Abstract
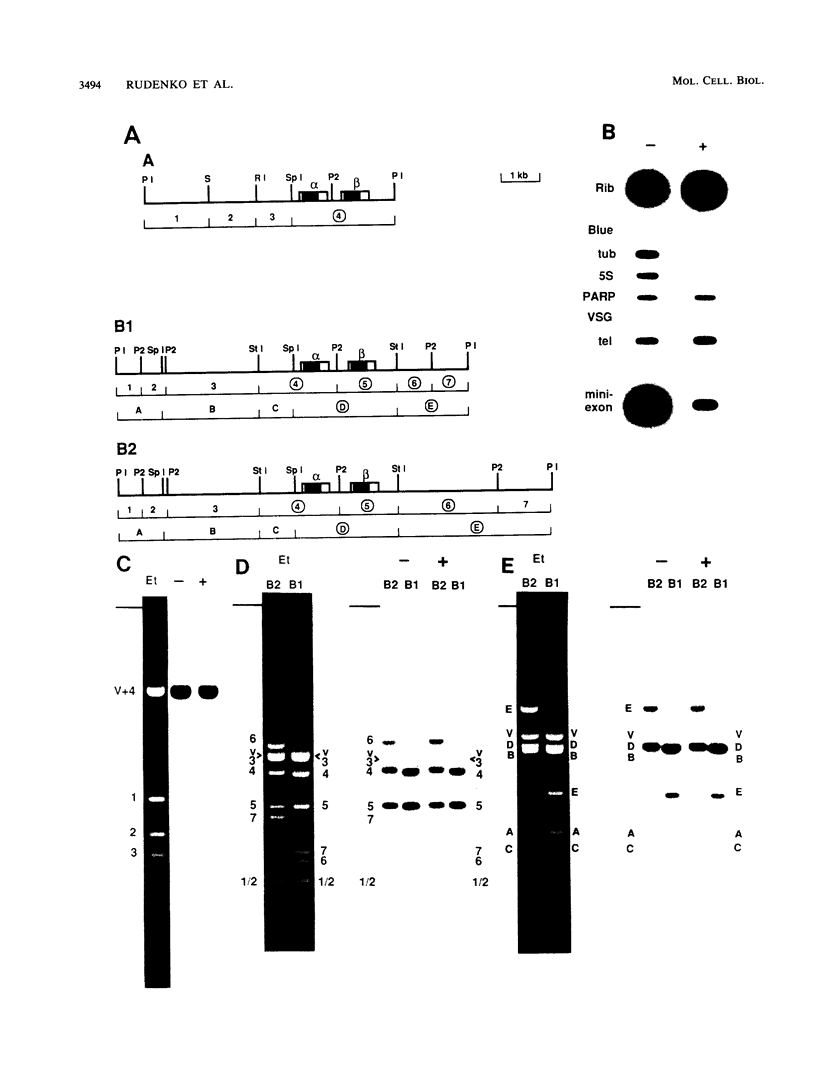
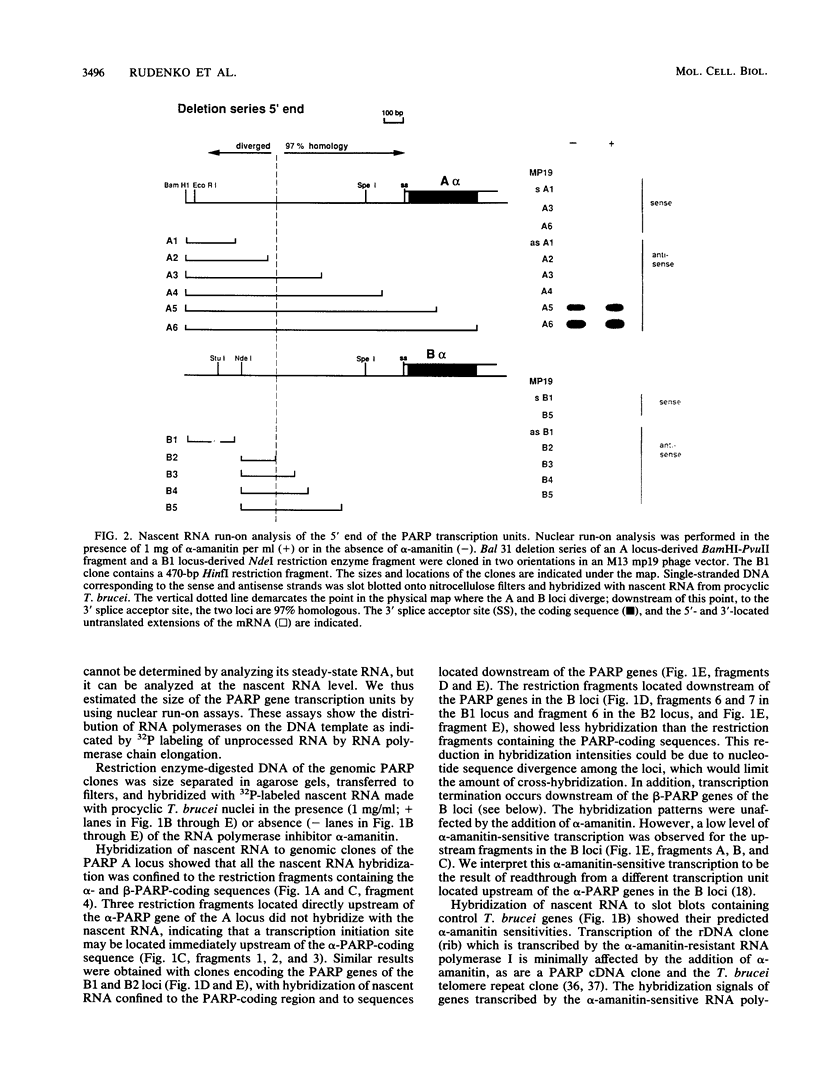
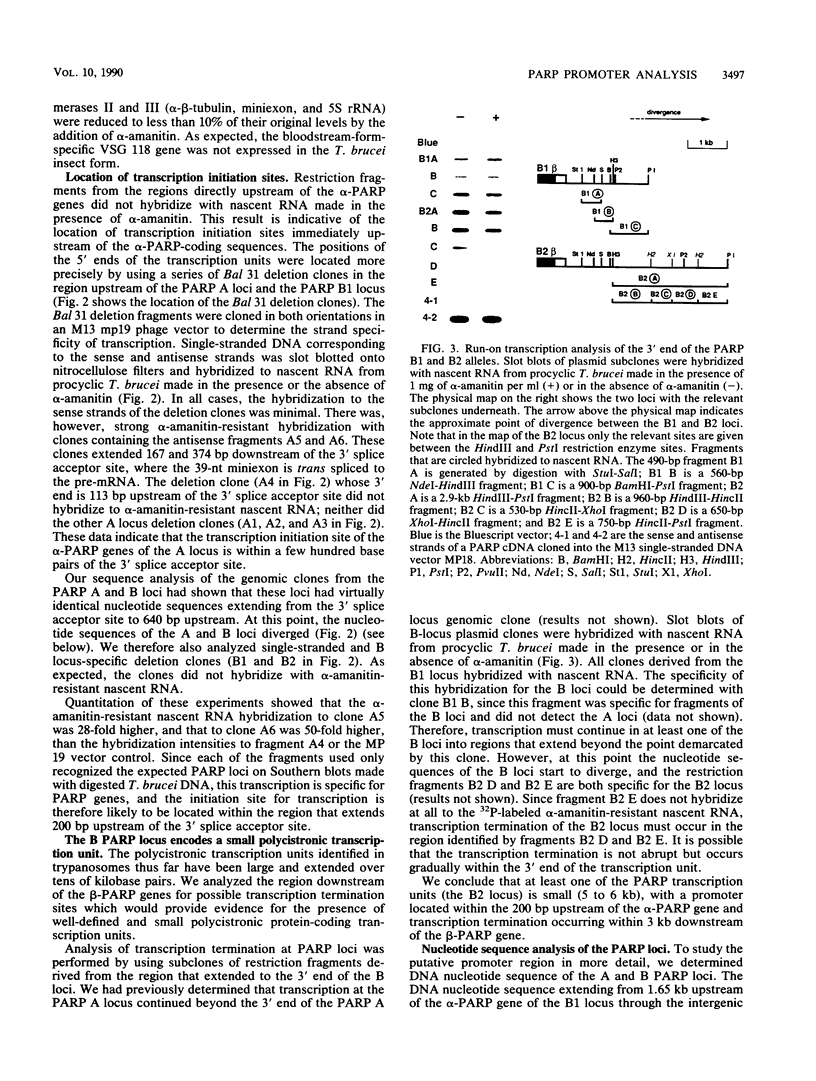
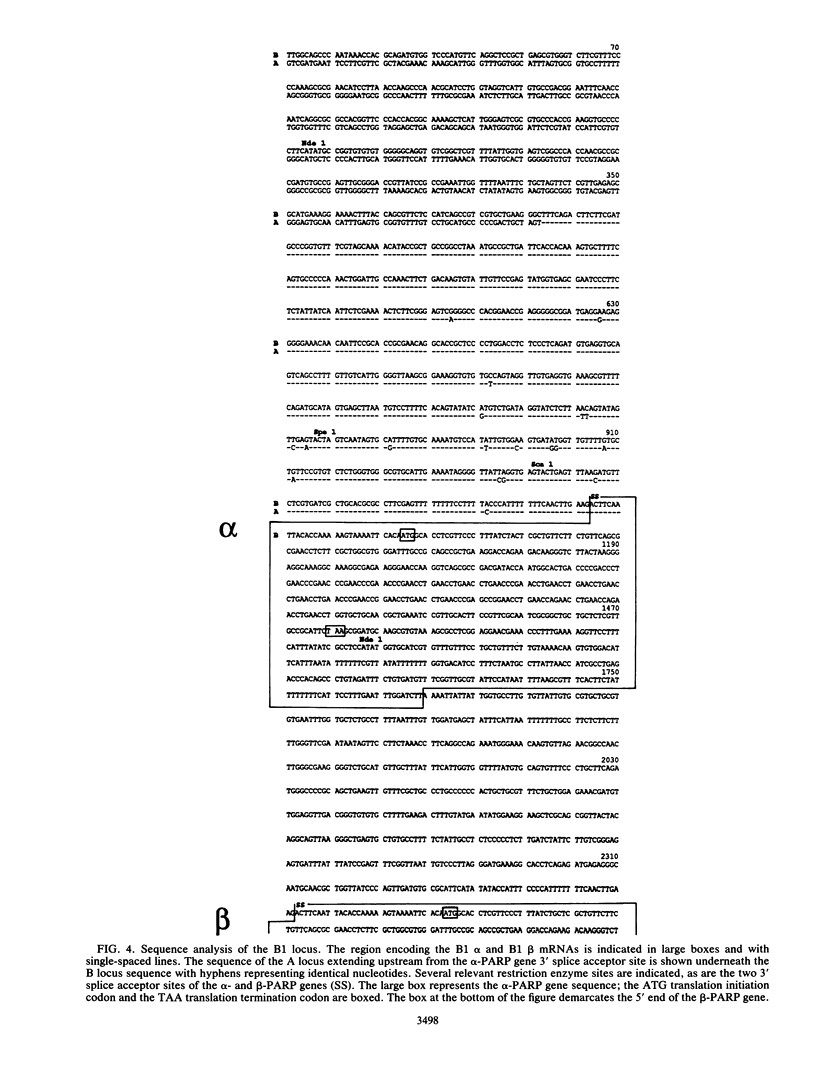
At least one of the procyclic acidic repetitive protein (PARP or procyclin) loci of Trypanosoma brucei is a small (5- to 6-kilobase) polycistronic transcription unit which is transcribed in an alpha-amanitin-resistant manner. Its single promoter, as mapped by run-on transcription analysis and UV inactivation of transcription, is located immediately upstream of the first alpha-PARP gene. Transcription termination occurs in a region approximately 3 kilobases downstream of the beta-PARP gene. The location of the promoter was confirmed by its ability to direct transcription of the bacterial chloramphenicol acetyltransferase gene in insect-form (procyclic) T. brucei. The putative PARP promoter is located in the region between the 3' splice acceptor site (nucleotide position 0) and nucleotide position -196 upstream of the alpha-PARP genes. Regulatory regions influencing the levels of PARP expression may be located further upstream. We conclude that a single promoter, which is located very close to the 3' splice acceptor site of the alpha-PARP genes, directs the transcription of a small, polycistronic, and alpha-amanitin-resistant transcription unit.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bellofatto V., Cross G. A. Expression of a bacterial gene in a trypanosomatid protozoan. Science. 1989 Jun 9;244(4909):1167–1169. doi: 10.1126/science.2499047. [DOI] [PubMed] [Google Scholar]
- Borst P. Discontinuous transcription and antigenic variation in trypanosomes. Annu Rev Biochem. 1986;55:701–732. doi: 10.1146/annurev.bi.55.070186.003413. [DOI] [PubMed] [Google Scholar]
- Brun R., Schönenberger Cultivation and in vitro cloning or procyclic culture forms of Trypanosoma brucei in a semi-defined medium. Short communication. Acta Trop. 1979 Sep;36(3):289–292. [PubMed] [Google Scholar]
- Bräutigam A. R., Sauerbier W. Transcription unit mapping in bacteriophage T7. I. In vivo transcription by Escherichia coli RNA polymerase. J Virol. 1973 Oct;12(4):882–886. doi: 10.1128/jvi.12.4.882-886.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton C. E., Mowatt M. R. The procyclic acidic repetitive proteins of Trypanosoma brucei. Purification and post-translational modification. J Biol Chem. 1989 Sep 5;264(25):15088–15093. [PubMed] [Google Scholar]
- Clayton C. E. The molecular biology of the Kinetoplastidae. Genet Eng. 1988;(7):1–56. [PubMed] [Google Scholar]
- Cornelissen A. W., Evers R., Köck J. Structure and sequence of genes encoding subunits of eukaryotic RNA polymerases. Oxf Surv Eukaryot Genes. 1988;5:91–131. [PubMed] [Google Scholar]
- Cornelissen A. W., Verspieren M. P., Toulmé J. J., Swinkels B. W., Borst P. The common 5' terminal sequence on trypanosome mRNAs: a target for anti-messenger oligodeoxynucleotides. Nucleic Acids Res. 1986 Jul 25;14(14):5605–5614. doi: 10.1093/nar/14.14.5605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Lange T., Berkvens T. M., Veerman H. J., Frasch A. C., Barry J. D., Borst P. Comparison of the genes coding for the common 5' terminal sequence of messenger RNAs in three trypanosome species. Nucleic Acids Res. 1984 Jun 11;12(11):4431–4443. doi: 10.1093/nar/12.11.4431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evers R., Hammer A., Köck J., Jess W., Borst P., Mémet S., Cornelissen A. W. Trypanosoma brucei contains two RNA polymerase II largest subunit genes with an altered C-terminal domain. Cell. 1989 Feb 24;56(4):585–597. doi: 10.1016/0092-8674(89)90581-3. [DOI] [PubMed] [Google Scholar]
- Gaffney D. F., McLauchlan J., Whitton J. L., Clements J. B. A modular system for the assay of transcription regulatory signals: the sequence TAATGARAT is required for herpes simplex virus immediate early gene activation. Nucleic Acids Res. 1985 Nov 11;13(21):7847–7863. doi: 10.1093/nar/13.21.7847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson W. C., Swinkels B. W., Borst P. Post-transcriptional control of the differential expression of phosphoglycerate kinase genes in Trypanosoma brucei. J Mol Biol. 1988 May 20;201(2):315–325. doi: 10.1016/0022-2836(88)90140-4. [DOI] [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackett P. B., Sauerbier W. Radiological mapping of the ribosomal RNA transcription unit in E. coli. Nature. 1974 Oct 18;251(5476):639–641. doi: 10.1038/251639a0. [DOI] [PubMed] [Google Scholar]
- Hackett P. B., Sauerbier W. The transcriptional organization of the ribosomal RNA genes in mouse L cells. J Mol Biol. 1975 Jan 25;91(3):235–256. doi: 10.1016/0022-2836(75)90378-2. [DOI] [PubMed] [Google Scholar]
- Johnson P. J., Kooter J. M., Borst P. Inactivation of transcription by UV irradiation of T. brucei provides evidence for a multicistronic transcription unit including a VSG gene. Cell. 1987 Oct 23;51(2):273–281. doi: 10.1016/0092-8674(87)90154-1. [DOI] [PubMed] [Google Scholar]
- Kooter J. M., Borst P. Alpha-amanitin-insensitive transcription of variant surface glycoprotein genes provides further evidence for discontinuous transcription in trypanosomes. Nucleic Acids Res. 1984 Dec 21;12(24):9457–9472. doi: 10.1093/nar/12.24.9457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kooter J. M., van der Spek H. J., Wagter R., d'Oliveira C. E., van der Hoeven F., Johnson P. J., Borst P. The anatomy and transcription of a telomeric expression site for variant-specific surface antigens in T. brucei. Cell. 1987 Oct 23;51(2):261–272. doi: 10.1016/0092-8674(87)90153-x. [DOI] [PubMed] [Google Scholar]
- König E., Delius H., Carrington M., Williams R. O., Roditi I. Duplication and transcription of procyclin genes in Trypanosoma brucei. Nucleic Acids Res. 1989 Nov 11;17(21):8727–8739. doi: 10.1093/nar/17.21.8727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laban A., Wirth D. F. Transfection of Leishmania enriettii and expression of chloramphenicol acetyltransferase gene. Proc Natl Acad Sci U S A. 1989 Dec;86(23):9119–9123. doi: 10.1073/pnas.86.23.9119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laird P. W., Kooter J. M., Loosbroek N., Borst P. Mature mRNAs of Trypanosoma brucei possess a 5' cap acquired by discontinuous RNA synthesis. Nucleic Acids Res. 1985 Jun 25;13(12):4253–4266. doi: 10.1093/nar/13.12.4253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee M. G., Van der Ploeg L. H. Frequent independent duplicative transpositions activate a single VSG gene. Mol Cell Biol. 1987 Jan;7(1):357–364. doi: 10.1128/mcb.7.1.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenardo M. J., Dorfman D. M., Reddy L. V., Donelson J. E. Characterization of the Trypanosoma brucei 5S ribosomal RNA gene and transcript: the 5S rRNA is a spliced-leader-independent species. Gene. 1985;35(1-2):131–141. doi: 10.1016/0378-1119(85)90165-9. [DOI] [PubMed] [Google Scholar]
- Lowndes N. F., Paul J., Wu J., Allan M. c-Ha-ras gene bidirectional promoter expressed in vitro: location and regulation. Mol Cell Biol. 1989 Sep;9(9):3758–3770. doi: 10.1128/mcb.9.9.3758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald R. J., Swift G. H., Przybyla A. E., Chirgwin J. M. Isolation of RNA using guanidinium salts. Methods Enzymol. 1987;152:219–227. doi: 10.1016/0076-6879(87)52023-7. [DOI] [PubMed] [Google Scholar]
- Mowatt M. R., Clayton C. E. Developmental regulation of a novel repetitive protein of Trypanosoma brucei. Mol Cell Biol. 1987 Aug;7(8):2838–2844. doi: 10.1128/mcb.7.8.2838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mowatt M. R., Clayton C. E. Polymorphism in the procyclic acidic repetitive protein gene family of Trypanosoma brucei. Mol Cell Biol. 1988 Oct;8(10):4055–4062. doi: 10.1128/mcb.8.10.4055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mowatt M. R., Wisdom G. S., Clayton C. E. Variation of tandem repeats in the developmentally regulated procyclic acidic repetitive proteins of Trypanosoma brucei. Mol Cell Biol. 1989 Mar;9(3):1332–1335. doi: 10.1128/mcb.9.3.1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pays E. Pseudogenes, chimaeric genes and the timing of antigen variation in African trypanosomes. Trends Genet. 1989 Dec;5(12):389–391. doi: 10.1016/0168-9525(89)90181-9. [DOI] [PubMed] [Google Scholar]
- Pays E., Tebabi P., Pays A., Coquelet H., Revelard P., Salmon D., Steinert M. The genes and transcripts of an antigen gene expression site from T. brucei. Cell. 1989 Jun 2;57(5):835–845. doi: 10.1016/0092-8674(89)90798-8. [DOI] [PubMed] [Google Scholar]
- Richardson J. P., Beecroft R. P., Tolson D. L., Liu M. K., Pearson T. W. Procyclin: an unusual immunodominant glycoprotein surface antigen from the procyclic stage of African trypanosomes. Mol Biochem Parasitol. 1988 Dec;31(3):203–216. doi: 10.1016/0166-6851(88)90150-8. [DOI] [PubMed] [Google Scholar]
- Roditi I., Carrington M., Turner M. Expression of a polypeptide containing a dipeptide repeat is confined to the insect stage of Trypanosoma brucei. Nature. 1987 Jan 15;325(6101):272–274. doi: 10.1038/325272a0. [DOI] [PubMed] [Google Scholar]
- Roditi I., Schwarz H., Pearson T. W., Beecroft R. P., Liu M. K., Richardson J. P., Bühring H. J., Pleiss J., Bülow R., Williams R. O. Procyclin gene expression and loss of the variant surface glycoprotein during differentiation of Trypanosoma brucei. J Cell Biol. 1989 Feb;108(2):737–746. doi: 10.1083/jcb.108.2.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudenko G., Bishop D., Gottesdiener K., Van der Ploeg L. H. Alpha-amanitin resistant transcription of protein coding genes in insect and bloodstream form Trypanosoma brucei. EMBO J. 1989 Dec 20;8(13):4259–4263. doi: 10.1002/j.1460-2075.1989.tb08611.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudenko G., Van der Ploeg L. H. Transcription of telomere repeats in protozoa. EMBO J. 1989 Sep;8(9):2633–2638. doi: 10.1002/j.1460-2075.1989.tb08403.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauerbier W., Millette R. L., Hackett P. B., Jr The effects of ultraviolet irradiation on the transcription of T4 DNA. Biochim Biophys Acta. 1970;209(2):368–386. doi: 10.1016/0005-2787(70)90735-5. [DOI] [PubMed] [Google Scholar]
- Seed B., Sheen J. Y. A simple phase-extraction assay for chloramphenicol acyltransferase activity. Gene. 1988 Jul 30;67(2):271–277. doi: 10.1016/0378-1119(88)90403-9. [DOI] [PubMed] [Google Scholar]
- Sharp P. A. Trans splicing: variation on a familiar theme? Cell. 1987 Jul 17;50(2):147–148. doi: 10.1016/0092-8674(87)90207-8. [DOI] [PubMed] [Google Scholar]
- Shea C., Lee M. G., Van der Ploeg L. H. VSG gene 118 is transcribed from a cotransposed pol I-like promoter. Cell. 1987 Aug 14;50(4):603–612. doi: 10.1016/0092-8674(87)90033-x. [DOI] [PubMed] [Google Scholar]
- Shea C., Van der Ploeg L. H. Stable variant-specific transcripts of the variant cell surface glycoprotein gene 1.8 expression site in Trypanosoma brucei. Mol Cell Biol. 1988 Feb;8(2):854–859. doi: 10.1128/mcb.8.2.854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith J. L., Levin J. R., Ingles C. J., Agabian N. In trypanosomes the homolog of the largest subunit of RNA polymerase II is encoded by two genes and has a highly unusual C-terminal domain structure. Cell. 1989 Mar 10;56(5):815–827. doi: 10.1016/0092-8674(89)90686-7. [DOI] [PubMed] [Google Scholar]
- Thomashow L. S., Milhausen M., Rutter W. J., Agabian N. Tubulin genes are tandemly linked and clustered in the genome of trypanosoma brucei. Cell. 1983 Jan;32(1):35–43. doi: 10.1016/0092-8674(83)90494-4. [DOI] [PubMed] [Google Scholar]
- Van der Ploeg L. H. Discontinuous transcription and splicing in trypanosomes. Cell. 1986 Nov 21;47(4):479–480. doi: 10.1016/0092-8674(86)90608-2. [DOI] [PubMed] [Google Scholar]
- Van der Ploeg L. H., Liu A. Y., Borst P. Structure of the growing telomeres of Trypanosomes. Cell. 1984 Feb;36(2):459–468. doi: 10.1016/0092-8674(84)90239-3. [DOI] [PubMed] [Google Scholar]
- Walder J. A., Eder P. S., Engman D. M., Brentano S. T., Walder R. Y., Knutzon D. S., Dorfman D. M., Donelson J. E. The 35-nucleotide spliced leader sequence is common to all trypanosome messenger RNA's. Science. 1986 Aug 1;233(4763):569–571. doi: 10.1126/science.3523758. [DOI] [PubMed] [Google Scholar]
- White T. C., Rudenko G., Borst P. Three small RNAs within the 10 kb trypanosome rRNA transcription unit are analogous to domain VII of other eukaryotic 28S rRNAs. Nucleic Acids Res. 1986 Dec 9;14(23):9471–9489. doi: 10.1093/nar/14.23.9471. [DOI] [PMC free article] [PubMed] [Google Scholar]







