Abstract
This randomized trial evaluated the impact of different exercise training modalities on the function and size of conduit arteries in healthy volunteers. Young (27 ± 5 years) healthy male subjects were randomized to undertake 6 months of either endurance training (ET; n= 10) or resistance training (RT; n= 13). High-resolution ultrasound was used to determine brachial, femoral and carotid artery diameter and wall thickness (IMT) and femoral and brachial flow-mediated dilatation (FMD) and glyceryl trinitrate (GTN)-mediated dilatation. Improvements in peak oxygen uptake occurred with ET (from 3.6 ± 0.7 to 3.8 ± 0.6 l min−1, P= 0.024) but not RT. Upper body muscular strength increased following RT (from 57.8 ± 17.7 to 69.0 ± 19.5 kg, P < 0.001), but not ET. Both groups exhibited increases in lean body mass (ΔET, 1.4 ± 1.8 kg and ΔRT, 2.3 ± 1.3 kg, P < 0.05). Resistance training increased brachial artery resting diameter (from 3.8 ± 0.5 to 4.1 ± 0.4 mm, P < 0.05), peak FMD diameter (+0.2 ± 0.2 mm, P < 0.05) and GTN-mediated diameter (+0.3 ± 0.3 mm, P < 0.01), as well as brachial FMD (from 5.1 ± 2.2 to 7.0 ± 3.9%, P < 0.05). No improvements in any brachial parameters were observed following ET. Conversely, ET increased femoral artery resting diameter (from 6.2 ± 0.7 to 6.4 ± 0.6 mm, P < 0.05), peak FMD diameter (+0.4 ± 0.4 mm, P < 0.05) and GTN-induced diameter (+0.3 ± 0.3 mm, P < 0.05), as well as femoral FMD-to-GTN ratio (from 0.6 ± 0.3 to 1.1 ± 0.8, P < 0.05). Resistance training did not induce changes in femoral artery parameters. Carotid artery IMT decreased in response to both forms of training. These findings indicate that 6 months of supervised exercise training induced changes in brachial and femoral artery size and function and decreased carotid artery IMT. These impacts of both RT and ET would be expected to translate to decreased cardiovascular risk.
Key points
We compared the effects of 6 months of randomly allocated endurance or resistance training on arterial dimensions.
Previous research suggests that arterial size increases with exercise, but this is based on cross-sectional comparisons or interventions that rarely exceeded 12 weeks.
Using high-resolution ultrasound, we demonstrated arterial size adaptations that are specific to the exercise mode. Resistance exercise increased diameter and function in the brachial artery. Femoral diameter and function increased after endurance exercise. Carotid arterial wall thickness decreased with training, while conduit arterial wall thicknesses remained unchanged.
This study directly addressed the question of differential impacts of exercise modality on vascular adaptations of conduit arteries in humans in response to a relatively prolonged training intervention period. We conclude that both endurance and resistance modalities have impacts on arterial size, function and wall thickness in vivo, which would be expected to translate to decreased cardiovascular risk.
Introduction
Regular exercise is associated with decreased cardiovascular risk in both primary and secondary prevention settings (Taylor et al. 2004; Blair & Morris, 2009). Whilst some of this impact relates to the beneficial effects of exercise on traditional cardiovascular risk factors (Thompson et al. 2003), there are also direct effects of repetitive exercise on arterial function and structure that are anti-atherogenic (Green, 2009; Joyner & Green, 2009).
Research pertaining to the effects of exercise on the vasculature in healthy asymptomatic subjects has been predominantly based on relatively short-term (4–12 weeks) training studies (Dinenno et al. 2001; Miyachi et al. 2001; Maiorana et al. 2001b; Thijssen et al. 2007; Tinken et al. 2008) or cross-sectional comparisons of trained athletes and control subjects (Schmidt-Trucksäss et al. 2000, 2003; Huonker et al. 2003; Naylor et al. 2006; Rowley et al. 2011). Whilst the former studies may not fully detect chronic adaptations, the latter are prone to well-established limitations relating to comparisons performed between individuals (Naylor et al. 2008; Spence et al. 2011). Whilst some longer-term interventional studies have been performed in clinical populations (Sigal et al. 2007; Schjerve et al. 2008), very few supervised exercise-training studies have evaluated vascular adaptations over time frames exceeding 12 weeks.
A further gap in the exercise training literature pertaining to vascular adaptation relates to a lack of direct comparison between distinct and commonly used training modalities, for example endurance (or aerobic) and resistance (or strength) exercise. Such comparison is valid because these modalities are associated with divergent haemodynamic responses, which can elicit differential adaptations in cardiac morphology (Morganroth et al. 1975; Spence et al. 2011). Surprisingly, we could not find any previous studies that have randomized subjects in order to make a direct comparison of the impact of endurance vs. resistance exercise training on vascular adaptation in asymptomatic humans.
We therefore undertook a randomized longitudinal study comparing the impact of 6 months of supervised endurance or resistance exercise training on arterial diameter, wall thickness and function in asymptomatic male volunteers. We hypothesized that these different training modalities would induce divergent vascular adaptations.
Methods
Ethical approval
This study complied with the Declaration of Helsinki, and the Human Research Ethics Committee of the University of Western Australia approved all experimental protocols. All subjects provided written, informed consent before participating in the study. Results relating to cardiac adaptation in these subjects have been published previously (Spence et al. 2011).
Subjects
Twenty-seven young, healthy men were recruited and randomly assigned to either an endurance training (ET) or a resistance training (RT) group. Subjects had no history of cardiovascular, musculoskeletal or metabolic disease and did not smoke or take medication for the duration of the study. Randomization was carried out using software available online (http://www.randomization.com) and prior to any baseline data collection. Three subjects withdrew from the ET group during the course of the experiment, citing work (n= 2) or study commitments. One subject withdrew from the RT group owing to interstate work transfer (n= 1). Results are therefore based on the remaining 23 subjects (27 ± 5 years old) who completed the 6 month intervention.
Experimental procedures
Subjects in both exercise groups completed a 6 month fully supervised and centre-based exercise intervention, attending three 1 h sessions per week. Baseline measures taken prior to programme commencement included assessment of body composition using dual-energy X-ray absorptiometry, aerobic fitness (peak oxygen consumption;  ) using a graded exercise test, muscular strength using the one-repetition maximum (1RM) protocol, and simultaneous vascular assessment of the right brachial, femoral and carotid arteries using high-resolution ultrasonography. Repeat measures were taken after 24 weeks of exercise training. Whilst our original intention was to collect detraining data on vascular outcomes, these have not been reported because a limited number of subjects returned.
) using a graded exercise test, muscular strength using the one-repetition maximum (1RM) protocol, and simultaneous vascular assessment of the right brachial, femoral and carotid arteries using high-resolution ultrasonography. Repeat measures were taken after 24 weeks of exercise training. Whilst our original intention was to collect detraining data on vascular outcomes, these have not been reported because a limited number of subjects returned.
Extensive details of our training programmes are included in a previous publication (Spence et al. 2011). Briefly, the ET intervention consisted of a progressively overloaded programme of walking, jogging and running, inclusive of specified training phases over the 24 week period. The focus of the periodized RT programme was Olympic weightlifting with incorporated supplemental exercises (e.g. dead-lift, back squat, front squat, bench press and overhead press) to develop overall strength and technique. Relative intensities for the ET and RT interventions were monitored throughout the sessions, individualized and progressed to ensure that subjects were exercising at prescribed percentages of 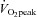 and 1RM, respectively.
and 1RM, respectively.
Experimental measures
Body composition
Prior to the graded exercise test, subjects underwent a whole-body dual-energy X-ray absorptiometry assessment (Lunar Prodigy; GE Medical Systems, Madison, WI, USA) to determine body composition; specifically, total fat mass, total lean body mass and body fat percentage.
Aerobic fitness
Graded exercise tests were performed on a treadmill to determine aerobic fitness as reported previously (Spence et al. 2011). The sum of the four highest consecutive 15 s oxygen uptake values, at each workload, was taken to represent peak oxygen consumption. Such data always occurred in the final 90 s of the workload stage.
Muscular strength
Using a typical 1RM protocol (Haykowsky et al. 2000), maximal upper and lower body strength was determined using the bench press and squat, respectively. Combined muscular strength was expressed as the summation of 1RM scores for the bench press and squat.
Vascular ultrasound assessment
Subjects reported to the laboratory in the morning in a fasted state and rested in the supine position for ∼10–15 min prior to any measurements being made. Systolic, diastolic and mean arterial blood pressures were determined from an automated sphygmomanometer (GE Pro 300V2; Dinamap, Tampa, FL, USA) on the left arm. A 10 MHz multifrequency linear array probe attached to a high-resolution ultrasound machine (T3000; Terason, Burlington, MA, USA) was used to assess simultaneously the femoral and brachial artery size and function as well as carotid arterial wall thickness and lumen diameter, in accordance with the methods we have previously described (Potter et al. 2007, 2008; Thijssen et al. 2011b). Briefly, the brachial artery was assessed with the right arm extended and supported at an angle of ∼80 deg from the torso, with the artery imaged in the distal third of the upper arm. The superficial femoral artery was assessed with the lower leg slightly elevated and the artery imaged in the proximal third of the thigh, at least 5 cm distal from the bifurcation, above the pneumatic cuff placement. Heart rate (HR) was monitored using an integrated three-lead ECG with the ultrasound machine.
Wall thickness of conduit arteries
Following the initial rest period and prior to performing the flow-mediated dilatation (FMD) technique (as described in the next subsection), the resting wall thickness and diameter of the brachial, femoral and carotid arteries were obtained using the high-resolution ultrasound machine. A longitudinal B-mode image of each artery was obtained with settings adjusted to focus on the far wall and recorded for 10 s. For the carotid artery, images were obtained approximately 2 cm proximal to the carotid bifurcation with three standardized probe angles (posterior, lateral and anterolateral) recorded over a 10 s period, in accordance with previous studies (Potter et al. 2007, 2008).
Endothelium-dependent dilatation of conduit arteries
To assess endothelium-dependent dilatation of the brachial and femoral arteries, the flow-mediated dilatation technique was used, which has been described in detail previously (Thijssen et al. 2011b). In brief, a rapid inflation–deflation pneumatic cuff (D. E. Hokanson Inc., Bellevue, WA, USA) was positioned on the right forearm, distal to the olecranon process, while a second cuff was positioned on the upper thigh ∼15 cm below the inguinal ligament. Ultrasound parameters were set to optimize longitudinal, B-mode images of the lumen–arterial wall interface. Continuous Doppler velocity assessment was also carried out using the lowest possible insonation angle (<60 deg), which was maintained for subsequent assessments. Dynamic range and Doppler gain settings were similar between the two machines (Potter et al. 2008). To assess resting vessel diameter and flow, a baseline scan was recorded for 1 min at the end of the rest period. The occluding cuffs were then simultaneously inflated to a suprasystolic pressure of ∼220 mmHg for 5 min. Diameter and flow recordings resumed 30 s before cuff deflation and continued for 5 min thereafter (Black et al. 2008).
Endothelium-independent dilatation of conduit arteries
To assess brachial and femoral artery peak dilatation, a 1 min baseline recording of resting arterial diameter and flow was collected. Subsequently, a single sublingual dose of glyceryl trinitrate (GTN; 400 μg) was administered. Measurements of arterial diameter and flow were recorded 3 min following GTN administration for a further 7 min to quantify endothelium-independent function of the conduit arteries.
Data analysis
All analyses were performed by a single investigator who was blinded to the group allocation and scan time points. Analysis of the diameter of conduit arteries was performed using custom-designed edge-detection and wall-tracking software, which is independent of investigator bias and has been fully described and validated previously (Woodman et al. 2001; Black et al. 2008). The software automatically detects the peak of the Doppler velocity waveform, synchronizing with the arterial diameter at 30 Hz. The peak diameter following cuff deflation was determined using an automated algorithm (Woodman et al. 2001; Black et al. 2008) so that FMD was calculated as the percentage rise from the preceding baseline diameter as a measure of endothelium-dependent vascular function (FMD%). Likewise, endothelium-independent vascular function was calculated as the percentage increase from its preceding baseline (GTN%). Both of these ‘preceding baseline’ diameters were used to calculate a study baseline for each subject at each visit.
Analysis of carotid, brachial and superficial femoral arterial wall thickness was also performed using a DICOM-based software package, which has proved to be observer independent, has been validated against phantoms and is described in detail elsewhere (Potter et al. 2007, 2008). Briefly, the initial video signal was encoded and stored as a digital DICOM file. Software analysis was performed at 30 Hz using an icon-based graphical programming language and toolkit (LabView 6.02; National Instruments, Austin, TX, USA). By identifying a region of interest on each first frame of every individual study, capturing both arterial walls, an automated calibration was made of diameters on the B-mode image. Within the identified region of interest in the diameter image, a pixel-density algorithm automatically identified the angle-corrected near and far wall lines for every pixel column for diameter assessment. The same algorithm was used to identify the far wall media–adventitia interface. Detection of the near and far wall lumen edges and the far wall media–adventitia interface was performed on every frame selected.
Statistical analysis
To examine the effects of training on measures of vascular structure and function, aerobic fitness, body composition and muscular strength, a two-way ANOVA was used. Main effects for time (pre-training vs. post-training) and exercise modality (ET vs. RT) were determined, along with interactions between these factors. Given recent evidence that prolonged training may result in resolution of functional adaptation, possibly dependent upon training duration and/or intensity (Tinken et al. 2008), we considered main effects for time and group to be physiologically relevant. Post hoc Student's paired t tests were conducted and P values reported. All statistical analyses were performed using Excel (Microsoft Office Excel 2007) and PASW Statistics for Windows version 18 (SPSS Inc., Chicago, IL, USA). All data are reported as means ± SD.
Results
Impact of exercise training on physiological outcomes
Data pertaining to physiological outcomes in response to exercise training are presented in Table 1. A significant main effect for time was observed for 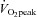 (F= 4.2, P= 0.05) which increased as a result of ET (P= 0.02) but remained unchanged following RT (P= 0.63). Upper body muscular strength analysis revealed a main effect for time (F= 20.62, P < 0.001) and an interaction effect (F= 6.64, P= 0.02) such that RT increased upper body strength (P= 0.001) but ET did not (P= 0.12). Lower body strength data revealed a main effect for time (F= 199.47, P < 0.001). Increases were observed in both groups (P < 0.001 for both ET and RT). Significant main effects for time (F= 205.29, P < 0.001) and interaction (F= 7.05, P= 0.02) were observed for combined strength (ET, P < 0.001 and RT, P < 0.001).
(F= 4.2, P= 0.05) which increased as a result of ET (P= 0.02) but remained unchanged following RT (P= 0.63). Upper body muscular strength analysis revealed a main effect for time (F= 20.62, P < 0.001) and an interaction effect (F= 6.64, P= 0.02) such that RT increased upper body strength (P= 0.001) but ET did not (P= 0.12). Lower body strength data revealed a main effect for time (F= 199.47, P < 0.001). Increases were observed in both groups (P < 0.001 for both ET and RT). Significant main effects for time (F= 205.29, P < 0.001) and interaction (F= 7.05, P= 0.02) were observed for combined strength (ET, P < 0.001 and RT, P < 0.001).
Table 1.
Physiological characteristics before (Pre) and after 6 months of endurance and resistance exercise training (Post)
| Endurance training (n= 10) | Resistance training (n= 13) | |||
|---|---|---|---|---|
| Parameter | Pre | Post | Pre | Post |
| Aerobic fitness | ||||
| Peak oxygen consumption (l min−1)‡ | 3.6 ± 0.7 | 3.8 ± 0.6* | 3.6 ± 0.9 | 3.6 ± 0.7 |
| Muscular strength | ||||
| Upper body strength (kg)‡§ | 57.9 ± 14.4 | 61.0 ± 14.3 | 57.8 ± 17.7 | 69.0 ± 19.5* |
| Lower body strength (kg)‡ | 89.0 ± 21.3 | 122.3 ± 14.6** | 96.7 ± 22.5 | 138.5 ± 15.9** |
| Combined strength (kg)‡§ | 146.9 ± 33.0 | 183.3 ± 37.1** | 154.5 ± 37.5 | 207.5 ± 30.8** |
| Body composition | ||||
| Lean body mass (kg)‡ | 56.9 ± 9.0 | 58.3 ± 9.5* | 59.7 ± 8.5 | 62.0 ± 8.0** |
| Fat mass (kg)‡ | 17.7 ± 9.1 | 16.7 ± 9.9 | 18.6 ± 8.5 | 17.8 ± 9.0 |
| Total body fat (%)‡ | 22.7 ± 7.4 | 21.1 ± 7.8 | 23.1 ± 7.4 | 21.4 ± 7.5* |
Values are means ± SD. *P < 0.05, **P < 0.001, significantly different from Pre. ‡P < 0.05, ANOVA main effect for time. §P < 0.05, ANOVA interaction effect.
In terms of body composition, there was a significant main effect for time (ANOVA, F= 33.39, P < 0.001) for measures of lean body mass, which increased in both groups (ET, P= 0.04 and RT, P < 0.001). Likewise, a main effect for time was observed for absolute fat mass (ANOVA, F= 4.12, P= 0.05), which decreased in both groups. Total body fat percentage decreased with training (ANOVA main effect for time, F= 11.68, P= 0.003) following RT (P= 0.02).
Impact of exercise training on size and function of the brachial artery
A summary of the effect of exercise training protocols on brachial and femoral vascular size is presented in Table 2. Data for two subjects were not available; hence, analysis was performed on n= 9 and n= 12 for the ET and RT groups, respectively. A significant interaction effect was observed for resting diameter of the brachial artery (ANOVA, F= 6.03, P= 0.03). Post hoc analysis revealed that following RT, resting diameter of the brachial artery increased by 5%, while no change was observed following ET (Fig. 1). Likewise, peak brachial FMD diameter increased with training (ANOVA main effect for time, F= 9.23, P= 0.007; ANOVA interaction, F= 5.79, P= 0.026). Specifically, after RT, peak FMD diameter increased (from 4.1 ± 0.3 to 4.4 ± 0.3 mm, P= 0.002), whereas this remained unchanged following ET (from 4.0 ± 0.5 to 4.0 ± 0.6 mm, P= 0.67). As a result, brachial FMD%, a measure of endothelium-dependent vascular function, increased with training (ANOVA main effect for time, F= 9.89, P= 0.005; Table 2).
Table 2.
Characteristics of conduit arteries before (Pre) and after 6 months of supervised endurance or resistance exercise training (Post)
| Endurance training | Resistance training | |||
|---|---|---|---|---|
| Parameter | Pre | Post | Pre | Post |
| Brachial artery | (n= 9) | (n= 12) | ||
| Diameter (mm)§ | 3.8 ± 0.6 | 3.9 ± 0.3 | 3.8 ± 0.5 | 4.1 ± 0.4* |
| Wall thickness (μm) | 680 ± 183 | 612 ± 93 | 674 ± 119 | 595 ± 88 |
| Wall-to-lumen ratio‡ | 0.19 ± 0.07 | 0.17 ± 0.04 | 0.18 ± 0.04 | 0.14 ± 0.02* |
| FMD (%)‡ | 5.5 ± 4.3 | 7.3 ± 3.7 | 5.1 ± 2.2 | 7.0 ± 3.9* |
| GTN (%) | 20.0 ± 4.7 | 15.8 ± 5.5 | 19.7 ± 4.6 | 20.0 ± 4.6 |
| FMD-to-GTN ratio‡ | 0.3 ± 0.2 | 0.5 ± 0.3 | 0.3 ± 0.1 | 0.4 ± 0.2* |
| Femoral artery | (n= 10) | (n= 13) | ||
| Diameter (mm)§ | 6.2 ± 0.6 | 6.4 ± 0.7* | 6.7 ± 0.5† | 6.6 ± 0.5 |
| Wall thickness (μm) | 788 ± 216 | 894 ± 333 | 807 ± 232 | 758 ± 177 |
| Wall-to-lumen ratio | 0.13 ± 0.03 | 0.14 ± 0.05 | 0.12 ± 0.03 | 0.11 ± 0.03 |
| FMD (%) | 4.5 ± 2.0 | 6.4 ± 2.2 | 5.8 ± 3.4 | 5.7 ± 2.4 |
| GTN (%) | 9.4 ± 3.00 | 8.6 ± 4.7 | 7.6 ± 3.2 | 7.9 ± 3.7 |
| FMD-to-GTN ratio‡§ | 0.6 ± 0.3 | 1.1 ± 0.8* | 0.9 ± 0.5 | 0.9 ± 0.5 |
| Carotid artery | ||||
| Diameter (mm) | 6.3 ± 0.8 | 6.3 ± 0.7 | 6.6 ± 0.4 | 6.7 ± 0.5 |
| Wall thickness (μm)‡ | 564 ± 78 | 507 ± 50 | 577 ± 63 | 522 ± 62 |
| Wall-to-lumen ratio‡ | 0.08 ± 0.03 | 0.07 ± 0.03 | 0.09 ± 0.01 | 0.08 ± 0.01 |
Values are means ± SD. *P < 0.05, significantly different from Pre. †P < 0.05, significantly different from endurance training. ‡P < 0.05, ANOVA main effect for time. §P < 0.05, ANOVA interaction effect. Abbreviations: FMD, endothelium-dependent vascular function (flow-mediated dilatation); and GTN, endothelium-independent vascular function (glyceryl trinitrate).
Figure 1. Resting brachial arterial diameter before (Pre) and after 6 months of supervised resistance or endurance exercise training (Post).
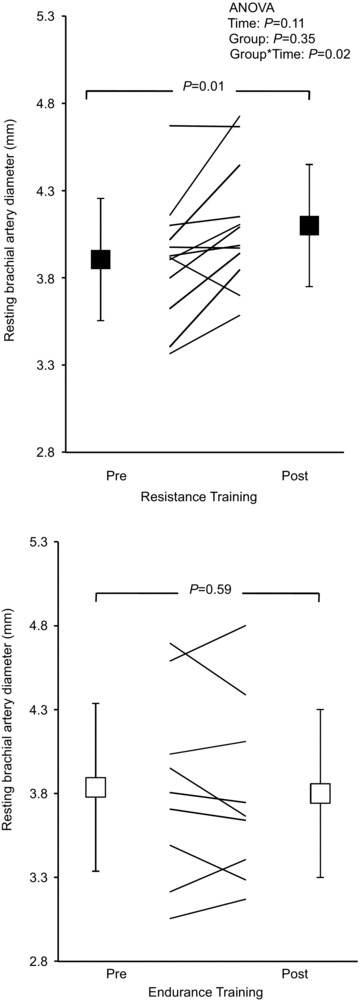
Values are means ± SD. The P values denote significance for the post hoc Student's t test, Pre vs. Post exercise training.
A significant interaction effect was observed for peak diameter after GTN (ANOVA, F= 14.67, P= 0.001) such that RT resulted in a 6% increase (from 4.7 ± 0.4 to 4.9 ± 0.4 mm, P= 0.008), while a 4% decrease was observed in response to ET (from 4.7 ± 0.6 to 4.5 ± 0.5 mm, P= 0.05). Consequently, GTN% was unchanged following either training protocol (ANOVA interaction, F= 3.99, P= 0.06; Table 2). Endothelial function expressed as FMD% is inclusive of vascular smooth muscle responsiveness, so the ratio of FMD to GTN was calculated to normalize FMD and indicate whether endothelial function had improved relative to smooth muscle vasodilator capacity. In the brachial artery, this ratio increased significantly as a result of training (ANOVA main effect for time, F= 9.80, P= 0.006).
Impact of exercise training on size and function of the femoral artery
A significant interaction effect was observed for resting diameter of the femoral artery (ANOVA, F= 5.47, P= 0.03), with an increase in resting diameter following ET, but not RT (Table 2 and Fig. 2). Likewise, peak FMD diameter was larger as a result of exercise training (ANOVA main effect for time, F= 6.43, P= 0.02; ANOVA interaction, F= 6.26, P= 0.02) whereby ET increased (from 6.5 ± 0.7 to 6.8 ± 0.7 mm, P= 0.01), while no change was observed following RT (from 7.0 ± 0.6 to 7.0 ± 0.5 mm, P= 0.98) (Fig. 2). Femoral artery FMD% (Table 2) did not change with either exercise training protocol. Femoral artery GTN% also remained unchanged with exercise, despite a significant interaction effect for peak GTN diameter (ANOVA, F= 6.59, P= 0.02). Peak GTN diameter increased with ET only (ET, from 6.8 ± 0.7 to 7.0 ± 0.7 mm, P= 0.04; and RT, from 7.3 ± 0.4 to 7.1 ± 0.5 mm, P= 0.15). We also analysed data as change from pre-intervention levels and found significant differences between ET (change, 0.3 mm) and RT (change, −0.1 mm) for resting femoral diameter (P= 0.02). The femoral artery FMD-to-GTN ratio was increased as a result of training (ANOVA main effect for time, F= 4.03, P= 0.05; ANOVA interaction, F= 4.07, P= 0.05). Specifically, ET resulted in an increased femoral artery FMD-to-GTN ratio (P= 0.03; Table 2), while there was no change observed in response to RT (P= 0.98).
Figure 2. Resting femoral arterial diameter before (Pre) and after 6 months of supervised resistance or endurance exercise training (Post).
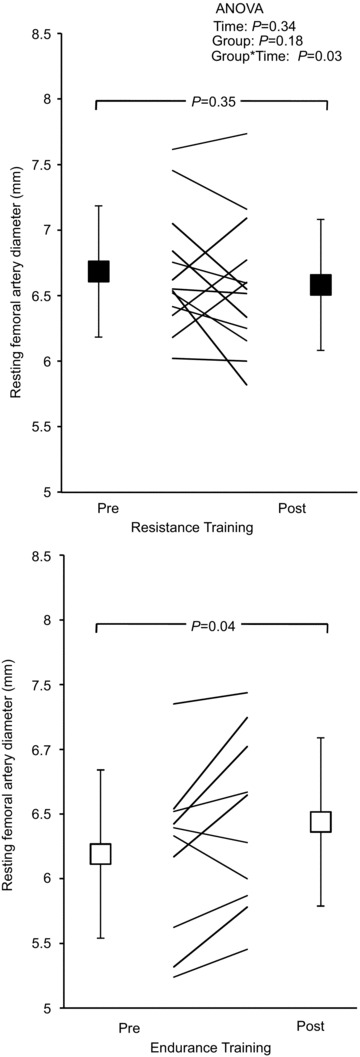
Values are mean ± SD. The P values denote significance for the post hoc Student's t test Pre vs. Post exercise training.
Impact of exercise training on arterial wall thickness
A significant main effect for time was observed for carotid artery intima–media thickness (IMT; ANOVA, F= 6.64, P= 0.02), which decreased from 564 ± 78 to 507 ± 50 μm following ET and from 577 ± 63 to 522 ± 62 μm after RT (Table 2). Wall-to-lumen ratio of the carotid artery also decreased with training (ANOVA main effect for time, F= 5.37, P= 0.03; Table 2). There were no changes in brachial or femoral arterial wall thickness following either exercise training intervention. However, there was a significant main effect for time (ANOVA, F= 4.93, P= 0.04) for brachial arterial wall-to-lumen ratio, which decreased with RT (P= 0.03; Table 2) but not ET (P= 0.41).
Discussion
Our results indicate that RT increased resting brachial artery diameter as well as peak diameter responses to both FMD and GTN stimuli. These upper limb vascular adaptations were not observed in the ET group. In contrast, the ET group exhibited increased resting and peak diameter response to FMD and GTN in the femoral artery after training. Taken together, these findings in healthy men indicate that 6 months of supervised exercise training induces differences in arterial size that are dependent upon exercise modality.
Recent studies have focused attention on the question of whether large muscle group dynamic exercise induces purely localized or systemic effects on arterial size, wall thickness and function (Rowley et al. 2012; Green et al. 2013). These studies, which relied upon cross-sectional comparisons of athletes, documented larger resting brachial diameters in the dominant, compared with non-dominant, arm of elite squash players, a finding that was not observed in matched control subjects (Rowley et al. 2011). Likewise, athletes participating in upper and lower limb sports demonstrated localized effects on arterial size (Green et al. 2013). In the present study, we also observed evidence for localized effects of exercise training on arterial diameter. Endurance training, predominantly involving exercise of the lower limbs, induced an increase in size of the femoral artery in the absence of adaptation of the brachial artery. Conversely, resistance training, which predominantly involved upper limb exercises and consistent reliance upon hand-gripping in this study, induced brachial but not femoral changes. These findings are in agreement with shorter-term interventions (Dinenno et al. 2001; Rakobowchuk, 2005; Thijssen et al. 2007). It is pertinent, however, to mention that RT, which involved some lower limb exercise, did not modify femoral parameters. Whilst this argues against the concept of regional specificity in conduit artery adaptation to training, it is possible that the magnitude of the leg exercise associated with RT was not great enough to induce adaptation. For example, shear stress, an acknowledged stimulus to adaptation in arterial function and size (Langille & O’Donnell, 1986), differs in quantum and pattern in response to different exercise modalities (Green, 2004; Thijssen et al. 2009). We therefore propose that our findings provide support for the notion that exercise training increases arterial lumen size in conduit arteries feeding active muscle beds, regardless of exercise modality.
As stated in the previous paragraph, localized changes in artery size are likely to be related to changes in arterial shear stress, a potent stimulus to nitric oxide production. It is well established that the endothelium, and specifically NO, mediates changes in arterial size (Langille & O’Donnell, 1986; Tronc et al. 1996). We recently presented evidence in humans pertaining to the relationship between changes in arterial function and structure (Tinken et al. 2008), which supports the conceptual framework first presented by Laughlin (1995). This suggests that initial improvements in NO-mediated vasodilator function as a result of exercise training can be transient and superseded by structural changes, which in turn facilitate normalization of function. These findings reinforce our interest in the present study in ANOVA main effects for time, as well as interactions between time and the group assignment. The present study, undertaken over a substantially longer time frame than most previous experiments (Dinenno et al. 2001; Rakobowchuk, 2005; Tinken et al. 2008; Schjerve et al. 2008; Birk et al. 2012), produced evidence of both localized increases in lumen dimensions and increases in function. That is, we observed enhanced FMD% in the brachial artery following RT. In addition, the FMD-to-GTN%, a measure reflecting global NO-dependent vasodilator function, increased in the femoral artery following ET. Adaptation also occurred in the size of both vessels. Based on the schema above, it might have been predicted that our longer intervention period would be associated with changes in arterial size, but normal function. One explanation for the concomitant increases in both function and size that we observed may relate to the nature of the exercise prescription. We diligently progressed the exercise stimulus in terms of intensity and volume (see Spence et al. 2011), which may have contributed to continued gains in arterial function in the presence of adaptations in artery size throughout the 24 weeks of training. We therefore suggest that vascular adaptation is highly dynamic and that set points that evolve to balance adaptations in function and structure can be adjusted continually in response to modifications in the exercise stimulus.
Previous studies detailing the impact of chronic exercise training on vascular adaptation have predominantly relied upon comparisons of athletes and control subjects (Schmidt-Trucksäss et al. 2000; Huonker et al. 2003). Several recent studies have suggested that, whilst athletes possess enlarged arterial diameters, consistent with our findings in response to 6 months of training, arterial function is not enhanced (Petersen et al. 2006; Rognmo et al. 2008; Green et al. 2013). The disparity between these findings may relate to the inherent limitations and assumptions associated with cross-sectional approaches. Furthermore, athletes may be exposed to higher levels of oxidative stress or related inflammation that can decrease FMD and NO-mediated vasodilatation (Cai & Harrison, 2000). Alternatively, it could be argued that function is normalized in athletes due the impact of substantive exercise on arterial structure, as evidenced by their greater arterial sizes (+0.8 mm vs. matched control subjects), whereas we induced an increase of +0.2 mm following 24 weeks of either ET or RT. It is therefore possible that longer periods of intensive training than those undertaken in the present study may have induced similar changes to those observed in elite resistance or endurance athletes. Further studies will be required to test this hypothesis.
The impacts of exercise training on arterial wall thickness were recently reviewed (Thijssen et al. 2011a). While it is suggested that physical activity levels are inversely associated with carotid IMT in healthy populations, studies addressing the impacts of exercise have produced disparate findings. Whilst some studies have reported higher carotid IMT in endurance athletes compared with control subjects (Abergel et al. 1998), others have observed no differences (Schmidt-Trucksäss et al. 2000; Moreau et al. 2002) or decreases (Rowley et al. 2012; Green et al. 2013). Several longitudinal studies have also reported decreased carotid IMT as a result of training (Rauramaa et al. 2004), although this is also not a consistent finding (Tanaka et al. 2002; Thijssen et al. 2007). The disparity in this literature may relate to the ‘load’ of the exercise intervention; our present and previous findings suggest that intensive longer-term training may be required to induce adaptations in carotid arterial wall size (Thijssen et al. 2011a). We also observed some evidence, although not significant, of effects on the wall thickness of peripheral conduit arteries. These findings concur with those of Rowley et al. (2011), who suggested that, while artery size changes were locally mediated, adaptations in wall thickness apparently occur systemically (Rowley et al. 2011, 2012; Green et al. 2013). In addition, our data add the novel observation that, regardless of exercise modality, changes in carotid wall thickness may be possible.
Apart from our vascular findings, we also measured body composition in this experiment. Predictably, RT resulted in increased lean body mass and muscular strength. Interestingly, ET also induced an increase in body lean mass. Upon closer inspection of our dual-energy X-ray absorptiometry data, this increase was confined to changes in the lower limbs, which is in agreement with the improvements in muscular strength that were also observed. Improvements in endothelial function (Green et al. 2011), body composition (Church et al. 2004), muscle strength (Jurca et al. 2004) and cardiorespiratory fitness (Stofan et al. 1998) have all been associated with enhanced prognosis, at least in older subjects and those with existing cardiovascular disease. Similar magnitudes of change in fat mass were also apparent in both groups, with the improvement in response to RT being somewhat surprising. Whilst recent studies have begun to address directly the implications for weight management and cardiometabolic health of distinct training modalities (Sigal et al. 2007; Dunstan, 2008; Church et al. 2010) in clinical populations, our data provide some insight into the preventive benefits of exercise of different modalities. Further, more detailed studies, will be required to address the mechanisms responsible for the changes we observed.
We did not include an inactive control group in the present experiment, which may be considered a limitation. However, recent debate has arisen regarding the universal relevance of intervention studies that involve such groups; it has been suggested that research should transition from determining efficacy (i.e. inclusion of a inactive control group), which has been consistently undertaken and is now considered well proven, to assessing comparative clinical effectiveness, that is comparison of active interventions (Huffman et al. 2011). In the present experiment, we considered an inactive control group redundant to the principal hypotheses relating to the relative benefits of commonly utilized and diverse modes of training. There have also been a number of previous parallel and cross-over designed studies of the impact of training on the vasculature in humans which have included a control group and observed no change in function or structure (Maiorana et al. 2000, 2001a, 2010; Tinken et al. 2008).
In summary, this study directly addressed the question of differential impacts of exercise modality on vascular adaptations of conduit arteries in humans in response to a relatively prolonged training intervention period. We conclude that both endurance and resistance modalities have impacts on arterial size, function and wall thickness in vivo, which would be expected to translate to decreased cardiovascular risk.
Acknowledgments
D.J.G. is supported by research funding from the Australian Research Council.
Glossary
- ET
endurance training
- FMD
flow-mediated dilatation
- GTN
glyceryl trinitrate
- HR
heart rate
- IMT
wall thickness
- 1RM
one-repetition maximum
- RT
resistance training
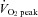
peak oxygen consumption
Author contributions
A.L.S., H.H.C. and D.J.G. were responsible for data collection, design and implementation of the exercise training programme, analysis and interpretation of data and drafting of the manuscript. L.H.N. was involved in data collection, analysis and interpretation as well as drafting of the manuscript. D.J.G. was responsible for the conception and study design, analysis and interpretation of results and drafting of the manuscript. All authors have revised and approved the final version of the manuscript.
References
- Abergel E, Linhart A, Chatellier G, Gariepy J, Ducardonnet A, Diebold B, Menard J. Vascular and cardiac remodeling in world class professional cyclists. Am Heart J. 1998;136:818–823. doi: 10.1016/s0002-8703(98)70126-7. [DOI] [PubMed] [Google Scholar]
- Birk GK, Dawson EA, Atkinson C, Haynes A, Cable NT, Thijssen DHJ, Green DJ. Brachial artery adaptation to lower limb exercise training: role of shear stress. J Appl Physiol. 2012;112:1653–1658. doi: 10.1152/japplphysiol.01489.2011. [DOI] [PubMed] [Google Scholar]
- Black MA, Cable NT, Thijssen DHJ, Green DJ. Importance of measuring the time course of flow-mediated dilatation in humans. Hypertension. 2008;51:203–210. doi: 10.1161/HYPERTENSIONAHA.107.101014. [DOI] [PubMed] [Google Scholar]
- Blair SN, Morris JN. Healthy hearts—and the universal benefits of being physically active: physical activity and health. Ann Epidemiol. 2009;19:253–256. doi: 10.1016/j.annepidem.2009.01.019. [DOI] [PubMed] [Google Scholar]
- Cai H, Harrison DG. Endothelial dysfunction in cardiovascular diseases: the role of oxidant stress. Circ Res. 2000;87:840–844. doi: 10.1161/01.res.87.10.840. [DOI] [PubMed] [Google Scholar]
- Church TS, Cheng YJ, Earnest CP, Barlow CE, Gibbons LW, Priest EL, Blair SN. Exercise capacity and body composition as predictors of mortality among men with diabetes. Diabetes Care. 2004;27:83–88. doi: 10.2337/diacare.27.1.83. [DOI] [PubMed] [Google Scholar]
- Church TST, Blair SNS, Cocreham SS, Johannsen NN, Johnson WW, Kramer KK, Mikus CRC, Myers VV, Nauta MM, Rodarte RQR, Sparks LL, Thompson AA, Earnest CPC. Effects of aerobic and resistance training on hemoglobin A1c levels in patients with type 2 diabetes: a randomized controlled trial. JAMA. 2010;304:2253–2262. doi: 10.1001/jama.2010.1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinenno F, Tanaka H, Monahan K, Clevenger C, Eskurze I, DeSouza C, Seals D. Regular endurance exercise induces expansive arterial remodelling in the trained limbs of healthy men. J Physiol. 2001;534:287–295. doi: 10.1111/j.1469-7793.2001.00287.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunstan DW. Aerobic exercise and resistance training for the management of type 2 diabetes mellitus. Nat Clin Pract Endocrinol Metab. 2008;4:250–251. doi: 10.1038/ncpendmet0790. [DOI] [PubMed] [Google Scholar]
- Green DJ. Comparison of forearm blood flow responses to incremental handgrip and cycle ergometer exercise: relative contribution of nitric oxide. J Physiol. 2004;562:617–628. doi: 10.1113/jphysiol.2004.075929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green DJ. Exercise training as vascular medicine: direct impacts on the vasculature in humans. Exerc Sport Sci Rev. 2009;37:196–202. doi: 10.1097/JES.0b013e3181b7b6e3. [DOI] [PubMed] [Google Scholar]
- Green DJ, Jones H, Thijssen D, Cable NT, Atkinson G. Flow-mediated dilation and cardiovascular event prediction: does nitric oxide matter. Hypertension. 2011;57:363–369. doi: 10.1161/HYPERTENSIONAHA.110.167015. [DOI] [PubMed] [Google Scholar]
- Green DJ, Rowley N, Spence AL, Carter HH, Whyte GP, George KP, Naylor LH, Cable NT, Dawson EA, Thijssen DH. Why isn't flow mediated dilation enhanced in athletes. Med Sci Sports Exerc. 2013;45:75–82. doi: 10.1249/MSS.0b013e318269affe. [DOI] [PubMed] [Google Scholar]
- Haykowsky M, Humen D, Teo K, Quinney A, Souster M, Bell G, Taylor D. Effects of 16 weeks of resistance training on left ventricular morphology and systolic function in healthy men >60 years of age. Am J Cardiol. 2000;85:1002–1006. doi: 10.1016/s0002-9149(99)00918-2. [DOI] [PubMed] [Google Scholar]
- Huffman KM, Slentz CA, Kraus WE. Control arms in exercise training studies: transitioning from an era of intervention efficacy to one of comparative clinical effectiveness research. J Appl Physiol. 2011;111:946–948. doi: 10.1152/japplphysiol.00323.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huonker M, Schmid A, Schmidt-Trucksass A, Grathwohl D, Keul J. Size and blood flow of central and peripheral arteries in highly trained able-bodied and disabled athletes. J Appl Physiol. 2003;95:685–691. doi: 10.1152/japplphysiol.00710.2001. [DOI] [PubMed] [Google Scholar]
- Joyner MJ, Green DJ. Exercise protects the cardiovascular system: effects beyond traditional risk factors. J Physiol. 2009;587:5551–5558. doi: 10.1113/jphysiol.2009.179432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurca R, Lamonte MJ, Church TS, Earnest CP, Fitzgerald SJ, Barlow CE, Jordan AN, Kampert JB, Blair SN. Associations of muscle strength and fitness with metabolic syndrome in men. Med Sci Sports Exerc. 2004;36:1301–1307. doi: 10.1249/01.mss.0000135780.88930.a9. [DOI] [PubMed] [Google Scholar]
- Langille BL, O’Donnell F. Reductions in arterial diameter produced by chronic decreases in blood flow are endothelium-dependent. Science. 1986;231:405–407. doi: 10.1126/science.3941904. [DOI] [PubMed] [Google Scholar]
- Laughlin MH. Endothelium-mediated control of coronary vascular tone after chronic exercise training. Med Sci Sports Exerc. 1995;27:1135–1144. [PubMed] [Google Scholar]
- Maiorana A, O’Driscoll G, Cheetham C, Dembo L, Stanton K, Goodman C, Taylor R, Green DJ. The effect of combined aerobic and resistance exercise training on vascular function in type 2 diabetes. J Am Coll Cardiol. 2001a;38:860–866. doi: 10.1016/s0735-1097(01)01439-5. [DOI] [PubMed] [Google Scholar]
- Maiorana A, O’Driscoll G, Dembo L, Cheetham C, Goodman C, Taylor R, Green DJ. Effect of aerobic and resistance exercise training on vascular function in heart failure. Am J Physiol Heart Circ Physiol. 2000;279:H1999–H2005. doi: 10.1152/ajpheart.2000.279.4.H1999. [DOI] [PubMed] [Google Scholar]
- Maiorana A, O’Driscoll G, Dembo L, Goodman C, Taylor R, Green DJ. Exercise training, vascular function, and functional capacity in middle-aged subjects. Med Sci Sports Exerc. 2001b;33:2022–2028. doi: 10.1097/00005768-200112000-00008. [DOI] [PubMed] [Google Scholar]
- Maiorana AJ, Naylor LH, Exterkate A, Swart A, Thijssen DHJ, Lam K, O’Driscoll G, Green DJ. The impact of exercise training on conduit artery wall thickness and remodeling in chronic heart failure patients. Hypertension. 2010;57:56–62. doi: 10.1161/HYPERTENSIONAHA.110.163022. [DOI] [PubMed] [Google Scholar]
- Miyachi M, Tanaka H, Yamamoto K, Yoshioka A, Takahashi K, Onodera S. Effects of one-legged endurance training on femoral arterial and venous size in healthy humans. J Appl Physiol. 2001;90:2439–2444. doi: 10.1152/jappl.2001.90.6.2439. [DOI] [PubMed] [Google Scholar]
- Moreau K, Donato A, Seals D, Dinenno F, Blackett S, Hoetzer G, DeSouza C, Tanaka H. Arterial intima-media thickness: site-specific associations with HRT and habitual exercise. Am J Physiol Heart Circ Physiol. 2002;283:H1409–H1417. doi: 10.1152/ajpheart.00035.2002. [DOI] [PubMed] [Google Scholar]
- Morganroth J, Maron BJ, Henry W, Epstein S. Comparative left ventricular dimensions in trained athletes. Annals Int Med. 1975;82:521–524. doi: 10.7326/0003-4819-82-4-521. [DOI] [PubMed] [Google Scholar]
- Naylor L, George KP, O’Driscoll G, Green DJ. The athlete's heart: a contemporary appraisal of the ‘Morganroth hypothesis’. Sports Med. 2008;38:69–90. doi: 10.2165/00007256-200838010-00006. [DOI] [PubMed] [Google Scholar]
- Naylor LH, O’Driscoll G, Fitzsimons M, Arnolda LF, Green DJ. Effects of training resumption on conduit arterial diameter in elite rowers. Med Sci Sports Exerc. 2006;38:86–92. doi: 10.1249/01.mss.0000181220.03855.1c. [DOI] [PubMed] [Google Scholar]
- Petersen SE, Wiesmann F, Hudsmith LE, Robson MD, Francis JM, Selvanayagam JB, Neubauer S, Channon KM. Functional and structural vascular remodeling in elite rowers assessed by cardiovascular magnetic resonance. J Am Coll Cardiol. 2006;48:790–797. doi: 10.1016/j.jacc.2006.04.078. [DOI] [PubMed] [Google Scholar]
- Potter K, Green DJ, Reed CJ, Woodman RJ, Watts GF, McQuillan BM, Burke V, Hankey GJ, Arnolda LF. Carotid intima-medial thickness measured on multiple ultrasound frames: evaluation of a DICOM-based software system. Cardiovasc Ultrasound. 2007;5:29. doi: 10.1186/1476-7120-5-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter K, Reed CJ, Green DJ, Hankey GJ, Arnolda LF. Ultrasound settings significantly alter arterial lumen and wall thickness measurements. Cardiovasc Ultrasound. 2008;6:6. doi: 10.1186/1476-7120-6-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakobowchuk M. Endothelial function of young healthy males following whole body resistance training. J Appl Physiol. 2005;98:2185–2190. doi: 10.1152/japplphysiol.01290.2004. [DOI] [PubMed] [Google Scholar]
- Rauramaa R, Halonen P, Väisänen SB, Lakka TA, Schmidt-Trucksäss A, Berg A, Penttilä IM, Rankinen T, Bouchard C. Effects of aerobic physical exercise on inflammation and atherosclerosis in men: the DNASCO Study: a six-year randomized, controlled trial. Ann Intern Med. 2004;140:1007–1014. doi: 10.7326/0003-4819-140-12-200406150-00010. [DOI] [PubMed] [Google Scholar]
- Rognmo Ø, Bjørnstad TH, Kahrs C, Tjønna AE, Bye A, Haram PM, Stølen T, Slørdahl SA, Wisloff U. Endothelial function in highly endurance-trained men: effects of acute exercise. J Strength Cond Res. 2008;22:535–542. doi: 10.1519/JSC.0b013e31816354b1. [DOI] [PubMed] [Google Scholar]
- Rowley NJ, Dawson EA, Birk GK, Cable NT, George KP, Whyte GP, Thijssen DHJ, Green DJ. Exercise and arterial adaptation in humans: uncoupling localized and systemic effects. J Appl Physiol. 2011;110:1190–1195. doi: 10.1152/japplphysiol.01371.2010. [DOI] [PubMed] [Google Scholar]
- Rowley NJ, Dawson EA, Hopman MTE, George KP, Whyte GP, Thijssen DH, Green DJ. Conduit diameter and wall remodeling in elite athletes and spinal cord injury. Med Sci Sports Exerc. 2012;44:844–849. doi: 10.1249/MSS.0b013e31823f6887. [DOI] [PubMed] [Google Scholar]
- Schjerve IE, Tyldum GA, Tjønna AE, Stølen T, Loennechen JP, Hansen HEM, Haram PM, Heinrich G, Bye A, Najjar SM, Smith GL, Slørdahl SA, Kemi OJ, Wisloff U. Both aerobic endurance and strength training programmes improve cardiovascular health in obese adults. Clin Sci (Lond) 2008;115:283. doi: 10.1042/CS20070332. [DOI] [PubMed] [Google Scholar]
- Schmidt-Trucksäss A, Schmid A, Bernd D, Huonker M. The relationship of left ventricular to femoral artery structure in male athletes. Med Sci Sports Exerc. 2003;35:214–219. doi: 10.1249/01.MSS.0000048637.26711.93. [DOI] [PubMed] [Google Scholar]
- Schmidt-Trucksäss A, Schmid A, Brunner C, Scherer N, Zäch G, Keul J, Huonker M. Arterial properties of the carotid and femoral artery in endurance-trained and paraplegic subjects. J Appl Physiol. 2000;89:1956–1963. doi: 10.1152/jappl.2000.89.5.1956. [DOI] [PubMed] [Google Scholar]
- Sigal RJ, Kenny GP, Boulé NG, Wells GA, Prud’homme D, Fortier M, Reid RD, Tulloch H, Coyle D, Phillips P, Jennings A, Jaffey J. Effects of aerobic training, resistance training, or both on glycemic control in type 2 diabetes: a randomized trial. Ann Intern Med. 2007;147:357–369. doi: 10.7326/0003-4819-147-6-200709180-00005. [DOI] [PubMed] [Google Scholar]
- Spence AL, Naylor LH, Carter HH, Buck CL, Dembo L, Murray CP, Watson P, Oxborough D, George KP, Green DJ. A prospective randomised longitudinal MRI study of left ventricular adaptation to endurance and resistance exercise training in humans. J Physiol. 2011;589:5443–5452. doi: 10.1113/jphysiol.2011.217125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stofan JR, DiPietro L, Davis D, Kohl HW, 3rd, Blair S. Physical activity patterns associated with cardiorespiratory fitness and reduced mortality: the Aerobics Center Longitudinal Study. Am J Public Health. 1998;88:1807–1813. doi: 10.2105/ajph.88.12.1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka H, Seals DR, Monahan KD, Clevenger CM, DeSouza CA, Dinenno FA. Regular aerobic exercise and the age-related increase in carotid artery intima-media thickness in healthy men. J Appl Physiol. 2002;92:1458–1464. doi: 10.1152/japplphysiol.00824.2001. [DOI] [PubMed] [Google Scholar]
- Taylor RS, Brown A, Ebrahim S, Jolliffe J, Noorani H, Rees K, Skidmore B, Stone JA, Thompson DR, Oldridge N. Exercise-based rehabilitation for patients with coronary heart disease: systematic review and meta-analysis of randomized controlled trials. Am J Med. 2004;116:682–692. doi: 10.1016/j.amjmed.2004.01.009. [DOI] [PubMed] [Google Scholar]
- Thijssen DH, Cable NT, Green DJ. Impact of exercise training on arterial wall thickness in humans. Clin Sci (Lond) 2011a;122:311–322. doi: 10.1042/CS20110469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thijssen DH, Dawson EA, Black MA, Hopman MTE, Cable NT, Green DJ. Brachial artery blood flow responses to different modalities of lower limb exercise. Med Sci Sports Exerc. 2009;41:1072–1079. doi: 10.1249/MSS.0b013e3181923957. [DOI] [PubMed] [Google Scholar]
- Thijssen DHJ, Black MA, Pyke KE, Padilla J, Atkinson G, Harris RA, Parker B, Widlansky ME, Tschakovsky ME, Green DJ. Assessment of flow-mediated dilation in humans: a methodological and physiological guideline. Am J Physiol Heart Circ Physiol. 2011b;300:H2–H12. doi: 10.1152/ajpheart.00471.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thijssen DHJ, de Groot PCE, Smits P, Hopman MTE. Vascular adaptations to 8-week cycling training in older men. Acta Physiol. 2007;190:221–228. doi: 10.1111/j.1748-1716.2007.01685.x. [DOI] [PubMed] [Google Scholar]
- Thompson PD, Buchner D, Pina IL, Balady GJ, Williams MA, Marcus BH, Berra K, Blair SN, Costa F, Franklin B, Fletcher GF, Gordon NF, Pate RR, Rodriguez BL, Yancey AK, Wenger NK American Heart Association Council on Clinical Cardiology Subcommittee on Exercise, Rehabilitation, and Prevention; American Heart Association Council on Nutrition, Physical Activity, and Metabolism Subcommittee on Physical Activity. Exercise and physical activity in the prevention and treatment of atherosclerotic cardiovascular disease: a statement from the Council on Clinical Cardiology (Subcommittee on Exercise, Rehabilitation, and Prevention) and the Council on Nutrition, Physical Activity, and Metabolism (Subcommittee on Physical Activity) Circulation. 2003;107:3109–3116. doi: 10.1161/01.CIR.0000075572.40158.77. [DOI] [PubMed] [Google Scholar]
- Tinken TM, Thijssen DHJ, Black MA, Cable NT, Green DJ. Time course of change in vasodilator function and capacity in response to exercise training in humans. J Physiol. 2008;586:5003–5012. doi: 10.1113/jphysiol.2008.158014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tronc F, Wassef M, Esposito B, Henrion D, Glagov S, Tedgui A. Role of NO in flow-induced remodeling of the rabbit common carotid artery. Arterioscler Thromb Vasc Biol. 1996;16:1256–1262. doi: 10.1161/01.atv.16.10.1256. [DOI] [PubMed] [Google Scholar]
- Woodman RJ, Playford DA, Watts GF, Cheetham C, Reed C, Taylor RR, Puddey IB, Beilin LJ, Burke V, Mori TA, Green DJ. Improved analysis of brachial artery ultrasound using a novel edge-detection software system. J Appl Physiol. 2001;91:929–937. doi: 10.1152/jappl.2001.91.2.929. [DOI] [PubMed] [Google Scholar]


