Abstract
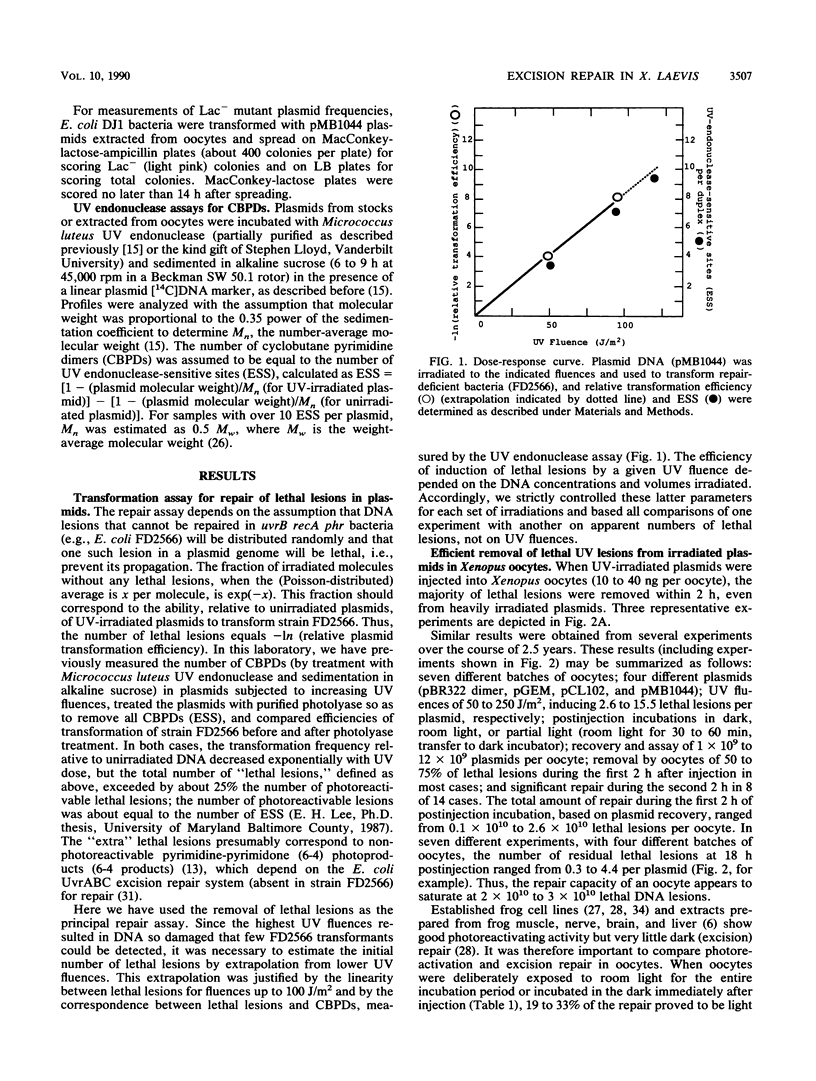
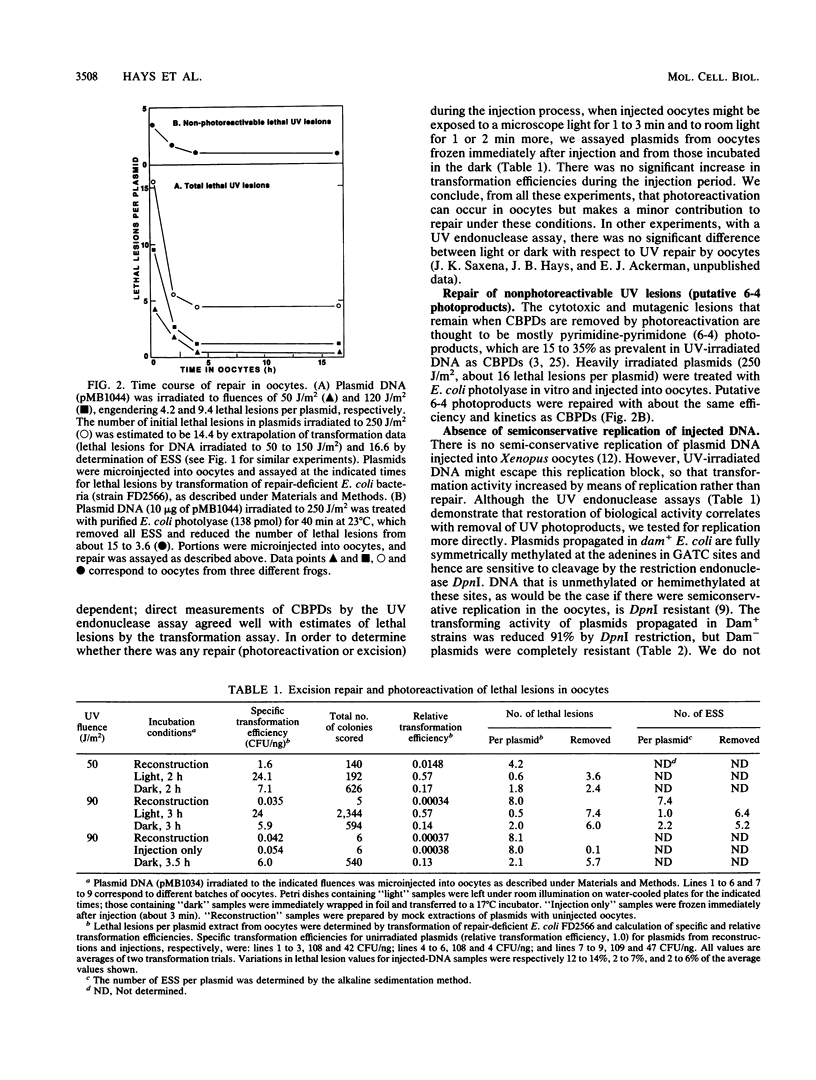
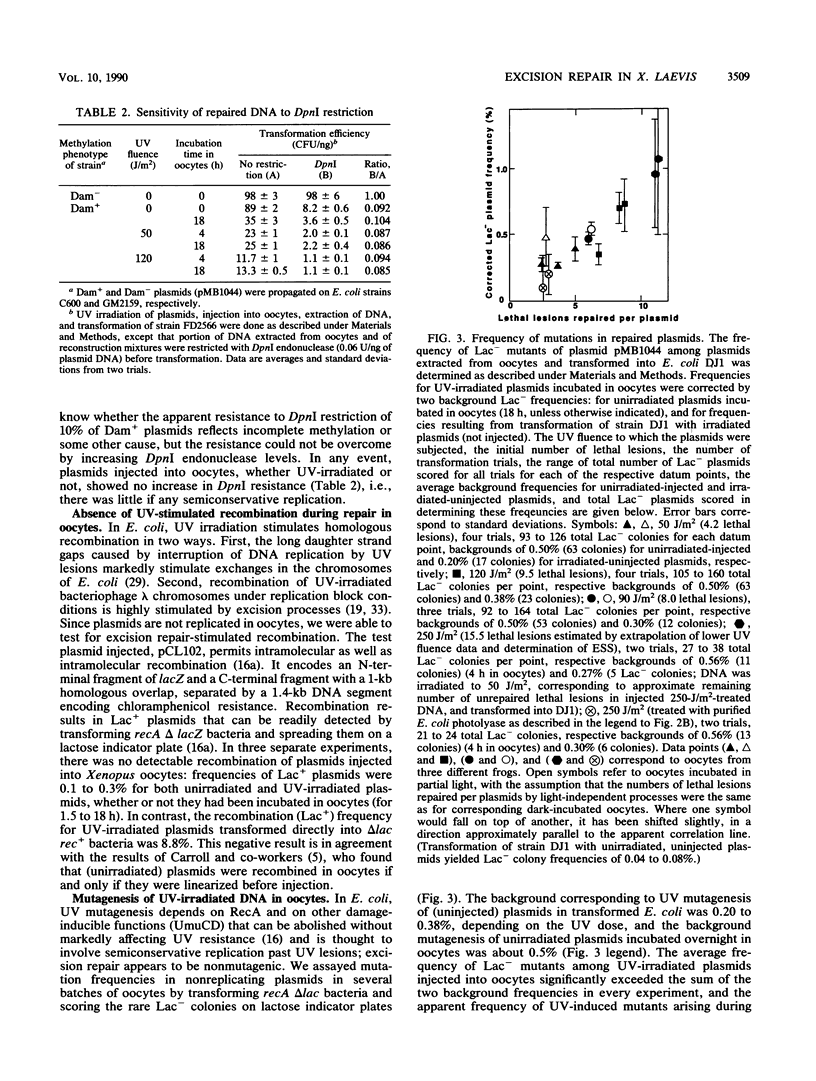
Repair of UV-irradiated plasmid DNA microinjected into frog oocytes was measured by two techniques: transformation of repair-deficient (delta uvrB delta recA delta phr) bacteria, and removal of UV endonuclease-sensitive sites (ESS). Transformation efficiencies relative to unirradiated plasmids were used to estimate the number of lethal lesions; the latter were assumed to be Poisson distributed. These estimates were in good agreement with measurements of ESS. By both criteria, plasmid DNA was efficiently repaired, mostly during the first 2 h, when as many as 2 x 10(10) lethal lesions were removed per oocyte. This rate is about 10(6) times the average for removal of ESS from repair-proficient human cells. Repair was slower but still significant after 2 h, but some lethal lesions usually remained after overnight incubation. Most repair occurred in the absence of light, in marked contrast to differentiated frog cells, previously shown to possess photoreactivating but no excision repair activity. There was no increase in the resistance to DpnI restriction of plasmids (methylated in Escherichia coli at GATC sites) incubated in oocytes; this implies no increase in hemimethylated GATC sites, and hence no semiconservative DNA replication. Plasmid substrates capable of either intramolecular or intermolecular homologous recombination were not recombined, whether UV-irradiated or not. Repair of Lac+ plasmids was accompanied by a significant UV-dependent increase in the frequency of Lac- mutants, corresponding to a repair synthesis error frequency on the order of 10(-4) per nucleotide.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bohr V. A., Smith C. A., Okumoto D. S., Hanawalt P. C. DNA repair in an active gene: removal of pyrimidine dimers from the DHFR gene of CHO cells is much more efficient than in the genome overall. Cell. 1985 Feb;40(2):359–369. doi: 10.1016/0092-8674(85)90150-3. [DOI] [PubMed] [Google Scholar]
- Brash D. E., Haseltine W. A. UV-induced mutation hotspots occur at DNA damage hotspots. Nature. 1982 Jul 8;298(5870):189–192. doi: 10.1038/298189a0. [DOI] [PubMed] [Google Scholar]
- Bredberg A., Kraemer K. H., Seidman M. M. Restricted ultraviolet mutational spectrum in a shuttle vector propagated in xeroderma pigmentosum cells. Proc Natl Acad Sci U S A. 1986 Nov;83(21):8273–8277. doi: 10.1073/pnas.83.21.8273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll D., Wright S. H., Wolff R. K., Grzesiuk E., Maryon E. B. Efficient homologous recombination of linear DNA substrates after injection into Xenopus laevis oocytes. Mol Cell Biol. 1986 Jun;6(6):2053–2061. doi: 10.1128/mcb.6.6.2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook J. S., McGrath J. R. Photoreactivating-enzyme activity in metazoa. Proc Natl Acad Sci U S A. 1967 Oct;58(4):1359–1365. doi: 10.1073/pnas.58.4.1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortese R., Harland R., Melton D. Transcription of tRNA genes in vivo: single-stranded compared to double-stranded templates. Proc Natl Acad Sci U S A. 1980 Jul;77(7):4147–4151. doi: 10.1073/pnas.77.7.4147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drake J. W. Comparative rates of spontaneous mutation. Nature. 1969 Mar 22;221(5186):1132–1132. doi: 10.1038/2211132a0. [DOI] [PubMed] [Google Scholar]
- Geier G. E., Modrich P. Recognition sequence of the dam methylase of Escherichia coli K12 and mode of cleavage of Dpn I endonuclease. J Biol Chem. 1979 Feb 25;254(4):1408–1413. [PubMed] [Google Scholar]
- Gurdon J. B., Melton D. A. Gene transfer in amphibian eggs and oocytes. Annu Rev Genet. 1981;15:189–218. doi: 10.1146/annurev.ge.15.120181.001201. [DOI] [PubMed] [Google Scholar]
- Gurdon J. B. Methods for nuclear transplantation in amphibia. Methods Cell Biol. 1977;16:125–139. doi: 10.1016/s0091-679x(08)60096-5. [DOI] [PubMed] [Google Scholar]
- Harland R. M., Laskey R. A. Regulated replication of DNA microinjected into eggs of Xenopus laevis. Cell. 1980 Oct;21(3):761–771. doi: 10.1016/0092-8674(80)90439-0. [DOI] [PubMed] [Google Scholar]
- Hauser J., Seidman M. M., Sidur K., Dixon K. Sequence specificity of point mutations induced during passage of a UV-irradiated shuttle vector plasmid in monkey cells. Mol Cell Biol. 1986 Jan;6(1):277–285. doi: 10.1128/mcb.6.1.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hays J. B., Martin S. J., Bhatia K. Repair of nonreplicating UV-irradiated DNA: cooperative dark repair by Escherichia coli uvr and phr functions. J Bacteriol. 1985 Feb;161(2):602–608. doi: 10.1128/jb.161.2.602-608.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato T., Ise T., Shinagawa H. Mutational specificity of the umuC mediated mutagenesis in Eschericha coli. Biochimie. 1982 Aug-Sep;64(8-9):731–733. doi: 10.1016/s0300-9084(82)80119-3. [DOI] [PubMed] [Google Scholar]
- Laufer C. S., Hays J. B., Windle B. E., Schaefer T. S., Lee E. H., Hays S. L., McClure M. R. Enhancement of Escherichia coli plasmid and chromosomal recombination by the Ref function of bacteriophage P1. Genetics. 1989 Nov;123(3):465–476. doi: 10.1093/genetics/123.3.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebkowski J. S., Clancy S., Miller J. H., Calos M. P. The lacI shuttle: rapid analysis of the mutagenic specificity of ultraviolet light in human cells. Proc Natl Acad Sci U S A. 1985 Dec;82(24):8606–8610. doi: 10.1073/pnas.82.24.8606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legerski R. J., Penkala J. E., Peterson C. A., Wright D. A. Repair of UV-induced lesions in Xenopus laevis oocytes. Mol Cell Biol. 1987 Dec;7(12):4317–4323. doi: 10.1128/mcb.7.12.4317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin P. F., Howard-Flanders P. Genetic exchanges caused by ultraviolet photoproducts in phage lambda DNA molecules: the role of DNA replication. Mol Gen Genet. 1976 Jul 23;146(2):107–115. doi: 10.1007/BF00268079. [DOI] [PubMed] [Google Scholar]
- Mandel M., Higa A. Calcium-dependent bacteriophage DNA infection. J Mol Biol. 1970 Oct 14;53(1):159–162. doi: 10.1016/0022-2836(70)90051-3. [DOI] [PubMed] [Google Scholar]
- Marinus M. G., Carraway M., Frey A. Z., Brown L., Arraj J. A. Insertion mutations in the dam gene of Escherichia coli K-12. Mol Gen Genet. 1983;192(1-2):288–289. doi: 10.1007/BF00327681. [DOI] [PubMed] [Google Scholar]
- Mellon I., Bohr V. A., Smith C. A., Hanawalt P. C. Preferential DNA repair of an active gene in human cells. Proc Natl Acad Sci U S A. 1986 Dec;83(23):8878–8882. doi: 10.1073/pnas.83.23.8878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell D. L. The relative cytotoxicity of (6-4) photoproducts and cyclobutane dimers in mammalian cells. Photochem Photobiol. 1988 Jul;48(1):51–57. doi: 10.1111/j.1751-1097.1988.tb02785.x. [DOI] [PubMed] [Google Scholar]
- Regan J. D., Cook J. S., Lee W. H. Photoreactivation of amphibian cells in culture. J Cell Physiol. 1968 Apr;71(2):173–176. doi: 10.1002/jcp.1040710208. [DOI] [PubMed] [Google Scholar]
- Rupp W. D., Wilde C. E., 3rd, Reno D. L., Howard-Flanders P. Exchanges between DNA strands in ultraviolet-irradiated Escherichia coli. J Mol Biol. 1971 Oct 14;61(1):25–44. doi: 10.1016/0022-2836(71)90204-x. [DOI] [PubMed] [Google Scholar]
- Ryoji M., Worcel A. Chromatin assembly in Xenopus oocytes: in vivo studies. Cell. 1984 May;37(1):21–32. doi: 10.1016/0092-8674(84)90297-6. [DOI] [PubMed] [Google Scholar]
- Sancar A., Rupp W. D. A novel repair enzyme: UVRABC excision nuclease of Escherichia coli cuts a DNA strand on both sides of the damaged region. Cell. 1983 May;33(1):249–260. doi: 10.1016/0092-8674(83)90354-9. [DOI] [PubMed] [Google Scholar]
- Seidman M. M., Bredberg A., Seetharam S., Kraemer K. H. Multiple point mutations in a shuttle vector propagated in human cells: evidence for an error-prone DNA polymerase activity. Proc Natl Acad Sci U S A. 1987 Jul;84(14):4944–4948. doi: 10.1073/pnas.84.14.4944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith T. A., Hays J. B. Repair and recombination of nonreplicating UV-irradiated phage DNA in E. coli II. Stimulation of RecF-dependent recombination by excision repair of cyclobutane pyrimidine dimers and of other photoproducts. Mol Gen Genet. 1985;201(3):393–401. doi: 10.1007/BF00331329. [DOI] [PubMed] [Google Scholar]
- van Zeeland A. A., Natarajan A. T., Verdegaal-Immerzeel E. A., Filon A. R. Photoreactivation of UV induced cell killing, chromosome aberrations, sister chromatid exchanges, mutations and pyrimidine dimers in Xenopus laevis fibroblasts. Mol Gen Genet. 1980;180(3):495–500. doi: 10.1007/BF00268052. [DOI] [PubMed] [Google Scholar]



