Abstract
Background/Aims
Endoscopic papillectomy is increasingly performed with curative intent for benign papillary tumors. This study was performed to identify factors that predict the presence of malignancy and affect endoscopic success.
Methods
We retrospectively analyzed the medical records of patients who received an endoscopic papillectomy for papillary adenoma from 2006 to 2009.
Results
A total of 43 patients received endoscopic papillectomy. The pathologic results after papillectomy revealed adenocarcinoma in five patients (12%), and the risk of malignancy was high in cases of large lesions, preprocedural pathology of high-grade dysplasia or high serum alkaline phosphatase. Endoscopic success was observed in 37 patients (86%) at the end of follow-up (mean duration, 10.4±9.6 months). The factor significantly affecting success was a complete resection at the initial papillectomy (p=0.007). Two patients experienced recurrence 10 and 32 months after the complete resection, but both achieved endoscopic success with repeated endoscopic treatment. Six patients with endoscopic failure received surgical resection.
Conclusions
Endoscopic papillectomy is a safe and effective method for the curative resection of benign papillary tumors, especially when complete resection is achieved at the initial papillectomy. Follow-up with surveillance should be performed for at least 3 years because of the possible recurrence of tumors during these periods.
Keywords: Endoscopic sphincterotomy, Benign papillary tumor, Adenocarcinoma, Endoscopic success
INTRODUCTION
Tumors arising from the duodenal papilla account for approximately 5% of gastrointestinal neoplasia,1 but, are identified more frequently with increasing use of upper endoscopic procedures and endoscopic retrograde cholangiopancreatography (ERCP).2,3 Most of these papillary tumors are benign adenomas, which are thought to undergo the same adenoma-carcinoma sequence as adenomas of the colon.4,5 Therefore, complete resection of a papillary tumor is mandatory even if it is not malignant at presentation.
A radical surgery such as pylorus-preserving pancreaticoduodenectomy (PPPD) is performed widely for the treatment of papillary tumors.3 However, because of high postoperative morbidity and mortality of radical surgery6,7 and more increasing recognition of papillary tumors at earlier stages with lower incidences of underlying malignancy,8 an application of this aggressive surgical approach to all frequently recognized benign tumors appears to be inappropriate.
Endoscopic snare papillectomy is increasingly performed with curative intent for benign papillary tumors and many studies about outcomes of the endoscopic treatment have been reported.2,3,7,9-14 However, because of high false-negative rate of endoscopic biopsy for malignancy ranging from 19% to 60%,15,16 the pretreatment distinction between benign and malignant papillary tumors is still challenging and the indication of the endoscopic treatment at clinical practice has not been well established yet.17 The current study was performed to evaluate the outcome of endoscopic papillectomy for benign papillary tumors at a single center and to identify the factors predicting the presence of malignancy and affecting the success of the endoscopic treatment.
MATERIALS AND METHODS
1. Patients
From January 2006 to December 2009, the data of patients who received endoscopic papillectomy abstracted from a prospectively maintained ERCP database at Seoul National University Hospital, Seoul, Korea. The data were classified into three categories according to previous study (Table 1),9 and were reviewed retrospectively.
Table 1.
Preprocedural, Procedural, and Postprocedural Data Points Collected on Patients Presenting with Benign Papillary Tumors
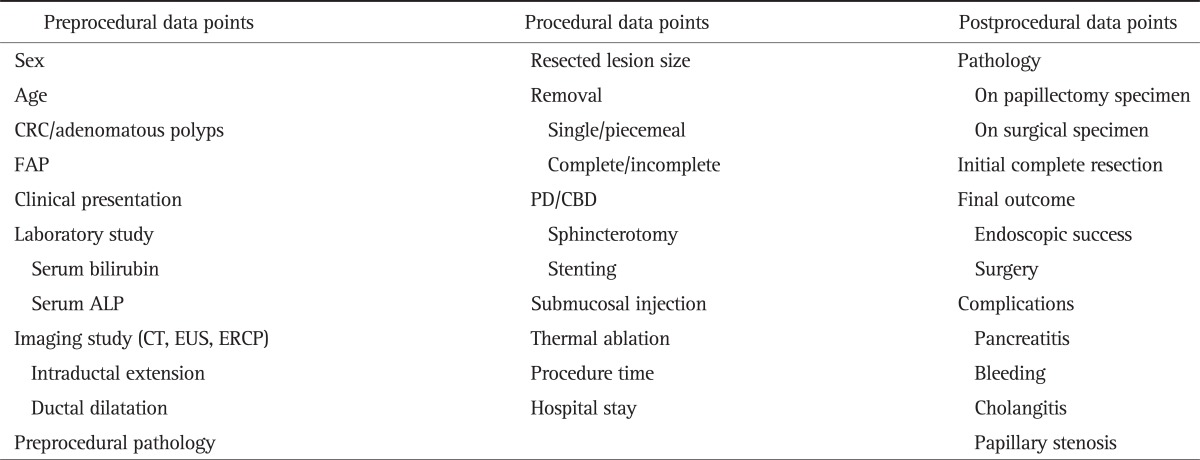
CRC, colorectal cancer; FAP, familial adenomatous polyposis; PD, pancreatic duct; CBD, common bile duct; ALP, alkaline phosphatase; CT, computed tomography; EUS, endoscopic ultrasonography; ERCP, endoscopic retrograde cholangiopancreatography.
Inclusion criteria were patients older than 18 years who underwent endoscopic papillectomy with curative intent for pathologically proven benign adenoma and who received follow-up endoscopy 4 to 8 weeks after endoscopic papillectomy. Exclusion criteria were patients whose endoscopic finding or ERCP showed features of unresectability. Features of unresectability were friability, ulceration, more than 50% lateral extension, and obvious duodenal infiltration at endoscopy or extensive intraductal involvement (more than 1 cm) at ERCP. The study protocol was reviewed and approved by the Institutional Review Board of Seoul National University Hospital.
2. Procedure
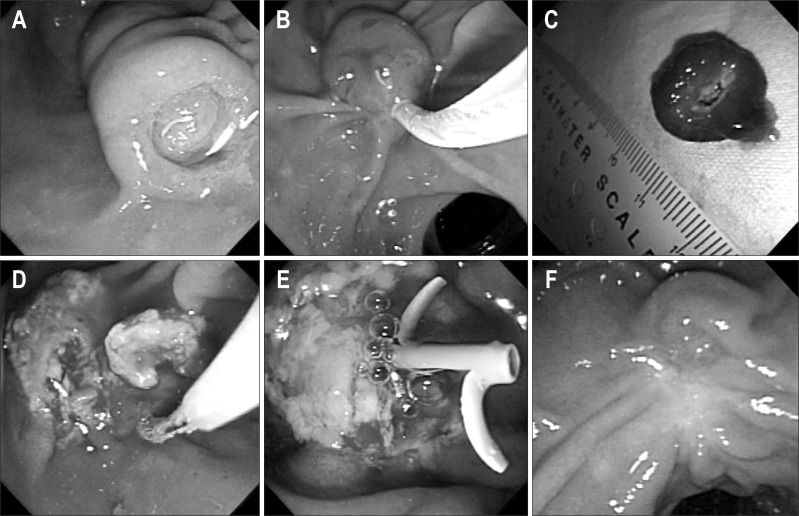
All endoscopic procedures at our institution were performed by two experienced endoscopists (Y.T.K. and J.K.R). Endoscopic ultrasonography (EUS, a radial ultrasound endoscope with 7.5 MHz and 12 MHz US frequencies, α5; ALOKA, Mitaka, Japan) and ERCP was performed before endoscopic papillectomy to evaluate intraductal extension and to determine resectability. The entire endoscopic procedure was performed under fluoroscopic guidance using a side-view duodenoscope (TJF 240; Olympus, Tokyo, Japan). If intraductal extension was suspected at EUS or ERCP, sphincterotomy was performed before and during papillectomy to quantify the intraductal extension and to permit a more aggressive treatment. After grasping the tumor at the base with a snare, resection was performed with using electrocautery (Fig. 1). For lesions that could not be grasped with a snare due to lateral extension, hypertonic saline mixed with epinephrine (1:50,000) was injected into the submucosal layer before resection. Lesions that did not permit en bloc resection were resected in a piecemeal fashion. The resected specimens were captured with a basket and measured with a ruler. The resection site was evaluated after each procedure for residual tumor and bleeding. Electrocautery was used for ablating residual tumor or managing bleeding. Pancreatic stent placement was performed unless a patient had pancreas divisum or a wide pancreatic orifice. Biliary stent placement was not performed routinely.
Fig. 1.
The case of a 57-year-old male patient who received endoscopic papillectomy for a benign papillary tumor of the major duodenal papilla. (A) Endoscopic view of a papillary tumor. (B) Papillectomy using a standard polypectomy snare. (C) Measurement of resected lesion size with a ruler after papillectomy. (D) Additional thermal ablation for a residual tumor after papillectomy. (E) Pancreatic stent placement for preventing postpapillectomy pancreatitis. (F) Eight weeks after papillectomy.
3. Outcome
After the procedure, pathologic results of resected tumors were classified according to the Vienna classification of gastrointestinal epithelial neoplasia.18
All patients received follow-up endoscopy with biopsy 4 to 8 weeks after endoscopic papillectomy. Complete resection at initial papillectomy was defined as absence of any residual tumor demonstrated by the 1st follow-up endoscopy with routine biopsy. Patients underwent follow-up endoscopy 3 to 12 months after the 1st follow-up endoscopy according to individual clinical situation. To be called a recurrence, at least one endoscopy with a biopsy specimen demonstrating no residual tissue was required, and a 3-month interval between the end of treatment and the diagnosis of a recurrence was required. Endoscopic success was defined as a complete excision of the tumor irrespective of the number of procedures required and absence of recurrence or a recurrence during the follow-up period, which was successfully treated with the endoscopic procedure. Endoscopic failure was defined as an inability to completely remove the lesion endoscopically regardless of the number of procedures, invasive malignancy with positive resection margin on histopathology, or a recurrence that was no longer endoscopically amenable. Pancreatitis was defined as a threefold increase in pancreatic enzymes with abdominal pain.19 Bleeding was defined only as a clinically evident bleeding requiring at least two packs of red blood cell transfusion. Statistical analysis was performed by using the chi-square test, Fisher's exact test, and the unpaired two-tailed test with p-values less than 0.05 regarded as significant.
RESULTS
1. Patients
From January 2006 to December 2009, 58 patients received endoscopic papillectomy for papillary tumors. Among them, the final pathologic results revealed nonadenomatous lesions (carcinoid tumor, neuroendocrine tumor, or heterotopic pancreas) in seven patients. In other six patients, endoscopic papillectomy with curative intent could not be performed because of the endoscopic findings of unresectability. These six patients received endoscopic papillectomy with diagnostic intent and the pathologic results of resected specimens revealed invasive adenocarcinoma. Other two patients could not be followed up after papillectomy. Therefore, total 43 patients received endoscopic papillectomy for pathologically proven benign adenoma with curative intent and underwent follow-up after papillectomy.
2. Endoscopic procedure and complications
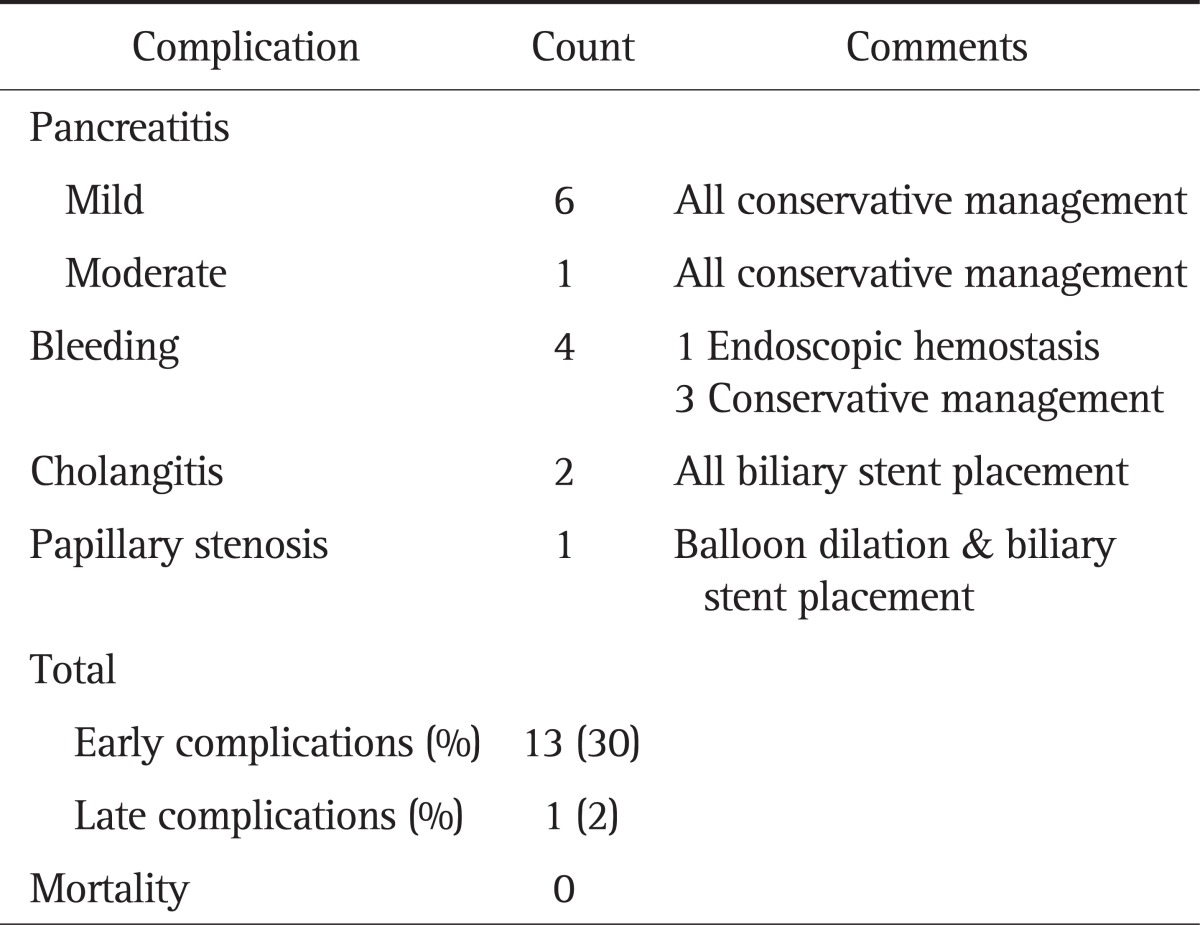
The endoscopic resection was performed by single en bloc resection in 39 patients and piecemeal resection in four patients. Pancreatic stent placement was performed in 32 patients (74%). The mean resected lesion size was 15±9 mm (range, 5 to 50 mm). Complications of endoscopic treatment occurred in 14 patients (32%), which are summarized in Table 2. There was no procedure-related mortality in our study.
Table 2.
Complications in Patients Undergoing Papillectomy for Benign Papillary Tumors
3. Preprocedural and final pathologic results
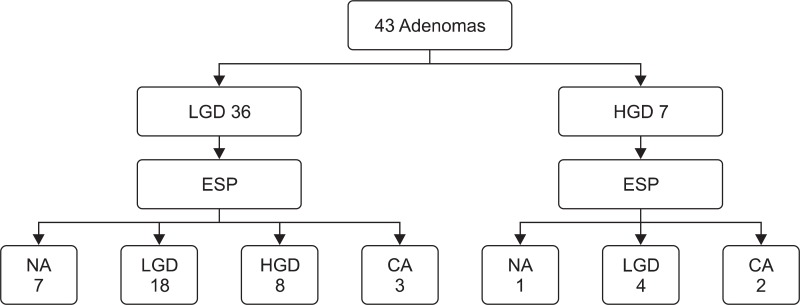
Preprocedural pathologic results revealed adenoma with low-grade dysplasia (LGD) and high-grade dysplasia (HGD) in 36 and seven patients, respectively. Pathologic results of the resected specimens (final pathologic results) revealed adenoma with LGD in 22 patients (51%), adenoma with HGD in eight patients (19%), and invasive adenocarcinoma in five patients (12%) (Fig. 2). In eight patients, tumor cells could not be found in the pathologic evaluation of resected specimens because of severe cautery artifact. These eight patients had no residual tumor on follow-up endoscopy with routine biopsy. Therefore, the final pathologic results of them could not be confirmed.
Fig. 2.
Preprocedural and final pathologic results.
LGD, low grade dysplasia; HGD, high grade dysplasia; ESP, endoscopic papillectomy; CA, invasive adenocarcinoma; NA, not available.
Compared with final pathologic results, underdiagnoses on preprocedural pathology were made in 13 patients (30%). The rate of malignancy on final pathology was significantly higher in patients with larger resected lesion (≥1.5 cm, p=0.013). Among other preprocedural and procedural factors, higher serum alkaline phosphatase (sALP) at presentation (≥120 IU/L) and the preprocedural pathology of HGD also tended to predict the presence of malignancy on final pathology (10% vs 40% and 10% vs 33%, respectively). However, these tendencies were not statistically significant (p>0.05). All patients with smaller lesion (<1.5 cm) were free of malignancy after papillectomy, whereas two of four patients (50%) with both larger lesion (≥1.5 cm) and preprocedural HGD and two of three patients (67%) with both larger lesion (≥1.5 cm) and higher sALP (≥120 IU/L) were diagnosed with malignancy.
4. Clinical outcomes after follow-up
Complete resection at initial papillectomy was noted on the 1st follow-up endoscopy in 32 patients (74%). After the follow-up period (mean duration of follow-up, 10.4±9.6 months), endoscopic success was seen in 37 patients (86%). Endoscopic failure was seen in six patients (invasive adenocarcinoma with positive resection margin, incomplete resection, and persistent residual tumor despite two sessions of endoscopic resections in three, one, and two patients, respectively).
During the follow-up period, two patients experienced recurrence 10 and 32 months after initial complete resection with pathology of LGD and HGD, respectively. These two patients received endoscopic thermal ablation for recurrent tumor, once and twice, respectively. Follow-up endoscopy with biopsy confirmed complete resection of tumor (endoscopic success) in both patients.
Among five patients diagnosed with invasive adenocarcinoma after papillectomy (Table 3), two patients, whose pathologic results revealed negative resection margin and negative lymphovascular invasion, could not receive surgical resection due to comorbidity and underwent only follow-up. Both patients achieved initial complete resection and did not experience recurrence during follow-up (10 and 17 months, respectively).
Table 3.
Pathologic Results and Clinical Outcomes of Patients with Invasive Adenocarcinoma after Papillectomy
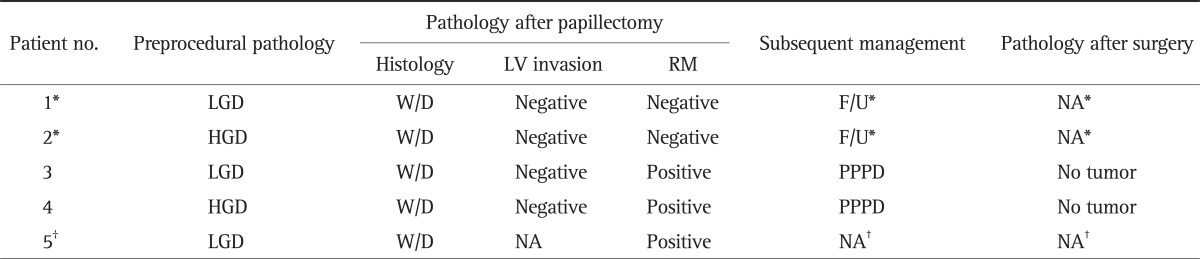
LV, lymphovascular; RM, resection margin; LGD, low-grade dysplasia; W/D, well-differentiated adenocarcinoma; F/U, follow-up with endoscopy; NA, not available; HGD, high-grade dysplasia; PPPD, pylorus-preserving pancreaticoduodenectomy.
*These two patients, who underwent only follow-up with endoscopy, achieved initial complete resection and did not experience recurrence during follow-up; †This patient refused to receive surgical resection and could not be followed up after discharge.
Among six patients with endoscopic failure, one patient diagnosed with invasive adenocarcinoma at papillectomy refused to receive surgical resection and could not be followed up after discharge. Other five patients received surgical resection (PPPD). The pathology after surgery revealed adenoma with LGD (5 cm sized tumor) in one patient, and interestingly, no residual tumor on surgical specimen in other four patients (Table 4).
Table 4.
Clinical and Pathologic Outcomes of Patients with Endoscopic Failure
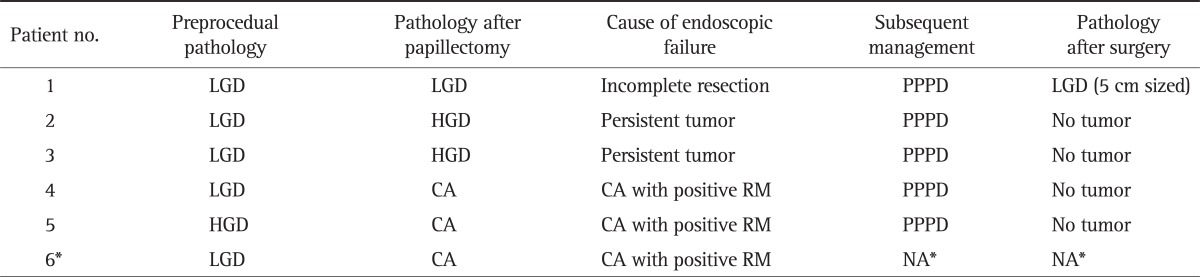
LGD, low-grade dysplasia; PPPD, pylorus-preserving pancreaticoduodenectomy; HGD, high-grade dysplasia; CA, invasive adenocarcinoma; NA, not available.
*This patient refused to receive surgical resection and could not be followed up after discharge.
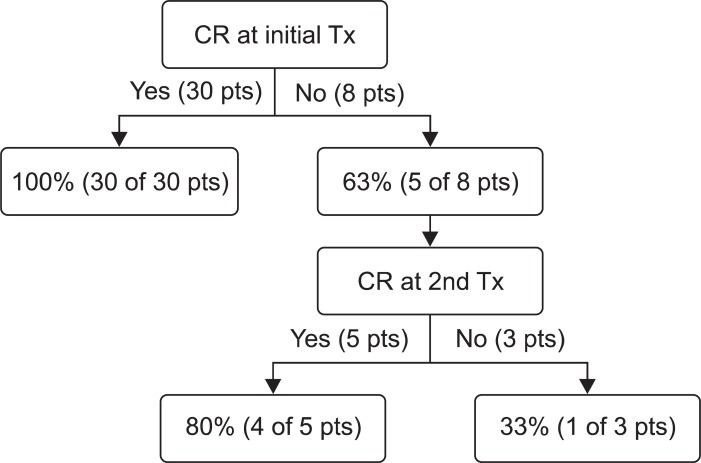
A statistical analysis was performed to identify factors affecting endoscopic success for patients without malignancy. Complete resection at initial papillectomy significantly affected endoscopic success (p=0.007). The rate of endoscopic success was 100% in patients with initial complete resection (and without malignancy) (Fig. 3). Even in patients with recurrence after complete resection, endoscopic success was achieved after repeated endoscopic treatment ultimately. However, in patients without initial complete resection, the rate of endoscopic success was only 63% (five of eight patients), and when a complete resection was not achieved even after two times of endoscopic treatment, the rate of endoscopic success fell to 33% (one of three patients) (Fig. 3). The rate of endoscopic failure was higher in patients with final pathology of HGD than LGD (25% vs 4%), but this difference was not significant (p>0.05).
Fig. 3.
The rate of final endoscopic success in case of complete or incomplete resection after endoscopic treatment (for patients without malignancy).
CR, complete resection; Tx, treatment; pts, patients.
DISCUSSION
Although endoscopic papillectomy has been established as a safe and effective alternative to surgery, an adequate patient selection for curative endoscopic papillectomy at clinical practice is still challenging because of the difficulty of accurate pretreatment diagnosis of malignancy with endoscopic findings alone17 and the high false-negative rate of endoscopic biopsy.15,16 In our study, the rate of malignancy after papillectomy was considerable (12%, similar to other reports)9-11,13,14 even after excluding patients with endoscopic findings suggesting malignancy. Therefore, the identification of factors predicting the presence of malignancy before papillectomy may help clinicians to determine the treatment option of papillary tumors. Several studies have reported these factors such as weight loss9 or tumor size.14 However the analyses in these studies were performed also in patients with preprocedural pathology of adenocarcinoma or with endoscopically unresectable tumors. To obtain more helpful information about management of papillary tumor at clinical practice, it may be more reasonable to perform this analysis only in patients with endoscopically amenable tumors. Our study, to the best of our knowledge, attempted this approach first. While the risk of malignancy, according to the results of our study, would be very low in patients with smaller lesion (<1.5 cm) irrespective of the presence of preprocedural HGD or higher sALP (≥120 IU/L), the possibility of presence of malignancy and subsequent radical surgery should be considered in patients with both larger lesion (≥1.5 cm) and preprocedural HGD or with both larger lesion (≥1.5 cm) and higher sALP (≥120 IU/L) because of the high rate of malignancy (50% and 67%, respectively).
In our study, the rate of endoscopic success was 86%, similar to that of other reports.9-13 Several studies have reported factors affecting the endoscopic success such as absence of intraductal lesions,10 smaller size,9,12 and absence of dilated ducts.9 All these analyses were performed in patients including malignancy cases. However, it is obvious these malignancy cases (invasive adenocarcinoma) should be classified as endoscopic failure, because a radical surgery such as PPPD should be considered in case of malignancy. Therefore, we evaluated the factors affecting the endoscopic success after excluding patients with malignancy. Based on the results of our study, when complete resection cannot be achieved after initial endoscopic treatment and endoscopically resected specimen shows the presence of HGD, subsequent radical surgery should be considered.
The option of subsequent surgery should be discussed if the endoscopically resected specimen shows the presence of HGD.10 Kim et al.14 reported the high recurrence rate in patients with preprocedural HGD and suggested that patients with preprocedural HGD should undergo radical surgery without endoscopic papillectomy, unless they have high operative risk. In our study, however, recurrence occurred in only one patient with HGD, and endoscopic success was achieved in this patient with repeated endoscopic treatment. Furthermore, all patients with initial complete resection achieved endoscopic success irrespective of the presence of HGD. Therefore, endoscopic papillectomy, in our opinion, is justified as a diagnostic and a potentially curative procedure in patients with preprocedural HGD and endoscopically benign-appearing papillary tumors, and when initial complete resection is achieved, follow-up with endoscopic surveillance is also justified. Other studies10,20 also reported that endoscopic papillectomy may be a curative treatment in patients with HGD. Further studies with the larger number of patients will be needed to clarify this issue.
Among six patients with endoscopic failure, four patients had pathologic results of positive microscopic resection margin after endoscopic papillectomy and eventually received PPPD. However, their pathologic results after PPPD revealed no residual tumor, which, to the best of our knowledge, is the first report in ampullary tumor, although similar cases have been reported in stomach and colon.21-24 The necrosis of residual tumors on the resection margin by the electrodiathermic burns might be one possible cause of the absence of residual tumor.21 However, because of lack of studies about this issue in ampullary tumor, supplemental radical surgery should be considered in case of positive resection margin after endoscopic papillectomy until further studies clarify this issue.
Interestingly, in all patients with invasive adenocarcinoma after papillectomy in our study (except one patient with loss of follow-up), no residual tumor was demonstrated after initial endoscopic papillectomy. This report suggests that endoscopic papillectomy might be a curative treatment option for certain subgroup of invasive adenocarcinoma. However, because of the considerable rate of lymph-node metastasis (10.0%) even in early stage (T1) ampullary cancer,25 the selection of the subgroup without lymph-node metastasis might be essential for the consideration of the curative endoscopic treatment.26 Several reports20,25,26 have discussed this issue to date. Our prior study26 reported the absence of lymph-node metastasis in patients with selected subgroup of T1 cancer (less than 2 cm in size and well-differentiated histology). Although further studies with the larger number of patients will be needed to clarify this endoscopically amenable subgroup, endoscopic papillectomy might be an alternative to surgery in patients with selected subgroup of T1 cancer who are not candidate for surgery due to severe comorbidities.
Several studies have suggested the guidelines of surveillance after endoscopic papillectomy. Catalano et al.12 proposed the guideline to include endoscopic treatment every 2 to 3 months until complete resection, followed by surveillance every 6 to 12 months for at least next 2 years. While we agree to this guideline, we additionally suggest the 1st follow-up endoscopy 4 to 8 weeks after initial papillectomy for confirmation of initial complete resection, because, in our study, initial complete resection was the most significant factor affecting endoscopic success. One patient experienced recurrence 32 months after initial complete resection with pathology of HGD. Therefore, we also suggest surveillance for at least 3 years after complete resection especially when the final pathology is HGD. Cheng et al.11 recommended annual surveillance for as long as 5 years after completion of the papillectomy.
Our study has several limitations. First, the small number of patients made several factors statistically insignificant. Second, the duration of follow-up was not sufficient to address the long term clinical outcome. Further studies with the large number of patients and long term follow-up will be needed.
In summary, endoscopic papillectomy is a safe and effective method for curative resection of benign papillary tumor. Initial complete resection is a strong predictor of endoscopic success. However, in case of the failure of complete resection after initial endoscopic treatment with the presence of HGD, a subsequent radical surgery should be considered. Endoscopic benign features cannot predict the presence of malignancy and the possibility of presence of malignancy should be considered in patients with both large lesion (≥1.5 cm) and preprocedural HGD or both large lesion (≥1.5 cm) and high sALP (≥120 IU/L). Follow-up with surveillance should be performed first 4 to 8 weeks after initial papillectomy and should be continued for at least 3 years in case of final pathology of HGD because of the possible recurrence of tumor during these periods.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Scarpa A, Capelli P, Zamboni G, et al. Neoplasia of the ampulla of Vater. Ki-ras and p53 mutations. Am J Pathol. 1993;142:1163–1172. [PMC free article] [PubMed] [Google Scholar]
- 2.Boix J, Lorenzo-Zúñiga V, Moreno de Vega V, Domènech E, Gassull MA. Endoscopic resection of ampullary tumors: 12-year review of 21 cases. Surg Endosc. 2009;23:45–49. doi: 10.1007/s00464-008-9866-3. [DOI] [PubMed] [Google Scholar]
- 3.Jung MK, Cho CM, Park SY, et al. Endoscopic resection of ampullary neoplasms: a single-center experience. Surg Endosc. 2009;23:2568–2574. doi: 10.1007/s00464-009-0464-9. [DOI] [PubMed] [Google Scholar]
- 4.Stolte M, Pscherer C. Adenoma-carcinoma sequence in the papilla of Vater. Scand J Gastroenterol. 1996;31:376–382. doi: 10.3109/00365529609006414. [DOI] [PubMed] [Google Scholar]
- 5.Seifert E, Schulte F, Stolte M. Adenoma and carcinoma of the duodenum and papilla of Vater: a clinicopathologic study. Am J Gastroenterol. 1992;87:37–42. [PubMed] [Google Scholar]
- 6.Allema JH, Reinders ME, van Gulik TM, et al. Results of pancreaticoduodenectomy for ampullary carcinoma and analysis of prognostic factors for survival. Surgery. 1995;117:247–253. doi: 10.1016/s0039-6060(05)80197-7. [DOI] [PubMed] [Google Scholar]
- 7.Norton ID, Gostout CJ, Baron TH, Geller A, Petersen BT, Wiersema MJ. Safety and outcome of endoscopic snare excision of the major duodenal papilla. Gastrointest Endosc. 2002;56:239–243. doi: 10.1016/s0016-5107(02)70184-3. [DOI] [PubMed] [Google Scholar]
- 8.Norton ID, Geller A, Petersen BT, Sorbi D, Gostout CJ. Endoscopic surveillance and ablative therapy for periampullary adenomas. Am J Gastroenterol. 2001;96:101–106. doi: 10.1111/j.1572-0241.2001.03358.x. [DOI] [PubMed] [Google Scholar]
- 9.Irani S, Arai A, Ayub K, et al. Papillectomy for ampullary neoplasm: results of a single referral center over a 10-year period. Gastrointest Endosc. 2009;70:923–932. doi: 10.1016/j.gie.2009.04.015. [DOI] [PubMed] [Google Scholar]
- 10.Bohnacker S, Seitz U, Nguyen D, et al. Endoscopic resection of benign tumors of the duodenal papilla without and with intraductal growth. Gastrointest Endosc. 2005;62:551–560. doi: 10.1016/j.gie.2005.04.053. [DOI] [PubMed] [Google Scholar]
- 11.Cheng CL, Sherman S, Fogel EL, et al. Endoscopic snare papillectomy for tumors of the duodenal papillae. Gastrointest Endosc. 2004;60:757–764. doi: 10.1016/s0016-5107(04)02029-2. [DOI] [PubMed] [Google Scholar]
- 12.Catalano MF, Linder JD, Chak A, et al. Endoscopic management of adenoma of the major duodenal papilla. Gastrointest Endosc. 2004;59:225–232. doi: 10.1016/s0016-5107(03)02366-6. [DOI] [PubMed] [Google Scholar]
- 13.Yamao T, Isomoto H, Kohno S, et al. Endoscopic snare papillectomy with biliary and pancreatic stent placement for tumors of the major duodenal papilla. Surg Endosc. 2010;24:119–124. doi: 10.1007/s00464-009-0538-8. [DOI] [PubMed] [Google Scholar]
- 14.Kim JH, Han JH, Yoo BM, Kim MW, Kim WH. Is endoscopic papillectomy safe for ampullary adenomas with high-grade dysplasia? Ann Surg Oncol. 2009;16:2547–2554. doi: 10.1245/s10434-009-0509-2. [DOI] [PubMed] [Google Scholar]
- 15.Beger HG, Treitschke F, Gansauge F, Harada N, Hiki N, Mattfeldt T. Tumor of the ampulla of Vater: experience with local or radical resection in 171 consecutively treated patients. Arch Surg. 1999;134:526–532. doi: 10.1001/archsurg.134.5.526. [DOI] [PubMed] [Google Scholar]
- 16.Clary BM, Tyler DS, Dematos P, Gottfried M, Pappas TN. Local ampullary resection with careful intraoperative frozen section evaluation for presumed benign ampullary neoplasms. Surgery. 2000;127:628–633. doi: 10.1067/msy.2000.106532. [DOI] [PubMed] [Google Scholar]
- 17.Adler DG, Qureshi W, Davila R, et al. The role of endoscopy in ampullary and duodenal adenomas. Gastrointest Endosc. 2006;64:849–854. doi: 10.1016/j.gie.2006.08.044. [DOI] [PubMed] [Google Scholar]
- 18.Schlemper RJ, Riddell RH, Kato Y, et al. The Vienna classification of gastrointestinal epithelial neoplasia. Gut. 2000;47:251–255. doi: 10.1136/gut.47.2.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cotton PB, Lehman G, Vennes J, et al. Endoscopic sphincterotomy complications and their management: an attempt at consensus. Gastrointest Endosc. 1991;37:383–393. doi: 10.1016/s0016-5107(91)70740-2. [DOI] [PubMed] [Google Scholar]
- 20.Yoon SM, Kim MH, Kim MJ, et al. Focal early stage cancer in ampullary adenoma: surgery or endoscopic papillectomy? Gastrointest Endosc. 2007;66:701–707. doi: 10.1016/j.gie.2007.02.049. [DOI] [PubMed] [Google Scholar]
- 21.Kim JH, Cheon JH, Kim TI, et al. Effectiveness of radical surgery after incomplete endoscopic mucosal resection for early colorectal cancers: a clinical study investigating risk factors of residual cancer. Dig Dis Sci. 2008;53:2941–2946. doi: 10.1007/s10620-008-0248-4. [DOI] [PubMed] [Google Scholar]
- 22.Chung YS, Park DJ, Lee HJ, et al. The role of surgery after incomplete endoscopic mucosal resection for early gastric cancer. Surg Today. 2007;37:114–117. doi: 10.1007/s00595-006-3328-0. [DOI] [PubMed] [Google Scholar]
- 23.Nagano H, Ohyama S, Fukunaga T, et al. Indications for gastrectomy after incomplete EMR for early gastric cancer. Gastric Cancer. 2005;8:149–154. doi: 10.1007/s10120-005-0328-5. [DOI] [PubMed] [Google Scholar]
- 24.Korenaga D, Orita H, Maekawa S, et al. Pathological appearance of the stomach after endoscopic mucosal resection for early gastric cancer. Br J Surg. 1997;84:1563–1566. [PubMed] [Google Scholar]
- 25.Lee SY, Jang KT, Lee KT, et al. Can endoscopic resection be applied for early stage ampulla of Vater cancer? Gastrointest Endosc. 2006;63:783–788. doi: 10.1016/j.gie.2005.09.015. [DOI] [PubMed] [Google Scholar]
- 26.Woo SM, Ryu JK, Lee SH, et al. Feasibility of endoscopic papillectomy in early stage ampulla of Vater cancer. J Gastroenterol Hepatol. 2009;24:120–124. doi: 10.1111/j.1440-1746.2008.05578.x. [DOI] [PubMed] [Google Scholar]