Abstract
Cardiac troponin T (cTnT) has a highly acidic extended N-terminus, the physiological role of which remains poorly understood. To decipher the physiological role of this unique region, we deleted specific regions within the N-terminus of mouse cTnT (McTnT) to create McTnT1-44Δ and McTnT45-74Δ proteins. Contractile function and dynamic force–length measurements were made after reconstituting the McTnT deletion proteins into detergent-skinned cardiac papillary fibres harvested from non-transgenic mice that expressed α-tropomyosin (Tm). To further understand how the functional effects of the N-terminus of cTnT are modulated by Tm isoforms, McTnT deletion proteins were reconstituted into detergent-skinned cardiac papillary fibres harvested from transgenic mice that expressed both α- and β-Tm. McTnT1-44Δ, but not McTnT45-74Δ, attenuated maximal activation of the thin filament. Myofilament Ca2+ sensitivity, as measured by pCa50 (−log of [Ca2+]free required for half-maximal activation), decreased in McTnT1-44Δ (α-Tm) fibres. The desensitizing effect of McTnT1-44Δ on pCa50 was ablated in β-Tm fibres. McTnT45-74Δ enhanced pCa50 in both α- and β-Tm fibres, with β-Tm having a bigger effect. The Hill coefficient of tension development was significantly attenuated by McTnT45-74Δ, suggesting an effect on thin-filament cooperativity. The rate of cross-bridge (XB) detachment and the strained XB-mediated impact on other XBs were augmented by McTnT1-44Δ in β-Tm fibres. The magnitude of the length-mediated recruitment of XBs was attenuated by McTnT1-44Δ in β-Tm fibres. Our data demonstrate that the 1−44 region of McTnT is essential for maximal activation, whereas the cardiac-specific 45−74 region of McTnT is essential for augmenting cooperativity. Moreover, our data show that α- and β-Tm isoforms have divergent effects on McTnT deletion mutant's ability to modulate cardiac thin-filament activation and Ca2+ sensitivity. Our results not only provide the first explicit evidence for the existence of two distinct functional regions within the N-terminus of cTnT, but also offer mechanistic insights into the divergent physiological roles of these regions in mediating cardiac contractile activation.
Key points
To elucidate the cardiac-specific role of the highly acidic extended N-terminus of cardiac troponin T (cTnT), the following deletions were made in the N-terminus of mouse cTnT (McTnT): McTnT1-44Δ and McTnT45-74Δ.
Thin-filament activation was assessed after reconstituting the deletion proteins into skinned non-transgenic mouse cardiac fibres expressing α-tropomyosin (Tm).
Because the N-terminus of cTnT interacts with the overlapping ends of Tm, we also sought to understand how Tm isoforms modulate the functional effects of the N-terminus of cTnT. Thus, the deletion proteins were reconstituted into skinned transgenic mouse cardiac fibres expressing β-Tm.
Maximal activation was decreased by McTnT1-44Δ irrespective of the type of Tm background. Cooperativity was decreased by McTnT45-74Δ, an effect that was more pronounced under β-Tm background.
We provide the first explicit evidence to show that the cardiac-specific extended N-terminus of cTnT contains two distinct regions that have divergent physiological roles in modulating cardiac thin-filament activation.
Introduction
In cardiac muscle contraction, the primary trigger for the movement of tropomyosin–troponin (Tm–Tn) complex on the actin filament comes from the binding of Ca2+ to cardiac troponin C (cTnC). In addition to cTnC, cardiac troponin T (cTnT) is also essential for the Ca2+-regulated activation of acto-myosin interactions that generate force in striated muscle (Malnic & Reinach, 1994; Malnic et al. 1998; Gordon et al. 2000; Kobayashi & Solaro, 2005). cTnT not only promotes the binding of Tm to actin, but also aids in the assembly of Tm on the actin filament by promoting head-to-tail interaction between two contiguous Tms (Heeley et al. 1987; Lehrer & Geeves, 1998). This action of cTnT on Tm is considered to be important for full cooperative activation of myofilaments (Malnic & Reinach, 1994; Chandra et al. 1999).
Direct evidence for the effect of cTnT–Tm interactions on thin-filament activation comes from previous studies, which showed that deletion of the first 76 or 96 amino acids in cTnT resulted in a greater inhibition of force and ATPase activity by Tm (Chandra et al. 1999; Communal et al. 2002). Based on our study (Chandra et al. 1999), we concluded that cross-bridge (XB) recruitment was inhibited by the effect of mutant cTnT on the overlapping ends of contiguous Tms. Interestingly, the removal of the overlapping ends of contiguous Tms inhibited myosin S1-ADP binding to fully-regulated thin filaments (Walsh et al. 1985; Heeley et al. 1989), and modifications to the end-to-end interactions of Tm altered the contractile dynamics (Gaffin et al. 2006) and resulted in a 10-fold weakening of its interaction with actin (Coulton et al. 2008). Collectively, these observations suggest that the tail domain of cTnT (residues 1−191 in mouse sequence) has a significant effect on how Tm–actin interactions modulate strong XB recruitment.
There is experimental evidence (Brisson et al. 1986) to suggest that the N-terminal end of the tail domain of fast skeletal TnT (fsTnT) in one structural unit (SU; Tm1–Tn1–actin7) may interact with the Tm from the neighbouring SU. Conclusions drawn from the study by Brisson et al. (1986) have major implications for cardiac muscle because the highly acidic tail domain of cTnT – in all species – has a unique cardiac-specific N-terminal extension (NTE) that extends deeper into the neighbouring SU (Gollapudi et al. 2012). For example, the NTE of cTnT extends by another 42 Å into the neighbouring SU, and is suggested to constrain the thin filaments more in the blocked state, thereby desensitizing the cardiac thin filaments to Ca2+ (Tobacman et al. 2002; Gollapudi et al. 2012). Our data also demonstrate that the NTE has an effect to modulate cooperative processes that govern non-linear effects during cardiac thin-filament activation (Gollapudi et al. 2012).
Because the extended N-terminus of cTnT is located around the head-to-tail overlapping region of two contiguous Tms, the presence of this highly acidic region in cTnT is strongly suggestive of a cardiac-specific functional role (Perry, 1998). The functional significance of the N-terminus of cTnT is further highlighted by the fact that numerous mutations, which are associated with familial hypertrophic cardiomyopathy, have been identified in the N-terminus of human cTnT (Willott et al. 2010). Moreover, some forms of human heart failure are associated with reexpression of embryonic isoforms that differ in the N-terminus of cTnT (Anderson et al. 1995). Furthermore, the existence of various Tm isoforms in different tissues suggests that the functional effect of the N-terminus of cTnT may be further modulated by the type of Tm isoform. For example, the α-Tm isoform predominates in the adult rat heart muscle, while the β-Tm isoform is expressed at low levels during cardiogenesis and in the hearts of neonates (Muthuchamy et al. 1993). In contrast, fast twitch skeletal muscle of adult rat contains both α- and β-isoforms of Tm, but the γ-isoform of Tm is restricted to slow twitch skeletal muscle (Wolska & Wieczorek, 2003). Interestingly, these Tm isoforms differ significantly from each other in or near the Tm–Tm overlap regions, suggesting a causal link between the type of Tm isoform and variation in cooperative activation of the thin filaments (Wolska et al. 1999).
Exactly what specific region of the N-terminus is functionally important, and how its functions are further modulated by Tm isoforms has not been studied. Based on our recent mechano-dynamic studies on transgenic (TG) mouse expressing a cTnT variant in the heart (Gollapudi et al. 2012), we identified two specific regions in the N-terminus of mouse cTnT (McTnT) as the potential candidates for the functional effects of the N-terminus of cTnT on cardiac thin filaments (see Fig. S3 in the Supplemental Material). We deleted specific regions within the N-terminus of McTnT to create McTnT1−44Δ and McTnT45−74Δ proteins. The impact of deletions in McTnT on cardiac function was tested by reconstituting the proteins into detergent-skinned cardiac papillary fibres isolated from non-transgenic (NTG) mice expressing α-Tm. Because the N-terminus of cTnT interacts with the head-to-tail overlapping region of Tm, it is important to understand how Tm isoforms modulate the functional effects of the N-terminus of cTnT. Therefore, to test how the effects of the N-terminus of cTnT are modulated by Tm isoforms, McTnT deletion proteins were reconstituted into detergent-skinned cardiac papillary fibres isolated from TG mice that expressed ∼55%β-Tm and ∼45%α-Tm. The rationale for choosing β-Tm TG mice is that α- and β-Tm differ by 39 amino acids, 25 of which are confined to the carboxyl domain (Muthuchamy et al. 1997); this region of Tm interacts with the N-terminus of TnT (region of TnT that is being investigated in this study).
Contractile function and dynamic force–length measurements were made to delineate the effects of cTnT deletion mutants, and to test how their effects were further modulated by α- and β-Tm isoforms. Data presented here provide the first explicit evidence to demonstrate that the 1−44 region of cTnT is essential for maximal activation, while the 45−74 region of cTnT is essential for cooperative activation of cardiac myofibres. Moreover, our study reveals the divergent effects of α- and β-Tm isoforms on cTnT-mediated contractile activation of cardiac myofibres.
Methods
Ethical approval/animal protocols
TG mice (FVBN strain), expressing ∼55%β-Tm and ∼45%α-Tm in the myocardium, used in this study were previously generated and well characterized (Muthuchamy et al. 1995). NTG mice (FVBN strain), expressing α-Tm in the myocardium, were used as controls. All animals used in this study were properly handled to minimize the pain and suffering, according to the established guidelines of the Washington State University Institutional Animal Care and Use Committee.
Preparation of detergent-skinned cardiac muscle fibre bundles
Detergent-skinned cardiac papillary muscle fibres were prepared as previously described (Chandra et al. 2006, 2007). In brief, mice were deeply anaesthetized by inhalation of isoflurane. The depth of the anaesthesia was assessed by a lack of pedal withdrawal reflex. Hearts were quickly removed and placed into an ice-cold high-relaxing (HR) solution of pCa (pCa =−log of [Ca2+]free) 9.0 containing (in mm): 2,3-butanedione monoxime, 20; N,N-bis(2-hydroxyethyl)-2-amino-ethanesulfonic acid (BES), 50; EGTA, 20; MgCl2, 6.29; Na2ATP, 6.09; potassium propionate, 30.83; sodium azide, 10; dithiothreitol (DTT), 1.0; benzamidine-HCl, 4. The pH of the solution was adjusted to 7.0 with KOH. A fresh cocktail of protease inhibitors (in μm: bestatin, 5; E-64, 2; leupeptin, 10; pepstatin, 1; phenylmethylsulfonyl fluoride (PMSF), 200) was added to the HR solution. Papillary muscle bundles collected from the left ventricles of the hearts were further dissected into smaller fibres of approximately 0.15 mm in width and 2 mm in length. Fibres were detergent-skinned overnight at 4°C in HR containing 1% Triton X-100.
Expression and purification of recombinant mouse cardiac Tn subunits
Recombinant c-myc-tagged McTnT (c-myc McTnT), mouse cardiac TnI (McTnI) and mouse cardiac TnC (McTnC), all cloned into pSBETa vector, were expressed in BL21*DE3 cells (Novagen, Madison, WI, USA) for protein synthesis (Chandra et al. 2006). McTnT 1−44 and McTnT 45−74 deletion (McTnT1−44Δ and McTnT45−74Δ) genes were synthesized (GenScript USA Inc, Piscataway, NJ, USA) after codon optimization for enhanced protein expression, cloned into pSBETa vector and were expressed in BL21*DE3 cells. For all protein preparations, BL21*DE3 cells cultured in 6 litres of terrific broth were spun down and sonicated, to lyse the cells and release the expressed proteins, in a buffer containing 50 mm Tris base, 6 m urea, 5 mm EDTA (pH 8.0 at 4°C). The sonication buffer also contained a cocktail of protease inhibitors: 0.2 mm PMSF, 5 mm benzamidine-HCl, 10 μm leupeptin, 1 μm pepstatin, 5 μm bestatin, 2 μm E-64 and 1 mm DTT. The sonicated preparation was then centrifuged to pellet out the insoluble cellular particulate material. The supernatant from the sonicated preparation was used for ammonium sulfate fractionation procedure. c-myc McTnT and McTnT45−74Δ proteins were purified as follows: The pellet from 60% ammonium sulfate fractionation was dissolved in 50 mm Tris base (pH 8.0 at 4°C), 6 m urea, 1 mm EDTA, 0.2 mm PMSF, 5 mm benzamidine-HCl, 0.01% sodium azide and 1 mm DTT, and then purified by ion-exchange chromatography on a DEAE-fast Sepharose column (GE Healthcare Biosciences, Pittsburgh, PA, USA). c-myc McTnT and McTnT45-74Δ proteins were eluted with a 0−0.4 m NaCl gradient. McTnT1-44Δ protein was purified using a similar procedure used for c-myc McTnT, except that a CM Sepharose column (GE Healthcare Biosciences) was used for ion-exchange chromatography. McTnI protein was purified as follows: the supernatant from the sonicated preparation was loaded onto a CM Sepharose column and eluted with a 0−0.3 m NaCl gradient. The eluted fractions containing McTnI protein were pooled and dialysed against a buffer containing 50 mm Tris-HCl, 6 m urea, 0.2 m NaCl, 3 mm CaCl2 and a cocktail of protease inhibitors (pH 8.0 at 4°C). The dialysed fractions were then loaded onto a cTnC affinity column. McTnI was eluted using a buffer containing 50 mm Tris-HCl, 6 m urea, 1 m NaCl, 2 mm EDTA and a cocktail of protease inhibitors (pH 8.0 at 4°C). McTnC protein was purified as follows: the supernatant from the sonicated preparation was loaded onto a DE-52 column (GE Healthcare Biosciences) and eluted with a 0–0.3 m NaCl gradient. The eluted fractions containing McTnC protein were pooled and dialysed against a buffer containing 50 mm Tris base, 6 m urea, 1 m NaCl, 5 mm CaCl2 and a cocktail of protease inhibitors (pH 7.5 at 4°C). The dialysed fractions were loaded onto a Phenyl Sepharose column and eluted with a buffer containing 50 mm Tris base, 1 m NaCl, 10 mm EDTA and a cocktail of protease inhibitors. For all the preparations, the eluted protein fractions were run on a 12.5% SDS gel to assess their purity. Fractions containing the pure protein were pooled and dialysed thoroughly against deionized water containing 15 mmβ-mercaptoethanol, lyophilized and stored at −80°C.
Reconstitution of recombinant mouse cardiac Tn subunits into detergent-skinned mouse cardiac muscle fibres
Reconstitution of recombinant Tn subunits into muscle fibres was performed as described previously (Chandra et al. 2007). c-myc-tagged wild-type McTnT (McTnTWT) was used as the control. In brief, the extraction of native Tn subunits was carried out using an extraction solution containing a mixture of McTnT (c-myc McTnT or McTnT1-44Δ or McTnT45-74Δ) and McTnI proteins. c-myc McTnTWT was used in our procedure so that the incorporation of exogenously added Tn subunits in the control reconstitution could be assessed by observing a differential SDS gel migration pattern of c-myc McTnT when compared with the endogenous McTnT. It has been shown that the use of McTnT with an 11 amino acid c-myc epitope at the N-terminus does not affect the normal cardiac function (Tardiff et al. 1998; Montgomery et al. 2001). Replacement of endogenous Tn subunits was carried out using an extraction solution containing McTnT (c-myc McTnT or McTnT1-44Δ or McTnT45-74Δ; 1.5 mg ml−1, W/V) and McTnI (1.0 mg ml−1, W/V) in 50 mm Tris-HCl (pH 8.0), 6 m urea, 1.0 m KCl, 10 mm DTT and a cocktail of protease inhibitors. High salt and urea in the extraction solution were removed according to a protocol described previously (Chandra et al. 2006). Finally, the McTnT–McTnI-treated fibres were incubated with McTnC to complete the reconstitution procedure. Detergent-skinned fibres reconstituted with McTnT1-44Δ+ McTnI + McTnC are referred to as ‘McTnT1-44Δ’, and those reconstituted with McTnT45-74Δ+ McTnI + McTnC are referred to as ‘McTnT45-74Δ’. Fibres reconstituted with c-myc McTnT + McTnI + McTnC are referred to as ‘McTnTWT’ and were used as controls.
The reconstituted fibres were resuspended in 2% SDS solution (10 μl fibre−1) for SDS–PAGE, as described previously (Mamidi et al. 2012). SDS-digested fibres were mixed with an equal volume of protein loading dye that contained 125 mm Tris-HCl (pH 6.8), 20% glycerol, 2% SDS, 0.01% bromophenol blue and 50 mmβ-mercaptoethanol. Equal quantities of protein (15 μg of total protein for NTG and 10 μg of total protein for TG samples) from the digested fibres were loaded and separated on a SDS–PAGE (4% acrylamide stacking gel and 10% acrylamide separating gel) and stained with Bio-Safe Coomassie blue G-250 (Bio-Rad Laboratories, Inc., Hercules, CA, USA) to visualize the differential migration pattern of exogenously added McTnT proteins when compared with endogenous McTnT.
Proteins separated on the SDS gel were transferred onto a polyvinylidene fluoride membrane for Western blot analysis using a Trans-Blot Turbo Transfer System (Bio-Rad Laboratories, Inc). The incorporation of c-myc McTnT, McTnT1-44Δ or McTnT45-74Δ was assessed using a monoclonal anti-TnT primary antibody (Sigma-Aldrich, ST Louis, MO, USA; clone JLT-12), followed by a HRP-labelled anti-mouse secondary antibody (Amersham Biosciences, Pittsburgh, PA, USA). Densitometric scanning of Western blots was performed using Image J software (acquired from NIH at: http://rsbweb.nih.gov/ij/).
Estimation of isoform levels and phosphorylation status of sarcomeric proteins in NTG and TG heart samples
Ventricles from NTG and TG mice were frozen in liquid nitrogen and thoroughly pulverized using a pestle and mortar as described previously (Ford et al. 2012). The pulverized tissue was resuspended in a protein extraction buffer (10 μl of buffer per 1 mg of tissue). The composition of the protein extraction buffers was: 2.5% SDS, 10% glycerol, 50 mm Tris base (pH 6.8 at 4°C), 1 mm DTT, 1 mm PMSF, 4 mm benzamidine-HCl, a cocktail of phosphatase inhibitors (PhosSTOP; Roche Applied Science, Indianapolis, IN, USA) and protease inhibitors (E-64, bestatin and leupeptin). The resuspended tissue was further homogenized using a tissue tearor and sonication in a water bath at 4°C. The sample was finally centrifuged at 10,000 rpm (7200g). Equal quantities (5 μg of total protein) of NTG and TG heart samples were loaded and separated on a 12.5% SDS gel. SDS gels were stained with either Coomassie blue or Pro-Q Diamond (Invitrogen, Carlsbad, CA, USA) to assess isoform expression levels or phosphorylation status of sarcomeric proteins.
Measurement of steady-state isometric tension and ATPase activity in reconstituted cardiac muscle fibres
Measurement of isometric steady-state tension and ATPase activity was according to a procedure described previously (Chandra et al. 2007, 2009). In brief, T-shaped aluminum clips were used to hold the muscle fibre between the motor arm (322C; Aurora Scientific Inc, Aurora, Ontario, Canada) and the force transducer (AE 801; Sensor One Technologies Corp, Sausalito, CA, USA). The resting sarcomere length (SL) was set to 2.3 μm using a laser diffraction pattern (Chandra et al. 2007, 2009). The muscle fibre was immersed in a constantly-stirred chamber containing Ca2+ solutions, the concentration of which ranged from pCa 4.3 to 9.0. The composition of various pCa solutions was calculated using methods described previously (Fabiato & Fabiato, 1979). The composition of the maximal Ca2+ activation solution (pCa 4.3) was (in mm): potassium propionate, 31; Na2ATP, 5.95; MgCl2, 6.61; EGTA, 10; CaCl2, 10.11; BES, 50; sodium azide, 5; phosphoenol pyruvate (PEP), 10. The composition of the relaxing solution (pCa 9.0) was (in mm): potassium propionate, 51.14; Na2ATP, 5.83; MgCl2, 6.87; EGTA, 10; CaCl2, 0.024; BES, 50; NaN3, 5; PEP, 10. The activation and relaxing solutions also contained 0.5 mg ml−1 pyruvate kinase (500 U mg−1), 0.05 mg ml−1 lactate dehydrogenase (870 U mg−1), 20 μm diadenosine pentaphosphate, 10 μm oligomycin and a cocktail of protease inhibitors. The pH of the Ca2+ solutions was adjusted to 7.0 using KOH. The ionic strength was 180 mm. After two cycles of maximal activation and relaxation, the SL of the muscle fibre was again monitored and set to 2.3 μm if necessary. The muscle fibre was then immersed in a series of Ca2+ solutions (pCa 4.3−9.0), and the isometric steady-state tensions were digitally recorded on a computer.
Steady-state isometric ATPase activity was measured according to a protocol described previously (de Tombe & Stienen, 1995; Chandra et al. 2007). Briefly, near-UV light (340 nm) was projected through the muscle chamber, then split (50:50) via a beam splitter and detected at 340 nm (sensitive to changes in NADH) and 400 nm (insensitive to changes in NADH). ATPase activity was measured as follows: ATP regeneration from ADP was coupled to the breakdown of PEP to pyruvate and ATP catalysed by pyruvate kinase, which was linked to the synthesis of lactate catalysed by lactate dehydrogenase. The breakdown of NADH during the synthesis of lactate was proportional to the ATP consumption and was measured by changes in UV absorbance at 340 nm. The signal for NADH was calibrated by multiple injections of 0.25 nmol of ADP.
Measurement of muscle fibre mechano-dynamics in reconstituted cardiac muscle fibres
Muscle fibres were activated in pCa 4.3 solution and, once a steady-state isometric activation was attained, force responses to step-like length perturbations (±0.5%, ±1.0%, ±1.5% and ±2.0% of the muscle length (ML)) were recorded as described previously (Ford et al. 2010). A non-linear recruitment distortion (NLRD) model (Ford et al. 2010) was fit to the force responses to estimate the following model parameters: b, the rate by which new XBs are recruited due to a change in ML; c, the rate by which the strain of distorted XBs was dissipated following a change in ML; γ, a parameter that characterizes the XB strain-mediated effect on the recruitment of strong XBs; ED, the instantaneous muscle fibre stiffness estimated from the immediate force response elicited due to a sudden change in ML; and ER, the magnitude of increase, from an initial steady-state to a new steady-state, in muscle fibre stiffness due to length-mediated recruitment of additional XBs. For additional details on the NLRD model, please refer to the Supplemental Material.
Measurement of rate of tension redevelopment (ktr) in reconstituted cardiac muscle fibres
Measurements of ktr in maximally activated (pCa 4.3) muscle fibres was according to a protocol (Brenner & Eisenberg, 1986) described in the Supplemental Material.
Data analysis
Data were analysed using two-way ANOVA. One factor in this analysis was McTnT variant (McTnTWT, McTnT1−44Δ or McTnT45−74Δ), and the second was Tm isoform (α-Tm or β-Tm). Therefore, we used two-way ANOVA to test the hypothesis that the effect of the McTnT variant on a given contractile parameter depended on the type of Tm isoform (interaction effect). When the interaction effect was significant, it showed that the differences between effects of McTnTWT, McTnT1−44Δ or McTnT45−74Δ were dissimilar in the presence of different Tm isoforms. When the interaction effect was not significant, we interpreted the main effect due to McTnT variant or Tm isoform. Planned multiple pairwise comparisons were made using Fisher's LSD method to test the effects of McTnT variants or Tm isoforms on contractile parameters. Hill's equation was fitted to normalized pCa–tension data to estimate pCa50 (−log of [Ca2+]free required for half-maximal activation) and Hill coefficient (nH) values. Values are reported as mean ± SEM. The criterion for statistical significance was set at P < 0.05. Asterisks in the figures represent statistical significance using post hoc (Fisher's LSD) comparisons.
Results
Rationale for the generation of McTnT1-44Δ and McTnT45-74Δ deletion mutants
Based on our recent work (Gollapudi et al. 2012), we identified two specific regions in McTnT as the potential candidates for the functional effects of the N-terminal region on cardiac thin filaments: (1) the highly acidic region of McTnT (1−44 amino acids), which shares 50% sequence homology with its counterpart (1−41 amino acids) from the mouse fast skeletal TnT (MfsTnT); and (2) the peptide sequence corresponding to 45−74 amino acids of McTnT, which is only present in the cTnT (Fig. 1A and Fig. S3 in the Supplemental Material; Perry, 1998). These specific regions were deleted from McTnT to create McTnT1−44Δ and McTnT45−74Δ (Fig. 1B).
Figure 1. Rationale for the generation of mouse cardiac troponin T (McTnT)1-44Δ and McTnT45-74Δ deletion mutants.
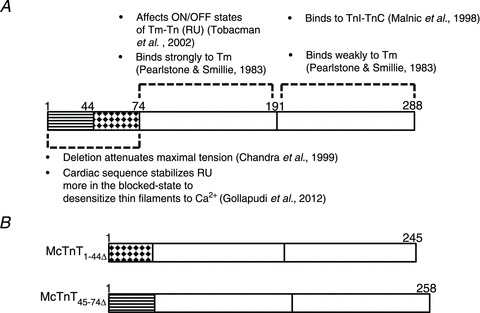
Based on our recent results from TG experiments (Gollapudi et al. 2012), we have identified two important regions within the N-terminus of McTnT (see Fig. S3 in the Supplemental Material for details). A, schematic representation of McTnT sequence depicting the functional significance of its N-terminal end (residues 1–74), N-terminal region (residues 75–191) and C-terminal region (residues 192–288). Regions of McTnT that have been deleted in the present study are indicated by striped (McTnT1-44Δ) and dotted (McTnT45-74Δ) patterns, respectively. B, schematic representation of McTnT1-44Δ and McTnT45-74Δ sequences. Tm, tropomyosin; Tn, troponin; RU, regulatory unit.
SDS–PAGE analysis of mouse cardiac muscle fibres to estimate the expression levels of β-Tm and the phosphorylation status of myofilament proteins
An equal quantity (5 μg of total protein) of samples from ventricular homogenates of NTG (α-Tm) and TG (β-Tm) mice was loaded and separated on a 12.5% SDS–PAGE, followed by staining with Coomassie blue. As shown in Fig. 2A, the amount of β-Tm was estimated to be ∼55% and the amount of α-Tm was ∼45% in the TG samples (Muthuchamy et al. 1995). No extra bands, apart from Tm, were found in TG samples, indicating that the isoform expression of other sarcomeric proteins such as myosin heavy chain, cardiac myosin binding protein-C, cTnT, cTnI and myosin light chain-1 were not different between NTG and TG samples. 12.5% SDS gels were stained with Pro-Q Diamond (Invitrogen) to estimate the phosphorylation status of sarcomeric proteins in NTG and TG samples (Ford et al. 2012). As shown in Fig. 2B, the phosphorylation status of sarcomeric proteins, apart from Tm, was not different between NTG and TG samples. TG samples had an extra phosphorylated band that corresponded to β-Tm.
Figure 2. SDS–PAGE analysis of non-transgenic (NTG) and transgenic (TG) ventricular homogenates to estimate the expression level of β-tropomyosin (Tm) and phosphorylation status of sarcomeric proteins.
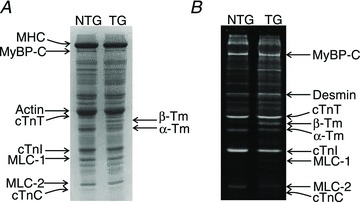
Samples from NTG and TG ventricular homogenates were run on a 12.5% SDS gel. A, Coomassie blue-stained SDS gel showing an expression of ∼55% of β-Tm and ∼45% of α-Tm in TG hearts. There are no extra bands in the TG sample, apart from Tm, indicating that there are no changes in the isoform expression of other sarcomeric proteins between the NTG and TG samples. B, Pro-Q Diamond-stained SDS gel indicating that the phosphorylation status of sarcomeric proteins is not significantly different between NTG and TG samples. As expected, there is an additional band corresponding to β-Tm in the TG fibre lane. cTnC, cardiac troponin C; cTnI, cardiac troponin I; cTnT, cardiac troponin T; MHC, myosin heavy chain; MLC-1, myosin light chain-1; MyBP-C, myosin binding protein-C.
SDS–PAGE and Western blot analysis of reconstituted fibres to estimate the extent of incorporation of exogenously added Tn subunits
McTnTWT was used as the control. In our reconstitution procedure, the exogenously added Tn complex consisted of McTnT (c-myc McTnT or McTnT1-44Δ or McTnT45-74Δ), McTnI and McTnC proteins. The incorporation of McTnTWT was detected by the differential migration pattern of c-myc-tagged McTnT, when compared with the endogenous cTnT. The incorporation of McTnT1-44Δ or McTnT45-74Δ was detected based on mobility differences when compared with the endogenous cTnT. As we have shown before (Chandra et al. 1999), the endogenous Tn complex is replaced as a whole when the molar excess of exogenously added cTnT competes with the endogenous cTnT. Therefore, the bands that correspond to McTnI and McTnC in the reconstituted fibres belong to the exogenously added components. First, the SDS-digested reconstituted fibre samples were run on an 8% SDS gel to visualize the mobility-based migration pattern of exogenously added McTnT proteins. Using SDS gels, we could not precisely quantify the extent of McTnT incorporation because the c-myc-tagged McTnT (WT) co-migrated with actin (lane c of Fig. 3A), while the presence of Tm bands hindered unambiguous quantification of McTnT1−44Δ or McTnT45−74Δ (lanes d and e of Fig. 3A); hence, we used the Western blot analysis. Image J software was used to accurately quantify the optical intensities of the protein bands. The total optical band intensity (i.e. the total amount of cTnT in a single lane) was assumed to be the sum of the optical band intensities of endogenous McTnT and the incorporated McTnT (c-myc-tagged McTnT, McTnT1-44Δ or McTnT45-74Δ) bands. The extent of incorporation was then determined by dividing the optical band intensity of the exogenously added McTnT with the total band intensity. This approach allowed us to unambiguously estimate the extent of incorporation of different proteins: it was 90% for both c-myc-tagged McTnT (lane c of Fig. 3B) and McTnT45-74Δ (lane b of Fig. 3C), but 97% for McTnT1-44Δ (lane c of Fig. 3C). The extent of replacement of endogenous McTnT in the reconstituted TG fibre groups was similar to that in the reconstituted NTG fibre groups.
Figure 3. SDS–PAGE and Western blot analysis of reconstituted NTG (α-tropomyosin (Tm)) mouse fibres.
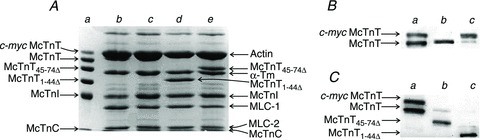
Reconstituted NTG fibres were digested using 2% SDS (Mamidi et al. 2012). Digested samples were run on a 10% SDS gel to estimate the extent of replacement of endogenous Tn subunits. c-myc-tagged wild-type McTnT (McTnTWT) was used as the control. Muscle fibre preparations from normal NTG hearts were used to show the extent of removal of endogenous cTnT in the reconstituted fibres. A, SDS gel showing the differential migration pattern of McTnTWT, McTnT1-44Δ or McTnT45-74Δ proteins in the reconstituted NTG fibre samples. Lane a represents purified recombinant proteins. Lanes b–e represent samples from normal NTG fibre, McTnTWT, McTnT1-44Δ or McTnT45-74Δ reconstituted fibres. Lane b shows the presence of the endogenous McTnT, whereas lanes c–e show a near-complete replacement of endogenous McTnT in the reconstituted samples. B, Western blot analysis of the reconstituted fibre samples. Lane a represents purified recombinant proteins. Lanes b and c represent samples from normal NTG and McTnTWT reconstituted samples, respectively. C, Western blot analysis of McTnT45-74Δ and McTnT1-44Δ reconstituted samples. Lane a represents purified recombinant proteins. Lanes b and c represent samples from McTnT45-74Δ and McTnT1-44Δ reconstituted fibres, respectively. MLC-1, myosin light chain-1.
Effect of McTnT deletion mutants on the Ca2+-activated maximal tension and ATPase activity in α- vs.β-Tm fibres
To assess the effect of McTnT deletion mutants on thin-filament activation, Ca2+-activated maximal tension was measured at pCa 4.3 in reconstituted fibres. Two-way ANOVA revealed no significant interaction effect, but showed a significant main effect (P < 0.0001) of McTnT deletion mutants on the Ca2+-activated maximal tension (Fig. 4A and B). To probe the determining factor for the significant main effect, subsequent post hoc tests were carried out. These post hoc tests using multiple planned pairwise comparisons revealed that McTnT1−44Δ, but not McTnT45−74Δ, had a significant effect of decreasing maximal tension under both α- and β-Tm backgrounds. McTnT1−44Δ decreased maximal tension by 36% in α-Tm fibres and by 30% in β-Tm fibres (Fig. 4A and B). Thus, whether it was under α- or β-Tm background, McTnT1−44Δ attenuated the Ca2+-activated maximal tension. In accord with our observations from Ca2+-activated tension experiments, McTnT1−44Δ attenuated the Ca2+-activated maximal ATPase activity in α- and β-Tm fibres (Table 1). In agreement with our observations from Ca2+-activated tension measurements, McTnT45−74Δ had no effect on maximal ATPase activity in α- or β-Tm fibres (Table 1).
Figure 4. Effect of mouse cardiac troponin T (McTnT) deletion mutants on Ca2+-activated maximal tension and muscle fibre stiffness (ED) in α- vs.β-tropomyosin (Tm) fibres.
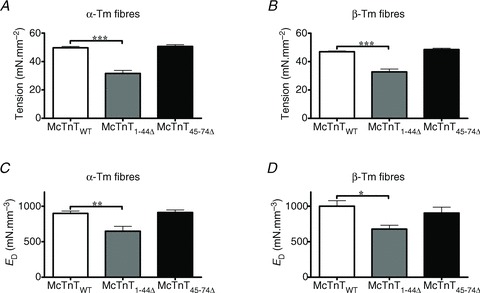
Ca2+-activated maximal tension was measured in reconstituted fibres at pCa 4.3 (de Tombe & Stienen, 1995). A, effect of McTnT deletions on Ca2+-activated maximal tension in NTG fibres containing α-Tm. The number of determinations was 14, 13 and 14 for McTnTWT, McTnT1-44Δ and McTnT45-74Δ groups, respectively. B, effect of McTnT deletions on Ca2+-activated maximal tension in TG fibres containing β-Tm. The number of determinations was 13, 14 and 14 for McTnTWT, McTnT1-44Δ and McTnT45-74Δ groups, respectively. Two-way ANOVA revealed no significant interaction effect, but revealed a significant main effect (P < 0.0001) of McTnT deletions on Ca2+-activated maximal tension. C, effect of McTnT deletions on ED in NTG fibres containing α-Tm. The number of determinations was 12, 7 and 13 for McTnTWT, McTnT1-44Δ and McTnT45-74Δ groups, respectively. D, effect of McTnT deletions on ED in TG fibres containing β-Tm. The number of determinations was 14, 7 and 12 for McTnTWT, McTnT1-44Δ and McTnT45-74Δ groups, respectively. ED represents muscle fibre stiffness and is an approximation of the number of strongly-bound XBs (Ford et al. 2010). ED was estimated by subjecting maximally activated muscle fibres to step-like length changes (Ford et al. 2010). Two-way ANOVA revealed no significant interaction effect, but showed a significant main effect (P < 0.001) of McTnT deletions on ED. Values are reported as mean ± SEM. ***P < 0.001; **P < 0.01; *P < 0.05.
Table 1.
ktr, b, ATPase and pCa50 (ATPase) values in NTG (α-Tm) and TG (β-Tm) fibres
| McTnTWT | McTnT1-44Δ | McTnT45-74Δ | |
|---|---|---|---|
| NTG (α-Tm) | |||
| ktr (s−1) | 12.87 ± 0.46 (14) | 14.18 ± 0.47 (13) | 14.05 ± 0.56 (14) |
| b (s−1) | 31.81 ± 1.41 (12) | 34.88 ± 3.20 (7) | 33.83 ± 1.61 (13) |
| ATPase (pmol mm−3 s−1) | 304.38 ± 9.36 (14) | 225.33 ± 10.67 (13)*** | 303.79 ± 7.97 (14) |
| pCa50 (ATPase) | 5.69 ± 0.02 (11) | 5.58 ± 0.03 (12)** | 5.99 ± 0.03 (7)*** |
| TG (β-Tm) | |||
| ktr (s−1) | 12.58 ± 0.50 (12) | 13.84 ± 0.65 (14) | 13.86 ± 0.52 (14) |
| b (s−1) | 31.94 ± 1.33 (14) | 35.42 ± 1.63 (7) | 34.79 ± 1.13 (12) |
| ATPase (pmol mm−3 s−1) | 258.01 ± 3.77 (10) | 224.16 ± 8.31 (14)*** | 278.02 ± 8.44 (14) |
| pCa50 (ATPase) | 5.74 ± 0.01 (8) | 5.71 ± 0.01 (10) | 6.18 ± 0.02 (7)*** |
The rate of tension redevelopment (ktr) was determined using a large release and restretch protocol (Brenner & Eisenberg, 1986). The rate of XB recruitment (b) was determined by fitting the NLRD model to the force responses elicited by step-like changes in ML (Ford et al. 2010). The Hill equation was fitted to the normalized pCa–ATPase relationships to derive pCa50 (ATPase) values. The number of determinations for each group is presented in the brackets beside each estimate. Values are reported as mean ± SEM. **P < 0.005; ***P < 0.001 when compared with McTnTWT within the respective groups. McTnT, mouse cardiac troponin T; McTnTWT, wild-type mouse cardiac troponin T; NTG, non-transgenic; TG, transgenic; Tm, tropomyosin.
Effect of McTnT deletion mutants on the muscle fibre stiffness in α- vs.β-Tm fibres
To examine if the decrease in maximal tension by McTnT1−44Δ was due to a decrease in the number of force-generating XBs, we measured the instantaneous muscle fibre stiffness (ED) by imposing step-like length perturbations in constantly-activated muscle fibres (Ford et al. 2010). ED is an approximate measure of stiffness of the population of strongly-bound XBs at the time of instantaneous ML change (refer to the Supplemental Material for details; and Ford et al. 2010). Two-way ANOVA showed no significant interaction effect, but revealed a significant main effect (P < 0.001) of McTnT deletion mutants on ED. Subsequent post hoc tests revealed that McTnT1−44Δ, but not McTnT45−74Δ, had a significant effect of decreasing ED in both α- and β-Tm fibres (Fig. 4C and D). For example, McTnT1−44Δ decreased ED by ∼28% in α-Tm fibres (Fig. 4C) and by ∼32% in β-Tm fibres (Fig. 4D). Thus, our data demonstrated that the decrease in Ca2+-activated maximal tension in fibres reconstituted with McTnT1−44Δ was due to reduced thin-filament activation caused by a decrease in the number of force-generating XBs. That the peptide segment 1−44 is essential for maximal activation is supported by our previous studies on the deletion of 1−76 amino acids in rat cTnT (RcTnT; Chandra et al. 1999) and the caspase-activated deletion of 1−96 amino acids in RcTnT (Communal et al. 2002). Although these two previous studies could not locate the specific segment responsible for the depression of thin-filament activation, our present study demonstrates that it is the first 44 amino acids of cTnT. This inference is supported by our observation that McTnT45−74Δ mutant showed no effect on maximal activation because the peptide segment essential for maximal activation (1−44 amino acids) is still intact in the McTnT45−74Δ mutant.
Effect of McTnT deletion mutants on myofilament Ca2+ sensitivity (pCa50) in α- vs.β-Tm fibres
Normalized tension values were plotted against a range of pCa to estimate the pCa–tension relationships in the reconstituted fibres (Fig. 5A and B). The Hill equation was fitted to the pCa–tension relationships to estimate the pCa50 (Fig. 5C). Two-way ANOVA revealed a significant interaction effect (P < 0.01) on pCa50, suggesting that the effect of McTnT deletions on pCa50 was influenced by the Tm isoform. In other words, these results demonstrate that the interplay between the effects of McTnT and Tm on thin filaments modulates Ca2+ sensitivity of tension development. In α-Tm fibres, McTnT1−44Δ caused a significant decrease (P < 0.001) in pCa50, as indicated by a rightward shift in the pCa–tension relationship (Fig. 5A). The [Ca2+]free needed to produce half-maximal tension was ∼23% more in α-Tm fibres containing McTnT1-44Δ when compared with the control McTnTWT. Irrespective of the type of reconstitution, β-Tm fibres showed a higher pCa50 when compared with the corresponding group of α-Tm fibres (Fig. 5C). An interesting observation was that the decrease in pCa50 induced by McTnT1−44Δ under an α-Tm background was ablated under a β-Tm background (indicated by arrows in Fig. 5B). This demonstrated that β-Tm maintained its ability to increase myofilament Ca2+ sensitivity even in the presence of a McTnT mutant protein that had the ability to decrease myofilament Ca2+ sensitivity – thus, resulting in attenuating the effect of McTnT1−44Δ on myofilament Ca2+ sensitivity under β-Tm background.
Figure 5. Effect of mouse cardiac troponin T (McTnT) deletion mutants on pCa–tension relationships, myofilament Ca2+-sensitivity (pCa50) and cooperativity of tension development (nH) in α- vs.β-tropomyosin (Tm) fibres.
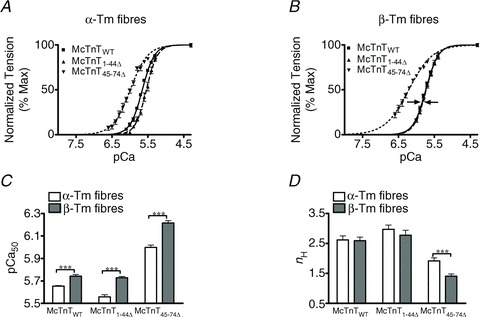
Normalized tension values were plotted against pCa to derive the pCa–tension relationships. The Hill equation was then fitted to the pCa–tension relationships to estimate pCa50 and nH. A, effect of McTnT deletion mutants on pCa–tension relationships in NTG fibres containing α-Tm. B, effect of McTnT deletion mutants on pCa–tension relationships in TG fibres containing β-Tm. C, effect of McTnT deletion mutants on pCa50 in α- and β-Tm fibres. D, effect of McTnT deletion mutants on nH in α- and β-Tm fibres. Two-way ANOVA revealed a significant interaction (P < 0.01) on pCa50, suggesting that the effect of McTnT deletion mutants on pCa50 was influenced by the type of Tm isoform present. The main contributing factor for the significant interaction effect was the ablation of McTnT1−44Δ-induced reduction in pCa50 under a β-Tm background (indicated by arrows in B). Irrespective of the type of reconstitution, TG (β-Tm) fibres exhibited a higher pCa50 when compared with the corresponding group of NTG (α-Tm) fibres. Two-way ANOVA revealed no significant interaction effect, but revealed significant main effects due to McTnT deletion mutants (P < 0.001) and Tm isoforms (P < 0.05) on nH. Subsequent post hoc tests revealed that McTnT45−74Δ led to a decreased nH under both α- and β-Tm backgrounds, with the decrease being more pronounced under a β-Tm background. The number of determinations for pCa–tension relationships, pCa50 and nH was 11, 12 and 14 for McTnTWT, McTnT1−44Δ and McTnT45−74Δ groups of α-Tm fibres, respectively. The number of determinations for pCa–tension relationships, pCa50 and nH was 13, 10 and 14 for McTnTWT, McTnT1−44Δ and McTnT45−74Δ groups of β-Tm fibres, respectively. Values are reported as mean ± SEM. ***P < 0.001.
In contrast, McTnT45−74Δ had an opposite effect on myofilament Ca2+ sensitivity of tension (Fig. 5). For example, McTnT45−74Δ induced a leftward shift in the pCa–tension relationship (Fig. 5A and B), causing a significant increase in pCa50 (Fig. 5C). The [Ca2+]free needed to produce half-maximal tension was ∼55% less in α-Tm fibres containing McTnT45−74Δ when compared with the control McTnTWT. Another significant observation was that the increase in pCa50 induced by McTnT45−74Δ, under an α-Tm background, was further enhanced under a β-Tm background (Fig. 5C). Thus, the [Ca2+]free needed to produce half-maximal tension was ∼67% less in β-Tm fibres containing McTnT45−74Δ when compared with the β-Tm fibres containing McTnTWT. Results from pCa–ATPase relationships also showed similar effects (Table 1). These observations highlight the ability of β-Tm to increase myofilament Ca2+ sensitivity under very different conditions (Fig. 5C). Collectively, these results demonstrate that the Tm isoforms have divergent effects on McTnT deletion mutant's ability to modulate myofilament Ca2+ sensitivity.
Effect of McTnT deletion mutants on cooperativity of tension development (nH) in α- vs.β-Tm fibres
The Hill equation was fitted to pCa–tension relationships to estimate nH in the reconstituted fibres (Fig. 5D). Two-way ANOVA revealed no significant interaction effect, but showed a significant main effect (P < 0.05) of McTnT deletion mutants and Tm isoforms on nH. Post hoc tests revealed that McTnT45-74Δ, but not McTnT1-44Δ, induced a significant decrease in nH under both α- and β-Tm backgrounds (Fig. 5D). McTnT45-74Δ decreased nH by ∼37% in α-Tm fibres, but the McTnT45-74Δ-induced decrease in nH was greater under β-Tm background, as suggested by a ∼46% decrease in nH (Fig. 5D). It is interesting to note that while McTnT1-44Δ showed no effect on nH, it significantly attenuated maximal tension. On the other hand, McTnT45-74Δ showed a significant attenuating effect on nH, but it had no impact on maximal tension. This further highlights our observation that these two regions (1-44 and 45-74) of cTnT have discrete functional properties.
Effect of McTnT deletion mutants on XB detachment kinetics in α- vs.β-Tm fibres
XB detachment kinetics in the reconstituted fibres was assessed by estimating the tension cost and the rate of XB distortion (c). To estimate tension cost values, the relationship between steady-state isometric tension and the corresponding ATPase activity was determined at various levels of Ca2+ activation (de Tombe & Stienen, 1995; Chandra et al. 2007). Two-way ANOVA revealed a significant interaction effect (P < 0.05), indicating the effect of McTnT deletion mutants on tension cost is influenced by the type of Tm isoform present. Subsequent post hoc tests revealed that McTnT1−44Δ, but not McTnT45−74Δ, had a significant effect of increasing tension cost under β-Tm background (Fig. 6A and B). McTnT1−44Δ increased tension cost by ∼29% in β-Tm fibres, when compared with the control β-Tm fibres reconstituted with McTnTWT (Fig. 6B). Because changes in tension cost reflect changes in the rate of XB detachment (Brenner, 1988), our data demonstrate that the rate of XB detachment is augmented by McTnT1−44Δ in TG fibres containing β-Tm (Fig. 6B).
Figure 6. Effect of mouse cardiac troponin T (McTnT) deletion mutants on tension cost and rate of XB distortion dynamics (c) in α- vs.β-tropomyosin (Tm) fibres.
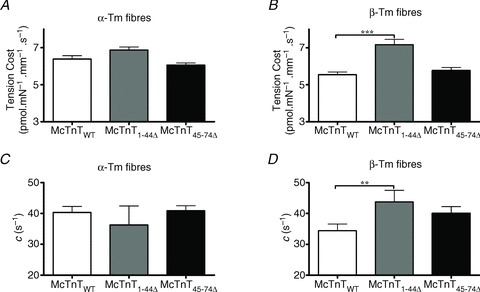
Steady-state isometric tension and ATPase activity were measured simultaneously (de Tombe & Stienen, 1995; Chandra et al. 2007). Tension cost was derived from ATPase/tension relationship, as described previously (Chandra et al. 2006). c was estimated by fitting the NLRD model to force responses elicited by step-like changes in ML (Ford et al. 2010). Both tension cost and c represent the rate of XB detachment (Campbell et al. 2004). A, effect of McTnT deletion mutants on tension cost in NTG fibres containing α-Tm. The number of determinations was 12, 13 and 14 for McTnTWT, McTnT1-44Δ and McTnT45−74Δ groups, respectively. B, effect of McTnT deletion mutants on tension cost in TG fibres containing β-Tm. The number of determinations was 13, 14 and 14 for McTnTWT, McTnT1−44Δ and McTnT45-74Δ groups, respectively. Two-way ANOVA revealed a significant interaction effect (P < 0.05), indicating that the effect of McTnT deletion mutants on tension cost is influenced by the type of Tm isoform present. Subsequent post hoc tests revealed that McTnT1-44Δ, but not McTnT45-74Δ, had a significant effect of increasing tension cost under a β-Tm background. C, effect of McTnT deletion mutants on c in NTG fibres containing α-Tm. The number of determinations was 12, 7 and 13 for McTnTWT, McTnT1-44Δ and McTnT45-74Δ groups, respectively. D, effect of McTnT deletion mutants on c in TG fibres containing β-Tm. The number of determinations was 14, 7 and 12 for McTnTWT, McTnT1-44Δ and McTnT45-74Δ groups, respectively. Two-way ANOVA revealed no significant interaction effect and no significant main effects on c. However, similar to the effects observed for tension cost data, post hoc tests revealed that McTnT1-44Δ, but not McTnT45-74Δ, had a significant effect of increasing c under a β-Tm background. Values are reported as mean ± SEM. **P < 0.05; ***P < 0.0001.
To substantiate our observations from tension cost experiments, we measured the rate of XB detachment kinetics, as estimated from XB distortion dynamics, c (Fig. 6C and D). Quick stretch-induced instantaneous increase in force (F1 in Fig. S1 of Supplemental Material) decreases rapidly to a minimum with a characteristic dynamic rate. This rapid force decay in Phase 2 (Fig. S1) represents the dynamic detachment of strained XBs (Piazzesi et al. 1997; Campbell et al. 2004; Stelzer et al. 2006), and has been shown to be a measure of XB detachment rate (Campbell et al. 2004; Chandra et al. 2006). Two-way ANOVA showed that both the interaction and main effects were not significant. However, just as we observed in the tension cost experiments (Fig. 6B), subsequent post hoc tests showed that McTnT1−44Δ, but not McTnT45-74Δ, had a significant effect (P < 0.001) of increasing c under β-Tm background (Fig. 6D). For example, β-Tm fibres reconstituted with McTnT1-44Δ showed a ∼27% increase in c, when compared with the control β-Tm fibres reconstituted with McTnTWT (Figs 6D and 8). Therefore, results from two independent and diverse experimental approaches demonstrate that the XB detachment kinetics is augmented by McTnT1-44Δ in β-Tm fibres, but not in α-Tm fibres.
Figure 8. Effect of mouse cardiac troponin T (McTnT)1−44Δ on c and γ in TG β-Tm fibres.
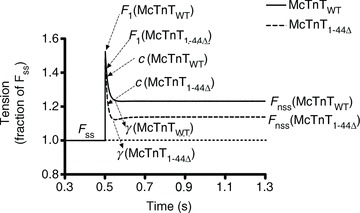
Force responses normalized to the steady-state isometric force, Fss, are shown for McTnTWT and McTnT1-44Δ reconstituted β-Tm fibres. Fnss represents the new steady-state force attained by the muscle fibre due to a change in ML. Force responses to 2% stretch in ML show that the instantaneous force (F1) is lower, while c is higher in β-Tm fibres reconstituted with McTnT1-44Δ. Force responses also show that γ is greater for McTnT1−44Δ reconstituted fibres as indicated by a greater dip in the force response. The new steady-state force attained by the muscle fibre, Fnss, was lower in McTnT1-44Δ reconstituted fibres, indicating that the ML-induced recruitment of additional XBs (ER) is reduced.
Effect of McTnT deletion mutants on XB strain-dependent impact on other XBs (γ) in α- vs.β-Tm fibres
Enhancement of XB detachment kinetics may affect the way strained XBs impact other XBs through an effect that is transduced via the thin-filament regulatory systems (Ford et al. 2010). As described in the Supplemental Material, the NLRD model parameter, γ, represents an effect by which strained XBs negatively impact other XBs via allosteric/cooperative mechanisms in the thin filament. Based on the augmenting effect of McTnT1-44Δ on XB detachment kinetics (Fig. 6), we predicted that McTnT1-44Δ would increase γ in the presence of β-Tm. Two-way ANOVA revealed no significant interaction effect, but showed a significant main effect (P < 0.05) of McTnT deletions on γ (Fig. 7A and B). The contributing factor for this significant main effect was determined by post hoc tests using multiple planned-pairwise comparisons. Post hoc analysis showed that McTnT1-44Δ, but not McTnT45-74Δ, induced a significant increase in γ under β-Tm background (Figs 7B and 8). For example, β-Tm fibres reconstituted with McTnT1-44Δ showed a ∼58% increase in γ when compared with the control β-Tm fibres reconstituted with McTnTWT (Fig. 7B). These observations again highlight our findings that α- and β-Tm isoforms have divergent impact on myofilament function.
Figure 7. Effect of mouse cardiac troponin T (McTnT) deletion mutants on γ and ER in α- vs.β-tropomyosin (Tm) fibres.
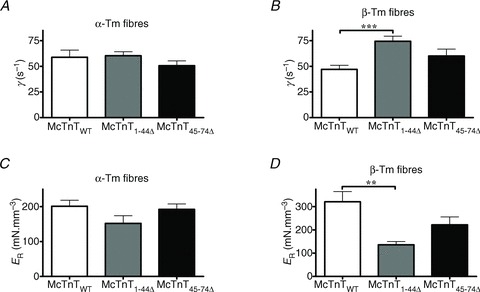
γ and ER were determined by fitting the NLRD model to the force responses elicited by the muscle fibres due to step-like length perturbations (Ford et al. 2010). A, effect of McTnT deletion mutants on γ in NTG fibres containing α-Tm. B, effect of McTnT deletion mutants on γ in TG fibres containing β-Tm. Two-way ANOVA revealed no significant interaction effect, and showed a main effect of McTnT deletions on γ. Subsequent post hoc tests showed that McTnT1-44Δ, but not McTnT45-74Δ, induced a significant increase in γ under a β-Tm background. C, effect of McTnT deletion mutants on ER in NTG fibres containing α-Tm. D, effect of McTnT deletion mutants on ER in TG fibres containing β-Tm. Two-way ANOVA revealed no significant interaction effect, and showed a significant main effect of McTnT deletions on ER. Subsequent post hoc tests revealed that McTnT1-44Δ lead to a significant decrease in ER under a β-Tm background. The number of determinations for γ and ER was 12, 7 and 13 for McTnTWT, McTnT1-44Δ and McTnT45-74Δ groups of α-Tm fibres, respectively. The number of determinations for γ and ER was 14, 7 and 12 for McTnTWT, McTnT1-44Δ and McTnT45-74Δ groups of β-Tm fibres, respectively. Values are reported as mean ± SEM. ***P < 0.001; **P < 0.01.
Effect of McTnT deletion mutants on the magnitude of the ML-mediated recruitment of new force-bearing XBs
McTnT1-44Δ-mediated effect on γ may decrease the magnitude of recruitment of new force-bearing XBs because its effect is to shift the equilibrium of XB recruitment away from the force-bearing XB population. Therefore, we measured the magnitude of increase in muscle fibre stiffness (ER), resulting from the recruitment of additional XBs in response to an increase in ML (Ford et al. 2010). Thus, changes in the estimates of ER can be correlated to changes in the SL-dependent activation of the muscle fibres. Stretch-activated force – resulting from a change in ML (Phase 3 of Fig. S1 in the Supplemental Material) – reaches a new steady-state level (Fnss). The difference between Fnss and the initial steady-state force (Fss) represents the magnitude of newly-recruited force-bearing XBs. Two-way ANOVA revealed no significant interaction effect, but showed a main effect (P < 0.01) of McTnT deletion mutants on ER. Subsequent post hoc tests revealed that ER was unaltered by McTnT1-44Δ under α-Tm background (Fig. 7C) and decreased significantly under β-Tm background (Fig. 7D). For example, McTnT1−44Δ decreased ER by ∼58% in β-Tm fibres (Fig. 7D). On the other hand, McTnT45-74Δ had no effect on ER in α- or β-Tm fibres (Fig. 7C and D).
Effect of McTnT deletion mutants on XB turnover rate in α- vs.β-Tm fibres
McTnT1-44Δ augmented the negative effect of strained XBs on other XBs (γ), which suggested that the equilibrium of XB recruitment was shifted away from the force-bearing XB population. Therefore, we assessed the XB turnover rate by estimating the rate of tension redevelopment (ktr) and the rate of XB recruitment (b) in maximally activated muscle fibres. As shown in Table 1, both McTnT1-44Δ and McTnT45−74Δ showed a general trend to increase ktr and b in α- or β-Tm fibres. Two-way ANOVA revealed no significant interaction effect, but showed a significant main effect (P < 0.05) of McTnT deletions on ktr. Post hoc tests using multiple planned-pairwise comparisons were performed to assess the contributing factors for the significant main effect of deletions in McTnT on ktr. Post hoc tests showed that the McTnT1-44Δ-induced increase in ktr under α-Tm background was only marginally not significant (P= 0.057), whereas McTnT45−74Δ had no effect on ktr in α- or β-Tm fibres. Two-way ANOVA revealed no significant interaction effect and no significant main effects on b. Post hoc tests showed that the McTnT deletion mutants had no effect on b in α- or β-Tm fibres. Because our post hoc tests showed marginal effects on ktr, it precluded us from determining which of the McTnT deletion mutants was responsible for the main effect observed for ktr.
Discussion
The dual role of cTnT in thin-filament activation by Ca2+ and XBs is most likely a function of its effects on Tm–actin interactions (Heeley et al. 1987; Malnic & Reinach, 1994; Gaffin et al. 2006; Coulton et al. 2008). However, very little is known as to which specific region of cTnT participates in modulating Tm–actin interactions within a SU (Tm1–Tn1–actin7) or possibly between neighbouring SUs. In this study, we identify two key regions in the N-terminus of cTnT that have divergent effects on Ca2+-activated maximal tension, myofilament Ca2+ sensitivity and cooperativity of tension development. Furthermore, we provide new evidence to show that the interplay between cTnT and Tm also plays a key role in modulating the outcome of cardiac myofilament activation.
McTnT1-44Δ and McTnT45−74Δ have divergent effects on Ca2+-activated maximal tension
Within the 1-74 region of cTnT, we identify the specific region that is responsible for attenuating the Ca2+-activated maximal activation in cardiac myofilaments (Fig. 4). For example, it was McTnT1-44Δ, but not McTnT45-74Δ, that caused a significant decrease in Ca2+-activated maximal tension and myofibre stiffness (ED). Because both Ca2+-activated maximal tension and ED are a function of the number of strongly-bound XBs, a decrease in maximal activation can be attributed to a decrease in the number of strongly-bound XBs. Therefore, our data demonstrate that the amino acids 1-44 of McTnT are essential for maximal activation of cardiac myofilaments. Interestingly, changing the Tm background from α- to β-Tm did not alter the effects of McTnT1-44Δ and McTnT45-74Δ on Ca2+-activated maximal tension (Fig. 4B) or myofibre stiffness (Fig. 4D). Thus, our data show that cTnT has a dominant effect on Ca2+-activated maximal tension development in cardiac myofilaments.
Our data from tension measurements raise two important questions: (1) why is it that Ca2+-activated maximal tension is attenuated by McTnT1-44Δ, but not by McTnT45-74Δ?; (2) why is the effect of McTnT1-44Δ on maximal tension similar under both α- and β-Tm backgrounds? The attenuating effect on maximal tension by McTnT1−44Δ, but not by McTnT45-74Δ, can be understood in terms of the charge-related impact on cTnT–Tm interactions. For example, it is known that the region containing 258-284 residues of Tm – a key region involved in the formation of head-to-tail overlap of Tm – interacts with the N-terminus of TnT (Pearlstone & Smillie, 1983; Muthuchamy et al. 1997; Jagatheesan et al. 2010b) and carries a net charge of −3 (Jagatheesan et al. 2010a). In our study, the deletion of 1−44 residues amounted to a removal of 23 negative charges from the N-terminus of McTnT. Attenuation of electrostatic repulsion – caused by the removal of high density of negative charges – would be expected to enhance cTnT–Tm interactions (Chandra et al. 1999). Such enhanced cTnT–Tm interactions may act to stabilize the cardiac thin filament in a sub-maximally activated state, attenuating the Ca2+-activated maximal tension. This cTnT effect on thin-filament activation may result from the effect of cTnT on the flexibility of Tm.
Recent studies (Li et al. 2012; Loong et al. 2012) suggested that an increase in the flexibility of Tm could lead to an increased activation of thin filaments. For example, the E180G Tm mutation-induced increase in myofilament Ca2+ sensitivity was shown to be associated with an increased Tm flexibility (Li et al. 2012; Loong et al. 2012). A flexible Tm would easily shift its azimuthal position on the actin filament to uncover the myosin binding sites: this would lead to a shift in the on/off equilibrium of the regulatory unit (RU; Tm–Tn) more toward the on-state, and thus cause an increase in Ca2+ sensitivity (Li et al. 2012). Therefore, a rigid Tm may shift the on/off equilibrium of the RU more toward the off-state, leading to a decreased activation of thin filaments. Because XB-mediated activation of thin filaments is more prominent in the cardiac muscle (Adhikari et al. 2004; Regnier et al. 2004), a rigid Tm-induced decrease in the number of strongly-bound XBs could further attenuate the activation of thin filaments. Thus, the net effect of a rigid Tm on the thin filament is that fewer RUs are turned on; leading to a decreased number of actin-bound XBs in McTnT1-44Δ reconstituted fibres.
Unlike in the case of McTnT1-44Δ, the deletion of residues 45-74 amounted to a net removal of only six negative charges from the N-terminus of McTnT. Thus, McTnT45-74Δ would be expected to have minor effects on the electrostatic interactions between McTnT and Tm. Both tension (Fig. 4A and B) and ED (Fig. 4C and D) of McTnT45-74Δ fibres remained unaltered, substantiating our observation that McTnT1-44Δ, but not McTnT45−74Δ, was responsible for attenuating the Ca2+-activated maximal tension. The effect of McTnT1-44Δ on maximal tension was similar under both α- and β-Tm backgrounds (Fig. 4A and B). Because both mouse α- and β-Tm isoforms possess a similar net charge of −3 in the region encompassing residues 258−284 (Jagatheesan et al. 2010a), McTnT1−44Δ would be expected to have a similar effect on tension under either α- or β-Tm background. Different Tm isoforms had no effect on the McTnT45-74Δ mutant's ability to generate normal tension, nor did they modulate the attenuating effect of McTnT1-44Δ, indicating that cTnT – but not Tm – had a dominant effect on the Ca2+-activated maximal tension.
McTnT1-44Δ and McTnT45-74Δ have divergent effects on myofilament Ca2+ sensitivity and cooperativity
McTnT1-44Δ and McTnT45-74Δ had contrasting effects on myofilament Ca2+ sensitivity under α- but not under β-Tm background. Under α-Tm background, McTnT1-44Δ showed a significant decrease in pCa50, while McTnT45-74Δ showed a significant increase in pCa50 (Fig. 5C). Thus, it is likely that a synergistic effect of deleting the entire 1−74 region would be to result in unaltered Ca2+ sensitivity. Indeed, findings from our previous study (Chandra et al. 1999) showed that the deletion of 1−76 amino acids in rat cTnT resulted in an unaltered myofilament Ca2+ sensitivity.
One possible reason for the attenuating effect of McTnT1-44Δ on pCa50 is that the thin filament harboring a rigid Tm would require more Ca2+ for full activation. On the other hand, McTnT45-74Δ augmented pCa50, with no effect on either the maximal tension or myofibre stiffness. The relationship of this work to our recent studies on a TG mouse – which expressed a McTnT N-terminal extension mutant (McTnTNTE) in the heart – requires brief consideration (Gollapudi et al. 2012). The effect of McTnT45−74Δ on myofilament Ca2+ sensitivity and tension has an uncanny similarity to the effect observed due to the deletion of McTnTNTE in which the first 1−73 amino acids of McTnT were replaced by 1−41 amino acids of MfsTnT (Gollapudi et al. 2012). In that study, we showed McTnTNTE increased myofilament Ca2+ sensitivity, without affecting the maximal tension or ED. Based on our observations, we concluded that the cardiac-specific NTE of cTnT was important for stabilizing the thin filament more in the blocked-state (Gollapudi et al. 2012). Our results from the present study demonstrate that it is the 45−74 peptide segment that is responsible for the cardiac-specific effect on the thin filament. Interestingly, this extra peptide segment, albeit with minor amino acid variations, is present only in the cardiac variant of cTnT across all species (Perry, 1998).
Irrespective of the type of McTnT deletion mutant, fibres containing β-Tm exhibited a higher pCa50 than fibres containing α-Tm (Fig. 5C). Our present results, in conjunction with previous studies (Palmiter et al. 1996; Wolska et al. 1999), demonstrate that β-Tm sensitizes cardiac myofibres to Ca2+. The Ca2+ sensitizing effect of β-Tm ablated the McTnT1-44Δ-induced decrease in pCa50, which was observed under α-Tm background (indicated by arrows in Fig. 5B, also see Fig. 5C). This finding is in good agreement with a previous study which showed that the protein kinase A-mediated decrease in pCa50, observed under α-Tm background, was abolished under β-Tm background (Palmiter et al. 1996). The Ca2+ sensitizing effect of β-Tm was also evident in fibres that contained McTnT45−74Δ. For example, McTnT45-74Δ-mediated increase in pCa50 (α-Tm fibres) was further enhanced under β-Tm background (Fig. 5C).
Regardless of the type of Tm isoform, the Hill coefficient (nH) was significantly attenuated by McTnT45-74Δ, suggesting an effect on thin-filament cooperativity (Fig. 5D). These results indicate that the region containing residues 45−74 in McTnT plays an important role in cooperative activation of thin filaments. Although the molecular basis of this altered cooperative mechanism is unclear, the source of this change is likely to be due to the deletion-induced effects on the Tm–Tn complex (RU), and possibly between two contiguous RUs. Because McTnT45-74Δ did not affect maximal tension or ED, it is likely that both Ca2+- and XB-mediated activation of thin filaments were unaffected. Likewise, McTnT45−74Δ did not affect the XB turnover rate as shown by an unaltered rate of tension redevelopment (ktr) and the rate of XB recruitment (b; Table 1). Because ktr is a measure of the rate of XB transition from the weak- to strong-binding states (Brenner & Eisenberg, 1986; Campbell, 1997), an unaltered ktr indicates that there is no change in the equilibrium between the closed- and open-states of thin filaments. Because of its lack of effect on maximal activation and XB turnover rate, but an augmenting effect on Ca2+ sensitivity, we predict that the deletion of 45−74 amino acids in cTnT shifts the equilibrium of thin filaments from the blocked- to closed-state, thereby sensitizing the thin filament to Ca2+. Supporting evidence for this conclusion comes from our recent data (Gollapudi et al. 2012), which demonstrated that McTnTNTE increased TG mouse cardiac fibre Ca2+ sensitivity, with no effect on maximal activation. Collectively, these observations demonstrate that the region 1−44 of McTnT is essential for maximal activation, whereas the region 45−74 of McTnT is essential for augmenting long-range cooperative/allosteric processes in the cardiac thin filament.
McTnT1-44Δ shifts the equilibrium of XB recruitment away from the force-bearing population
The attenuating effect of McTnT1-44Δ on maximal activation (Ca2+-activated maximal tension and ED) was similar under α- and β-Tm backgrounds (Fig. 4), suggesting that McTnT1-44Δ shifted the equilibrium of XB recruitment away from the force-bearing population. But the mechanism by which McTnT1-44Δ shifted the equilibrium differed depending on the type of Tm isoform present. For example, both tension cost (Fig. 6B) and c (Fig. 6D) were significantly greater in McTnT1−44Δ reconstituted fibres under β-Tm background, but not under α-Tm background (Fig. 6A and C). These observations, in conjunction with experimental evidences presented above, are strongly suggestive of differential effects of α- and β-Tm on XB detachment dynamics and contractile function. Faster XB detachment kinetics (Figs 6D and 8) and a greater negative effect of distorted XBs (γ) on other bound XBs (Figs 7B and 8) suggest that McTnT1−44Δ enhances the detachment of XBs from actin in β-Tm fibres. This effect of McTnT1−44Δ on XBs would shift the equilibrium of XB recruitment away from the force-bearing population, leading to the attenuation of: (1) maximal tension by ∼30% (Fig. 4B); (2) ED– which is an approximation of a number of strongly-bound XBs – by ∼32% (Fig. 4D); and (3) ER– which reflects the ML-mediated effect on the recruitment of new XBs – by ∼58% (Figs 7D and 8). The decrease in ER suggests that McTnT1-44Δ blunts the mechanisms that underlie the SL-dependent recruitment of XBs under a β-Tm background. Regardless of the mode of action, McTnT1-44Δ had an attenuating effect on thin-filament maximal activation. These data not only highlight the physiological significance of residues 1−44 in cTnT, but also demonstrate the divergent effects of Tm isoforms on cTnT-mediated cardiac function.
Conclusions
We provide the first explicit evidence to demonstrate the existence of two new functional regions within the N-terminus of cTnT and ascribe divergent physiological roles that were previously unknown: (1) amino acids 1-44 of McTnT modulate maximal activation of thin filaments, acting through a charge-dependent mechanism on cTnT–Tm interactions; (2) amino acids 45-74 of McTnT desensitize cardiac thin filaments to Ca2+ through an effect on stabilizing the thin filament in the blocked-state; (3) cTnT, but not Tm, has a dominant effect on Ca2+-activated maximal tension in cardiac myofilaments; and (4) interplay between the effects of cTnT and Tm on the thin filament modulates myofilament Ca2+ sensitivity of tension development. The physiological significance of amino acids 1-44 of cTnT stems from the observation that the N-terminus of cTnT undergoes changes – through alternative splicing – not only during development (Jin & Lin, 1988), but also in some forms of heart diseases (Akella et al. 1995; Saba et al. 1996). The functional relevance of our current study is further highlighted by the observation that the majority of the differences between the TnT isoforms − which are expressed in cardiac, slow and skeletal muscle fibre types − lie within the N-terminus of TnT (Wei & Jin, 2011). This suggests that structural differences in the N-terminus of TnT confer a regulatory mechanism to meet varied functional demands of different muscle fibre types. Our data also suggest that the presence of cardiac-specific residues 45-74 of cTnT – which is conserved across species – may be essential for augmenting long-range cooperative effects in cardiac thin filaments. Enhanced cooperative allosteric processes, combined with a desensitizing effect of cardiac-specific residues on Ca2+ sensitivity, may slow XB recruitment mechanisms such that it aids in tuning the dynamics of contraction to heart rate. Our study provides novel mechanistic insights that have significant implications for tissue- and disease-related changes in TnT isoform expression and muscle function.
Acknowledgments
This work was funded by National Heart, Lung and Blood Institute Grant R01-HL-075643 (to Murali Chandra), R01-HL-081680 (to David F. Wieczorek) and Poncin fellowship (to Ranganath Mamidi). We thank Dr. Sampath Gollapudi for helping us to generate figures using MATLAB. There are no conflicts of interest.
Glossary
- cTnC
cardiac troponin C
- cTnI
cardiac troponin I
- cTnT
cardiac troponin T
- DTT
dithiothreitol
- fsTnT
fast skeletal troponin T
- HR
high-relaxing
- McTnC
mouse cardiac troponin C
- McTnI
mouse cardiac troponin I
- McTnT
mouse cardiac troponin T
- McTnTNTE
mouse cardiac troponin T N-terminal extension
- McTnTWT
wild-type mouse cardiac troponin T
- MfsTnT
mouse fast skeletal troponin T
- ML
muscle length
- NLRD
non-linear recruitment distortion
- NTE
N-terminal extension
- NTG
non-transgenic
- PEP
phosphoenol pyruvate
- PMSF
phenylmethylsulfonyl fluoride
- RcTnT
rat cardiac troponin T
- RU
regulatory unit
- SL
sarcomere length
- SU
structural unit
- TG
transgenic
- Tm
tropomyosin
- Tn
troponin
- XB
cross-bridge
Author contributions
R.M. and S.L.M. were responsible for experimental design, data collection, analysing data, interpreting data, drafting and revising the manuscript. D.F.W. and M.C. were responsible for experimental design, analysing data, interpreting data, drafting and revising the manuscript. The experiments were carried out in the Department of VCAPP at Washington State University, Pullman, WA, USA. All authors approved the final version of the manuscript.
Supplementary material
Supplementry Material
References
- Adhikari BB, Regnier M, Rivera AJ, Kreutziger KL, Martyn DA. Cardiac length dependence of force and force redevelopment kinetics with altered cross-bridge cycling. Biophys J. 2004;87:1784–1794. doi: 10.1529/biophysj.103.039131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akella AB, Ding XL, Cheng R, Gulati J. Diminished Ca2+ sensitivity of skinned cardiac muscle contractility coincident with troponin T-band shifts in the diabetic rat. Circ Res. 1995;76:600–606. doi: 10.1161/01.res.76.4.600. [DOI] [PubMed] [Google Scholar]
- Anderson PA, Greig A, Mark TM, Malouf NN, Oakeley AE, Ungerleider RM, Allen PD, Kay BK. Molecular basis of human cardiac troponin T isoforms expressed in the developing, adult, and failing heart. Circ Res. 1995;76:681–686. doi: 10.1161/01.res.76.4.681. [DOI] [PubMed] [Google Scholar]
- Brenner B. Effect of Ca2+ on cross-bridge turnover kinetics in skinned single rabbit psoas fibers: implications for regulation of muscle contraction. Proc Natl Acad Sci U S A. 1988;85:3265–3269. doi: 10.1073/pnas.85.9.3265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner B, Eisenberg E. Rate of force generation in muscle: correlation with actomyosin ATPase activity in solution. Proc Natl Acad Sci U S A. 1986;83:3542–3546. doi: 10.1073/pnas.83.10.3542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brisson JR, Golosinska K, Smillie LB, Sykes BD. Interaction of tropomyosin and troponin T: a proton nuclear magnetic resonance study. Biochemistry. 1986;25:4548–4555. doi: 10.1021/bi00364a014. [DOI] [PubMed] [Google Scholar]
- Campbell K. Rate constant of muscle force redevelopment reflects cooperative activation as well as cross-bridge kinetics. Biophys J. 1997;72:254–262. doi: 10.1016/S0006-3495(97)78664-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell KB, Chandra M, Kirkpatrick RD, Slinker BK, Hunter WC. Interpreting cardiac muscle force-length dynamics using a novel functional model. Am J Physiol Heart Circ Physiol. 2004;286:H1535–H1545. doi: 10.1152/ajpheart.01029.2003. [DOI] [PubMed] [Google Scholar]
- Chandra M, Mamidi R, Ford S, Hidalgo C, Witt C, Ottenheijm C, Labeit S, Granzier H. Nebulin alters cross-bridge cycling kinetics and increases thin filament activation: a novel mechanism for increasing tension and reducing tension cost. J Biol Chem. 2009;284:30889–30896. doi: 10.1074/jbc.M109.049718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandra M, Montgomery DE, Kim JJ, Solaro RJ. The N-terminal region of troponin T is essential for the maximal activation of rat cardiac myofilaments. J Mol Cell Cardiol. 1999;31:867–880. doi: 10.1006/jmcc.1999.0928. [DOI] [PubMed] [Google Scholar]
- Chandra M, Tschirgi ML, Ford SJ, Slinker BK, Campbell KB. Interaction between myosin heavy chain and troponin isoforms modulate cardiac myofiber contractile dynamics. Am J Physiol Regul Integr Comp Physiol. 2007;293:R1595–R1607. doi: 10.1152/ajpregu.00157.2007. [DOI] [PubMed] [Google Scholar]
- Chandra M, Tschirgi ML, Rajapakse I, Campbell KB. Troponin T modulates sarcomere length-dependent recruitment of cross-bridges in cardiac muscle. Biophys J. 2006;90:2867–2876. doi: 10.1529/biophysj.105.076950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Communal C, Sumandea M, de Tombe P, Narula J, Solaro RJ, Hajjar RJ. Functional consequences of caspase activation in cardiac myocytes. Proc Natl Acad Sci U S A. 2002;99:6252–6256. doi: 10.1073/pnas.092022999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulton AT, Koka K, Lehrer SS, Geeves MA. Role of the head-to-tail overlap region in smooth and skeletal muscle beta-tropomyosin. Biochemistry. 2008;47:388–397. doi: 10.1021/bi701144g. [DOI] [PubMed] [Google Scholar]
- de Tombe PP, Stienen GJ. Protein kinase A does not alter economy of force maintenance in skinned rat cardiac trabeculae. Circ Res. 1995;76:734–741. doi: 10.1161/01.res.76.5.734. [DOI] [PubMed] [Google Scholar]
- Fabiato A, Fabiato F. Calculator programs for computing the composition of the solutions containing multiple metals and ligands used for experiments in skinned muscle cells. J Physiol (Paris) 1979;75:463–505. [PubMed] [Google Scholar]
- Ford SJ, Chandra M, Mamidi R, Dong W, Campbell KB. Model representation of the nonlinear step response in cardiac muscle. J Gen Physiol. 2010;136:159–177. doi: 10.1085/jgp.201010467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford SJ, Mamidi R, Jimenez J, Tardiff JC, Chandra M. Effects of R92 mutations in mouse cardiac troponin T are influenced by changes in myosin heavy chain isoform. J Mol Cell Cardiol. 2012;53:542–551. doi: 10.1016/j.yjmcc.2012.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaffin RD, Gokulan K, Sacchettini JC, Hewett TE, Klevitsky R, Robbins J, Sarin V, Zawieja DC, Meininger GA, Muthuchamy M. Changes in end-to-end interactions of tropomyosin affect mouse cardiac muscle dynamics. Am J Physiol Heart Circ Physiol. 2006;291:H552–H563. doi: 10.1152/ajpheart.00688.2005. [DOI] [PubMed] [Google Scholar]
- Gollapudi SK, Mamidi R, Mallampalli SL, Chandra M. The N-terminal extension of cardiac troponin T stabilizes the blocked state of cardiac thin filament. Biophys J. 2012;103:940–948. doi: 10.1016/j.bpj.2012.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon AM, Homsher E, Regnier M. Regulation of contraction in striated muscle. Physiol Rev. 2000;80:853–924. doi: 10.1152/physrev.2000.80.2.853. [DOI] [PubMed] [Google Scholar]
- Heeley DH, Golosinska K, Smillie LB. The effects of troponin T fragments T1 and T2 on the binding of nonpolymerizable tropomyosin to F-actin in the presence and absence of troponin I and troponin C. J Biol Chem. 1987;262:9971–9978. [PubMed] [Google Scholar]
- Heeley DH, Smillie LB, Lohmeier-Vogel EM. Effects of deletion of tropomyosin overlap on regulated actomyosin subfragment 1 ATPase. Biochem J. 1989;258:831–836. doi: 10.1042/bj2580831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jagatheesan G, Rajan S, Ahmed RP, Petrashevskaya N, Boivin G, Arteaga GM, Tae HJ, Liggett SB, Solaro RJ, Wieczorek DF. Striated muscle tropomyosin isoforms differentially regulate cardiac performance and myofilament calcium sensitivity. J Muscle Res Cell Motil. 2010a;31:227–239. doi: 10.1007/s10974-010-9228-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jagatheesan G, Rajan S, Wieczorek DF. Investigations into tropomyosin function using mouse models. J Mol Cell Cardiol. 2010b;48:893–898. doi: 10.1016/j.yjmcc.2009.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin JP, Lin JJ. Rapid purification of mammalian cardiac troponin T and its isoform switching in rat hearts during development. J Biol Chem. 1988;263:7309–7315. [PubMed] [Google Scholar]
- Kobayashi T, Solaro RJ. Calcium, thin filaments, and the integrative biology of cardiac contractility. Annu Rev Physiol. 2005;67:39–67. doi: 10.1146/annurev.physiol.67.040403.114025. [DOI] [PubMed] [Google Scholar]
- Lehrer SS, Geeves MA. The muscle thin filament as a classical cooperative/allosteric regulatory system. J Mol Biol. 1998;277:1081–1089. doi: 10.1006/jmbi.1998.1654. [DOI] [PubMed] [Google Scholar]
- Li XE, Suphamungmee W, Janco M, Geeves MA, Marston SB, Fischer S, Lehman W. The flexibility of two tropomyosin mutants, D175N and E180G, that cause hypertrophic cardiomyopathy. Biochem Biophys Res Commun. 2012;424:493–496. doi: 10.1016/j.bbrc.2012.06.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loong CK, Zhou HX, Bryant Chase P. Familial hypertrophic cardiomyopathy related E180G mutation increases flexibility of human cardiac alpha-tropomyosin. FEBS Lett. 2012;586:3503–3507. doi: 10.1016/j.febslet.2012.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malnic B, Farah CS, Reinach FC. Regulatory properties of the NH2- and COOH-terminal domains of troponin T. ATPase activation and binding to troponin I and troponin C. J Biol Chem. 1998;273:10594–10601. doi: 10.1074/jbc.273.17.10594. [DOI] [PubMed] [Google Scholar]
- Malnic B, Reinach FC. Assembly of functional skeletal muscle troponin complex in Escherichia coli. Eur J Biochem. 1994;222:49–54. doi: 10.1111/j.1432-1033.1994.tb18840.x. [DOI] [PubMed] [Google Scholar]
- Mamidi R, Gollapudi SK, Mallampalli SL, Chandra M. Alanine or aspartic acid substitutions at serine23/24 of cardiac troponin I decrease thin filament activation, with no effect on crossbridge detachment kinetics. Arch Biochem Biophys. 2012;525:1–8. doi: 10.1016/j.abb.2012.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montgomery DE, Tardiff JC, Chandra M. Cardiac troponin T mutations: correlation between the type of mutation and the nature of myofilament dysfunction in transgenic mice. J Physiol. 2001;536:583–592. doi: 10.1111/j.1469-7793.2001.0583c.xd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthuchamy M, Grupp IL, Grupp G, O’Toole BA, Kier AB, Boivin GP, Neumann J, Wieczorek DF. Molecular and physiological effects of overexpressing striated muscle beta-tropomyosin in the adult murine heart. J Biol Chem. 1995;270:30593–30603. doi: 10.1074/jbc.270.51.30593. [DOI] [PubMed] [Google Scholar]
- Muthuchamy M, Pajak L, Howles P, Doetschman T, Wieczorek DF. Developmental analysis of tropomyosin gene expression in embryonic stem cells and mouse embryos. Mol Cell Biol. 1993;13:3311–3323. doi: 10.1128/mcb.13.6.3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthuchamy M, Rethinasamy P, Wieczorek DF. Tropomyosin structure and function new insights. Trends Cardiovasc Med. 1997;7:124–128. doi: 10.1016/S1050-1738(97)00004-2. [DOI] [PubMed] [Google Scholar]
- Palmiter KA, Kitada Y, Muthuchamy M, Wieczorek DF, Solaro RJ. Exchange of beta- for alpha-tropomyosin in hearts of transgenic mice induces changes in thin filament response to Ca2+, strong cross-bridge binding, and protein phosphorylation. J Biol Chem. 1996;271:11611–11614. doi: 10.1074/jbc.271.20.11611. [DOI] [PubMed] [Google Scholar]
- Pearlstone JR, Smillie LB. Effects of troponin-I plus-C on the binding of troponin-T and its fragments to alpha-tropomyosin. Ca2+ sensitivity and cooperativity. J Biol Chem. 1983;258:2534–2542. [PubMed] [Google Scholar]
- Perry SV. Troponin T: genetics, properties and function. J Muscle Res Cell Motil. 1998;19:575–602. doi: 10.1023/a:1005397501968. [DOI] [PubMed] [Google Scholar]
- Piazzesi G, Linari M, Reconditi M, Vanzi F, Lombardi V. Cross-bridge detachment and attachment following a step stretch imposed on active single frog muscle fibres. J Physiol. 1997;498:3–15. doi: 10.1113/jphysiol.1997.sp021837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regnier M, Martin H, Barsotti RJ, Rivera AJ, Martyn DA, Clemmens E. Cross-bridge versus thin filament contributions to the level and rate of force development in cardiac muscle. Biophys J. 2004;87:1815–1824. doi: 10.1529/biophysj.103.039123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saba Z, Nassar R, Ungerleider RM, Oakeley AE, Anderson PA. Cardiac troponin T isoform expression correlates with pathophysiological descriptors in patients who underwent corrective surgery for congenital heart disease. Circulation. 1996;94:472–476. doi: 10.1161/01.cir.94.3.472. [DOI] [PubMed] [Google Scholar]
- Stelzer JE, Larsson L, Fitzsimons DP, Moss RL. Activation dependence of stretch activation in mouse skinned myocardium: implications for ventricular function. J Gen Physiol. 2006;127:95–107. doi: 10.1085/jgp.200509432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tardiff JC, Factor SM, Tompkins BD, Hewett TE, Palmer BM, Moore RL, Schwartz S, Robbins J, Leinwand LA. A truncated cardiac troponin T molecule in transgenic mice suggests multiple cellular mechanisms for familial hypertrophic cardiomyopathy. J Clin Invest. 1998;101:2800–2811. doi: 10.1172/JCI2389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobacman LS, Nihli M, Butters C, Heller M, Hatch V, Craig R, Lehman W, Homsher E. The troponin tail domain promotes a conformational state of the thin filament that suppresses myosin activity. J Biol Chem. 2002;277:27636–27642. doi: 10.1074/jbc.M201768200. [DOI] [PubMed] [Google Scholar]
- Walsh TP, Trueblood CE, Evans R, Weber A. Removal of tropomyosin overlap and the co-operative response to increasing calcium concentrations of the acto-subfragment-1 ATPase. J Mol Biol. 1985;182:265–269. doi: 10.1016/0022-2836(85)90344-4. [DOI] [PubMed] [Google Scholar]
- Wei B, Jin JP. Troponin T isoforms and posttranscriptional modifications: evolution, regulation and function. Arch Biochem Biophys. 2011;505:144–154. doi: 10.1016/j.abb.2010.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willott RH, Gomes AV, Chang AN, Parvatiyar MS, Pinto JR, Potter JD. Mutations in Troponin that cause HCM, DCM and RCM: what can we learn about thin filament function. J Mol Cell Cardiol. 2010;48:882–892. doi: 10.1016/j.yjmcc.2009.10.031. [DOI] [PubMed] [Google Scholar]
- Wolska BM, Keller RS, Evans CC, Palmiter KA, Phillips RM, Muthuchamy M, Oehlenschlager J, Wieczorek DF, de Tombe PP, Solaro RJ. Correlation between myofilament response to Ca2+ and altered dynamics of contraction and relaxation in transgenic cardiac cells that express beta-tropomyosin. Circ Res. 1999;84:745–751. doi: 10.1161/01.res.84.7.745. [DOI] [PubMed] [Google Scholar]
- Wolska BM, Wieczorek FM. The role of tropomyosin in the regulation of myocardial contraction and relaxation. Pflugers Arch. 2003;446:1–8. doi: 10.1007/s00424-002-0900-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


