Abstract
We identified a series of immunodominant and subdominant epitopes from α fetoprotein (AFP), restricted by HLA-A*0201, which are recognized by the human T cell repertoire. The four immunodominant epitopes have been tested for immunogenicity in vivo, in HLA-A*0201+AFP+ advanced stage hepatocellular cancer (HCC) patients, and have activated and expanded AFP-specific IFN-γ-producing T cells in these patients, despite high serum levels of this self Ag. Here, we have examined the frequency, function, and avidity of the T cells specific for subdominant epitopes from AFP. We find that T cells specific for several of these epitopes are of similar or higher avidity than those specific for immunodominant epitopes. We then tested the peripheral blood of subjects ex vivo with different levels of serum AFP for the hierarchy of response to epitopes from this Ag and find that HCC patients have detectable frequencies of circulating IFN-γ-producing AFP-specific CD8+ T cells to both immunodominant and subdominant epitopes. We find the immunodominant and subdominant peptide-specific T cells to be differentially expanded with different modes of Ag presentation. Whereas spontaneous and AFP protein-stimulated responses show evidence for immunodominance, AdVhAFP-transduced dendritic cell-stimulated responses were broader and not skewed. Importantly, these data identify subdominant epitopes from AFP that can activate high-avidity T cells, and that can be detected and expanded in HCC subjects. These subdominant epitope-specific T cells can also recognize tumor cells and may be important therapeutically.
Hepatocellular carcinoma (HCC)4 is one of the main causes of cancer deaths with a global incidence of over 500,000 new cases per year. Once diagnosed, HCC has no effective systemic therapy and an overall 6% probability of 5-year survival. We are investigating the use of α fetoprotein (AFP) as a T cell-mediated tumor rejection Ag for HCC. We identified HLA-A*0201-restricted peptide epitopes that were immunogenic and naturally processed and presented, and then confirmed that three of them were present on the surface of HLAA*0201+AFP+ HCC cells by mass spectrometry (1–4). Four of the peptides were designated as “immunodominant” based on the finding that they showed the strongest and most reproducible IFN-γ production and cytotoxic function in vitro in T cell cultures from healthy donor cells (with either adenovirus human AFP (AdVhAFP)-transduced DC stimulation or peptide-pulsed PBMC stimulation). The same four peptides were identified as immunodominant based on immunization of HLA-A*0201 transgenic mice, although the exact order of immunodominance was not identical (2). In the majority of healthy donor T cell cultures, AFP542 was immunodominant to AFP158, and these were followed by AFP325 and AFP137. In the HLA-A*0201 transgenic mice, the order was AFP158, AFP542, AFP325, and AFP137. Subsequent murine studies, in which different modes of immunization (plasmid DNA, peptides in adjuvant, AdV) were compared with IFN-γ ELISPOT readouts, indicated that, although AFP158 remained most immunodominant, the immunodominance order varied with immunization strategy for the other three peptides (3). This was not unexpected, because each method of immunization uses different AFP Ag presentation modes. In addition, the binding of each peptide to MHC class I has different stabilization and kinetic properties (2).
Based on the preclinical data, six AFP+HLA-A*0201+ subjects were treated with four immunodominant AFP-derived peptides emulsified in Montanide, and we found that after vaccination, the frequency of circulating AFP-specific T cells increased (by MHC tetramer assay) and the frequency of IFN-γ-producing AFP-specific T cells also increased (by ELISPOT assay) (5). However, responses to AFP158 and AFP542 no longer appeared immunodominant in this cohort of patients. We recently completed treatment of 10 AFP+HLA-A*0201+ HCC subjects with AFP peptide-pulsed autologous dendritic cells (DC). As in the pilot peptide/Montanide trial, we have seen increased frequencies of circulating AFP-specific T cells and of IFN-γ-producing AFP-specific T cells after vaccination (18). In this trial, none of the four immunizing peptides appears to be immunodominant to any other.
When we originally screened candidate AFP epitopes, we identified 10 subdominant epitopes in addition to the 4 immunodominant (Table I). The responses to these epitopes have not been studied further. As suggested by others (6, 7), responses to subdominant epitopes might be more clinically relevant due to reduced effects of central and peripheral tolerance on such T cells. The effects of fetal Ag expression and the presence of tumor-derived Ag presence in serum on hierarchy are unknown. Here, we have investigated the avidity of subdominant epitope-specific T cells for peptide-pulsed and tumor targets and the hierarchy of CD8 T cell responses to the previously identified immunodominant and subdominant epitopes in subjects with different levels of high serum AFP. We wanted to determine whether HCC subjects have altered hierarchy of responses to this self tumor associated Ag, and more importantly, to determine whether “subdominant” epitopes could be more potent immunogens in these subjects and be the focus of future immunotherapy vaccines.
Table I.
Dominant and subdominant peptidesa
| Amino Acid Start | Length | Sequence | Anchors | Designation |
|---|---|---|---|---|
| 1 | 9 | MKWVESIFL | 1 | Subdominant |
| 137 | 9 | PLFQVPEPV | 2 | Immunodominant |
| 158 | 9 | FMNKFIYEI | 2 | Immunodominant |
| 178 | 9 | ILLWAARYD | 1 | Subdominant |
| 218 | 9 | LLNQHACAV | 2 | Subdominant |
| 235 | 9 | FQAITVTKL | 1 | Subdominant |
| 306 | 10 | TTLERGQCII | 1 | Subdominant |
| 325 | 10 | GLSPNLNRFL | 2 | Immunodominant |
| 485 | 9 | CIRHEMTPV | 2 | Subdominant |
| 492 | 9 | PVNPGVGQC | 1 | Subdominant |
| 507 | 10 | NRRPCFSSLV | 1 | Subdominant |
| 542 | 9 | GVALQTMKQ | 1 | Immunodominant |
| 547 | 10 | TMKQEFLINL | 2 | Subdominant |
| 555 | 9 | NLVKQKPQI | 2 | Subdominant |
| 549 | 9 | KQEFLINLV | 1 | Cryptic |
Shown are the starting amino acid for the peptide, the total length in amino acids, the peptide sequence, the number of anchor residues for HLA-A*0201, and the designation as immunodominant, subdominant, or cryptic/negative from the original peptide description (2). Data on immunodominant peptides are set in bold type.
Materials and Methods
HCC patients and healthy donors
All donors were HLA-A*0201 positive according to standard HLA serotyping and/or genotyping procedures. Informed consent for participating in this study was obtained from all donors (HCC patients, UCLA IRB no. 00-01-026; healthy donors, UPCI no. 04-001). The donors include six HCC patients and two healthy donors. The HCC patient characteristics are shown in Table II.
Table II.
HCC patient characteristicsa
| Patient ID | Age | Sex | Race | Risk Factor | Stage | Previous Treatments | Sites of Disease | Serum AFP Concentration (ng/ml) |
|---|---|---|---|---|---|---|---|---|
| A3 | 69 | M | Caucasian | Alcohol | IVa | RFA | Liver | 2,705 |
| A4 | 62 | M | Asian | HCV | IVb | None | Liver | 10,800 |
| B1 | 67 | M | Caucasian | HBV | IVb | None | Liver Lung | 77,000 |
| B10 | 59 | M | Caucasian | HCV | IVa | RFA | Liver | 463,040 |
| B11 | 60 | M | Caucasian | Porphyria | IVa | Chemoembo | Liver | 4,340 |
| B12 | 52 | M | Caucasian | Unknown | III | Chemoembo RFA | Liver | 74 |
Shown are the characteristics of the HCC patients whose cells were used in this study, including the patient identification code, age at enrollment, sex, race, HCC risk factor, stage of disease, HCC treatments received prior to enrollment (radiofrequency ablation (RFA); chemoembolization (chemoembo)), sites of HCC, and the level of serum AFP.
HCV, hepatitis C virus; HBV, hepatitis B virus.
Cell lines
The human CML cell line K562 stably transfected with HLA-A*0201 (K562/A2.1) was generously provided by W. Herr (Mainz, Germany) and the HLA-A2+ AFP-expressing HCC cell line HepG2 was obtained from American Type Culture Collection.
Generation of DC and DC culture
DC were prepared as described (8, 9) with some modifications. Peripheral blood was drawn by venous puncture or leukapheresis, and lymphocytes were purified by Ficoll (Pharmacia) gradient separation. Mononuclear cells (3–4 × 107) were plated in T-25 flasks (Costar) in RPMI 1640/penicillin-streptomycin-Fungizone/5–10% human AB serum for 2 h at 37°C in a humidified CO2 incubator. The nonadherent cells were removed by gentle rinsing with PBS, and the loosely adherent cells were cultured in medium with 800 U/ml GM-CSF (Immunex) and 500 U/ml IL-4 (R&D Systems) for 7 days. The nonadherent and loosely adherent DC were harvested by vigorous washing. These cells generally consisted of 30–50% DC as assessed by morphology and phenotyping. No further maturation treatments were performed to avoid potential Th1/Th2 skewing of T cell responses (10).
CTL generation from peptide-pulsed and AdV-transduced DCs
Peptide-specific CTL were generated using a protocol by Plebanski (1, 11, 12). Briefly, DC from HLA-A*0201 donors were pulsed with AFP peptides (prepared by the peptide synthesis facility at the University of Pittsburgh Medical Center) at 10 μg/ml peptide, in serum-free RPMI 1640, at room temperature for 120 min. The DC were centrifuged and counted, and then plated in wells of a 24-well plate at a 1:20 ratio with autologous nonadherent PBMC in 5–10% AB serum/RPMI 1640/penicillin-streptomycin-Fungizone with 10 ng/ml IL-7 (Biosource) for 1 wk, and supplemented with IL-2 (Hoffman-La Roche) at 10 U/ml every 3–4 days. After 1-wk culture, the nonadherent CTL were counted and restimulated weekly with fresh or thawed DC pulsed with the same peptide. After two restimulations, the CTL were harvested and the CD8+ cells were isolated and tested.
DC (prepared as described from PBMC incubated with GM-CSF and IL-4) were transduced with AdVhAFP at a multiplicity of infection of 1000 for 2 h in RPMI 1640/2% AB. Transduced DC were washed, and plated at 1–2 × 105 cells/ml to serve as stimulators for PBMC. CTL were restimulated weekly with fresh, autologous AdV-transduced DC at a ratio of 1 DC to 20 CTL. CTL were plated with the transduced DC at 2 × 106 cells/ml in 10% AB medium plus IL-7 at 10–25 ng/ml. Cultures were supplemented with IL-2 at 10 U/ml every 3–4 days. For the experiments presented, cells were harvested after 7 or 21 days of culture.
Selection of CD8+ lymphocytes from CTL
Before assays, the CD8+ T cells were positively isolated with the use of immunomagnetic beads following the manufacturer's instructions (Miltenyi Biotec). Resulting cell populations were >98% CD8-positive according to FACS analysis.
IFN-γ ELISPOT assay
Multiscreen HA plates (Millipore) were coated with 4 μg/ml mAb anti-human IFN-γ (BD Biosciences) in PBS overnight at 4°C. Unbound Ab was removed by three washings with PBS. After blocking the plates with PBS/10% FBS (2 h, 37°C), CD8 T cells were seeded at 105 cells/well in duplicate wells. K562/A2.1 cells (1 × 105 well) were pulsed with different AFP peptides, rinsed, and seeded. Control wells contained CD8+ T cells (alone or with 10–100 μg/ml PHA), CD8+ T cells in the presence of unloaded APC (“no peptide”) or negative (cryptic) peptide (AFP549)-pulsed APC. Culture medium was X-VIVO medium (Cambrex) at a final volume of 200 μl/well. Cells were incubated at 37°C in 5% CO2 in a humidified atmosphere. After a culture period of 20–24 h, cells were removed by six washings with PBS/0.05% Tween 20 (PBS/T). Captured cytokine was detected by incubation for 2 h at 37°C with biotinylated mAb anti-hIFN-γ (BD Biosciences) at 2 μg/ml in PBS/0.5% BSA. After washing the wells three times with PBS/T and three times with PBS at room temperature, avidin peroxidase complex (1/100; Vectastain Elite kit; Vector) was added for 1 h at room temperature. Unbound complex was removed by three successive washings with PBS/T and three with PBS alone. Peroxidase staining was performed with 3-amino-9-ethyl-carbazole (AEC; Sigma-Aldrich) and stopped by rinsing the plates under warm running tap water. Spot numbers were both manually and automatically determined with the imaging system from Cellular Technology. To calculate the number of CD8+ T cells responding to a particular peptide, the mean numbers of spots with APC alone were subtracted from mean spot numbers induced by peptide-loaded APC. PHA-stimulated CD8+ cells often yielded >200 spots per 105 cells and are listed as “200” so that lower frequency spots can be more clearly shown. PHA-stimulated spots with fewer than 200 spots per 105 cells are labeled with the actual spot numbers counted.
Peptide synthesis
Peptides were synthesized at the University of Pittsburgh Peptide Synthesis Facility using standard f-moc technology. Stock solutions were prepared in DMSO (10 mg/ml) and were kept at –80°C until use.
Statistical analysis
For statistical evaluation, two-sided Wilcoxon tests, stratified by subject, was used. Values of p < 0.05 were considered significant.
Results
As we showed previously, healthy donor DC that are engineered to express the entire AFP Ag activate IFN-γ-producing CD8+ T cells specific for a variety of AFP-derived peptides. These T cells expanded during the in vitro culture period (3 wk) at expected frequencies with some T cells at higher frequencies being dominant in the culture (immunodominant), whereas others were reproducibly detected, but at lower frequencies (subdominant). We obtained similar data with AdVhAFP-transduced DC immunization of HLA*0201/Kb transgenic mice (2). Despite different physical characteristics (peptide length, number of anchor residues, binding affinity, off-kinetics; Ref. 2; Table I), the same four immunodominant peptides were generally seen, whether the healthy donor was of human or murine origin.
Responses in healthy donors
To reconfirm previous data, we tested 3-wk AdVhAFP/DC expanded CD8 T cells from healthy donors by IFN-γ ELISPOT for reactivity to 15 AFP peptides (Table I), and found the expected immunodominant, subdominant, and negative-peptide responses (Fig. 1A). The immunodominant peptide responses (for the two healthy donors tested) were statistically significantly higher then the subdominant peptide responses ( p < 0.001). To look more closely at the issue of immunodominance and to reduce the effects of culture expansion, we also tested healthy donor cells in a direct ex vivo ELISPOT as well as after only 7 days of in vitro stimulation (IVS). For 7-day IVS, we chose two methods of Ag presentation. First, AFP protein-fed GM-CSF/IL-4 DC (not matured), which would approximate AFP-Ag presentation in HCC patients with high levels of serum AFP in whom immature DC have taken up circulating protein. Second, AdVhAFP-transduced DC (AdVhAFP/DC) were used, which allows for longer AFP Ag processing and presentation on a more mature DC (9, 13).
FIGURE 1.
Hierarchy of responses to AFP peptides in healthy donors. A, PBMC were cultured for 3 wk with autologous DC transduced with AdVhAFP and tested for peptide epitope recognition by IFN-γ ELISPOT. A representative experiment from one donor is shown. Responses to immunodominant epitopes AFP137, AFP158, AFP325, and AFP542 (left side) exceed those to subdominant epitopes (p < 0.001; two donors pooled). Response to PHA serves as a positive control; no spots were detected to K562/A2.1 cells (which presented peptides), and background spots to K562/A2.1 cells without peptide (“no peptide”) were low and were subtracted. B, PBMC were tested directly ex vivo, or after 7 days of IVS with AFP protein-fed DC or AdVhAFP-transduced DC in an IFN-γ ELISPOT for peptide epitope recognition. A representative experiment from one donor is shown. Responses to immunodominant epitopes (left side) exceed those to subdominant epitopes (p < 0.001, two donors pooled), and responses to AFP presented by AdVhAFP-transduced DC exceed those in response to AFP protein-fed DC. The same donor is shown in A and B to allow direct comparison between conditions.
These data (Fig. 1B) show that, whereas 7 days of AdVhAFP/DC stimulation begins to preferentially expand the four immunodominant peptide-specific T cells ( p < 0.001 for two donors combined), immunodominance after 7 days of protein-fed DC is less obvious (and less stimulatory in general) but significant ( p < 0.001 for two donors combined). Few spots were detected in direct ex vivo analysis but the relative immunodominance was still detected ( p < 0.001 for two donors combined).
Subdominant epitopes and avidity
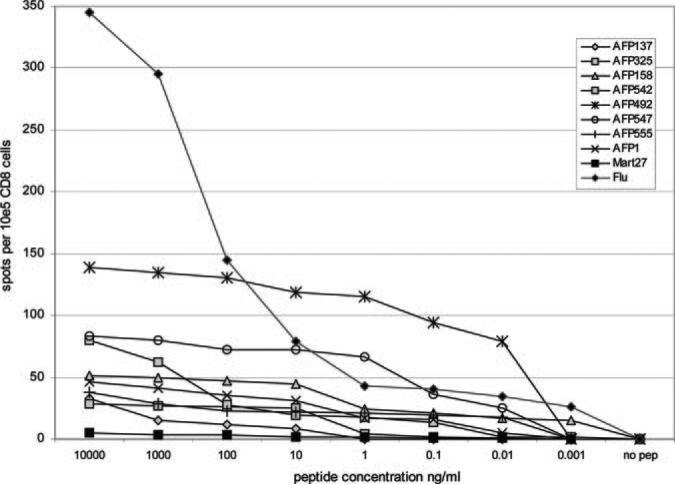
Higher avidity T cells have been shown to exhibit superior in vitro and in vivo efficacy against tumor (14). It might be expected that two-anchor, high affinity peptides might be best equipped to expand T cells that exhibit the highest avidity. Conversely, there have been studies that suggest that T cells specific for less effectively presented subdominant self Ag peptides might be of higher avidity due to reduced effects of tolerance. In this model, T cells with the highest avidity for the immunodominant epitopes would be most likely to be deleted, whereas subdominant epitopes specific for less well-presented peptides would be less affected. To examine this question in healthy donor cells (presumably exposed to AFP only during fetal development), we expanded peptide-specific CD8 T cells (cultured for 3 wk) and tested their avidity to decreasing concentration of peptide-pulsed targets. We found that several of these subdominant peptides (AFP1, AFP492, AFP547, and AFP555) have avidity that is equal to or greater than that observed for the immunodominant peptides AFP137, AFP158, and AFP325 (Fig. 2). As expected, the positive control viral peptide Flu M158 – 66 specific T cells recognize peptide concentrations <0.01 ng/ml, whereas the one-anchor, poorly binding, self peptide MART-127–35 requires peptide concentrations of ≥10 μg/ml for recognition. We tested each immunodominant and subdominant epitope at least twice and the resultant data also show that subdominant peptides AFP218, AFP306, and AFP507 are less avid than AFP137 and AFP325 (requiring >10 ng/ml), AFP178 requires >100 ng/ml, and AFP235 and AFP542 are similar to MART-127–35 (≥10 μg/ml needed to detect responses).
FIGURE 2.
Avidity of AFP peptide-specific CD8 T cells for peptide-pulsed targets. AFP peptide-specific T cells were expanded with 3 wk of culture with peptide-pulsed autologous DC. The T cells were plated in the IFN-γ ELISPOT with decreasing doses of peptide pulsed onto K562/A2.1 cells to determine the lowest amount of peptide required for IFN-γ production. Data are shown for the four immunodominant peptides and four subdominant peptides, as well as the positive control Flu M1 and negative control MART-1 peptide; cell cultures were from healthy donors.
Together, these data show that healthy donors can expand T cells specific for subdominant AFP peptides at a range of avidities and that several subdominant epitopes (AFP1, AFP492, AFP547, AFP555) can be recognized at >10 pg/ml, similar to immunodominant peptide AFP158. Therefore, healthy donors have high-avidity T cell precursors to these subdominant peptides, which can be activated and expanded by Ag-bearing DC.
Hierarchy of peptide responses in HCC patients
We have tested the four immunodominant peptides in two HCC immunotherapy clinical trials (5, 18). In the first trial, we found that these peptides, delivered emulsified in Montanide adjuvant, were immunogenic and able to stimulate the expansion (by MHC tetramer) and activation (by IFN-γ ELISPOT) of AFP-specific T cells (5). This was somewhat unexpected, as HCC patients have high serum levels of this protein, up to 1 mg/ml, which might lead to tolerance of the circulating AFP-specific T cells. The subjects in the first trial had an average AFP level of 25,517 ng/ml (range, 162–105,617 ng/ml). Similarly, in the second trial, when these same four peptides were delivered on autologous DC, we observed expansion of and cytokine production by AFP-specific T cells (18). These vaccinated subjects had AFP levels averaging 2,469 ng/ml (range, 96–6,310 ng/ml). We also performed a more in-depth analysis of these CD8 T cells and found that, prevaccine, they were of a naive and/or central memory phenotype, were capable of limited, partial differentiation postvaccination, and made IFN-γ, but not other cytokines tested (L. H. Butterfield, A. Ribas, D. M. Potter, and J. S. Economou, submitted for publication). We did not detect AFP-specific CD4 T cells in these subjects when CD4 T cells were restimulated with autologous DC pulsed with purified AFP protein.
Here, we wanted to determine whether high levels of circulating, tumor-derived AFP influenced the hierarchy of AFP-specific T cells. We assessed the hierarchy of response to the immunodominant and subdominant epitopes in several HCC subjects with high serum AFP. Study of several patients should also demonstrate whether the hierarchy of response is consistent across subjects, which might point to specific subdominant epitopes for immunization. The patient characteristics are shown in Table II. They were advanced stage III-IVa, were all HLA-A2.1+, and had levels of serum AFP ranging from 74 to 463,040 ng/ml (average, 92,993).
Ex vivo direct responses to AFP peptides
We first used unstimulated PBMC to determine whether the AFP responses could be directly detected without the potential skewing from in vitro culture and expansion. Examples of these data for individual patients are shown in Fig. 3, A and B. Patient A3 (Fig. 3A) had low level, but detectable CD8+ T cells (10/105 CD8+ to 90/105 CD8+ cells) specific for many AFP peptides. Notably, three of the four highest frequency T cells were specific for subdominant peptides. In patient B1 (Fig. 3B), the responses were lower frequency (maximum 24/105) and specific for fewer peptides. T cells were detected to two immunodominant (AFP137 and AFP542) and two subdominant peptides (AFP1 and AFP306). Overall, six HCC patients were tested for the hierarchy of responses to the AFP peptides, and the statistical analysis of these data is summarized in Fig. 3C. This demonstrates that HCC patients have a broad range of AFP-specific T cells that are at a higher frequency than healthy donors. Interestingly, when responses to the 4 immunodominant peptides were compared with those to the other 11 peptides, patient T cells were in fact skewed toward the immunodominant peptides ( p = 0.023), but the specific hierarchy is not the same across patients.
FIGURE 3.
Hierarchy of HCC patient responses to AFP peptides ex vivo. A, The hierarchy of response to AFP-derived peptides is shown for HCC patient A3. PBMC were thawed, and separated CD8 cells were plated directly in the IFN-γ ELISPOT with K562/A2.1 cells pulsed with individual peptides to determine the frequencies of peptide-specific T cells. T cells were detected to most of the peptides, with the highest frequency responses to AFP1, AFP218, and AFP158. B, The hierarchy of response to AFP-derived peptides is shown for HCC patient B1. Cells were plated as above and the only responses were to epitopes AFP137, AFP1, AFP306, and AFP542. C, The median frequencies to each AFP peptide, for all six HCC patients (A3, A4, B1, B10, B11, B12) are shown; error bars indicate the first and fourth quartiles. The two-sided Wilcoxon test shows that, together, the six HCC patients have higher frequencies of T cells to the four immunodominant peptides, compared with the sum of the subdominant peptide frequencies (p = 0.023).
AFP protein stimulated responses
We then investigated the function of these T cells after a single 7-day IVS with AFP protein-fed autologous DC. This culture condition simulates the natural mode of AFP Ag presentation: soluble Ag taken up, processed, and presented by DC that have not undergone maturation or been otherwise activated. Examples of these data are shown in Fig. 4, A and B. Patient A3 (Fig. 4A) showed increases in the frequency of several AFP peptide-specific T cells compared with ex vivo analysis, as expected. The immunodominant peptides were among those that expanded best. In contrast, B1 (Fig. 4B) showed substantial increases in both immunodominant and subdominant peptides. The statistical analysis of the data with 7-day IVS for all six HCC patients with protein-fed DC is shown in Fig. 4C. As expected, the IVS allowed for expansion of many T cell specificities. When the 4 immunodominant peptides were compared with the 11 others across all six patients, the frequencies of these cells were again skewed toward immunodominant peptides ( p = 0.0072).
FIGURE 4.
Hierarchy of HCC patient responses to AFP peptides after 7 days of IVS with AFP protein-fed DC. A, The hierarchy of response to AFP-derived peptides is shown for HCC patient A3. PBMC were cultured for 7 days, and separated CD8 cells were plated directly in the IFN-γ ELISPOT with K562/A2.1 cells pulsed with individual peptides to determine the frequencies of peptide-specific T cells. T cells were detected to many of the peptides, with the highest frequency responses to AFP137, AFP158, and AFP235. B, The hierarchy of response to AFP-derived peptides is shown for HCC patient B1. Cells were plated as above, and the highest frequency responses were to subdominant epitopes AFP218, AFP306, AFP485, and AFP507. C, The median frequencies to each AFP peptide (after 7-day IVS) for all six HCC patients (A3, A4, B1, B10, B11, B12) are shown; error bars indicate the first and fourth quartiles. The two-sided Wilcoxon test shows that together, the six HCC patients have higher frequencies of T cells to the four immunodominant peptides, compared with the sum of the subdominant peptide frequencies, after 7-day IVS with protein-fed DC (p = 0.0072).
These data show that these subjects have a diverse repertoire of AFP-specific T cells capable of producing IFN-γ upon recognition of cognate peptide, and that many of the most potent responses in specific patients are to subdominant epitopes. These data also highlight the fact that Ag presentation by protein-fed immature DC leads to further skewing of the hierarchy toward the four immunodominant epitopes.
AdVhAFP transduced DC responses
We then wanted to examine the differences in T cell hierarchy and function when these cells undergo 7-day IVS stimulated by AdVhAFP Ag-engineered DC. This mode of Ag presentation results in high levels of Ag expression in DC as well as an intermediate level of DC phenotypic and functional maturation (13). This culture condition simulates the mode of Ag presentation for patients enrolled in a clinical trial of AdVhAFP-engineered DC (J. S. Economou and A. Ribas, unpublished data). Again, patient A3 is shown (Fig. 5A) after 7-day IVS with AdVhAFP-transduced DC, and responses to all tested dominant and subdominant were similarly increased. This is in contrast to what was seen in 7-day protein-fed DC stimulation and with direct ex vivo analysis, both of which were more focused. In Fig. 5B, patient B1 is shown, where the AdVhAFP-transduced DC stimulation also resulted in a strong expansion of T cells of many specificities. The statistical analysis of the data from all six patients is shown in Fig. 5C, which demonstrates that higher frequencies of T cells were observed for the peptides tested. Importantly, there was no significant difference between responses to immunodominant and subdominant epitopes ( p = 0.56) among these patients, indicating that Ag presentation via AdVhAFP engineered DC allows for stimulation of a broad range of T cell specificities without skewing of the hierarchy.
FIGURE 5.
Hierarchy of HCC patient responses to AFP peptides after 7 days of IVS with AdVhAFP-transduced DC. A, The hierarchy of response to AFP-derived peptides is shown for HCC patient A3. PBMC were cultured for 7 days, and separated CD8 cells were plated directly in the IFN-γ ELISPOT with K562/A2.1 cells pulsed with individual peptides to determine the frequencies of peptide-specific T cells. T cells were detected to all of the peptides, with similar high-frequency responses. B, The hierarchy of response to AFP-derived peptides is shown for HCC patient B1. Cells were plated as above, and the highest frequency responses were to epitopes AFP158, AFP507, AFP1, and AFP485. C, The median frequencies to each AFP peptide (after 7-day IVS) for all six HCC patients (A3, A4, B1, B10, B11, B12) are shown; error bars indicate the first and fourth quartiles. The two-sided Wilcoxon test shows that, together, the six HCC patients do not have higher frequencies of T cells to the four immunodominant peptides after 7-day IVS with AdVhAFP-transduced DC (p = 0.56).
Responses to an HCC tumor cell line
Although the studies described above have used peptide-pulsed tumor cell line targets (K562/A2.1) to investigate function, the most important endpoint of AFP T cell activation is the ability of the T cells to recognize and respond to AFP-expressing tumor. We therefore tested the AFP immunodominant and subdominant peptide-specific T cell cultures for reactivity to the HLA-A2.1+, AFP-expressing HCC cell line HepG2.
Fig. 6A shows a summary of responses to HepG2 by IFN-γ ELISPOT, showing the level of reactivity of different peptide-specific T cells to HepG2. Interestingly, the AFP542-specific T cells, which we previously showed exhibit low levels of MHC binding affinity, but a slow off-rate (2), and which we showed here do not stimulate high-avidity T cells for peptide-pulsed targets, nonetheless, had the ability to recognize HepG2 cells well. The subdominant peptide AFP178 also showed strong HepG2 reactivity, as did other immunodominant (AFP158) and subdominant peptides (AFP1). This demonstrates that several of the subdominant epitope specific T cells were capable of recognizing Ag-expressing tumor cells in addition to the immunodominant epitope-specific T cells.
FIGURE 6.
AFP T cell responses to the AFP-expressing, HLA-A2+ HCC cell line HepG2. A, Expanded AFP peptide-specific T cells (from healthy donor cultures shown in Fig. 2) were plated in the IFN-γ ELISPOT assays to determine the frequency of CD8 cells recognizing the HCC cell line HepG2, which endogenously produces AFP. Each AFP peptide-specific T cell culture recognized the tumor cells by producing IFN-γ. B, PBMC from each HCC patient were tested for recognition of HepG2 cells by ELISPOT. Data are shown for direct ex vivo analysis (from the experiments shown in Fig. 3), for 7-day IVS with protein-fed DC (“AFP/DC” (from the experiments shown in Fig. 4)), and for 7-day IVS with AdVhAFP-transduced DC (“AdVhAFP/DC” (from the experiments shown in Fig. 5)).
In the ex vivo and 7-day IVS studies with HCC patient cells, reactivity to HepG2 was also assessed (shown in Fig. 6B). These data show that brief activation with Ag-expressing DC can stimulate CD8+ T cells, which also recognize HCC cells, and that AdVhAFP-transduced DC were superior for activation in each of the six HCC patient cells tested.
Discussion
A critical aspect of T cell response activation is Ag presentation. Indeed, immunotherapy vaccination efforts are designed to provide adequate Ag presentation and to overcome the lack of presentation or inhibitory presentation provided by tumor. One might predict that constant exposure to high dose systemic Ag, the common situation in AFP-expressing HCC, would quickly lead to exhaustion and/or deletion of AFP-specific T cells. Our clinical immunotherapy trials have found this not to be the case. To optimally activate effective antitumor lymphocytes, we must characterize the natural state of immunity to the target Ag, and identify ways to activate the Ag-reactive cells present in patients.
Hierarchy of T cell responses can be influenced by many aspects of Ag presentation (15). These influences include the efficiency of Ag processing and presentation, the frequency and duration of Ag encounter, the nature of the APC, competition for Ag-bearing APC, and the available repertoire of responding T cells. In the HCC subjects here, the initiating event for HCC development varies (hepatitis B virus, hepatitis C virus, EtOH, etc.), as does the serum concentration of Ag and the duration of disease, and, thus, Ag exposure. In the context of immunotherapy of cancer, the cancer vaccine is delivered many years after the tumor-immune system interaction has begun; hence it is the available repertoire that must be assessed for potential tumor reactivity and tested for optimal modes of activation.
We have analyzed AFP responses by pulsing DC with synthetic class I peptides, pulsing with purified AFP protein or transducing with AdVhAFP. In patients, circulating soluble AFP may also be taken up by cells with AFP-specific cell surface receptors (up-regulated by the presence of AFP) on the surface of some hemopoietic cells (16). The immunological outcomes of these modes of Ag processing and presentation may be very different. Indeed, our short-term stimulation with protein-fed DC or Ag engineered DC, chosen to approximate tumor-derived circulating AFP taken up by resident immature DC or a candidate DC-based cancer vaccine, respectively, result in different levels and hierarchies of responses.
Our finding that spontaneous CD8 cell responses to AFP exist prevaccination (at a level detectable ex vivo by MHC tetramers) (5, 18) indicates that there is some level of endogenous presentation of AFP. The detection of IFN-γ production ex vivo by some of these T cells indicates that not all of these T cells are nonfunctional or Th2-skewed. Here, we have investigated responses to subdominant as well as immunodominant epitopes. Unexpectedly, we were able to detect expanded pools of T cells specific for the subdominant epitopes, capable of producing IFN-γ ex vivo, although overall, the immunodominant peptides were at higher frequencies. After 7-day IVS with AFP protein-fed DC, the frequencies of functional Th1 cells increased, as did skewing of the population toward immunodominant epitopes. It was 7-day IVS with the AdVhAFP-transduced DC that was capable of the strongest expansion and greatest breadth of T cell stimulation, with improved recognition of AFP-expressing HCC cells. This further supports Ag engineering of DC with AdV vectors to potently and broadly stimulate multiple T cell specificities.
Lastly, we show that healthy donors expand high-avidity CD8 T cells for a variety of AFP peptides including the two-anchor, strong binding immunodominant peptides AFP158, AFP137, and AFP325, as expected. We also demonstrate that several subdominant epitopes (AFP1, AFP492, AFP547, AFP555) can also be recognized at low peptide concentrations by T cells, indicating that they are capable of activating high-avidity T cells.
Together, we find that many subdominant epitopes might be more clinically relevant in HCC patients due to their frequency in the peripheral blood, ability to be activated to produce IFN-γ, and avidity relative to the immunodominant peptides. Vaccines based upon full-length Ag engineering should be more potent inducers of antitumor responses, due in part to the ability to harness these additional T cell specificities. In addition, identification of epitopes as immunodominant in many cases has been based on culture of healthy donor cells with Ag or by screening HLA-A2.1 transgenic mice (1, 2, 17). The data presented here suggest that hierarchy of response is not necessarily the same in patients exposed to tumor-derived Ag and that epitopes that are subdominant in healthy donors can be equivalent or superior to those identified as immunodominant, in cancer patients.
Footnotes
This work was supported by Scientist Development Grant from the American Heart Association (0330102N), the University of Pittsburgh Cancer Institute, and the Henry L. Hillman Foundation.
Abbreviations used in this paper: HCC, hepatocellular carcinoma; AFP, α fetoprotein; DC, dendritic cell; IVS, in vitro stimulation.
Disclosures
L. H. Butterfield is a coinventor on patents covering the AFP peptides used in this study. The University of California, Los Angeles, CA is also involved with these patents.
References
- 1.Butterfield LH, Koh A, Meng W, Vollmer CM, Ribas A, Dissette V, Lee E, Glaspy JA, McBride WH, Economou JS. Generation of human T-cell responses to an HLA-A2.1-restricted peptide epitope derived from α-fetoprotein. Cancer Res. 1999;59:3134–3142. [PubMed] [Google Scholar]
- 2.Butterfield LH, Meng WS, Koh A, Vollmer CM, Ribas A, Dissette VB, Faull K, Glaspy JA, McBride WH, Economou JS. T cell responses to HLA-A*0201-restricted peptides derived from human α fetoprotein. J. Immunol. 2001;166:5300–5538. doi: 10.4049/jimmunol.166.8.5300. [DOI] [PubMed] [Google Scholar]
- 3.Meng WS, Butterfield LH, Ribas A, Dissette VB, Heller JB, Miranda GA, Glaspy JA, McBride WH, Economou JS. α-Fetoprotein-specific tumor immunity induced by plasmid prime-adenovirus boost genetic vaccination. Cancer Res. 2001;61:8782–876. [PubMed] [Google Scholar]
- 4.Meng WS, Butterfield LH, Ribas A, Heller JB, Dissette VB, Glaspy JA, McBride WH, Economou JS. Fine specificity analysis of an HLA-A2.1-restricted immunodominant T cell epitope derived from human α-fetoprotein. Mol. Immunol. 2000;37:943–950. doi: 10.1016/s0161-5890(01)00017-7. [DOI] [PubMed] [Google Scholar]
- 5.Butterfield LH, Ribas A, Meng WS, Dissette VB, Amarnani S, Vu HT, Seja E, Todd K, Glaspy JA, McBride WH, Economou JS. T-cell responses to HLA-A*0201 immunodominant peptides derived from α-fetoprotein in patients with hepatocellular cancer. Clin. Cancer Res. 2003;9:5902–5908. [PubMed] [Google Scholar]
- 6.Gross DA, Graff-Dubois S, Opolon P, Cornet S, Alves P, Bennaceur-Griscelli A, Faure O, Guillaume P, Firat H, Chouaib S, et al. High vaccination efficiency of low-affinity epitopes in antitumor immunotherapy. J. Clin. Invest. 2004;113:425–433. doi: 10.1172/JCI19418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Otahal P, Hutchinson SC, Mylin LM, Tevethia MJ, Tevethia SS, Schell TD. Inefficient cross-presentation limits the CD8+ T cell response to a subdominant tumor antigen epitope. J. Immunol. 2005;175:700–712. doi: 10.4049/jimmunol.175.2.700. [DOI] [PubMed] [Google Scholar]
- 8.Romani N, Gruner S, Brang D, Kampgen E, Lenz A, Trockenbacher B, Konwalinka G, Fritsch PO, Steinman RM, Schuler G. Proliferating dendritic cell progenitors in human blood. J. Exp. Med. 1994;180:83–93. doi: 10.1084/jem.180.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arthur JF, Butterfield LH, Roth MD, Bui LA, Kiertscher SM, Lau R, Dubinett S, Glaspy J, McBride WH, Economou JS. A comparison of gene transfer methods in human dendritic cells. Cancer Gene Ther. 1997;4:17–25. [PubMed] [Google Scholar]
- 10.Tatsumi T, Kierstead LS, Ranieri E, Gesualdo L, Schena FP, Finke JH, Bukowski RM, Mueller-Berghaus J, Kirkwood JM, Kwok WW, Storkus WJ. Disease-associated bias in T helper type 1 (Th1)/Th2 CD4+ T cell responses against MAGE-6 in HLA-DRB10401+ patients with renal cell carcinoma or melanoma. J. Exp. Med. 2002;196:619–628. doi: 10.1084/jem.20012142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Butterfield LH, Jilani SM, Chakraborty NG, Bui LA, Ribas A, Dissette VB, Lau R, Gamradt SC, Glaspy JA, McBride WH, et al. Generation of melanoma-specific cytotoxic T lymphocytes by dendritic cells transduced with a MART-1 adenovirus. J. Immunol. 1998;161:5607–5613. [PubMed] [Google Scholar]
- 12.Plebanski M, Allsopp CE, Aidoo M, Reyburn H, Hill AV. Induction of peptide-specific primary cytotoxic T lymphocyte responses from human peripheral blood. Eur. J. Immunol. 1995;25:1783–1787. doi: 10.1002/eji.1830250645. [DOI] [PubMed] [Google Scholar]
- 13.Schumacher L, Ribas A, Dissette VB, McBride WH, Mukherji B, Economou JS, Butterfield LH. Human dendritic cell maturationby adenovirus transduction enhances tumor antigen-specific T-cell responses. J. Immunother. 2004;27:191–200. doi: 10.1097/00002371-200405000-00003. [DOI] [PubMed] [Google Scholar]
- 14.Zeh HJ, III, Perry-Lalley D, Dudley ME, Rosenberg SA, Yang JC. High avidity CTLs for two self-antigens demonstrate superior in vitro and in vivo antitumor efficacy. J. Immunol. 1999;162:989–994. [PubMed] [Google Scholar]
- 15.Yewdell JW, Bennink JR. Immunodominance in major histocompatibility complex class I-restricted T lymphocyte responses. Annu. Rev. Immunol. 1999;17:51–88. doi: 10.1146/annurev.immunol.17.1.51. [DOI] [PubMed] [Google Scholar]
- 16.Esteban C, Trojan J, Macho A, Mishal Z, Lafarge-Frayssinet C, Uriel J. Activation of an α-fetoprotein/receptor pathway in human normal and malignant peripheral blood mononuclear cells. Leukemia. 1993;7:1807–1816. [PubMed] [Google Scholar]
- 17.Lu J, Celis E. Recognition of prostate tumor cells by cytotoxic T lymphocytes specific for prostate-specific membrane antigen. Cancer Res. 2002;62:5807–5812. [PubMed] [Google Scholar]
- 18.Butterfield LH, Ribas A, Dissette VB, Lee Y, Yang JQ, De la Rocha P, Duran SD, Hernandez J, Seja E, Potter DM, et al. A phase I/II trial testing immunization of hepatocellular carcinoma patients with dendritic cells pulsed with four α-fetoprotein peptides. Clin. Cancer Res. 2006;12:2817–2825. doi: 10.1158/1078-0432.CCR-05-2856. [DOI] [PubMed] [Google Scholar]