Abstract
Large-conductance, voltage- and Ca2+-activated K+ (BK) channels display near linear current–voltage (I-V) plots for voltages between −100 and +100 mV, with an increasing sublinearity for more positive potentials. As is the case for many types of channels, BK channels are blocked at positive potentials by intracellular Ca2+ and Mg2+. This fast block progressively reduces single-channel conductance with increasing voltage, giving rise to a negative slope in the I-V plots beyond about +120 mV, depending on the concentration of the blockers. In contrast to these observations of pronounced differences in the magnitudes and shapes of I-V plots in the absence and presence of intracellular blockers, Schroeder and Hansen (2007. J. Gen. Physiol. http://dx.doi.org/10.1085/jgp.200709802) have reported identical I-V plots in the absence and presence of blockers for BK channels, with both plots having reduced conductance and negative slopes, as expected for blockers. Schroeder and Hansen included both Ca2+ and Mg2+ in the intracellular solution rather than a single blocker, and they also studied BK channels expressed from α plus β1 subunits, whereas most previous studies used only α subunits. Although it seems unlikely that these experimental differences would account for the differences in findings between previous studies and those of Schroeder and Hansen, we repeated the experiments using BK channels comprised of α plus β1 subunits with joint application of 2.5 mM Ca2+ plus 2.5 mM Mg2+, as Schroeder and Hansen did. In contrast to the findings of Schroeder and Hansen of identical I-V plots, we found marked differences in the single-channel I-V plots in the absence and presence of blockers. Consistent with previous studies, we found near linear I-V plots in the absence of blockers and greatly reduced currents and negative slopes in the presence of blockers. Hence, studies of conductance mechanisms for BK channels should exclude intracellular Ca2+/Mg2+, as they can reduce conductance and induce negative slopes.
INTRODUCTION
Large-conductance, voltage- and Ca2+-activated K+ (BK) channels are widely distributed and involved in many physiological processes (Pallotta et al., 1981; Golowasch et al., 1986; Cox et al., 1997a; Gribkoff et al., 1997; Vergara et al., 1998; Xia et al., 2002; Magleby, 2003; Cox, 2005; Cui, 2010; Latorre et al., 2010). Depending on the tissue, functional BK channels can be comprised of four pore-forming α subunits alone, or four α subunits plus accessory β subunits, with the β1 subunit increasing the apparent Ca2+ sensitivity of BK channels in smooth muscle (Knaus et al., 1995; McManus et al., 1995; Nimigean and Magleby, 1999; Lu et al., 2006). The conductance properties of BK channels have been of considerable interest because BK channels have the highest single-channel conductance of K+-selective channels, of 200–300 pS in symmetrical 150-mM K+ solutions (Pallotta et al., 1981; Eisenman et al., 1986; Cox et al., 1997b; Hille, 2001; Brelidze et al., 2003; Nimigean et al., 2003; Geng et al., 2011). Our study was undertaken to address contradictory observations concerning the shape of I-V curves for BK channels.
Schroeder and Hansen (2006, 2007, 2008) report that the single-channel current amplitudes of BK channels comprised of α plus β1 subunits increase as the membrane voltage is increased from 0 to +100 mV, and then progressively decrease with increasing voltage, leading to a negative slope in the I-V plots for large positive potentials. The intracellular solutions for the experiments in their papers all contained 2.5 mM Ca2+ and 2.5 mM Mg2+. As a control experiment to test for the possible effects of intracellular Ca2+ and Mg2+, Schroeder and Hansen (2007) obtained I-V plots in the absence of Ca2+ and Mg2+ and reported I-V plots with current amplitudes and negative slope identical to those they obtained in the presence of Ca2+ and Mg2+ (their Fig. 7 B).
Schroeder and Hansen’s (2007) observations of reduced currents and negative slope at positive potentials in the presence of intracellular Ca2+ and Mg2+ are consistent with previous studies on fast block by Ca2+ and Mg2+ in BK channels (Marty, 1983; Morales et al., 1996; Cox et al., 1997b; Zhang et al., 2006). However, Schroeder and Hansen’s (2007) observation of the same reduced currents and negative slopes in the absence and presence of intracellular Ca2+ and Mg2+ contrasts with many previous studies that show near linear I-V plots between −100 and +100 mV and a small sublinearity for more positive potentials, and this was the case for BK channels with and without β1 subunits (Marty, 1981; Wong et al., 1982; Yellen, 1984a,b; Villarroel et al., 1988; Ferguson, 1991; McManus et al., 1995; Morales et al., 1996; Zeng et al., 2003 [in supplemental material]; Brelidze and Magleby, 2004; Zhang et al., 2006; Carvacho et al., 2008; Geng et al., 2011). Nevertheless, in many of the previous studies, a sufficiently positive voltage range was not always explored to reveal a negative slope if present, and most studies were done in the absence of the β1 subunit.
Whether reduced currents and negative slope can be observed for BK channels in the absence of intracellular blockers has profound implications for the gating of BK channels. Schroeder and Hansen (2007) propose that the negative slope comes from fast gating, whereas previous studies propose that it arises from fast cation block. Resolving this question is important, as further studies have built upon the work of Schroeder and Hansen by assuming that the negative slope arises from fast gating rather than from Ca2+ and Mg2+ block (Schroeder and Hansen, 2008, 2009; Abenavoli et al., 2009; Nelson, 2011). In this Communication, we further examine the shape of the I-V plot for BK channels expressed with α plus β1 subunits. We find a near linear I-V plot at positive voltages in the absence of blockers and reduced currents and negative slope at more positive potentials with intracellular Ca2+ and Mg2+. Hence, studies of normal conductance mechanisms for BK channels should be based on data obtained in the absence of intracellular Ca2+/Mg2+, as these blockers can reduce conductance and induce negative slopes at positive potentials.
MATERIALS AND METHODS
The patch-clamp technique (Hamill et al., 1981) was used to record single-channel currents from inside-out patches of membrane obtained from Xenopus laevis oocytes expressing BK channels after injection of cRNAs coding for both α and β1 subunits. The mouse Slo1 α subunit (same as GenBank accession no. U09383) was provided by Merck Pharmaceuticals, and the β1 subunit (identical to NCBI Protein database accession no. gi:31981659) was provided by R. Brenner (The University of Texas Health Science Center at San Antonio, San Antonio, TX). The extracellular solution contained 150 mM KCl and 10 mM HEPES, with pH adjusted to 7.2 with KOH. The intracellular solution in the absence of Ca2+ and Mg2+ contained 150 mM KCl, 10 mM HEPES, and 10 mM EDTA, with pH adjusted to 7.2 with KOH. The intracellular solution with Ca2+ and Mg2+ contained 150 mM KCl, 10 mM HEPES, 2.5 mM CaCl2, and 2.5 mM MgCl2, with pH adjusted to 7.2 with KOH. The single-channel records were collected and analyzed with a cutoff frequency of 10 kHz (Axopatch 200B; Molecular Devices) and filtered at 5 kHz for display. Single-channel currents were sampled at 200,000/s with pClamp9 software (Molecular Devices). Single-channel current amplitudes were determined from all-points histograms as the distance between the peaks of histograms fitted to the open and closed current levels. Similar I-V plots were found when the single-channel currents were measured by eye with cursor lines through the open and closed current levels. Detailed descriptions of the methods have been presented previously (Nimigean and Magleby, 1999; Brelidze and Magleby, 2004).
RESULTS
To reexamine whether a negative slope is observed at large positive potentials in the absence of intracellular Ca2+ and Mg2+, we studied BK channels comprised of mouse α plus β1 subunits expressed in Xenopus oocytes using single-channel recording from excised inside-out patches of membrane so that the intracellular solution could be changed. Data were collected up to sufficiently high potentials (+200 mV) so that a negative slope would be observed if present.
Near linear I-V plots in the absence of intracellular blockers with a small increasing sublinearity at higher positive potentials
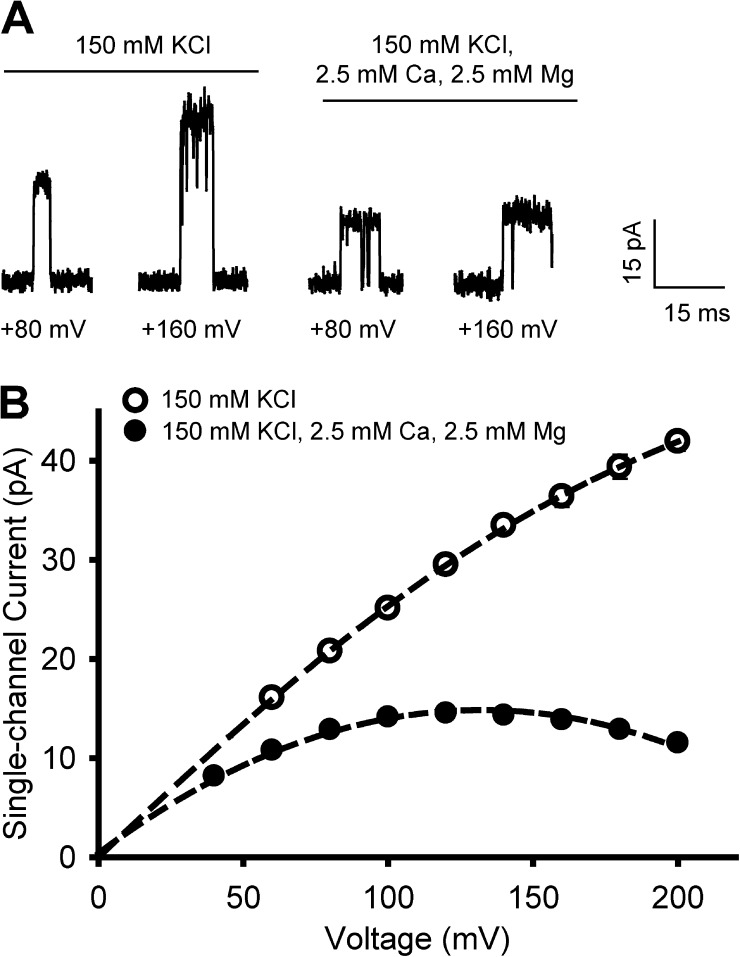
In the absence of intracellular blockers, a near linear I-V curve was observed for positive potentials up to +100 mV, with a small but increasing sublinearity for higher potentials up to +200 mV (Fig. 1 B, open circles). As is typical for BK channels, a large single-channel conductance (256 pS at +80 mV) was observed in the absence of blockers. These observations are consistent with those of Zeng et al., (2003, Fig. S1 C) who also studied BK channels comprised of α plus β1 subunits at high positive potentials in the absence of blockers. The observations in Fig. 1 B (open circles) are also consistent with other previous studies exploring a smaller range of positive potentials in the absence of blockers for BK channels comprised of α plus β1 subunits and of α subunits alone (Marty, 1981; Pallotta et al., 1981; Wong et al., 1982; Yellen, 1984a,b; Villarroel et al., 1988; Ferguson, 1991; McManus et al., 1995; Morales et al., 1996; Zeng et al., 2003 [in supplemental material]; Brelidze and Magleby, 2004; Zhang et al., 2006; Carvacho et al., 2008; Geng et al., 2011).
Figure 1.
Intracellular Ca2+ and Mg2+ induce a negative slope at high positive potentials for I-V plots from BK channels that is not observed in their absence. (A) Representative single-channel current records from BK channels at the indicated voltages without and with 2.5 mM Ca2+ and Mg2+ in the intracellular solution. The solutions also contained 150 mM KCl and 10 mM HEPES, pH 7.2. The presented current recordings were filtered at 5 kHz for display, but the data were collected and analyzed with 10 kHz low-pass filtering for the I-V plots. The divalent cation blockers reduce the outward single-channel current amplitudes, with a greater fractional decrease at +160 mV than at +80 mV. (B) I-V plots of single-channel current amplitudes indicate that 2.5 mM of intracellular Ca2+ and Mg2+ induce a negative slope for potentials greater than +120 mV. A negative slope is not observed in the absence of Ca2+ and Mg2+ (open circles). The dashed lines are cubic spline fits constrained to pass through the origin. Each plotted point is the mean from five or more patches. The absence of visible error bars indicates that the SEM is less than the symbol size.
The observations in Fig. 1 B (open circles) and previous studies of near linear I-V curves with a small sublinearity at more positive potentials in the absence of blockers differ from those of Schroeder and Hansen (2007) who report reduced conductance and negative slopes in the absence of blockers. It should be noted that in some cases with very low concentrations of intracellular blockers, the sublinearity can be greater than in Fig. 1 B (open circles), but, in contrast to Schroeder and Hansen (2007), there was not a marked reduction in conductance for voltages less than +100 mV (Cox et al., 1997b).
Schroeder and Hansen (2007) used less filtering in their analysis than we used for Fig. 1, or has typically been used in previous studies. However, this difference in filtering would not explain why they observed a negative slope of the observed mean current amplitude in the absence of Ca2+ and Mg2+ whereas we did not, as differences in filtering should not change the mean single-channel current amplitude (Fig. 9 in Blatz and Magleby, 1986). Schroeder and Hansen (2007) studied human BK channels stably expressed in HEK293 cells, whereas for most previous studies, BK channels were expressed from transfection with DNA or injected cRNA. It cannot be ruled out, but seems highly unlikely, that a different expression system would induce identical I-V plots with reduced conductance and negative slope both in the absence and presence of intracellular Ca2+ and Mg2+.
The intracellular blockers Ca2+ and Mg2+ reduce the single-channel conductance and induce a negative slope at positive potentials
Changing the intracellular solution in the previous experiment to one containing 2.5 mM Ca2+ and 2.5 mM Mg2+ reduced the single-channel currents in a voltage-dependent manner, giving rise to a reduced conductance at positive potentials and a negative slope in the I-V plots beyond about +120 mV (Fig. 1 B, closed circles). The reduction in conductance by the blockers was apparent well before the appearance of the negative slope, with a conductance at +80 mV of 161 pS compared with 259 pS in the absence of blockers. The reduced conductance and negative slope in the I-V plots in Fig. 1 B (closed circles) is a characteristic feature of intracellular block, as reported previously (Marty, 1983; Golowasch et al., 1986; Morales et al., 1996; Cox et al., 1997b; Zhang et al., 2006). The reduced conductance and negative slope in the presence of intracellular Ca2+ and Mg2+ in Fig. 1 B (closed circles) are essentially the same as reported by Schroeder and Hansen (2007) both in the presence and absence of intracellular blockers.
DISCUSSION
In contrast to the marked differences in I-V plots in the absence and presence of intracellular Ca2+ and Mg2+ in Fig. 1 and in the literature, Schroeder and Hansen (2007) have reported essentially identical I-V plots in the absence and presence of intracellular blockers, with both I-V plots displaying the same reduced conductance and negative slope as would be expected if intracellular blockers were present under both conditions. We are not aware of reports other than Schroeder and Hansen’s in which essentially identical I-V plots have been observed in the absence and presence of millimolar concentrations of intracellular Ca2+ and Mg2+ with 150 mM KCl in the solutions. High concentrations of intracellular K+ (added as KCl) can reduce, but not eliminate, the action of intracellular blockers through apparent competitive inhibition of K+ displacing the blockers (Ferguson, 1991; Zhang et al., 2006).
Schroeder and Hansen (2006, 2007, 2009) used a β-distribution analysis to examine very fast flickering in the single-channel current to characterize the negative slope in the presence of Ca2+ and Mg2+. They suggested that the negative slope may arise from the depletion of K+ from the selectivity filter at higher voltages, leading to instability and fast gating of the selectivity filter. An alternative explanation for the negative slope is that it may arise from fast Ca2+ and Mg2+ block, as a negative slope is not observed in the absence of these ions (Fig. 1 B, open circles). Support for such a possibility is the observation that the characteristic concentration and voltage dependence of Ca2+ and Mg2+ block, including the negative slope, are well described by quantitative blocking models (Zhang et al., 2006). Nevertheless, this does not establish the mechanism.
An electrostatic displacement of K+ from the inner cavity by cation blockers would reduce single-channel currents by reducing the K+ available to enter the selectivity filter. Such a reduction in K+ might then induce fast flickery gating, leading to a further decrease in current. If this were the case, Ca2+, Mg2+, and H+ block should be associated with increased open-channel noise. Such increased noise is not apparent with 5–10-kHz filtering when millimolar Ca2+ is added to the intracellular solution (Zhang et al., 2006) or when currents are reduced 40% from H+ block by changing the pH from 9 to 5 (Brelidze and Magleby, 2004). However, 5–10-kHz filtering could prevent detection of the very high frequency selectivity filter gating proposed by Schroeder and Hansen (2007). A detailed study of Ca2+ and Mg2+ block by high frequency β analysis may be able to resolve this question.
Schroeder and Hansen (2007) have performed such an analysis and found that the fractional reduction in current caused by very fast flickering is the same with and without divalent cations (their Fig. 7 C). Based on this observation, they rule out that the negative slope arises from Ca2+ and Mg2+ block. However, because the I-V plots in their data had identical reduced conductance and negative slope with and without blockers, in contrast to the observations of others and in Fig. 1, it seems that their data should not be used to test whether Ca2+ and Mg2+ can induce a negative slope. To determine whether Ca2+ and Mg2+ can induce a high frequency flickery gating, a comparison should be made between data obtained from BK channels that display normal conductance and near linear I-V plots in the absence of blockers to data obtained with intracellular blockers that display reduced conductance and negative slopes.
Unlike fast block by Ca2+, Mg2+, and H+, which does not appear to increase the open-channel noise at 5–10-kHz filtering, intracellular Na+ (5 mM) gives a pronounced flickery block with mean blocked and unblocked lifetimes of ∼10 µs at +80 mV (Yellen, 1984a,b). If the Na+-blocking and -unblocking lifetimes are exponentially distributed, there would be many blocking and unblocking events of sufficient duration to produce flickery block with the 4-kHz filtering in the experiments of Yellen (1984a,b). In addition to a flickery block, Na+ block also differs from Ca2+ and Mg2+ block in that for membrane potentials greater than +140 mV, the I-V inflects upwards giving an N shape, attributed to Na+ passing through the channel (punch through) at the higher membrane potentials (French and Shoukimas, 1985; Nimigean and Miller, 2002).
As shown in Fig. 1 B (closed circles), Ca2+ and Mg2+ block does reduce single-channel current amplitudes. Is this observation then sufficient to exclude that rapid selectivity filter gating reduces the observed single-channel currents, as proposed by Schroeder and Hansen (2007)? In the absence of intracellular Ca2+, Mg2+, and also the near absence of H+ (pH 9), a small sublinearity still remains in the I-V plots at high voltages (Brelidze and Magleby, 2004). If the (perhaps unwarranted) assumption is made that the I-V plot would be linear in the absence of blockers and rapid selectivity filter gating, then the deviation from linearity in the observed currents in the absence of blockers might reflect rapid selectivity filter gating, but other factors could also contribute to the sublinearity (see discussions in Nimigean et al., 2003; Brelidze and Magleby, 2004, 2005; Geng et al., 2011).
In conclusion, previous studies and Fig. 1 show that I-V curves from BK channels display reduced conductance and a negative slope at high positive potentials in the presence of intracellular blockers such as Ca2+ and Mg2+, but do not display reduced conductance and negative slopes in the absence of such blockers. These findings differ from those of Schroeder and Hansen (2007) of a negative slope both in the absence and presence of blockers.
Acknowledgments
This work is supported in part by National Institutes of Health (grant AR032805 to K.L Magleby).
Lawrence G. Palmer served as editor.
Footnotes
Abbreviation used in this paper:
- BK
- large-conductance, voltage- and Ca2+-activated K+
References
- Abenavoli A., DiFrancesco M.L., Schroeder I., Epimashko S., Gazzarrini S., Hansen U.P., Thiel G., Moroni A. 2009. Fast and slow gating are inherent properties of the pore module of the K+ channel Kcv. J. Gen. Physiol. 134:219–229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blatz A.L., Magleby K.L. 1986. Quantitative description of three modes of activity of fast chloride channels from rat skeletal muscle. J. Physiol. 378:141–174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brelidze T.I., Magleby K.L. 2004. Protons block BK channels by competitive inhibition with K+ and contribute to the limits of unitary currents at high voltages. J. Gen. Physiol. 123:305–319 10.1085/jgp.200308951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brelidze T.I., Magleby K.L. 2005. Probing the geometry of the inner vestibule of BK channels with sugars. J. Gen. Physiol. 126:105–121 10.1085/jgp.200509286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brelidze T.I., Niu X., Magleby K.L. 2003. A ring of eight conserved negatively charged amino acids doubles the conductance of BK channels and prevents inward rectification. Proc. Natl. Acad. Sci. USA. 100:9017–9022 10.1073/pnas.1532257100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvacho I., Gonzalez W., Torres Y.P., Brauchi S., Alvarez O., Gonzalez-Nilo F.D., Latorre R. 2008. Intrinsic electrostatic potential in the BK channel pore: Role in determining single channel conductance and block. J. Gen. Physiol. 131:147–161 10.1085/jgp.200709862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox D.H. 2005. The BKCa channel’s Ca2+-binding sites, multiple sites, multiple ions. J. Gen. Physiol. 125:253–255 10.1085/jgp.200509270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox D.H., Cui J., Aldrich R.W. 1997a. Allosteric gating of a large conductance Ca-activated K+ channel. J. Gen. Physiol. 110:257–281 10.1085/jgp.110.3.257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox D.H., Cui J., Aldrich R.W. 1997b. Separation of gating properties from permeation and block in mslo large conductance Ca-activated K+ channels. J. Gen. Physiol. 109:633–646 10.1085/jgp.109.5.633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui J. 2010. BK-type calcium-activated potassium channels: coupling of metal ions and voltage sensing. J. Physiol. 588:4651–4658 10.1113/jphysiol.2010.194514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenman G., Latorre R., Miller C. 1986. Multi-ion conduction and selectivity in the high-conductance Ca++-activated K+ channel from skeletal muscle. Biophys. J. 50:1025–1034 10.1016/S0006-3495(86)83546-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson W.B. 1991. Competitive Mg2+ block of a large-conductance, Ca2+-activated K+ channel in rat skeletal muscle. Ca2+, Sr2+, and Ni2+ also block. J. Gen. Physiol. 98:163–181 10.1085/jgp.98.1.163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- French R.J., Shoukimas J.J. 1985. An ion’s view of the potassium channel. The structure of the permeation pathway as sensed by a variety of blocking ions. J. Gen. Physiol. 85:669–698 10.1085/jgp.85.5.669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geng Y.Y., Niu X.W., Magleby K.L. 2011. Low resistance, large dimension entrance to the inner cavity of BK channels determined by changing side-chain volume. J. Gen. Physiol. 137:533–548 10.1085/jgp.201110616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golowasch J., Kirkwood A., Miller C. 1986. Allosteric effects of Mg2+ on the gating of Ca2+-activated K+ channels from mammalian skeletal muscle. J. Exp. Biol. 124:5–13 [DOI] [PubMed] [Google Scholar]
- Gribkoff V.K., Starrett J.E., Jr, Dworetzky S.I. 1997. The pharmacology and molecular biology of large-conductance calcium-activated (BK) potassium channels. Adv. Pharmacol. 37:319–348 10.1016/S1054-3589(08)60954-0 [DOI] [PubMed] [Google Scholar]
- Hamill O.P., Marty A., Neher E., Sakmann B., Sigworth F.J. 1981. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 391:85–100 10.1007/BF00656997 [DOI] [PubMed] [Google Scholar]
- Hille B. 2001. Ion Channels of Excitable Membranes. Third edition. Sinauer Associates, Inc., Sunderland, MA: 814 pp [Google Scholar]
- Knaus H.G., Eberhart A., Koch R.O., Munujos P., Schmalhofer W.A., Warmke J.W., Kaczorowski G.J., Garcia M.L. 1995. Characterization of tissue-expressed alpha subunits of the high conductance Ca2+-activated K+ channel. J. Biol. Chem. 270:22434–22439 10.1074/jbc.270.38.22434 [DOI] [PubMed] [Google Scholar]
- Latorre R., Morera F.J., Zaelzer C. 2010. Allosteric interactions and the modular nature of the voltage- and Ca2+-activated (BK) channel. J. Physiol. 588:3141–3148 10.1113/jphysiol.2010.191999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu R., Alioua A., Kumar Y., Eghbali M., Stefani E., Toro L. 2006. MaxiK channel partners: physiological impact. J. Physiol. 570:65–72 10.1113/jphysiol.2005.098913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magleby K.L. 2003. Gating mechanism of BK (Slo1) channels: So near, yet so far. J. Gen. Physiol. 121:81–96 10.1085/jgp.20028721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marty A. 1981. Ca-dependent K channels with large unitary conductance in chromaffin cell membranes. Nature. 291:497–500 10.1038/291497a0 [DOI] [PubMed] [Google Scholar]
- Marty A. 1983. Blocking of large unitary calcium-dependent potassium currents by internal sodium ions. Pflugers Arch. 396:179–181 10.1007/BF00615524 [DOI] [PubMed] [Google Scholar]
- McManus O.B., Helms L.M., Pallanck L., Ganetzky B., Swanson R., Leonard R.J. 1995. Functional role of the beta subunit of high conductance calcium-activated potassium channels. Neuron. 14:645–650 10.1016/0896-6273(95)90321-6 [DOI] [PubMed] [Google Scholar]
- Morales E., Cole W.C., Remillard C.V., Leblane N. 1996. Block of large conductance Ca2+-activated K+ channels in rabbit vascular myocytes by internal Mg2+ and Na+. J. Physiol. 495:701–716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson P.H. 2011. A permeation theory for single-file ion channels: One- and two-step models. J. Chem. Phys. 134:165102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nimigean C.M., Magleby K.L. 1999. The β subunit increases the Ca2+ sensitivity of large conductance Ca2+-activated potassium channels by retaining the gating in the bursting states. J. Gen. Physiol. 113:425–440 10.1085/jgp.113.3.425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nimigean C.M., Miller C. 2002. Na+ block and permeation in a K+ channel of known structure. J. Gen. Physiol. 120:323–335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nimigean C.M., Chappie J.S., Miller C. 2003. Electrostatic tuning of ion conductance in potassium channels. Biochemistry. 42:9263–9268 10.1021/bi0348720 [DOI] [PubMed] [Google Scholar]
- Pallotta B.S., Magleby K.L., Barrett J.N. 1981. Single channel recordings of Ca2+-activated K+ currents in rat muscle cell culture. Nature. 293:471–474 10.1038/293471a0 [DOI] [PubMed] [Google Scholar]
- Schroeder I., Hansen U.P. 2006. Strengths and limits of Beta distributions as a means of reconstructing the true single-channel current in patch clamp time series with fast gating. J. Membr. Biol. 210:199–212 10.1007/s00232-006-0858-8 [DOI] [PubMed] [Google Scholar]
- Schroeder I., Hansen U.P. 2007. Saturation and microsecond gating of current indicate depletion-induced instability of the MaxiK selectivity filter. J. Gen. Physiol. 130:83–97 10.1085/jgp.200709802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder I., Hansen U.P. 2008. Tl+-induced micros gating of current indicates instability of the MaxiK selectivity filter as caused by ion/pore interaction. J. Gen. Physiol. 131:365–378 10.1085/jgp.200809956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder I., Hansen U.P. 2009. Using a five-state model for fitting amplitude histograms from MaxiK channels: beta-distributions reveal more than expected. Eur. Biophys. J. 38:1101–1114 [DOI] [PubMed] [Google Scholar]
- Vergara C., Latorre R., Marrion N.V., Adelman J.P. 1998. Calcium-activated potassium channels. Curr. Opin. Neurobiol. 8:321–329 10.1016/S0959-4388(98)80056-1 [DOI] [PubMed] [Google Scholar]
- Villarroel A., Alvarez O., Oberhauser A., Latorre R. 1988. Probing a Ca2+-activated K+ channel with quaternary ammonium ions. Pflugers Arch. 413:118–126 10.1007/BF00582521 [DOI] [PubMed] [Google Scholar]
- Wong B.S., Lecar H., Adler M. 1982. Single calcium-dependent potassium channels in clonal anterior pituitary cells. Biophys. J. 39:313–317 10.1016/S0006-3495(82)84522-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia X.M., Zeng X., Lingle C.J. 2002. Multiple regulatory sites in large-conductance calcium-activated potassium channels. Nature. 418:880–884 10.1038/nature00956 [DOI] [PubMed] [Google Scholar]
- Yellen G. 1984a. Ionic permeation and blockade in Ca2+-activated K+ channels of bovine chromaffin cells. J. Gen. Physiol. 84:157–186 10.1085/jgp.84.2.157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yellen G. 1984b. Relief of Na+ block of Ca2+-activated K+ channels by external cations. J. Gen. Physiol. 84:187–199 10.1085/jgp.84.2.187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng X.H., Xia X.M., Lingle C.J. 2003. Redox-sensitive extracellular gates formed by auxiliary beta subunits of calcium-activated potassium channels. Nat. Struct. Biol. 10:448–454 10.1038/nsb932 [DOI] [PubMed] [Google Scholar]
- Zhang Y., Niu X., Brelidze T.I., Magleby K.L. 2006. Ring of negative charge in BK channels facilitates block by intracellular Mg2+ and polyamines through electrostatics. J. Gen. Physiol. 128:185–202 10.1085/jgp.200609493 [DOI] [PMC free article] [PubMed] [Google Scholar]