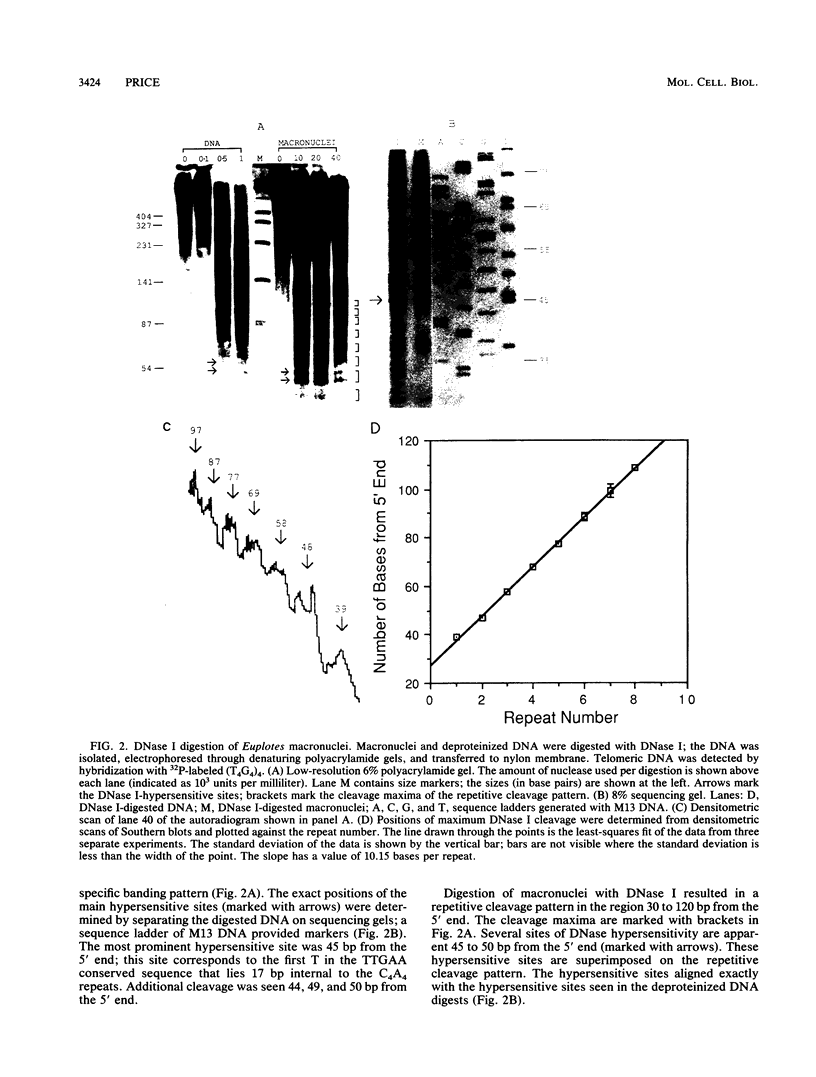
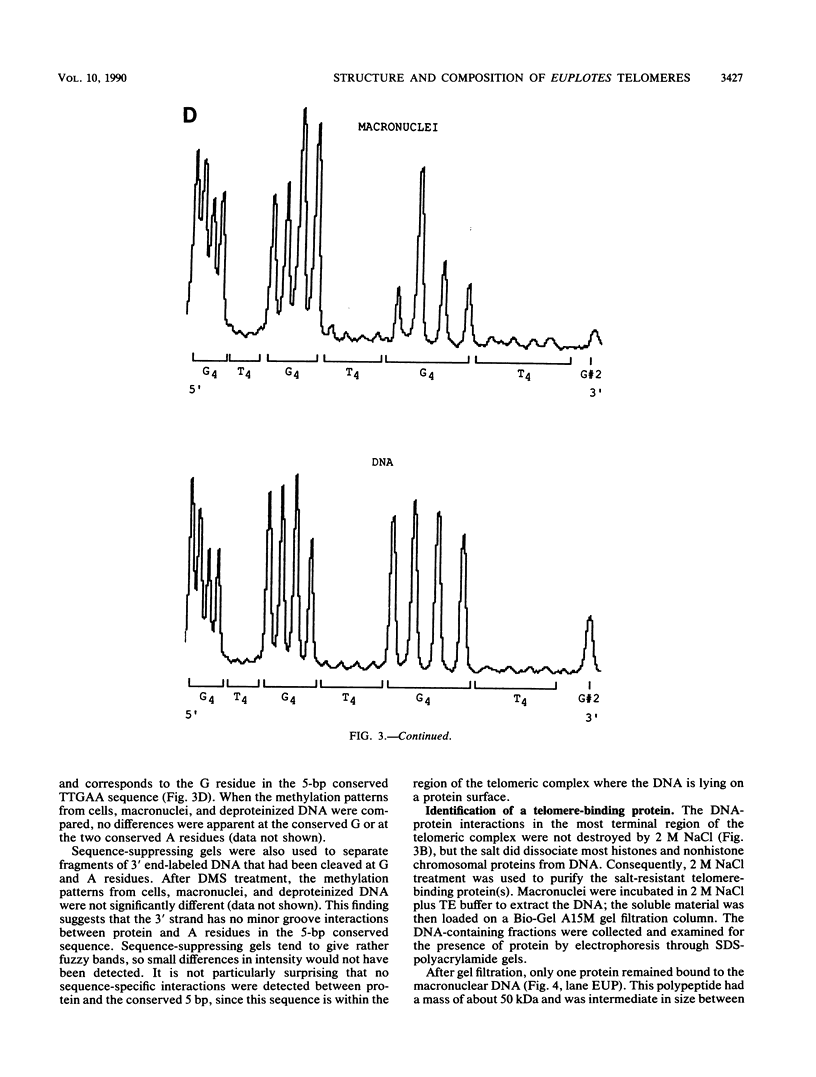
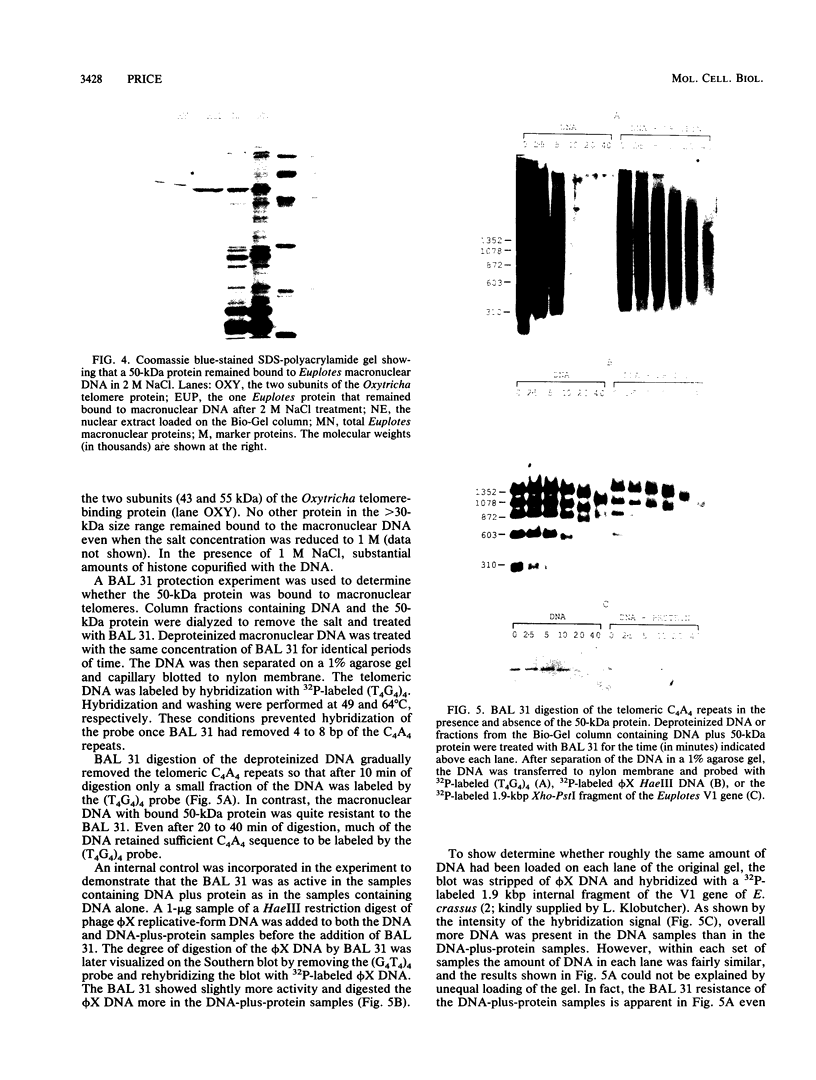
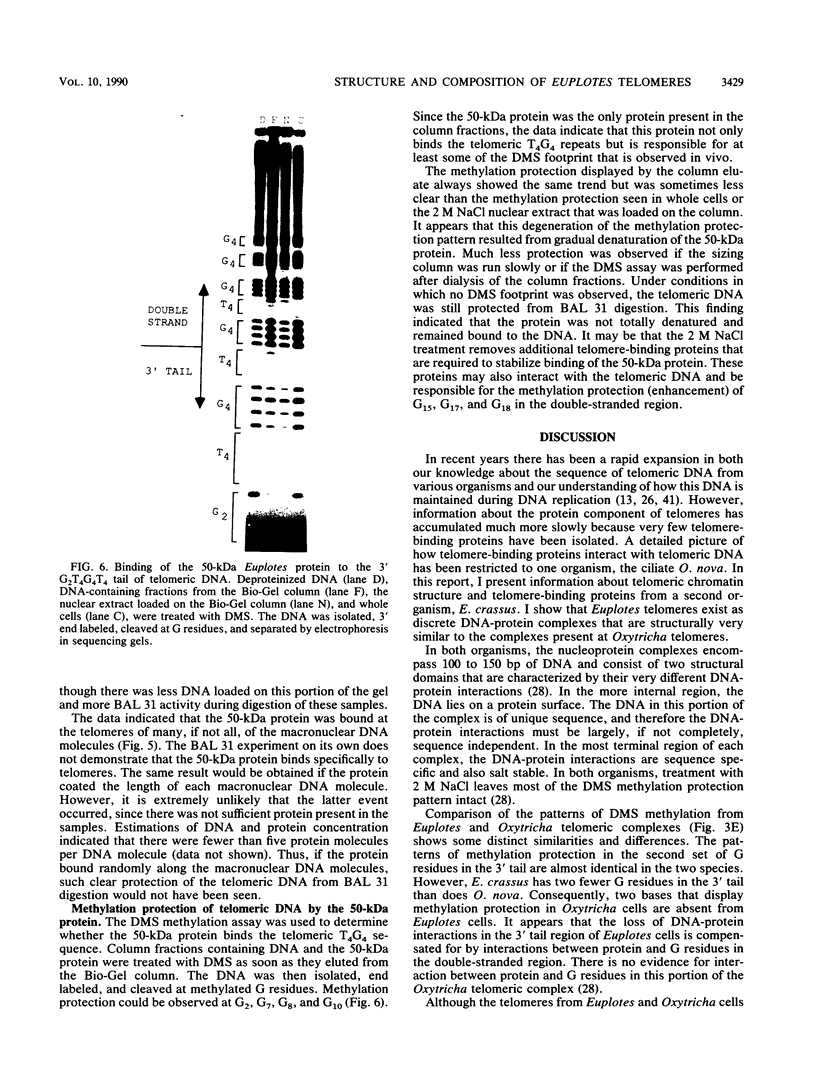
The nucleoprotein structure of telomeres from Euplotes crassus was studied by using nuclease and chemical footprinting. The macronuclear telomeres were found to exist as DNA-protein complexes that are resistant to micrococcal nuclease digestion. Each complex encompassed 85 to 130 base pairs of macronuclear DNA and appeared to consist of two structural domains that are characterized by dissimilar DNA-protein interactions. Dimethyl sulfate footprinting demonstrated that very sequence-specific and salt-stable interactions occur in the most terminal region of each complex. DNase I footprinting indicated that DNA in the region 30 to 120 base-pairs from the 5' end lies on a protein surface; the interactions in this region of the complex are unlikely to be sequence specific. A 50-kilodalton telomere-binding protein was isolated. Binding of this protein protected telomeric DNA from BAL 31 digestion and gave rise to many of the sequence-specific DNA-protein interactions that were observed in vivo. The telomeric complexes from E. crassus were very similar in overall structure to the complexes found at Oxytricha telomeres. However, telomeric complexes from the two ciliates showed significant differences in internal organization. The telomeric DNA, the telomere-binding proteins, and the resultant DNA-protein interactions were all somewhat different. The telomere-binding proteins from the two ciliates were found to be less closely conserved than might have been expected. It appears that the proteins are tailored to match their cognate telomeric DNA.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allshire R. C., Gosden J. R., Cross S. H., Cranston G., Rout D., Sugawara N., Szostak J. W., Fantes P. A., Hastie N. D. Telomeric repeat from T. thermophila cross hybridizes with human telomeres. Nature. 1988 Apr 14;332(6165):656–659. doi: 10.1038/332656a0. [DOI] [PubMed] [Google Scholar]
- Baird S. E., Klobutcher L. A. Characterization of chromosome fragmentation in two protozoans and identification of a candidate fragmentation sequence in Euplotes crassus. Genes Dev. 1989 May;3(5):585–597. doi: 10.1101/gad.3.5.585. [DOI] [PubMed] [Google Scholar]
- Berman J., Tachibana C. Y., Tye B. K. Identification of a telomere-binding activity from yeast. Proc Natl Acad Sci U S A. 1986 Jun;83(11):3713–3717. doi: 10.1073/pnas.83.11.3713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackburn E. H., Chiou S. S. Non-nucleosomal packaging of a tandemly repeated DNA sequence at termini of extrachromosomal DNA coding for rRNA in Tetrahymena. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2263–2267. doi: 10.1073/pnas.78.4.2263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackburn E. H. The molecular structure of centromeres and telomeres. Annu Rev Biochem. 1984;53:163–194. doi: 10.1146/annurev.bi.53.070184.001115. [DOI] [PubMed] [Google Scholar]
- Buchman A. R., Lue N. F., Kornberg R. D. Connections between transcriptional activators, silencers, and telomeres as revealed by functional analysis of a yeast DNA-binding protein. Mol Cell Biol. 1988 Dec;8(12):5086–5099. doi: 10.1128/mcb.8.12.5086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadilla C. L., Harp J., Flanagan J. M., Olins A. L., Olins D. E. Preparation and characterization of soluble macronuclear chromatin from the hypotrich Euplotes eurystomus. Nucleic Acids Res. 1986 Jan 24;14(2):823–841. doi: 10.1093/nar/14.2.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung M. K., Drivas D. T., Littau V. C., Johnson E. M. Protein tightly bound near the termini of the Physarum extrachromosomal rDNA palindrome. J Cell Biol. 1981 Oct;91(1):309–314. doi: 10.1083/jcb.91.1.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drew H. R. Structural specificities of five commonly used DNA nucleases. J Mol Biol. 1984 Jul 15;176(4):535–557. doi: 10.1016/0022-2836(84)90176-1. [DOI] [PubMed] [Google Scholar]
- Gottschling D. E., Cech T. R. Chromatin structure of the molecular ends of Oxytricha macronuclear DNA: phased nucleosomes and a telomeric complex. Cell. 1984 Sep;38(2):501–510. doi: 10.1016/0092-8674(84)90505-1. [DOI] [PubMed] [Google Scholar]
- Gottschling D. E., Zakian V. A. Telomere proteins: specific recognition and protection of the natural termini of Oxytricha macronuclear DNA. Cell. 1986 Oct 24;47(2):195–205. doi: 10.1016/0092-8674(86)90442-3. [DOI] [PubMed] [Google Scholar]
- Greider C. W., Blackburn E. H. A telomeric sequence in the RNA of Tetrahymena telomerase required for telomere repeat synthesis. Nature. 1989 Jan 26;337(6205):331–337. doi: 10.1038/337331a0. [DOI] [PubMed] [Google Scholar]
- Harper D. S., Jahn C. L. Differential use of termination codons in ciliated protozoa. Proc Natl Acad Sci U S A. 1989 May;86(9):3252–3256. doi: 10.1073/pnas.86.9.3252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson E. R., Blackburn E. H. An overhanging 3' terminus is a conserved feature of telomeres. Mol Cell Biol. 1989 Jan;9(1):345–348. doi: 10.1128/mcb.9.1.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klobutcher L. A., Swanton M. T., Donini P., Prescott D. M. All gene-sized DNA molecules in four species of hypotrichs have the same terminal sequence and an unusual 3' terminus. Proc Natl Acad Sci U S A. 1981 May;78(5):3015–3019. doi: 10.1073/pnas.78.5.3015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lutter L. C. Precise location of DNase I cutting sites in the nucleosome core determined by high resolution gel electrophoresis. Nucleic Acids Res. 1979 Jan;6(1):41–56. doi: 10.1093/nar/6.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martindale D. W. Codon usage in Tetrahymena and other ciliates. J Protozool. 1989 Jan-Feb;36(1):29–34. doi: 10.1111/j.1550-7408.1989.tb02679.x. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. A new method for sequencing DNA. Proc Natl Acad Sci U S A. 1977 Feb;74(2):560–564. doi: 10.1073/pnas.74.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Miceli C., La Terza A., Melli M. Isolation and structural characterization of cDNA clones encoding the mating pheromone Er-1 secreted by the ciliate Euplotes raikovi. Proc Natl Acad Sci U S A. 1989 May;86(9):3016–3020. doi: 10.1073/pnas.86.9.3016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moyzis R. K., Buckingham J. M., Cram L. S., Dani M., Deaven L. L., Jones M. D., Meyne J., Ratliff R. L., Wu J. R. A highly conserved repetitive DNA sequence, (TTAGGG)n, present at the telomeres of human chromosomes. Proc Natl Acad Sci U S A. 1988 Sep;85(18):6622–6626. doi: 10.1073/pnas.85.18.6622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pluta A. F., Zakian V. A. Recombination occurs during telomere formation in yeast. Nature. 1989 Feb 2;337(6206):429–433. doi: 10.1038/337429a0. [DOI] [PubMed] [Google Scholar]
- Price C. M., Cech T. R. Properties of the telomeric DNA-binding protein from Oxytricha nova. Biochemistry. 1989 Jan 24;28(2):769–774. doi: 10.1021/bi00428a053. [DOI] [PubMed] [Google Scholar]
- Price C. M., Cech T. R. Telomeric DNA-protein interactions of Oxytricha macronuclear DNA. Genes Dev. 1987 Oct;1(8):783–793. doi: 10.1101/gad.1.8.783. [DOI] [PubMed] [Google Scholar]
- Raghuraman M. K., Dunn C. J., Hicke B. J., Cech T. R. Oxytricha telomeric nucleoprotein complexes reconstituted with synthetic DNA. Nucleic Acids Res. 1989 Jun 12;17(11):4235–4253. doi: 10.1093/nar/17.11.4235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes D., Klug A. Helical periodicity of DNA determined by enzyme digestion. Nature. 1980 Aug 7;286(5773):573–578. doi: 10.1038/286573a0. [DOI] [PubMed] [Google Scholar]
- Richards E. J., Ausubel F. M. Isolation of a higher eukaryotic telomere from Arabidopsis thaliana. Cell. 1988 Apr 8;53(1):127–136. doi: 10.1016/0092-8674(88)90494-1. [DOI] [PubMed] [Google Scholar]
- Roth M., Lin M., Prescott D. M. Large scale synchronous mating and the study of macronuclear development in Euplotes crassus. J Cell Biol. 1985 Jul;101(1):79–84. doi: 10.1083/jcb.101.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Runge K. W., Zakian V. A. Introduction of extra telomeric DNA sequences into Saccharomyces cerevisiae results in telomere elongation. Mol Cell Biol. 1989 Apr;9(4):1488–1497. doi: 10.1128/mcb.9.4.1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sogin M. L., Elwood H. J. Primary structure of the Paramecium tetraurelia small-subunit rRNA coding region: phylogenetic relationships within the Ciliophora. J Mol Evol. 1986;23(1):53–60. doi: 10.1007/BF02100998. [DOI] [PubMed] [Google Scholar]
- Sogin M. L., Swanton M. T., Gunderson J. H., Elwood H. J. Sequence of the small subunit ribosomal RNA gene from the hypotrichous ciliate Euplotes aediculatus. J Protozool. 1986 Feb;33(1):26–29. doi: 10.1111/j.1550-7408.1986.tb05550.x. [DOI] [PubMed] [Google Scholar]
- Solomon M. J., Strauss F., Varshavsky A. A mammalian high mobility group protein recognizes any stretch of six A.T base pairs in duplex DNA. Proc Natl Acad Sci U S A. 1986 Mar;83(5):1276–1280. doi: 10.1073/pnas.83.5.1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanton M. T., Greslin A. F., Prescott D. M. Arrangement of coding and non-coding sequences in the DNA molecules coding for rRNAs in Oxytricha sp. DNA of ciliated protozoa. VII. Chromosoma. 1980;77(2):203–215. doi: 10.1007/BF00329545. [DOI] [PubMed] [Google Scholar]
- Swanton M. T., Heumann J. M., Prescott D. M. Gene-sized DNA molecules of the macronuclei in three species of hypotrichs: size distributions and absence of nicks. DNA of ciliated protozoa. VIII. Chromosoma. 1980;77(2):217–227. doi: 10.1007/BF00329546. [DOI] [PubMed] [Google Scholar]
- Zahler A. M., Prescott D. M. DNA primase and the replication of the telomeres in Oxytricha nova. Nucleic Acids Res. 1989 Aug 11;17(15):6299–6317. doi: 10.1093/nar/17.15.6299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahler A. M., Prescott D. M. Telomere terminal transferase activity in the hypotrichous ciliate Oxytricha nova and a model for replication of the ends of linear DNA molecules. Nucleic Acids Res. 1988 Jul 25;16(14B):6953–6972. doi: 10.1093/nar/16.14.6953. [DOI] [PMC free article] [PubMed] [Google Scholar]