Abstract
Background
Regional lymph node involvement is the most important prognostic factor in cutaneous melanoma. Since only 20% of melanoma patients have occult nodal disease and would benefit from a regional lymphadenectomy, the sentinel lymph node (SLN) biopsy was introduced. NIR fluorescence has been hypothesized to improve SLN mapping.
Objectives
To assess the potential of intraoperative near-infrared (NIR) fluorescence imaging to improve SLN mapping in melanoma patients and to examine the optimal dose of indocyanine green adsorbed to human serum albumin (ICG:HSA).
Methods
Fifteen consecutive cutaneous melanoma patients underwent the standard SLN procedure using 99mtechnetium-nancolloid and patent blue. In addition, intraoperative NIR fluorescence imaging was performed after injection of 1.6 mL of 600, 800, 1000 or 1200 μM of ICG:HSA in four quadrants around the primary excision scar.
Results
NIR fluorescence SLN mapping was successful in 93% of patients. In one patient, no SLN could be identified using either conventional methods or NIR fluorescence. A total of 30 SLNs (average 2.0, range 1-7) were detected, 30 radioactive (100%), 27 blue (73%), and 30 NIR fluorescent (100%). With regard to the effect of concentration on SBR a trend (P = 0.066) was found favouring the 600, 800 and 1000 μM groups over the 1200 μM group.
Conclusion
This study demonstrates feasibility and accuracy of SLN mapping using ICG:HSA. Considering safety, cost, and pharmacological characteristics, an ICG:HSA concentration of 600 μM appears optimal for SLN mapping in cutaneous melanoma, though lower doses need to be assessed.
INTRODUCTION
Regional lymph node involvement is the most important prognostic factor in cutaneous melanoma patients and complete lymph node dissection is the current standard treatment for patients with nodal metastasis.1 Only 20% of melanoma patients have occult nodal disease and therefore will benefit from a regional lymphadenectomy.2 However, the remaining 80% of patients without nodal involvement will be exposed to the risk of morbidity without offering a therapeutic advantage when performing a lymphadenectomy. The sentinel lymph node (SLN) biopsy was introduced by Morton et al.3 to obtain a minimal invasive assessment of regional nodal involvement and is now regarded as standard-of-care in staging cutaneous melanoma patients with primary lesions of at least 1 mm thick.
The SLN biopsy in melanoma patients is generally performed using a combination of radioactive colloid and blue dye. The use of this combination facilitates detection rates of over 90% and false-negative rates of approximately 5%.3-5 However, the use of radioactive colloid comes with certain disadvantages, such as the involvement of a nuclear physician, high costs, and the lack of visual information. In addition, blue dye staining can result in hampered visibility of the surgical field and results in tattooing of the injection site lasting for several months. Consequently, blue dye staining is omitted in some institutions when the primary tumour is located in the facial region. The blue dye cannot be seen through skin and fatty tissue.
The use of invisible near-infrared (NIR) light (700-900 nm) has several characteristics that can be advantageous in the SLN procedure, which include relatively high penetration into living tissue (several millimetres) and the lack of ionizing radiation.6 Indocyanine green (ICG) is one of only two clinically available NIR fluorescent agents and is currently the most optimal agent for SLN mapping.7 In several studies, intraoperative imaging systems in combination with ICG have been used for the SLN procedure for various types of cancer.8-13 The lymphatic channels and SLNs in cutaneous melanoma patients are often relatively superficially located. Therefore, NIR fluorescence imaging could be particularly useful for this indication. Indeed, several groups reported on the successful use of NIR fluorescence and ICG for SLN mapping in melanoma patients. 14-18
Preclinical evidence demonstrated that premixing of ICG with human serum albumin (HSA, complex is ICG:HSA) increases the fluorescence intensity and hydrodynamic diameter of ICG, resulting in better retention in the SLN.19 The aims of the current study were to assess the use of NIR fluorescence imaging using ICG:HSA and the Mini-FLARE intraoperative imaging system for the SLN procedure in melanoma patients. Furthermore, the optimal dose of ICG:HSA to perform a NIR fluorescence SLN procedure was assessed.
MATERIALS AND METHODS
Preparation of Indocyanine Green Adsorbed to Human Serum Albumin
ICG (25 mg vials) was purchased from Pulsion Medical Systems (Munich, Germany) and resuspended in 10 mL of sterile water for injection for the 600 μM group, or in 5 mL of sterile water for injection for the 800 μM, 1000 μM and 1200 μM groups, to yield stock solutions of 3.2 mM and 6.4 mM, respectively. Various amounts of this stock solution were transferred to a 50 cc vial of Cealb (20% human serum albumin [HSA] solution; Sanquin, Amsterdam, The Netherlands) to yield ICG in HSA (ICG:HSA) at a final concentration of 600 μM, 800 μM, 1000 μM or 1200 μM.
Intraoperative NIR Fluorescence Imaging
SLN mapping was performed using the Mini-FLARE image-guided surgery system as described in detail previously.9 Briefly, the system consists of 2 wavelength separated light sources: a “white” LED light source, generating 26,600 lx of 400 to 650 nm light to illuminate the surgical field and an NIR LED light source, generating 7.7 mW / cm2 of 760 nm fluorescence excitation light. White light and NIR fluorescence images are acquired simultaneously and displayed in real time, using custom designed optics and software. A pseudo-coloured (lime green) image of NIR fluorescence superimposed over the white light image is also displayed, to provide the NIR fluorescence signal in proper anatomical context.
Clinical Trial
The current dose escalation clinical trial was approved by the Medical Ethics Committee of the Leiden University Medical Center and was performed in concordance with the ethical standards of the Helsinki Declaration of 1975. Fifteen consecutive patients that were planned to undergo a therapeutic re-excision and a SLN procedure for cutaneous melanoma were included in this study between April 2010 and June 2011. Exclusion criteria were pregnancy, lactation or an allergy to iodine, shellfish, or indocyanine green.
All patients gave informed consent and were anonymized. Patients received the standard-of-care SLN procedure. For our institution, this implies injections at four quadrants around the primary excision scar of 60-100 MBq 99mtechnetium-nanocolloid on the afternoon of the day before, or the morning prior to surgery. Before the start of the operation, approximately 1 mL total of patent blue V (Guerbet, France) was injected at four quadrants around the primary excision scar. Immediately after injection of patent blue, a total of 1.6 mL of ICG:HSA was injected at the same four sites as the patent blue injections. ICG:HSA was injected at a concentration of 600, 800, 1000 and 1200 μM. In each concentration group, 3 patients were included (N = 12 patients). Subsequently, 3 patients were included at the optimal ICG:HSA concentration.
After surgical scrub, the Mini-FLARE imaging head was positioned at approximately 30 cm above the surgical field. The NIR fluorescence signal was measured percutaneously, prior to skin incision, and continuously during the surgical procedure. Throughout the procedure, the surgeon was continuously provided with real-time NIR fluorescence image guidance. When the SLN could not be found easily by NIR fluorescence, the handheld gamma probe could be used for the localization of SLNs. Relative brightness of the SLNs was determined by measuring signal-to-background ratios (SBRs); that is the NIR fluorescence signal of the SLN divided by the (auto)fluorescent signal of a directly adjacent region. Excised sentinel lymph nodes were analyzed ex vivo for NIR fluorescence and radioactivity and were routinely analyzed by histopathology. SLNs were fixed in formalin and embedded in paraffin for haematoxylin, eosin, and immunohistopathological staining using the S-100 and MART-1 markers at six levels, with an interval of 50-150 μm.
Statistical Analysis
For statistical analysis, SPSS statistical software package (Version 17.0, Chicago, IL) was used. Graphs were generated using GraphPad Prism Software (Version 5.01, La Jolla, CA). Signal-to-background ratios were reported as median and range. To test differences between groups, the Kruskal-Wallis one-way analysis of variance test and the Dunn's Multiple Comparison Test were used to test for differences between time and dose groups. Statistical tests were two-tailed and P < 0.05 was considered significant.
RESULTS
Patient and Tumour Characteristics
Fifteen consecutive patients with cutaneous melanoma undergoing SLN mapping were included in this study. Patients and tumour characteristics are described in Table 1. Median body mass index (BMI) was 24 (range 18 - 32), median age was 55 years (range 21 - 70 years), and median Breslow's depth was 1.9 mm (range 0.7 - 7 mm). In 1 patient, the melanoma was located in the head and neck region, in 4 patients at the higher extremities, in 9 patients at the trunk and in 1 patient at the lower extremities.
Table 1.
Patient and Tumour Characteristics
| Characteristic | N | % |
|---|---|---|
| Age (median, range) | 55 (21 - 70) | |
| Gender (% male) | 53 | |
| Body Mass Index (median, range) | 24 (18 - 32) | |
| Skin type | ||
| -II | 8 | 53 |
| -III | 7 | 47 |
| Breslow (median, range) | 1,9 (0,7 - 7) | |
| Clark classification | ||
| -3 | 4 | 27 |
| -4 | 10 | 67 |
| -N/A | 1 | 6 |
| Tumour localization | ||
| - Head and neck | 1 | 6 |
| - Upper extremities | 4 | 27 |
| - Ventral trunk | 4 | 27 |
| - Dorsal trunk | 5 | 34 |
| - Lower extremities | 1 | 6 |
| Type of Surgery | ||
| - Primary excision | 1 | 6 |
| - Re-excision | 14 | 94 |
Intraoperative NIR Fluorescence Imaging
Average time between ICG:HSA injection and skin incision was 12.6 ± 3.6 minutes. In 14 patients, one or more SLNs were identified. A total of 30 SLNs were detected, all of which were radioactive and fluorescent (Table 2). Twenty-two out of 30 SLNs (73%) were stained blue. A typical example of NIR fluorescence imaging using the Mini-FLARE imaging system is shown in figure 1. In one patient, no SLN could be identified using either conventional methods or NIR fluorescence. Average time between skin incision and resection of the first SLN was 10.2 ± 3.9 minutes. The location of the SLNs is summarized in Table 2. In each patient, following resection of all NIR fluorescent nodes, the surgical field was systematically inspected for remaining radioactivity or blue nodes. No additional nodes were found that were not detected by NIR fluorescence. No adverse reactions associated with the use of ICG:HSA or the Mini-FLARE imaging system were observed.
Table 2.
SLN Identification Results
| Characteristic | N | % |
|---|---|---|
| SLN Detection | ||
| - Number of SLNs Identified | 30 | |
| - Average Number of SLNs Identified (S.D.) | 2.0 ± 1.6 | |
| Method of Detection | ||
| - Radioactive | 30 | 100 |
| - Blue | 22 | 73 |
| - Near-Infrared Fluorescence | 30 | 100 |
| Average Time between Injection of ICG:HSA and Skin Incision (S.D.) | 12.6 ± 3.6 | |
| Average Time between Skin Incision and SLN Resection (S.D.) | 10.2 ± 3.9 | |
| Pecutaneous identification | ||
| - Lymphatic vessels (no. of patients) | 13 | 87 |
| - Sentinel lymph nodes (no. of nodes) | 8 | 27 |
| Sentinel lymph node localization | ||
| - Axilla | 18 | 60 |
| - Neck | 2 | 7 |
| - Groin | 4 | 13 |
| - Ventral trunk | 5 | 17 |
| - Dorsal trunk | 1 | 3 |
| Histology* | ||
| - Negative | 11 | 72 |
| - Macrometastases | 1 | 7 |
| - Micrometastases | 1 | 7 |
| - Isolated Tumour Cells and Micrometastases | 1 | 7 |
| Adjuvant treatment | ||
| - None | 12 | 80 |
| - Axillary Lymph Node Dissection | 2 | 13 |
| - Follow up | 1 | 7 |
| Complications | ||
| - No | 15 | 100 |
| -Yes | 0 | 0 |
No SLNs detected in 1 patient using both conventional techniques and NIR fluorescence
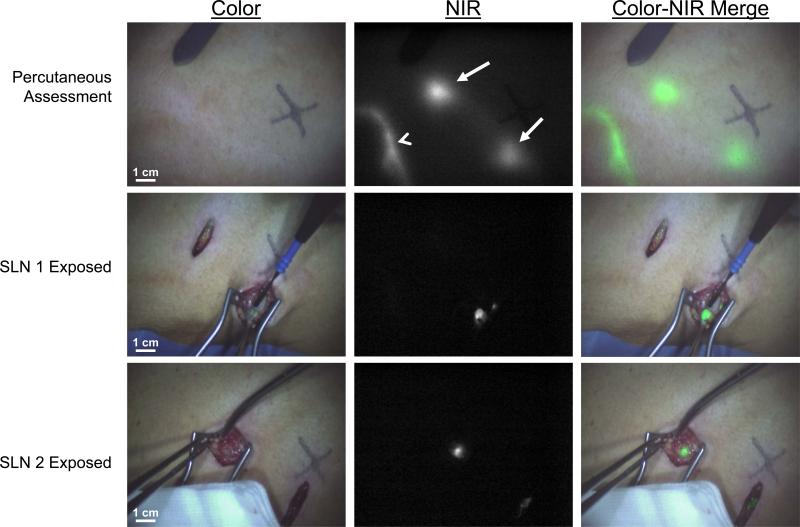
Figure 1. Sentinel lymph node mapping using NIR fluorescence imaging in cutaneous melanoma.
Lymphatic channel (arrowhead) and SLNs (arrows) can be clearly identified percutaneously (top row). Identification of the first SLN (middle row) and second SLN (bottom row) is demonstrated using NIR fluorescence imaging at 15 min after injection of 1.6 mL of 1000 μM ICG:HSA around the excision scar.
The Effect of Lymphatic Tracer Dose on SLN Brightness
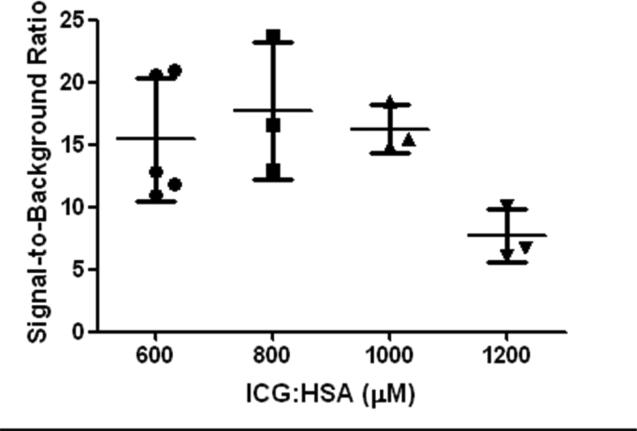
The effect of injected lymphatic tracer dose on fluorescence brightness was determined by comparing median SBRs between concentration groups (Fig. 2). Median SBRs of the SLNs were 12.33 (range: 10.90 – 20.92), 16.59 (range: 12.94 – 23.73), 15.47 (range: 14.87 – 18.42) and 6.76 (range: 6.14 – 10.14) for the 600, 800, 1000 and 1200 μM concentration groups, respectively. The independent samples Kruskal-Wallis Test to test for difference in signal-to-background ratios showed a trend favouring the 600,800 and 1000 μM dose groups over the 1200 μM dose group (P = 0.066).
Figure 2. Optimization of ICG:HSA dose.
Signal-to-background ratio (mean ± S.D.) of melanoma SLNs (ordinate) is plotted as a function of injected dose of ICG:HSA (abscissa). A trend was found favouring the 600, 800 and 1000 μM over 1200 μM (P = 0.066).
Percutaneous Visualization of Lymphatic Channels and Sentinel Lymph Nodes
Prior to the incision, the ability to identify lymphatic vessels and SLNs using NIR fluorescence was assessed. In 13 of 15 patients (87%), lymphatic vessels running away from the site of injection could be identified percutaneously (Fig. 1). In 3 of 15 patients (20%), the location of a total of 8 SLNs could be identified prior to incising the skin. These SLNs were all located in the neck and on the ventral trunk.
DISCUSSION
The current study demonstrates feasibility of NIR fluorescence SLN mapping using ICG:HSA and the Mini-FLARE imaging system in melanoma patients. The accuracy of the SLN procedure using NIR fluorescence was similar to that of radiocolloids and superior to that of blue dye staining. The use of both NIR fluorescence and radiocolloids lead to a 100% SLN detection rate. Whereas only 73% of all SLNs were stained blue. These results are in concordance with previous studies performed by our group on NIR fluorescence SLN mapping in breast and vulvar cancer patients.9;20;21
An advantage of using NIR fluorescent light is that it is capable of penetrating several millimetres into living tissue. In cutaneous melanoma, there is a high variability in localization of the SLN mostly depending on the site of the primary tumour. Not infrequently, different SLNs can be found at completely different sites in the human body, which can lead to a prolonged search and even the need of displacing the patient on the operating table. In these cases, real-time percutaneous assessment of lymphatic drainage, from the primary tumour to the SLN will have a substantial added value. Percutaneous assessment of lymphatic vessels was possible in 87% of patients in the current study. Moreover, in 20% of patients, the SLNs could be identified through the skin. SLNs in cutaneous melanoma patients are often superficially located, making NIR fluorescence imaging highly suitable for this indication. Percutaneous visualization can be influenced by the skin type of patients, as melanin and hemoglobin determine skin color and have high scattering and absorption properties in the NIR spectral range. In this study, no differences were observed as all patients had skin type II/III. Moreover, when located deeper, for example in the axilla, no SLNs could be identified percutaneously using NIR fluorescence. The advantages of NIR fluorescence using ICG:HSA could allow more efficient detection of the SLN, which can result in less damage of healthy tissue, and possibly increase sensitivity.22 Though, in the current study, in one patient, no SLN could be identified using NIR fluorescence or the conventional technique, for which no explanation could be appointed.
For future clinical use of this new technique it is important to determine the dose of ICG:HSA that provides optimal imaging results. We observed a trend in SBR differences between the ascending concentrations of ICG:HSA leading to a decline of signal in the 1200 μM group. This is in line with previously reported results in breast cancer.9 A decrease of fluorescence signal with an increase in concentration can likely be explained by fluorescence quenching.7 To assess the possibility of using lower concentrations for fluorescence SLN mapping in melanoma patients, a dose-finding study with concentrations ranging from 50 μM – 500 μM of ICG:HSA will be reported separately (Clinicaltrials.gov identifier: NCT01121718).
In the process of optimization of the SLN procedure using NIR fluorescence in breast cancer patients, we have previously demonstrated in a randomized trial that there is no advantage of using ICG premixed with HSA over ICG alone20 and that patent blue can be omitted when NIR fluorescence is used23. For optimization of this technique in melanoma patients, future studies investigating the additional value of premixing ICG with HSA and the necessity to use patent blue have to be performed. Moreover, in the current study SLN localization was performed directly after tracer administration. In a previous study by our group in SLN biopsy in oral cavity and oropharyngeal cancers, rapid migration of ICG:HSA beyond the SLN was observed (van der Vorst et al, 2012, in press). The migration beyond the SLN requires localization of SLN directly after injection of the tracer, to prevent detection of many second-tier lymph nodes.
In conclusion, our current study demonstrates the feasibility of SLN mapping in cutaneous melanoma patients using ICG:HSA and the mini-FLARE image-guided surgery system. With respect to logistical and safety issues, a dose of 600μM of ICG:HSA seems to be optimal. NIR fluorescence has a 100% accuracy in SLN detection and adds the value of percutaneous lymphatic drainage and in some cases SLN assessment.
Bulleted statements.
Although near-infrared fluorescence imaging for sentinel lymph node mapping in melanoma has been reported, our study is the first to combine the near-infrared fluorophore with human serum albumin to improve lymph node retention and the first to optimize contrast agent dosing and performance in patients.
ACKNOWLEDGMENTS
The authors thank Lindsey Gendall for editing. This work was supported in part by NIH grants R01-CA-115296 and R21-CA-130297 and the Dutch Cancer Society grant UL2010-4732. This research was performed within the framework of CTMM, the Center for Translational Molecular Medicine, project MUSIS (grant 03O-202). Joost van der Vorst is an MD-medical research trainee funded by The Netherlands Organisation for Health Research and Development (grant 92003593).
Footnotes
J.R. van der Vorst and B.E. Schaafsma contributed equally to the study and share first authorship.
Conflicts of interest: FLARE™ technology is owned by Beth Israel Deaconess Medical Center, a teaching hospital of Harvard Medical School. It has been licensed to the FLARE™ Foundation, a non-profit organization focused on promoting the dissemination of medical imaging technology for research and clinical use. Dr. Frangioni is the founder and chairman of the FLARE™ Foundation. The Beth Israel Deaconess Medical Center will receive royalties for sale of FLARE™ Technology. Dr. Frangioni has elected to surrender post-market royalties to which he would otherwise be entitled as inventor, and has elected to donate pre-market proceeds to the FLARE™ Foundation.
Reference List
- 1.Balch CM, Soong SJ, Atkins MB, et al. An evidence-based staging system for cutaneous melanoma. CA Cancer J Clin. 2004;54:131–49. doi: 10.3322/canjclin.54.3.131. [DOI] [PubMed] [Google Scholar]
- 2.Morton DL, Wanek L, Nizze JA, et al. Improved long-term survival after lymphadenectomy of melanoma metastatic to regional nodes. Analysis of prognostic factors in 1134 patients from the John Wayne Cancer Clinic. Ann Surg. 1991;214:491–9. doi: 10.1097/00000658-199110000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Morton DL, Wen DR, Wong JH, et al. Technical details of intraoperative lymphatic mapping for early stage melanoma. Arch Surg. 1992;127:392–9. doi: 10.1001/archsurg.1992.01420040034005. [DOI] [PubMed] [Google Scholar]
- 4.Thompson JF, McCarthy WH, Bosch CM, et al. Sentinel lymph node status as an indicator of the presence of metastatic melanoma in regional lymph nodes. Melanoma Res. 1995;5:255–60. doi: 10.1097/00008390-199508000-00008. [DOI] [PubMed] [Google Scholar]
- 5.Gershenwald JE, Ross MI. Sentinel-lymph-node biopsy for cutaneous melanoma. N Engl J Med. 2011;364:1738–45. doi: 10.1056/NEJMct1002967. [DOI] [PubMed] [Google Scholar]
- 6.Frangioni JV. In vivo near-infrared fluorescence imaging. Curr Opin Chem Biol. 2003;7:626–34. doi: 10.1016/j.cbpa.2003.08.007. [DOI] [PubMed] [Google Scholar]
- 7.Gioux S, Choi HS, Frangioni JV. Image-guided surgery using invisible near-infrared light: fundamentals of clinical translation. Mol Imaging. 2010;9:237–55. [PMC free article] [PubMed] [Google Scholar]
- 8.Hojo T, Nagao T, Kikuyama M, et al. Evaluation of sentinel node biopsy by combined fluorescent and dye method and lymph flow for breast cancer. Breast. 2010;19:210–3. doi: 10.1016/j.breast.2010.01.014. [DOI] [PubMed] [Google Scholar]
- 9.Mieog JS, Troyan SL, Hutteman M, et al. Towards Optimization of Imaging System and Lymphatic Tracer for Near-Infrared Fluorescent Sentinel Lymph Node Mapping in Breast Cancer. Ann Surg Oncol. 2011 doi: 10.1245/s10434-011-1566-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Troyan SL, Kianzad V, Gibbs-Strauss SL, et al. The FLARE intraoperative near-infrared fluorescence imaging system: a first-in-human clinical trial in breast cancer sentinel lymph node mapping. Ann Surg Oncol. 2009;16:2943–52. doi: 10.1245/s10434-009-0594-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Murawa D, Hirche C, Dresel S, et al. Sentinel lymph node biopsy in breast cancer guided by indocyanine green fluorescence. Br J Surg. 2009;96:1289–94. doi: 10.1002/bjs.6721. [DOI] [PubMed] [Google Scholar]
- 12.Schaafsma BE, Mieog JSD, Hutteman M, et al. The clinical use of indocyanine green as a near-infrared fluorescent contrast agent for image-guided oncologic surgery. J Surg Oncol. 2011 doi: 10.1002/jso.21943. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Crane LM, Themelis G, Pleijhuis RG, et al. Intraoperative multispectral fluorescence imaging for the detection of the sentinel lymph node in cervical cancer: a novel concept. Mol Imaging Biol. 2011;13:1043–9. doi: 10.1007/s11307-010-0425-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fujisawa Y, Nakamura Y, Kawachi Y, et al. A Custom-Made, Low-Cost Intraoperative Fluorescence Navigation System with Indocyanine Green for Sentinel Lymph Node Biopsy in Skin Cancer. Dermatology. 2011 doi: 10.1159/000327080. [DOI] [PubMed] [Google Scholar]
- 15.Fujiwara M, Mizukami T, Suzuki A, et al. Sentinel lymph node detection in skin cancer patients using real-time fluorescence navigation with indocyanine green: preliminary experience. J Plast Reconstr Aesthet Surg. 2009;62:e373–e378. doi: 10.1016/j.bjps.2007.12.074. [DOI] [PubMed] [Google Scholar]
- 16.Tanaka R, Nakashima K, Fujimoto W. Sentinel lymph node detection in skin cancer using fluorescence navigation with indocyanine green. J Dermatol. 2009;36:468–70. doi: 10.1111/j.1346-8138.2009.00679.x. [DOI] [PubMed] [Google Scholar]
- 17.Mizukami T, Fujiwara M, Suzuki A, et al. Sentinel lymph node detection by indocyanine green fluorescence imaging in skin cancer patients: technical refinement. The Open Surgical Oncology Journal. 2010;2:57–61. [Google Scholar]
- 18.Polom K, Murawa D, Rho YS, et al. Skin melanoma sentinel lymph node biopsy using real-time fluorescence navigation with indocyanine green and indocyanine green with human serum albumin. Br J Dermatol. 2011 doi: 10.1111/j.1365-2133.2011.10634.x. [DOI] [PubMed] [Google Scholar]
- 19.Ohnishi S, Lomnes SJ, Laurence RG, et al. Organic alternatives to quantum dots for intraoperative near-infrared fluorescent sentinel lymph node mapping. Mol Imaging. 2005;4:172–81. doi: 10.1162/15353500200505127. [DOI] [PubMed] [Google Scholar]
- 20.Hutteman M, Mieog JS, van der Vorst JR, et al. Randomized, double-blind comparison of indocyanine green with or without albumin premixing for near-infrared fluorescence imaging of sentinel lymph nodes in breast cancer patients. Breast Cancer Res Treat. 2011;127:163–70. doi: 10.1007/s10549-011-1419-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hutteman M, van der Vorst JR, Gaarenstroom KN, et al. Optimization of near-infrared fluorescent sentinel lymph node mapping for vulvar cancer. Am J Obstet Gynecol. 2011 [Google Scholar]
- 22.Fujisawa Y, Nakamura Y, Kawachi Y, et al. Indocyanine green fluorescence-navigated sentinel node biopsy showed higher sensitivity than the radioisotope or blue dye method, which may help to reduce false-negative cases in skin cancer. J Surg Oncol. 2012 doi: 10.1002/jso.23045. [DOI] [PubMed] [Google Scholar]
- 23.van der Vorst JR, Schaafsma BE, Verbeek FP, et al. Randomized Comparison of Near-infrared Fluorescence Imaging Using Indocyanine Green and 99(m) Technetium With or Without Patent Blue for the Sentinel Lymph Node Procedure in Breast Cancer Patients. Ann Surg Oncol. 2012 doi: 10.1245/s10434-012-2466-4. [DOI] [PMC free article] [PubMed] [Google Scholar]