Abstract
Cardiac arrest is a major public health problem affecting thousands of individuals each year in both the before hospital and in-hospital settings. However, although the scope of the problem is large, the quality of care provided during resuscitation attempts frequently does not meet quality of care standards, despite evidence-based cardiopulmonary resuscitation (CPR) guidelines, extensive provider training, and provider credentialing in resuscitation medicine. Although this fact may be disappointing, it should not be surprising. Resuscitation of the cardiac arrest victim is a highly complex task requiring coordination between various levels and disciplines of care providers during a stressful and relatively infrequent clinical situation. Moreover, it requires a targeted, high-quality response to improve clinical outcomes of patients. Therefore, solutions to improve care provided during resuscitation attempts must be multifaceted and targeted to the diverse number of care providers to be successful.
Keywords: Cardiopulmonary resuscitation, Cardiac arrest, Feedback, Debriefing
In the “2010 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care (AHA Guidelines),”1,2 the focus of resuscitation priorities during cardiac arrest has shifted from early airway and breathing management toward providing high quality uninterrupted chest compressions and early defibrillation for shockable rhythms, which is exemplified in the acronym change from Airway-Breathing-Circulation, or ABC, to Circulation-Airway-Breathing, or CAB. There have been numerous studies supporting this simplified approach, including a recent clinical trial demonstrating that even administration of advanced cardiac life support (ACLS) medications may not provide a survival benefit to cardiac arrest patients.3,4 Consequently, it seems that providing high quality CPR (summarized in the AHA Guidelines catchphrase “Push Hard, Push Fast”) with minimal interruptions and prompt defibrillation may be the most important actions during cardiac arrest that will translate into a survival benefit.
Given the complexity of the care required during cardiac arrest resuscitation, it should not be surprising that, even though in many locales cardiac arrest survival rates have improved, overall, strategies to improve resuscitation quality and outcomes are not fully implemented. An adult in-hospital cardiac arrest (IHCA) registry study has documented a rate of survival to discharge for adult in-hospital arrest at 19%; pediatric rates of survival are slightly higher, exceeding 25%.5–10 Out-of-hospital cardiac arrest (OHCA) survival rates are much lower for both groups at less than 10%, with survival depending on location of arrest, initial rhythm, as well as patient and rescuer factors.11,12 The variability of survival rates among different locations given the same rhythm and same setting suggests that resuscitation performance may be a contributing factor.11 This variability in performance can be explained by several factors. These events occur infrequently from the perspective of any given rescuer, most rescuers are uncomfortable in these highly stressful situations,13 and this feeling of unease is only magnified by the existing training programs that follow a low-frequency paradigm (ie, certification every 2–4 years). Clearly, new approaches, both technological and educational, are needed. In this article, we review some of the new approaches to improving cardiac arrest resuscitation performance. The focus will be on a continuous quality improvement paradigm (ie, before, during, after): to improve resuscitation outcomes, we must improve training methods before actual cardiac arrest events, monitor quality during resuscitation attempts, and feedback care deficiencies to frontline care providers after the events using quantitative debriefing programs.
CURRENT STATE OF RESUSCITATION PERFORMANCE
Recent resuscitation literature, assisted by CPR-recording devices, large cardiac arrest event registries, and high-fidelity ACLS simulation studies, have focused on and provide a significant amount of objective data regarding rescuer performance during actual and simulated cardiac arrests. Unfortunately, a common theme from these studies was that resuscitation performance frequently does not meet established care guidelines during IHCA, OHCA, and simulated cardiac arrests. Even more troubling, these deficiencies in care spanned the literature on both pediatric and adult patients.14–16 To illustrate, Wik and colleagues15 reported that during adult OHCA resuscitations, 33% of chest compressions were too shallow and were being delivered only 48% of the time during the arrest (ie, nearly half the time when the heart had stopped and there was little or no cardiac output, no chest compressions were being performed). Although one might expect such care deficiencies during the sometimes chaotic resuscitation of OHCA victims, similar deficiencies (23% of chest compressions with incorrect rates; 36% of chest compressions too shallow) were also seen during adult in-hospital arrest care.14 Sutton and colleagues,16 in the only pediatric report of actual arrest resuscitation quality to date, demonstrated that even with the provision of defibrillator automated corrective feedback during the arrest, resuscitation efforts still did not consistently meet established care guidelines. However, this pediatric study did seem to demonstrate improved care compliance in comparison to previous adult investigations. The investigators hypothesized that their improved care was related to a bedside CPR training program instituted at their institution,17 highlighting a possible target for improving resuscitation outcomes (see later discussion).
In addition to difficulties with chest compression delivery, ventilation rates exceeding AHA recommendations have also been problematic.18,19 Why are incorrect ventilation rates troubling? During the low-flow state of CPR, cardiac output and pulmonary blood flow are approximately 25% to 50% of that during normal sinus rhythm. Therefore, much less ventilation is necessary for adequate gas exchange from the blood traversing the pulmonary circulation. Furthermore, both laboratory and clinical data indicate that a rapid rate of assisted ventilation (“over-ventilation” from aggressive rescue breathing) during CPR is common and can substantially compromise venous return and cardiac output by increasing intrathoracic pressure. 18,19 These detrimental hemodynamic effects are compounded when one considers the effect of interruptions in CPR to provide airway management and rescue breathing.20–22 Several studies have supported these results during adult resuscitation attempts23,24 and, as a result, the AHA now recommends the CAB approach, emphasizing that the rescuer should focus on providing high quality chest compressions with minimal interruptions. However, given that most pediatrics arrests are actually asphyxial in nature, controlled ventilation is still recommended. A recent large pediatric series from Japan supported the need for ventilation in pediatric arrest victims. In this study, favorable neurologic outcome 1 month after arrest was improved in patients who received conventional CPR compared with compressiononly CPR for an arrest that was noncardiac in nature.25 In short, the resuscitation technique should be titrated to the physiology of the patient to optimize patient outcome.
Performance of actual chest compressions and ventilations is only one aspect of resuscitation quality. In addition to deficiencies in these psychomotor skills, appropriate recognition and treatment of cardiac arrest rhythms has also been shown to be problematic in actual practice. The treatment of choice for short-duration ventricular fibrillation (VF) is prompt defibrillation. Nevertheless, a large recent registry study showed that defibrillation was delayed beyond 2 minutes in nearly one-third of inhospital VF-ventricular tachycardia (VT) arrests. In general, as the mortality rate increases by 7% to 10% per minute of delay to defibrillation, such delays in treatment must be avoided.26 Furthermore, the wrong treatment decisions are frequently made with respect to defibrillation. In a study involving emergency medical providers (EMS) providers and medical residents, although manual defibrillation decreased pauses in chest compressions compared with semiautomatic defibrillation, more inappropriate shocks were delivered (26%) with a manual approach. In this study, nearly 80% of these shocks were delivered for an organized cardiac rhythm.27 Currently, the resuscitation literature is lacking a report of rhythm recognition and treatment during real pediatric cardiac arrest. However, during simulated resuscitations, pediatric residents at an academic teaching hospital delayed defibrillation by greater than 3 minutes after onset of pulseless VT over half of the time.28 This is particularly troubling because recent studies indicate that VF and VT (ie, shockable rhythms) occur in 27% of inhospital pediatric cardiac arrests at some time during the resuscitation,7 with as many as 41% of pediatric cardiac intensive care arrests associated with VF or VT.29 Programs to improve rhythm recognition and treatment are needed in both the adult and pediatric realm.
IMPROVING PERFORMANCE IMPROVES OUTCOMES
Although numerous studies have documented that resuscitation quality frequently does not meet established care guidelines, it also appears that this substandard care is adversely affecting hemodynamics during, and outcomes from, cardiac arrest resuscitation. For example, increasing chest compression depth to the AHA Guideline standard results in favorable hemodynamic changes, such as an increased arterial blood pressure, in adult humans30 and an increase in coronary blood flow in mature pigs.31 In addition, in both human and animal studies of adult subjects, the minimal interruption of chest compressions seems to be a critically important element of CPR quality because even short pauses in chest compressions (4–5 seconds) decreases coronary perfusion pressure, short-term clinical outcome (eg, defibrillation success), and survival.23,32 Most importantly, studies in adults have established that aggressive implementation of the AHA Guidelines substantially improves adult cardiac arrest survival outcomes, including more favorable neurologic outcomes.33,34 Thus, there seems to be evidence that improving resuscitation quality will translate to improved outcomes for patients.
BEFORE: RESUSCITATION TRAINING
Because the quality of CPR is directly related to survival outcomes,23,35,36 several studies have implicated the existing educational programs for teaching CPR skills as a prime target for interventions to improve survival after cardiac arrest. Although most hospitals in the United States require either basic life support or ACLS certification for most care providers, this is often the only resuscitation training practitioners receive, and there is a growing body of literature supporting the notion that basic life support and ACLS certification may not necessarily even translate to adequate performance of these resuscitation skills during actual arrest events, especially given that most providers have poor retention of these skills 3 to 6 months after traditional training. Deficiencies with not only operational performance in simulated scenarios,37–40 but also with self-perceived rescuer confidence,13 are all too common. Better programs to improve training success are desirable with the expectation that this would translate into higher quality CPR performed during actual resuscitation attempts.
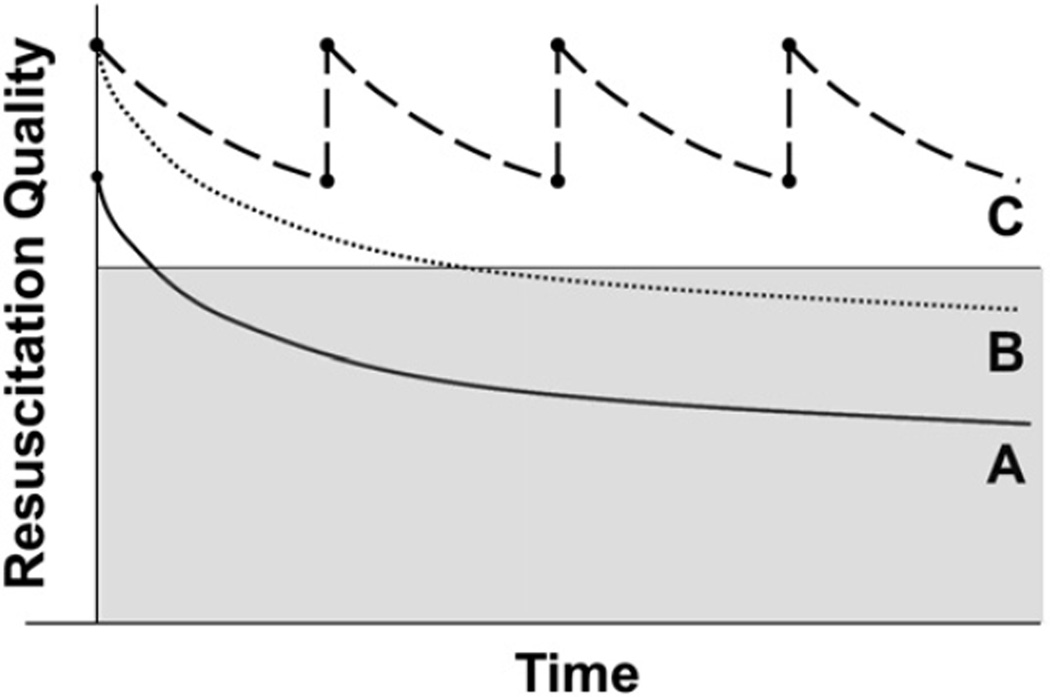
A multifaceted approach is needed to improve existing resuscitation training methods. Alternative training strategies in addition to the standard certification courses should be used to supplement existing resuscitation training. Techniques, such as higher fidelity simulation,41–43 automated quantitative feedback during training,44 postevent debriefing,45 and regular refresher training17,46,47 have shown promise. Individually or together, these techniques can be used to augment resuscitation performance (Fig. 1) and will be discussed in more detail.
Fig. 1.
Resuscitation quality after training. Curve A depicts quality decline after traditional instruction. Note fall into gray shaded zone of poor quality several months after initial training. Curve B represents the theoretical addition of high realism simulation and expert debriefing. Although there is no change in rate of psychomotor skill quality decrement over time, resuscitation quality is maintained longer owing to higher level of initial skill acquisition. Curve C represents addition of frequent refresher training in addition to simulation to prevent decrement to poor quality.
Simulation has shown to be an effective tool to teach resuscitation skills.41–43 Moreover, there is a growing body of literature supporting that higher fidelity training methods and scenarios achieve superior training targets (ie, the more realistic the manikin and scenario, the better the educational outcomes).41 Importantly, the superiority of high fidelity training is not limited to simulated scenarios. For example, in the realm of critical care and/or emergency medicine, recent simulation science has demonstrated that training in central line insertion and daily maintenance not only improves patient outcomes (eg, decreased complications with insertion48 and catheter-related infections49) but, also, the cost50 associated with performance errors. Furthermore, one recent study confirmed that simulation-based education could, in fact, result in higher quality of care provided during an actual resuscitation events43 (ie, these studies have demonstrated that improving operational performance on manikins can improve operational performance in real life). Why does simulation work? First, it provides the benefit of enhancing team work and increasing familiarity with resuscitation equipment, thereby avoiding more frequent errors. As previously mentioned, cardiac arrest resuscitations are relatively uncommon events for a given care provider. Simulation provides the opportunity to make these stressful clinical situations more “common,” in a protected educational environment. Although the literature regarding simulation and improving team work is still evolving, it is likely that simulation is among the best-suited instruments to observe and improve on team dynamics and other human factors during rare-occurring, stressful situations, such as cardiac arrests.51
In a study by Verplancke and colleagues,52 noncritical-care nurses reported an average of 59 months since their last actual delivery of CPR and 18 months since their last CPR training. It is likely that this gap in CPR training and experience is present in other hospitals and care settings, which ultimately leads to a decline in resuscitation performance. As a result, brief, but more frequent training “refreshers” may offer one solution to this problem. Three pediatric studies, all evaluating health care providers in both ICUs and general inpatient floors, have established that brief, intermittent “refresher” CPR training can improve both CPR skill acquisition as well as skill retention in a simulated cardiac arrest scenario.17,46,47 The idea that a brief, relatively infrequent training can improve CPR performance may seem illogical considering that the high-intensity, standard, AHA programs demonstrate poor retention rates. The success of these refresher training programs is grounded in educational precepts and it takes into account the principles of adult learning. Adult learning theory states that there are certain characteristics common to successful adult educational programs: they must be focused, practical, and the need for obtaining the information must be apparent.53–55 Because the education can be concentrated in refresher trainings (<2 minutes in these pediatric investigations), participants do not have to attend formal classroom instruction, making the program both practical and relevant. However, although refreshers may improve CPR skill acquisition and CPR performance, the optimal frequency of these refreshers, length of refresher training modules, and content of training still remains undetermined. Short and frequent refreshers may be more effective than more extensive refresher trainings on a less frequent basis. However, requiring recertification at a shorter time interval could be time consuming and impractical. It is likely that a multicenter trial will be needed to fully evaluate this promising educational technique.
DURING: MONITORING CPR QUALITY WITH TITRATION TO PATIENT PHYSIOLOGY
The evaluation of the effectiveness of ongoing CPR efforts has proven difficult. Several methods that are used commonly (eg, presence of femoral or carotid pulsations, pulse oximetry) have not correlated with successful resuscitation and may even mislead rescuers. The following is a discussion of real-time audiovisual feedback systems, arterial blood pressure monitoring, and end-tidal carbon dioxide (CO2) capnography as methods to guide resuscitation quality.
Real-time Audiovisual Feedback
Interest in improving CPR quality through real-time feedback devices has been evolving since the early 1990s. Human,56,57 animal,58 and manikin studies59–62 have shown improvement in quantitative measures of CPR quality and surrogates of survival outcomes (eg, end-tidal CO2) when CPR feedback devices were used. Given the improvements seen in previous investigations, these technologies offer promise as we look for ways to strengthen our training methods, particularly in light of the fact that one of the problems highlighted concerning existing educational programs has been the poor ability of instructors to actually perceive CPR error in class participants.63 As a result, the AHA now suggests that training programs consider use of automated real-time feedback devices to improve overall training efficacy by providing a quantitative assessment of the CPR performed by trainees.64
During the past decade, innovative technologies have extended the ability to monitor real-time CPR process from manikins used for training purposes to use in actual cardiac arrest victims. Using force transducer and/or accelerometer technology through pads placed between the rescuer’s hands and the patient’s chest, quantitative CPR quality information can be recorded, analyzed, and fed back to the rescuer in an effort to correct CPR deficiencies. Feedback can be given on chest compressions rate, depth, ventilation rate, pauses, and incomplete chest wall recoil (leaning). Feedback-enabled defibrillators, in before-and-after design trials (ie, studies with retrospective controls) have shown to improve CPR quality delivered by EMS providers and in-hospital care providers.36,65 In one study of adult OHCA, feedback increased the mean compression depth from 34 mm to 38 mm and increased the percentage of compressions within AHA Guidelines recommendations for depth from 24% to 53%.36 In similar fashion, another clinical study demonstrated that feedback improved in-hospital CPR quality by reducing the variability of CPR, conforming more to the AHA Guidelines recommendations.65 In the pediatric environment, two studies from a single institution have further confirmed the positive effect of feedback in improving CPR quality. In the study by Sutton and colleagues,16 compliance rates for chest compression depth and rate approached 70%. Unfortunately, this study was observational and lacked a before-period control group to fully evaluate the effect of feedback technology. However, the compliance rates far exceed those published in the adult-care and pediatric-care literature to date. Furthermore, in a small subset of patients from this same cohort, the investigators demonstrated a marked reduction in leaning because of feedback.66 In accordance with the 2010 International Liaison Committee on Resuscitation recommendations, feedback technologies can improve quantitative measure of CPR quality, in training and real cardiac arrest situations.
However, although automated feedback devices can support improvements in CPR quality, questions have been raised regarding whether these devices actually improve patient outcomes.44 None of the studies mentioned so far showed a significant improvement in any type of survival, but they were also not powered to do so. So, although it is clear that feedback technologies can coach providers to achieve quantitative feedback targets, whether achieving these targets through automated feedback technologies improves outcomes remains in question. A recent British Medical Journal publication by Hostler and colleagues,67 using a cluster randomized design from three sites within the Resuscitation Outcomes Consortium in the United States and Canada, although demonstrating improvement in CPR quality, did not show a difference in survival outcomes. Does this mean that CPR quality is not related to clinical outcome? Unfortunately, the feedback targets used in this study were based on 2005 guidelines and, as a result, even with feedback, the average chest compression depth reached only 40 mm. Currently the AHA Guidelines recommend a depth of at least 50 mm to improve outcomes from adult cardiac arrest. In short, it seems that feedback technologies are effective at getting providers to achieve the programmed quality targets. It is the responsibility of resuscitation scientists to determine the best targets for CPR quality that will translate into improved clinical outcomes.
A particularly helpful technology used in feedback enabled defibrillators is the ability to display a signal that filters CPR artifact from the ECG tracing so that rhythm analysis can occur during chest compressions. The obvious benefit is that a rescuer would no longer have to pause chest compressions every 2 minutes to analyze cardiac rhythms. As a result, with fewer interruptions, there would be improved coronary and cerebral perfusion and likelihood of return of spontaneous circulation (ROSC). To date, there is no published literature regarding the clinical use of this feature incorporated into defibrillators. There is a modicum of literature regarding the accuracy of similar technology. 68 If this approach is shown to be reliable and not lead to incorrect rhythm interpretations, it has the potential to enhance CPR performance by decreasing interruptions in chest compressions.
Arterial Blood Pressure and End-tidal CO2
Although CPR quality monitoring defibrillators have been highlighted in recent literature, older technology, such as monitoring of arterial blood pressure and end-tidal CO2, during resuscitation can provide the rescuer with CPR quality information. Why monitor arterial blood pressure? Diastolic blood pressure is a major determinant of myocardial perfusion pressure (MPP), the driving force for myocardial blood flow during CPR.69–72 Aortic diastolic pressure (AoDP) is related to MPP by the following equation: MPP = AoDP – right atrial diastolic pressure (RADP). Because the right atrial diastolic pressure does not change substantially during CPR,73,74 arterial diastolic blood pressure is the most important variable affecting MPP and myocardial blood flow. Because adequate myocardial blood flow is necessary for successful resuscitation from cardiac arrests,75–77 it follows that increasing arterial diastolic pressure will improve resuscitation outcomes. Evidence supporting diastolic blood pressure augmentation to improve the chance of resuscitation comes from numerous studies demonstrating that provision of vasoactive agents, such as epinephrine or vasopressin, or the application of abdominal binders, by raising AoDP, improve MPP and resuscitation success.78–83 These laboratory investigations show that arterial diastolic pressures of at least 30 mmHg during CPR are typically necessary for adequate myocardial blood flow and successful resuscitation. Animals with arterial diastolic pressures less than 25 mmHg rarely survived. There is also supporting evidence from clinical adult arrest studies that diastolic pressures greater than 30 mmHg are associated with return of spontaneous circulation.84 Therefore, an approach of “goal-directed” CPR, where a provider monitors arterial blood pressure and titrates chest compression force and vasoactive agents to achieve hemodynamic goals, seems reasonable.
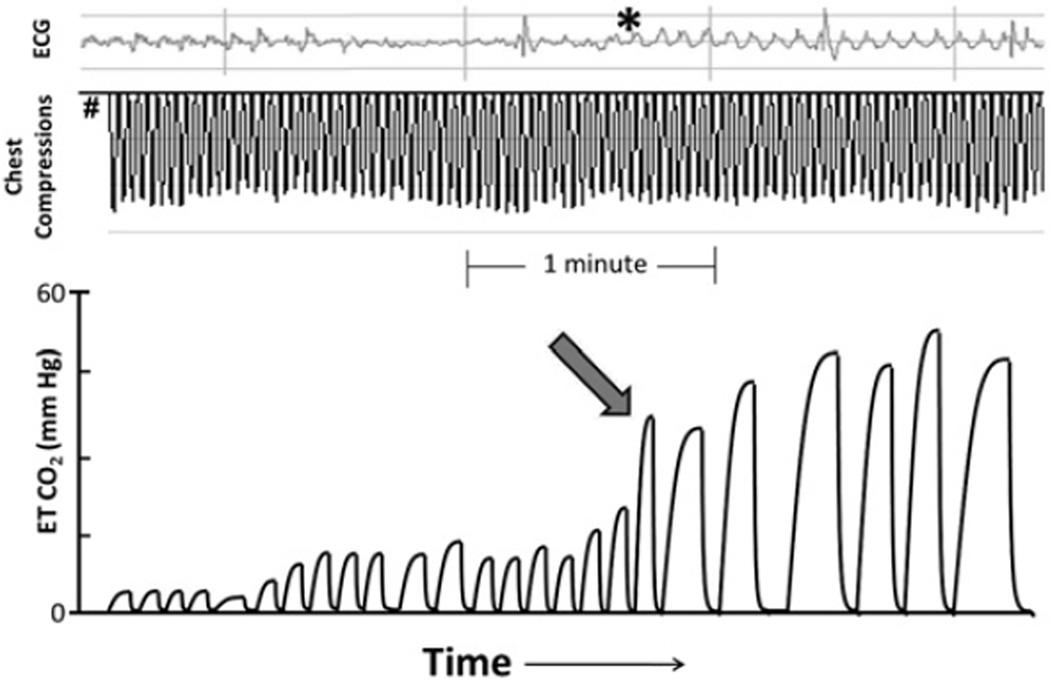
Given the unexpected nature of many cardiac arrests and the sometimes chaotic nature of resuscitation, particularly during OHCA, placement of invasive arterial monitoring is not always feasible. In these situations, continuous end-tidal monitoring CO2 (ie, capnography) can be used as an alternative to monitor CPR quality. In numerous experimental models, noninvasive end-tidal CO2 correlates well with cardiac output, MPP, and resuscitation success.85–89 Furthermore, the utility of end-tidal CO2 monitoring during clinical investigations is not a new discovery and has been described since the 1970s when Kalenda90 described three patients who were monitored for expired CO2 during cardiac arrest. He described using CO2 levels to monitor for rescuer fatigue and saw improvement in end-tidal CO2 levels when a new rescuer started (presumably because the new provider was performing better CPR). He was also the first to show that ROSC could be recognized by a sudden rise in expired CO2. By recognizing ROSC without having to interrupt chest compressions to check for a pulse or arterial blood pressure, one can anticipate that interruptions in chest compressions can be minimized (Fig. 2). Finally, building on this work, other studies have documented differences in end-tidal CO2 levels between survivors and nonsurvivors after adult cardiac arrest, suggesting that end-tidal CO2 can also be used as a prognostic tool during cardiac arrest.86 As a result, continuous end-tidal CO2 monitoring is now recommended during cardiac arrest resuscitation when available.
Fig. 2.
Using end-tidal (ET) CO2 to detect ROSC. From onset of arrest (#), note slow increase in end-tidal CO2 as compressions are delivered. With ROSC (arrow), organized ECG rhythm begins to appear under chest compression artifact (asterisk) and end-tidal CO2 rises suddenly to greater than 50 mmHg. Providers could have used the rapid rise in end-tidal CO2 as a clinical guide that there was a return of spontaneous circulation, without having to pause chest compressions and risk interruption of CPR for a rhythm check.
In conclusion, several technologies, some old and some rather new, are available to providers in both OHCA and IHCA settings. Although there can be arguments made about the superiority of a given technology, the first step in developing plans to improve resuscitation quality is to monitor the care provided during the arrest so that targeted treatment plans can be developed.
Matching Cardiac Arrest Physiology to Resuscitation
Beginning in 2005, the AHA and European Resuscitation Council guidelines for CPR were adjusted to better match the physiologic needs of the cardiac arrest victim, focusing on the delivery of high quality chest compressions with provision of “adequate” ventilation. What defines adequate ventilation? Once cardiac arrest has ensued and the heart has stopped beating, there is little to no blood flow throughout the body. At that point, during the low-flow state of CPR, cardiac output and blood flow are approximately 25% to 50% of that during normal sinus rhythm. As a result, less ventilation is needed for adequate gas exchange. In addition, there is the concern that excessive ventilation from rescuers may have detrimental effects on hemodynamics during resuscitation and survival outcomes.18,19 These detrimental hemodynamic effects are compounded when one considers the effect of interruptions in CPR to provide airway management and rescue breathing. As a result, the chest compression to ventilation ratio was increased from 15:2 to 30:2 in 2005 to ensure chest compressions were being delivered for a greater proportion of the time during CPR. In 2009, a large prospective observational study corroborated the association between increased chest compression fraction (the proportion of resuscitation time without spontaneous circulation during which chest compressions are administered) and improved outcome, with two highest groups of chest compression fraction more than twice as likely to survive.91
The next obvious question raised by the developing body of literature supporting increased chest compression fraction was whether ventilation was needed at all (ie, compression-only resuscitation). Between 2005 and 2010, several studies were focused on investigating an alternative resuscitation strategy, known as cardiocerebral resuscitation (CCR).92–95 CCR entails providing more uninterrupted chest compressions to ensure optimal cerebral and cardiac perfusion. In Arizona, Bobrow and colleagues94 described a variant of CCR termed minimally interrupted cardiac resuscitation, which minimizes interruptions in chest compressions by delaying endotracheal intubation and positive pressure ventilations, instead initially providing passive oxygen insufflation via an oral pharyngeal airway and non-rebreather face mask. The study demonstrated a significant improvement in survival to discharge for OHCA. Since then, several other EMS systems have demonstrated comparable improvements in survival by implementing similar protocols that emphasized uninterrupted chest compressions and delayed intubation.93,95
During the same period that CCR was being investigated for OHCA by EMS providers, resuscitation scientists began to establish whether compression-only CPR was preferable to standard CPR for bystanders. Because bystander CPR is one of the most important determinants of resuscitation outcome,96 the hope was by removing the need for ventilation delivery, more bystander CPR would be provided and outcomes from OHCA would be improved. After several studies demonstrated the efficacy of bystander-initiated compression-only CPR, this technique was endorsed in the 2010 AHA and European Resuscitation Council guidelines for CPR as a reasonable alternative to conventional CPR for adult OHCA.97,98 Most recently, using survival to hospital discharge as the primary outcome, a meta-analysis was recently published in Lancet and concluded that compression-only CPR is preferably to conventional CPR with rescue breathing for adult OHCA.99 However, in pediatrics, given that most arrests are actually asphyxial in nature, controlled ventilation is still recommended. A recent large pediatric series from Japan supported this approach and found that favorable neurologic outcome 1 month after arrest was improved in patients who received conventional CPR compared with compression-only CPR for an arrest that was noncardiac in nature.25 In short, this is one of the take-home points of this article: resuscitation technique and quality should be monitored and titrated to the physiology of the patient to optimize outcome.
Mechanical CPR Devices
As high-quality chest compressions with minimal interruptions seem to be a determinant of IHCA and OHCA survival, it follows that mechanical compression devices may be useful during resuscitation attempts. Piston-type devices and circumferential constriction band devices have been evaluated during cardiac arrest resuscitation and they have shown promise in improving hemodynamic and short-term clinical outcomes.100–102 Although these devices can easily deliver high-quality chest compressions, rescuers must be cautious to limit interruptions in the deployment of said devices.103 See discussion of these devices elsewhere in this issue.
AFTER: PERFORMANCE DEBRIEFING
Health care debriefing is defined as a facilitator-led participant discussion of events with reflection and assimilation of learning into practice. Structured debriefing can trace its origins back to the military in World War II. General George Marshall ordered soldiers under his command to give an account of their experience on return home from a mission. Although the initial intent was to gather tactical information or strategize for future battles, he noticed that debriefings were also spiritually healing and morale building for his soldiers. The technique was further refined in the military and aviation industries, and although initially used as a means to minimize the stress response and improve psychological outcomes from traumatic and infrequent situations, 104–108 currently debriefing is conceptualized as a method to improve care during rare and stressful events.
The value of debriefing starts with resuscitation training. Structured debriefing has been established as a useful tool to improve compliance of in-hospital adult care providers during simulated cardiac arrest. Although debriefing or automated feedback alone improved CPR quality modestly in a study from the University of Pennsylvania, the combination led to a more considerable improvement in quality.109 Similarly, debriefing with pediatric in-hospital care providers has also shown the positive effects of debriefing during resuscitation training. In a study from the Children’s Hospital of Philadelphia, the combination of instructor-led training and debriefing with automated defibrillator feedback improved CPR quality compared with either the training or debriefing method alone.46 Therefore, in addition to quantitative monitoring of trainee performance, it seems prudent to ensure that performance is fed back to trainees in an attempt to achieve the best educational outcomes.
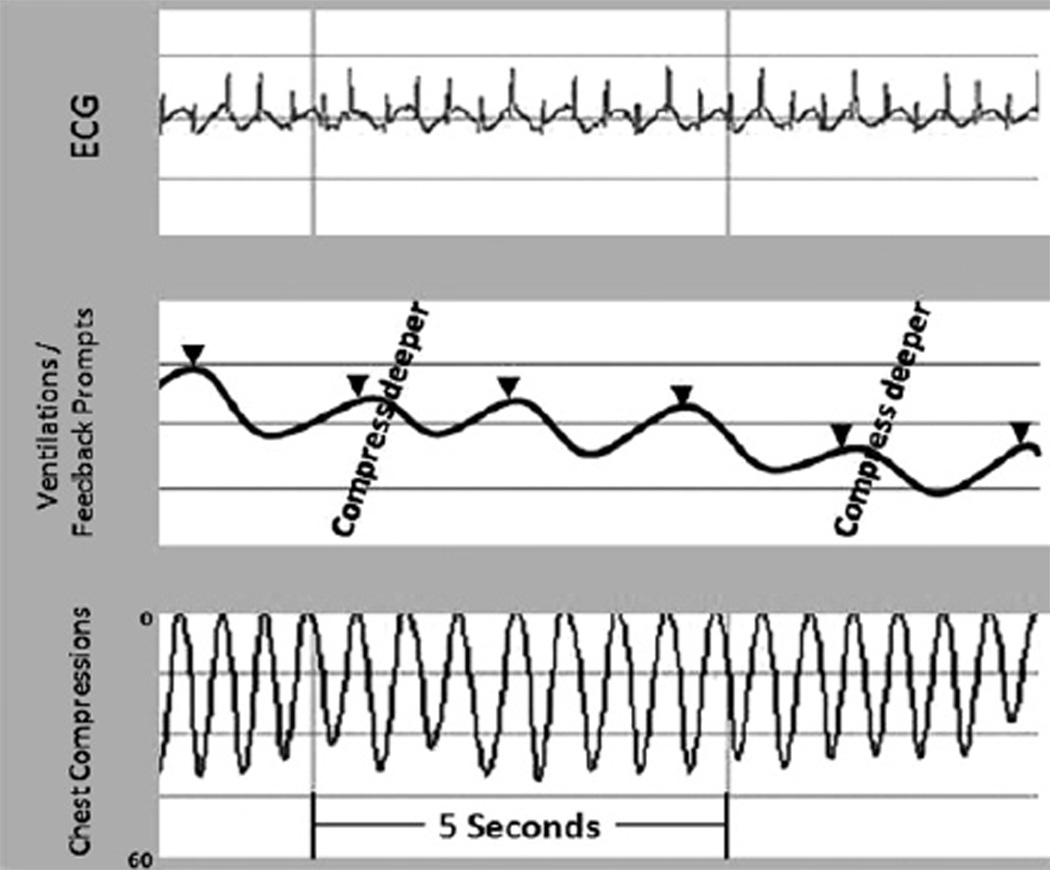
The first study published demonstrating efficacy of debriefing to improve outcomes from cardiac arrest came from the University of Chicago where the combination of resuscitation debriefing interventions and audiovisual feedback via defibrillators produced a marked improvement in resuscitation performance and a 33% increase in ROSC.45 This particular debriefing program consisted of weekly sessions that reviewed transcripts of quantitative data downloaded from defibrillators, including CPR-quality ECG data (Fig. 3). Although this study was a designed before-after study using historical controls, the benefit of structured debriefing was apparent with improved CPR quality target compliance and ROSC.
Fig. 3.
Representative CPR quantitative recording. Provides ability to review ECG, ventilation, and chest compression data after events to improve future resuscitation quality. Note prompts given to rescuers to “compress deeper” when the chest compressions are too shallow. The arrow heads indicate ventilations, in this recording provided at a rate of approximately 60 per minute (too fast!). These recordings can be used to provide a structured quantitative postevent review for rescuers who participated in the resuscitation.
Although these investigations reported positive findings with the addition of debriefing, a European study in the pre-hospital setting failed to show any benefit after incorporating performance evaluation.110 However, this study should not deter resuscitation scientists from recommending performance debriefing. Instead, this study highlighted an important aspect of successful debriefing: the process must be completed with front-line care providers. In the European study, CPR performance data were presented to EMS leadership or local CPR instructors, not to front-line care providers. This prohibited the “self-reflection” and “assimilation” that is of paramount importance to debriefing success. Therefore, this study highlights the importance of having a highly structured debriefing process performed with front-line providers.
EVIDENCE THAT PUTTING IT ALL TOGETHER IMPROVES OUTCOMES
Although this article is focused on techniques to improve resuscitation performance (eg, innovative training methods, monitors to enable providers to titrate the resuscitation to arrest physiology, real-time feedback-enabled CPR monitoring defibrillators, and a systematic post-cardiac arrest debriefing process), it is likely that a bundled approach incorporating two to several of these techniques will be necessary to improve long-term patient outcomes. As a promising recent example, the “Take Heart America” program was a comprehensive, community-wide, systems-based approach to the treatment of cardiac arrest.111 This program consisted of widespread cardiopulmonary resuscitation skills training in schools and businesses, retraining of all EMS personnel in methods to deliver high quality CPR, deploying additional automated external defibrillators in schools and public places (ie, enabling prompt defibrillation when needed), and establishing treatment protocols regarding transport to and treatment by cardiac arrest centers. As a result of this intensive program, bystander CPR rates increased from 20% to 29% (P = .086, odds ratio 1.7, 95% confidence interval 0.96–2.89), hypothermia therapy for admitted out-of-hospital cardiac arrest victims increased from 0% to 45%, and, most importantly, survival to hospital discharge for all patients after out-of-hospital cardiac arrest in these two sites improved from 8.5% to 19% (P = .011, odds ratio 2.60, confidence interval 1.19–6.26). Although this study used historical controls, the magnitude of improvement in survival outcomes provides strong evidence that initiation of a resuscitation care bundle or system will be effective to improve outcomes from cardiac arrest.
SUMMARY
In spite of the remarkable progress made in resuscitation science since Kouwenhoven’s112 original description of closed chest cardiac massage, survival from cardiac arrest continues to be very low. The reader should be convinced that this could be attributed, in part, to the poor performance of resuscitation care. Furthermore, it should be clear that resuscitation of the cardiac arrest victim is a highly complex task requiring coordination between multiple levels and disciplines of care providers. In short, resuscitation is not easy and, despite improvements in care over the past 50 years, there is substantial work to be done. The authors argue that using a continuous quality improvement bundle (ie, improving training before, monitoring and titrating quality during, and debriefing after events) seems to hold promise as the resuscitation community strives to improve the care that we deliver to cardiac arrest victims. In future investigations, with this approach, we expect resuscitation scientists to begin to establish that improvements in performance will subsequently translate into better survival rates for victims of sudden cardiac arrest.
ACKNOWLEDGMENTS
The authors would like to thank Dr Robert A. Berg and Dana Niles for their help in preparation of this article. We would also like to acknowledge Marion Leary, Lori Boyle, and Jessica Leffelman, who have supported resuscitation science at the University of Pennsylvania and Children’s Hospital of Philadelphia.
Financial Disclosures or Conflicts of Interest: Vinay Nadkarni receives unrestricted research grant support from the Laerdal Foundation for Acute Care Medicine. Robert Sutton is supported through a career development award from the Eunice Kennedy Shriver National Institute of Child Health & Human Development (K23HD062629). Benjamin Abella receives research funding from Philips Healthcare, the American Heart Association, and the Doris Duke Foundation, and has received speaking honoraria from Philips Healthcare.
REFERENCES
- 1.Berg RA, Hemphill R, Abella BS, et al. Part 5: adult basic life support: 2010 American Heart Association guidelines for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation. 2010;122(18 Suppl 3):S685–S705. doi: 10.1161/CIRCULATIONAHA.110.970939. [DOI] [PubMed] [Google Scholar]
- 2.Berg MD, Schexnayder SM, Chameides L, et al. Part 13: pediatric basic life support: 2010 American Heart Association guidelines for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation. 2010;122(18 Suppl 3):S862–75. doi: 10.1161/CIRCULATIONAHA.110.971085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Olasveengen TM, Sunde K, Brunborg C, et al. Intravenous drug administration during out-of-hospital cardiac arrest: a randomized trial. JAMA. 2009;302(20):2222–2229. doi: 10.1001/jama.2009.1729. [DOI] [PubMed] [Google Scholar]
- 4.van Walraven C, Stiell IG, Wells GA, et al. Do advanced cardiac life support drugs increase resuscitation rates from in-hospital cardiac arrest? The OTAC study group. Ann Emerg Med. 1998;32(5):544–553. doi: 10.1016/s0196-0644(98)70031-9. [DOI] [PubMed] [Google Scholar]
- 5.Lloyd-Jones D, Adams R, Carnethon M, et al. Heart disease and stroke statistics—2009 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2009;119(3):480–486. doi: 10.1161/CIRCULATIONAHA.108.191259. [DOI] [PubMed] [Google Scholar]
- 6.Tibballs J, Kinney S. A prospective study of outcome of in-patient paediatric cardiopulmonary arrest. Resuscitation. 2006;71(3):310–318. doi: 10.1016/j.resuscitation.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 7.Samson RA, Nadkarni VM, Meaney PA, et al. Outcomes of in-hospital ventricular fibrillation in children. N Engl J Med. 2006;354(22):2328–2339. doi: 10.1056/NEJMoa052917. [DOI] [PubMed] [Google Scholar]
- 8.Nadkarni VM, Larkin GL, Peberdy MA, et al. First documented rhythm and clinical outcome from in-hospital cardiac arrest among children and adults. JAMA. 2006;295(1):50–57. doi: 10.1001/jama.295.1.50. [DOI] [PubMed] [Google Scholar]
- 9.Meaney PA, Nadkarni VM, Cook EF, et al. Higher survival rates among younger patients after pediatric intensive care unit cardiac arrests. Pediatrics. 2006;118(6):2424–2433. doi: 10.1542/peds.2006-1724. [DOI] [PubMed] [Google Scholar]
- 10.de Mos N, van Litsenburg RR, McCrindle B, et al. Pediatric in-intensive-care-unit cardiac arrest: incidence, survival, and predictive factors. Crit Care Med. 2006;34(4):1209–1215. doi: 10.1097/01.CCM.0000208440.66756.C2. [DOI] [PubMed] [Google Scholar]
- 11.Nichol G, Thomas E, Callaway CW, et al. Regional variation in out-of-hospital cardiac arrest incidence and outcome. JAMA. 2008;300(12):1423–1431. doi: 10.1001/jama.300.12.1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Donoghue AJ, Nadkarni V, Berg RA, et al. Out-of-hospital pediatric cardiac arrest: an epidemiologic review and assessment of current knowledge. Ann Emerg Med. 2005;46(6):512–522. doi: 10.1016/j.annemergmed.2005.05.028. [DOI] [PubMed] [Google Scholar]
- 13.Hayes CW, Rhee A, Detsky ME, et al. Residents feel unprepared and unsupervised as leaders of cardiac arrest teams in teaching hospitals: a survey of internal medicine residents. Crit Care Med. 2007;35(7):1668–1672. doi: 10.1097/01.CCM.0000268059.42429.39. [DOI] [PubMed] [Google Scholar]
- 14.Abella BS, Alvarado JP, Myklebust H, et al. Quality of cardiopulmonary resuscitation during in-hospital cardiac arrest. JAMA. 2005;293(3):305–310. doi: 10.1001/jama.293.3.305. [DOI] [PubMed] [Google Scholar]
- 15.Wik L, Kramer-Johansen J, Myklebust H, et al. Quality of cardiopulmonary resuscitation during out-of-hospital cardiac arrest. JAMA. 2005;293(3):299–304. doi: 10.1001/jama.293.3.299. [DOI] [PubMed] [Google Scholar]
- 16.Sutton RM, Niles D, Nysaether J, et al. Quantitative analysis of CPR quality during in-hospital resuscitation of older children and adolescents. Pediatrics. 2009;124(2):494–499. doi: 10.1542/peds.2008-1930. [DOI] [PubMed] [Google Scholar]
- 17.Niles D, Sutton RM, Donoghue A, et al. “Rolling refreshers”: a novel approach to maintain CPR psychomotor skill competence. Resuscitation. 2009;80(8):909–912. doi: 10.1016/j.resuscitation.2009.04.021. [DOI] [PubMed] [Google Scholar]
- 18.Aufderheide TP, Lurie KG. Death by hyperventilation: a common and life-threatening problem during cardiopulmonary resuscitation. Crit Care Med. 2004;32(Suppl 9):S345–S351. doi: 10.1097/01.ccm.0000134335.46859.09. [DOI] [PubMed] [Google Scholar]
- 19.Aufderheide TP, Sigurdsson G, Pirrallo RG, et al. Hyperventilation-induced hypotension during cardiopulmonary resuscitation. Circulation. 2004;109(16):1960–1965. doi: 10.1161/01.CIR.0000126594.79136.61. [DOI] [PubMed] [Google Scholar]
- 20.Yannopoulos D, Aufderheide TP, Gabrielli A, et al. Clinical and hemodynamic comparison of 15: 2 and 30: 2 compression-to-ventilation ratios for cardiopulmonary resuscitation. Crit Care Med. 2006;34(5):1444–1449. doi: 10.1097/01.CCM.0000216705.83305.99. [DOI] [PubMed] [Google Scholar]
- 21.Ewy GA. Continuous-chest-compression cardiopulmonary resuscitation for cardiac arrest. Circulation. 2007;116(25):2894–2896. doi: 10.1161/CIRCULATIONAHA.107.751065. [DOI] [PubMed] [Google Scholar]
- 22.Ewy GA, Zuercher M, Hilwig RW, et al. Improved neurological outcome with continuous chest compressions compared with 30:2 compressions-to-ventilations cardiopulmonary resuscitation in a realistic swine model of out-of-hospital cardiac arrest. Circulation. 2007;116(22):2525–2530. doi: 10.1161/CIRCULATIONAHA.107.711820. [DOI] [PubMed] [Google Scholar]
- 23.Edelson DP, Abella BS, Kramer-Johansen J, et al. Effects of compression depth and pre-shock pauses predict defibrillation failure during cardiac arrest. Resuscitation. 2006;71(2):137–145. doi: 10.1016/j.resuscitation.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 24.Valenzuela TD, Kern KB, Clark LL, et al. Interruptions of chest compressions during emergency medical systems resuscitation. Circulation. 2005;112(9):1259–1265. doi: 10.1161/CIRCULATIONAHA.105.537282. [DOI] [PubMed] [Google Scholar]
- 25.Kitamura T, Iwami T, Kawamura T, et al. Conventional and chest-compression-only cardiopulmonary resuscitation by bystanders for children who have out-of-hospital cardiac arrests: a prospective, nationwide, population-based cohort study. Lancet. 2010;375(9723):1347–1354. doi: 10.1016/S0140-6736(10)60064-5. [DOI] [PubMed] [Google Scholar]
- 26.Chan PS, Krumholz HM, Nichol G, et al. American Heart Association National Registry of Cardiopulmonary Resuscitation Investigators. Delayed time to defibrillation after in-hospital cardiac arrest. N Engl J Med. 2008;358(1):9–17. doi: 10.1056/NEJMoa0706467. [DOI] [PubMed] [Google Scholar]
- 27.Kramer-Johansen J, Edelson DP, Abella BS, et al. Pauses in chest compression and inappropriate shocks: a comparison of manual and semi-automatic defibrillation attempts. Resuscitation. 2007;73(2):212–220. doi: 10.1016/j.resuscitation.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 28.Hunt EA, Vera K, Diener-West M, et al. Delays and errors in cardiopulmonary resuscitation and defibrillation by pediatric residents during simulated cardiopulmonary arrests. Resuscitation. 2009;80(7):819–825. doi: 10.1016/j.resuscitation.2009.03.020. [DOI] [PubMed] [Google Scholar]
- 29.Rhodes JF, Blaufox AD, Seiden HS, et al. Cardiac arrest in infants after congenital heart surgery. Circulation. 1999;100(19 Suppl):194–199. doi: 10.1161/01.cir.100.suppl_2.ii-194. [DOI] [PubMed] [Google Scholar]
- 30.Ornato JP, Levine RL, Young DS, et al. The effect of applied chest compression force on systemic arterial pressure and end-tidal carbon dioxide concentration during CPR in human beings. Ann Emerg Med. 1989;18(7):732–737. doi: 10.1016/s0196-0644(89)80005-8. [DOI] [PubMed] [Google Scholar]
- 31.Bellamy RF, DeGuzman LR, Pedersen DC. Coronary blood flow during cardiopulmonary resuscitation in swine. Circulation. 1984;69(1):174–180. doi: 10.1161/01.cir.69.1.174. [DOI] [PubMed] [Google Scholar]
- 32.Berg RA, Sanders AB, Kern KB, et al. Adverse hemodynamic effects of interrupting chest compressions for rescue breathing during cardiopulmonary resuscitation for ventricular fibrillation cardiac arrest. Circulation. 2001;104(20):2465–2470. doi: 10.1161/hc4501.098926. [DOI] [PubMed] [Google Scholar]
- 33.Aufderheide TP, Yannopoulos D, Lick CJ, et al. Implementing the 2005 American Heart Association guidelines improves outcomes after out-of-hospital cardiac arrest. Heart Rhythm. 2010;7(10):1357–1362. doi: 10.1016/j.hrthm.2010.04.022. [DOI] [PubMed] [Google Scholar]
- 34.Thigpen K, Davis SP, Basol R, et al. Implementing the 2005 American Heart Association guidelines, including use of the impedance threshold device, improves hospital discharge rate after in-hospital cardiac arrest. Respir Care. 2010;55(8):1014–1019. [PubMed] [Google Scholar]
- 35.Abella BS, Sandbo N, Vassilatos P, et al. Chest compression rates during cardiopulmonary resuscitation are suboptimal: a prospective study during in-hospital cardiac arrest. Circulation. 2005;111(4):428–434. doi: 10.1161/01.CIR.0000153811.84257.59. [DOI] [PubMed] [Google Scholar]
- 36.Kramer-Johansen J, Myklebust H, Wik L, et al. Quality of out-of-hospital cardiopulmonary resuscitation with real time automated feedback: a prospective interventional study. Resuscitation. 2006;71(3):283–292. doi: 10.1016/j.resuscitation.2006.05.011. [DOI] [PubMed] [Google Scholar]
- 37.Arshid M, Lo TY, Reynolds F. Quality of cardio-pulmonary resuscitation (CPR) during paediatric resuscitation training: time to stop the blind leading the blind. Resuscitation. 2009;80(5):558–560. doi: 10.1016/j.resuscitation.2009.02.017. [DOI] [PubMed] [Google Scholar]
- 38.Perkins GD, Boyle W, Bridgestock H, et al. Quality of CPR during advanced resuscitation training. Resuscitation. 2008;77(1):69–74. doi: 10.1016/j.resuscitation.2007.10.012. [DOI] [PubMed] [Google Scholar]
- 39.DeVita MA, Schaefer J, Lutz J, et al. Improving medical crisis team performance. Crit Care Med. 2004;32(Suppl 2):S61–S65. doi: 10.1097/01.ccm.0000110872.86812.1c. [DOI] [PubMed] [Google Scholar]
- 40.Smith KK, Gilcreast D, Pierce K. Evaluation of staff’s retention of ACLS and BLS skills. Resuscitation. 2008;78(1):59–65. doi: 10.1016/j.resuscitation.2008.02.007. [DOI] [PubMed] [Google Scholar]
- 41.Donoghue AJ, Durbin D, Nadel F, et al. Effect of high-fidelity simulation on pediatric advanced life support training in pediatric housestaff: a randomized trial. Pediatr Emerg Care. 2009;25(3):139–144. doi: 10.1097/PEC.0b013e31819a7f90. [DOI] [PubMed] [Google Scholar]
- 42.Wayne DB, Butter J, Siddall VJ, et al. Simulation-based training of internal medicine residents in advanced cardiac life support protocols: a randomized trial. Teach Learn Med. 2005;17(3):210–216. doi: 10.1207/s15328015tlm1703_3. [DOI] [PubMed] [Google Scholar]
- 43.Wayne DB, Didwania A, Feinglass J, et al. Simulation-based education improves quality of care during cardiac arrest team responses at an academic teaching hospital: a case-control study. Chest. 2008;133(1):56–61. doi: 10.1378/chest.07-0131. [DOI] [PubMed] [Google Scholar]
- 44.Yeung J, Meeks R, Edelson D, et al. The use of CPR feedback/prompt devices during training and CPR performance: a systematic review. Resuscitation. 2009;80(7):743–751. doi: 10.1016/j.resuscitation.2009.04.012. [DOI] [PubMed] [Google Scholar]
- 45.Edelson DP, Litzinger B, Arora V, et al. Improving in-hospital cardiac arrest process and outcomes with performance debriefing. Arch Intern Med. 2008;168(10):1063–1069. doi: 10.1001/archinte.168.10.1063. [DOI] [PubMed] [Google Scholar]
- 46.Sutton RM, Niles D, Meaney PM, et al. “Booster” training; evaluation of instructor-led bedside cardiopulmonary resuscitation skill training and automated corrective feedback to improve cardiopulmonary resuscitation compliance of pediatric basic life support providers during simulated cardiac arrest. Pediatr Crit Care Med. 2011;12(3):e116–e121. doi: 10.1097/PCC.0b013e3181e91271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sutton RM, Niles D, Meaney PA, et al. Low-dose, high-frequency CPR training improves skill retention of in-hospital pediatric providers. Pediatrics. 2011;128(1):e145–e151. doi: 10.1542/peds.2010-2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Barsuk JH, McGaghie WC, Cohen ER, et al. Simulation-based mastery learning reduces complications during central venous catheter insertion in a medical intensive care unit. Crit Care Med. 2009;37(10):2697–2701. [PubMed] [Google Scholar]
- 49.Barsuk JH, Cohen ER, Feinglass J, et al. Use of simulation-based education to reduce catheter-related bloodstream infections. Arch Intern Med. 2009;169(15):1420–1423. doi: 10.1001/archinternmed.2009.215. [DOI] [PubMed] [Google Scholar]
- 50.Cohen ER, Feinglass J, Barsuk JH, et al. Cost savings from reduced catheter-related bloodstream infection after simulation-based education for residents in a medical intensive care unit. Simul Healthc. 2010;5(2):98–102. doi: 10.1097/SIH.0b013e3181bc8304. [DOI] [PubMed] [Google Scholar]
- 51.Hunt EA, Shilkofski NA, Stavroudis TA, et al. Simulation: translation to improved team performance. Anesthesiol Clin. 2007;25(2):301–319. doi: 10.1016/j.anclin.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 52.Verplancke T, De Paepe P, Calle PA, et al. Determinants of the quality of basic life support by hospital nurses. Resuscitation. 2008;77(1):75–80. doi: 10.1016/j.resuscitation.2007.10.006. [DOI] [PubMed] [Google Scholar]
- 53.Knowles M. The adult learner: a neglected species. 4th edition. Houston (TX): Gulf Publishing Company; 1990. [Google Scholar]
- 54.Kaufman DM. Applying educational theory in practice. BMJ. 2003;326(7382):213–216. doi: 10.1136/bmj.326.7382.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.DeWitt TG. The application of social and adult learning theory to training in community pediatrics, social justice, and child advocacy. Pediatrics. 2003;112(3 Part 2):755–757. [PubMed] [Google Scholar]
- 56.Berg RA, Sanders AB, Milander M, et al. Efficacy of audio-prompted rate guidance in improving resuscitator performance of cardiopulmonary resuscitation on children. Acad Emerg Med. 1994;1(1):35–40. [PubMed] [Google Scholar]
- 57.Kern KB, Sanders AB, Raife J, et al. A study of chest compression rates during cardiopulmonary resuscitation in humans. The importance of rate-directed chest compressions. Arch Intern Med. 1992;152(1):145–149. [PubMed] [Google Scholar]
- 58.Milander MM, Hiscok PS, Sanders AB, et al. Chest compression and ventilation rates during cardiopulmonary resuscitation: the effects of audible tone guidance. Acad Emerg Med. 1995;2(8):708–713. doi: 10.1111/j.1553-2712.1995.tb03622.x. [DOI] [PubMed] [Google Scholar]
- 59.Sutton RM, Donoghue AJ, Myklebust H, et al. The voice advisory manikin (VAM): an innovative approach to pediatric lay provider basic life support skill education. Resuscitation. 2007;75(1):161–168. doi: 10.1016/j.resuscitation.2007.02.007. [DOI] [PubMed] [Google Scholar]
- 60.Wik L, Thowsen J, Steen PA. An automated voice advisory manikin system for training in basic life support without an instructor. A novel approach to CPR training. Resuscitation. 2001;50(2):167–172. doi: 10.1016/s0300-9572(01)00331-8. [DOI] [PubMed] [Google Scholar]
- 61.Wik L, Myklebust H, Auestad BH, et al. Retention of basic life support skills 6 months after training with an automated voice advisory manikin system without instructor involvement. Resuscitation. 2002;52(3):273–279. doi: 10.1016/s0300-9572(01)00476-2. [DOI] [PubMed] [Google Scholar]
- 62.Wik L, Myklebust H, Auestad BH, et al. Twelve-month retention of CPR skills with automatic correcting verbal feedback. Resuscitation. 2005;66(1):27–30. doi: 10.1016/j.resuscitation.2004.12.022. [DOI] [PubMed] [Google Scholar]
- 63.Kaye W, Rallis SF, Mancini ME, et al. The problem of poor retention of cardiopulmonary resuscitation skills may lie with the instructor, not the learner or the curriculum. Resuscitation. 1991;21(1):67–87. doi: 10.1016/0300-9572(91)90080-i. [DOI] [PubMed] [Google Scholar]
- 64.Mancini ME, Soar J, Bhanji F, et al. Education, Implementation, Teams Chapter Collaborators. Part 12: education, implementation, and teams: 2010 international consensus on cardiopulmonary resuscitation and emergency cardiovascular care science with treatment recommendations. Circulation. 2010;122(16 Suppl 2):S539–S581. doi: 10.1161/CIRCULATIONAHA.110.971143. [DOI] [PubMed] [Google Scholar]
- 65.Abella BS, Edelson DP, Kim S, et al. CPR quality improvement during in-hospital cardiac arrest using a real-time audiovisual feedback system. Resuscitation. 2007;73(1):54–61. doi: 10.1016/j.resuscitation.2006.10.027. [DOI] [PubMed] [Google Scholar]
- 66.Niles D, Nysaether J, Sutton R, et al. Leaning is common during in-hospital pediatric CPR, decreased with automated corrective feedback. Resuscitation. 2009;80(5):553–557. doi: 10.1016/j.resuscitation.2009.02.012. [DOI] [PubMed] [Google Scholar]
- 67.Hostler D, Everson-Stewart S, Rea TD, et al. Effect of real-time feedback during cardiopulmonary resuscitation outside hospital: prospective, cluster-randomised trial. BMJ. 2011;342:512. doi: 10.1136/bmj.d512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Berger RD, Palazzolo J, Halperin H. Rhythm discrimination during uninterrupted CPR using motion artifact reduction system. Resuscitation. 2007;75(1):145–152. doi: 10.1016/j.resuscitation.2007.03.007. [DOI] [PubMed] [Google Scholar]
- 69.Voorhees WD, Babbs CF, Tacker WA., Jr Regional blood flow during cardiopulmonary resuscitation in dogs. Crit Care Med. 1980;8(3):134–136. doi: 10.1097/00003246-198003000-00008. [DOI] [PubMed] [Google Scholar]
- 70.Michael JR, Guerci AD, Koehler RC, et al. Mechanisms by which epinephrine augments cerebral and myocardial perfusion during cardiopulmonary resuscitation in dogs. Circulation. 1984;69(4):822–835. doi: 10.1161/01.cir.69.4.822. [DOI] [PubMed] [Google Scholar]
- 71.Halperin HR, Tsitlik JE, Guerci AD, et al. Determinants of blood flow to vital organs during cardiopulmonary resuscitation in dogs. Circulation. 1986;73(3):539–550. doi: 10.1161/01.cir.73.3.539. [DOI] [PubMed] [Google Scholar]
- 72.Schleien CL, Dean JM, Koehler RC, et al. Effect of epinephrine on cerebral and myocardial perfusion in an infant animal preparation of cardiopulmonary resuscitation. Circulation. 1986;73(4):809–817. doi: 10.1161/01.cir.73.4.809. [DOI] [PubMed] [Google Scholar]
- 73.Dean JM, Koehler RC, Schleien CL, et al. Improved blood flow during prolonged cardiopulmonary resuscitation with 30% duty cycle in infant pigs. Circulation. 1991;84(2):896–904. doi: 10.1161/01.cir.84.2.896. [DOI] [PubMed] [Google Scholar]
- 74.Berkowitz ID, Gervais H, Schleien CL, et al. Epinephrine dosage effects on cerebral and myocardial blood flow in an infant swine model of cardiopulmonary resuscitation. Anesthesiology. 1991;75(6):1041–1050. doi: 10.1097/00000542-199112000-00017. [DOI] [PubMed] [Google Scholar]
- 75.Ralston SH, Voorhees WD, Babbs CF. Intrapulmonary epinephrine during prolonged cardiopulmonary resuscitation: improved regional blood flow and resuscitation in dogs. Ann Emerg Med. 1984;13(2):79–86. doi: 10.1016/s0196-0644(84)80566-1. [DOI] [PubMed] [Google Scholar]
- 76.Ditchey RV, Lindenfeld J. Failure of epinephrine to improve the balance between myocardial oxygen supply and demand during closed-chest resuscitation in dogs. Circulation. 1988;78(2):382–389. doi: 10.1161/01.cir.78.2.382. [DOI] [PubMed] [Google Scholar]
- 77.Pellis T, Weil MH, Tang W, et al. Evidence favoring the use of an alpha2-selective vasopressor agent for cardiopulmonary resuscitation. Circulation. 2003;108(21):2716–2721. doi: 10.1161/01.CIR.0000096489.40209.DD. [DOI] [PubMed] [Google Scholar]
- 78.Redding JS, Pearson JW. Resuscitation from ventricular fibrillation. Drug therapy. JAMA. 1968;203(4):255–260. [PubMed] [Google Scholar]
- 79.Redding JS, Pearson JW. Evaluation of drugs for cardiac resuscitation. Anesthesiology. 1963;24:203–207. doi: 10.1097/00000542-196303000-00008. [DOI] [PubMed] [Google Scholar]
- 80.Redding JS. Abdominal compression in cardiopulmonary resuscitation. Anesth Analg. 1971;50(4):668–675. [PubMed] [Google Scholar]
- 81.Yakaitis RW, Otto CW, Blitt CD. Relative importance of alpha and beta adrenergic receptors during resuscitation. Crit Care Med. 1979;7(7):293–296. doi: 10.1097/00003246-197907000-00001. [DOI] [PubMed] [Google Scholar]
- 82.Pearson JW, Redding JS. The role of epinephrine in cardiac resuscitation. Anesth Analg. 1963;42:599–606. [PubMed] [Google Scholar]
- 83.Pearson JW, Redding JS. Peripheral vascular tone on cardiac resuscitation. Anesth Analg. 1965;44(6):746–752. [PubMed] [Google Scholar]
- 84.Paradis NA, Martin GB, Rivers EP, et al. Coronary perfusion pressure and the return of spontaneous circulation in human cardiopulmonary resuscitation. JAMA. 1990;263(8):1106–1113. [PubMed] [Google Scholar]
- 85.Weil MH, Bisera J, Trevino RP, et al. Cardiac output and end-tidal carbon dioxide. Crit Care Med. 1985;13(11):907–909. doi: 10.1097/00003246-198511000-00011. [DOI] [PubMed] [Google Scholar]
- 86.Sanders AB, Kern KB, Otto CW, et al. End-tidal carbon dioxide monitoring during cardiopulmonary resuscitation. A prognostic indicator for survival. JAMA. 1989;262(10):1347–1351. [PubMed] [Google Scholar]
- 87.Sanders AB, Ewy GA, Bragg S, et al. Expired PCO2 as a prognostic indicator of successful resuscitation from cardiac arrest. Ann Emerg Med. 1985;14(10):948–952. doi: 10.1016/s0196-0644(85)80235-3. [DOI] [PubMed] [Google Scholar]
- 88.Sanders AB, Atlas M, Ewy GA, et al. Expired PCO2 as an index of coronary perfusion pressure. Am J Emerg Med. 1985;3(2):147–149. doi: 10.1016/0735-6757(85)90039-7. [DOI] [PubMed] [Google Scholar]
- 89.Gudipati CV, Weil MH, Bisera J, et al. Expired carbon dioxide: a noninvasive monitor of cardiopulmonary resuscitation. Circulation. 1988;77(1):234–239. doi: 10.1161/01.cir.77.1.234. [DOI] [PubMed] [Google Scholar]
- 90.Kalenda Z. The capnogram as a guide to the efficacy of cardiac massage. Resuscitation. 1978;6(4):259–263. doi: 10.1016/0300-9572(78)90006-0. [DOI] [PubMed] [Google Scholar]
- 91.Christenson J, Andrusiek D, Everson-Stewart S, et al. Chest compression fraction determines survival in patients with out-of-hospital ventricular fibrillation. Circulation. 2009;120(13):1241–1247. doi: 10.1161/CIRCULATIONAHA.109.852202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kellum MJ, Kennedy KW, Ewy GA. Cardiocerebral resuscitation improves survival of patients with out-of-hospital cardiac arrest. Am J Med. 2006;119(4):335–340. doi: 10.1016/j.amjmed.2005.11.014. [DOI] [PubMed] [Google Scholar]
- 93.Kellum MJ, Kennedy KW, Barney R, et al. Cardiocerebral resuscitation improves neurologically intact survival of patients with out-of-hospital cardiac arrest. Ann Emerg Med. 2008;52(3):244–252. doi: 10.1016/j.annemergmed.2008.02.006. [DOI] [PubMed] [Google Scholar]
- 94.Bobrow BJ, Clark L, Ewy GA, et al. Minimally interrupted cardiac resuscitation by emergency medical services for out-of-hospital cardiac arrest. JAMA. 2008;299(10):1158–1165. doi: 10.1001/jama.299.10.1158. [DOI] [PubMed] [Google Scholar]
- 95.Garza AG, Gratton MC, Salomone JA, et al. Improved patient survival using a modified resuscitation protocol for out-of-hospital cardiac arrest. Circulation. 2009;119(19):2597–2605. doi: 10.1161/CIRCULATIONAHA.108.815621. [DOI] [PubMed] [Google Scholar]
- 96.Gilmore CM, Rea TD, Becker LJ, et al. Three-phase model of cardiac arrest: time-dependent benefit of bystander cardiopulmonary resuscitation. Am J Cardiol. 2006;98(4):497–499. doi: 10.1016/j.amjcard.2006.02.055. [DOI] [PubMed] [Google Scholar]
- 97.Bohm K, Rosenqvist M, Herlitz J, et al. Survival is similar after standard treatment and chest compression only in out-of-hospital bystander cardiopulmonary resuscitation. Circulation. 2007;116(25):2908–2912. doi: 10.1161/CIRCULATIONAHA.107.710194. [DOI] [PubMed] [Google Scholar]
- 98.SOS-KANTO study group. Cardiopulmonary resuscitation by bystanders with chest compression only (SOS-KANTO): an observational study. Lancet. 2007;369(9565):920–926. doi: 10.1016/S0140-6736(07)60451-6. [DOI] [PubMed] [Google Scholar]
- 99.Hupfl M, Selig HF, Nagele P. Chest-compression-only versus standard cardiopulmonary resuscitation: a meta-analysis. Lancet. 2010;376(9752):1552–1557. doi: 10.1016/S0140-6736(10)61454-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Dickinson ET, Verdile VP, Schneider RM, et al. Effectiveness of mechanical versus manual chest compressions in out-of-hospital cardiac arrest resuscitation: a pilot study. Am J Emerg Med. 1998;16(3):289–292. doi: 10.1016/s0735-6757(98)90105-x. [DOI] [PubMed] [Google Scholar]
- 101.Ward KR, Menegazzi JJ, Zelenak RR, et al. A comparison of chest compressions between mechanical and manual CPR by monitoring end-tidal PCO2 during human cardiac arrest. Ann Emerg Med. 1993;22(4):669–674. doi: 10.1016/s0196-0644(05)81845-1. [DOI] [PubMed] [Google Scholar]
- 102.Casner M, Andersen D, Isaacs SM. The impact of a new CPR assist device on rate of return of spontaneous circulation in out-of-hospital cardiac arrest. Prehosp Emerg Care. 2005;9(1):61–67. doi: 10.1080/10903120590891714. [DOI] [PubMed] [Google Scholar]
- 103.Wang HC, Chiang WC, Chen SY, et al. Video-recording and time-motion analyses of manual versus mechanical cardiopulmonary resuscitation during ambulance transport. Resuscitation. 2007;74(3):453–460. doi: 10.1016/j.resuscitation.2007.01.018. [DOI] [PubMed] [Google Scholar]
- 104.Troiani TA, Boland RT. Critical incident stress debriefing: keeping your flight crew healthy. J Air Med Transp. 1992;11(10):21–24. doi: 10.1016/s1046-9095(05)80202-8. [DOI] [PubMed] [Google Scholar]
- 105.FitzGerald ML, Braudaway CA, Leeks D, et al. Debriefing: a therapeutic intervention. Mil Med. 1993;158(8):542–545. [PubMed] [Google Scholar]
- 106.Samter J, Fitzgerald ML, Braudaway CA, et al. Debriefing: from military origin to therapeutic application. J Psychosoc Nurs Ment Health Serv. 1993;31(2):23–27. doi: 10.3928/0279-3695-19930201-09. [DOI] [PubMed] [Google Scholar]
- 107.Mitchell AM, Sakraida TJ, Kameg K. Critical incident stress debriefing: implications for best practice. Disaster Manag Response. 2003;1(2):46–51. doi: 10.1016/s1540-2487(03)00008-7. [DOI] [PubMed] [Google Scholar]
- 108.Ireland S, Gilchrist J, Maconochie I. Debriefing after failed paediatric resuscitation: a survey of current UK practice. Emerg Med J. 2008;25(6):328–330. doi: 10.1136/emj.2007.048942. [DOI] [PubMed] [Google Scholar]
- 109.Dine CJ, Gersh RE, Leary M, et al. Improving cardiopulmonary resuscitation quality and resuscitation training by combining audiovisual feedback and debriefing. Crit Care Med. 2008;36(10):2817–2822. doi: 10.1097/CCM.0b013e318186fe37. [DOI] [PubMed] [Google Scholar]
- 110.Olasveengen TM, Tomlinson AE, Wik L, et al. A failed attempt to improve quality of out-of-hospital CPR through performance evaluation. Prehosp Emerg Care. 2007;11(4):427–433. doi: 10.1080/10903120701536628. [DOI] [PubMed] [Google Scholar]
- 111.Lick CJ, Aufderheide TP, Niskanen RA, et al. Take Heart America: a comprehensive, community-wide, systems-based approach to the treatment of cardiac arrest. Crit Care Med. 2011;39(1):26–33. doi: 10.1097/CCM.0b013e3181fa7ce4. [DOI] [PubMed] [Google Scholar]
- 112.Kouwenhoven WB, Jude JR, Knickerbocker GG. Closed-chest cardiac massage. JAMA. 1960;173:1064–1067. doi: 10.1001/jama.1960.03020280004002. [DOI] [PubMed] [Google Scholar]