Abstract
Phthalate esters are commonly used plasticizers found in many household items, personal care products, and medical devices. Animal studies have shown that in utero exposure to di-(n-butyl) phthalate (DBP) within a critical window during gestation causes male reproductive tract abnormalities resembling testicular dysgenesis syndrome (TDS). Our studies utilized p53-deficient mice for their ability to display greater resistance to apoptosis during development. This model was chosen to determine whether multi-nucleated germ cells (MNGs) induced by gestational DBP exposure could survive postnatally and evolve into testicular germ cell cancer. Pregnant dams were exposed to DBP (500 mg/kg/day) by oral gavage from gestational day (GD) 12 until birth. Perinatal effects were assessed on GD 19 and postnatal days 1, 4, 7, and 10 for the number of MNGs present in control and DBP-treated p53-heterozygote and null animals. As expected, DBP exposure induced MNGs, with greater numbers found in p53 null mice. Additionally, there was a time-dependent decrease in the incidence of MNGs during the early postnatal period. Histological examination of adult mice exposed in utero to DBP revealed persistence of abnormal germ cells only in DBP-treated p53-null mice, not in p53-heterozygote or wild-type mice. Immunohistochemical staining of perinatal MNGs and adult abnormal germ cells was negative for both octamer binding protein-3/4 and placental alkaline phosphatase. This unique model identifies a role for p53 in the perinatal apoptosis of DBP-induced MNGs, and provides insight into the long-term effects of gestational DBP exposure within a p53-null environment.
Keywords: Testis, phthalate, fetal testis, p53, testicular germ cell cancer (TGCC), multi-nucleated germ cells (MNGs)
Introduction
The worldwide incidence of testicular cancer has been increasing steadily, with the highest prevalence currently found in northern European countries (Richiardi et al, 2004). In the United States, testicular germ cell cancer (TGCC) is the most common neoplasia in men between the ages of 15–34 years old, predominantly affecting Caucasians (McGlynn et al, 2003, Skakkebaek et al, 2007, Swerdlow et al, 1999). Unfortunately, the etiology of this disease is unknown (Lacerda et al, 2009), with only a few established risk factors, including cryptorchidism, age, and family history (Neale et al, 2008). Other factors, such as low birth weight (Ahlgren et al, 2007), high estrogen levels during pregnancy (Akre et al, 1996), advanced maternal age at conception (Michos et al, 2007), length of gestation (Michos et al, 2007), and birth order (Akre et al, 1996, Michos et al, 2007, Richiardi et al, 2003) may also play a role. The young age of TGCC presentation raises the question of whether early life exposures to environmental factors have an influence on its development (Brown et al, 1986, Main et al, 2010, Rasmussen et al, 2003, Skakkebaek et al, 2007).
In 2001, Skakkebaek et al. proposed Testicular Dysgenesis Syndrome (TDS), a hypothesis to explain the etiology of TGCC and other male reproductive tract abnormalities. TDS suggests that a combination of genetic, lifestyle, or environmental factors may affect the normal development and function of fetal Leydig and Sertoli cells and their precursors. This altered development may predispose the developing male reproductive tract to subsequent abnormalities including hypospadias, cryptorchidism, low sperm counts, and testicular cancer (Sharpe and Skakkebaek, 2008, Skakkebaek et al, 2003, Skakkebaek et al, 2001). Interestingly, many of the features described by the TDS hypothesis are observed in rats developmentally exposed to phthalates, a family of antiandrogenic chemical contaminants (Foster, 2006, Sharpe and Skakkebaek, 2008, Witorsch and Thomas, 2010).
Di-(n-butyl) phthalate (DBP) is an endocrine disrupting chemical (EDC) and a ubiquitous plasticizer utilized in the production of flexible polyvinyl chloride (PVC) materials. As phthalates are non-covalently bound within PVC, they leach out, becoming available for biological exposure (Heudorf et al, 2007, Lottrup et al, 2006, Swan, 2008). Studies in fetal male rats have shown that in utero exposure to DBP results in dysgenesis within the testis characterized by the induction of cryptorchidism and hypospadias (Fisher et al, 2003, Mylchreest et al, 2002), abnormal Leydig cell aggregation (Barlow and Foster, 2003, Mahood et al, 2005), reduced steroidogenic gene expression and testosterone production (Lehmann et al, 2004), and altered seminiferous cords, including the induction of multinucleated germ cells (MNGs) (Ferrara et al, 2006, Fisher et al, 2003, Kleymenova et al, 2005, Mahood et al, 2007).
DBP-induced formation of MNGs has been of particular interest, as it is speculated that these developmentally impaired germ cells may develop into carcinoma in situ (CIS) cells, the known precursor to TGCC in humans (Ferrara et al, 2006). TGCC has followed clinical detection of testicular CIS cells in approximately 50% of patients five years after the initial diagnoses (Hoei-Hansen et al, 2007).
Two immunohistochemical markers are commonly used to characterize the development and neoplastic transformation of testicular germ cells. Octamer binding protein -3/4 (Oct-3/4) is an essential octamer-binding transcription factor that is required for early germ cell development. It is found in totipotent and pluripotent embryonic stem cells as well as primordial germ cells (Okumura-Nakanishi et al, 2005, Pesce et al, 1998, Rajpert-De Meyts et al, 2004), and is a useful diagnostic tool in the identification of neoplasms of germ cell origin (Cheng et al, 2007, Jones et al, 2004). Placental alkaline phosphatase (PLAP) is commonly used to identify CIS cells in adult males and is normally found in primordial germ cells, gonocytes, placental syncytiotrophoblasts and oogonia (Hoei-Hansen, 2008, Hoei-Hansen et al, 2007, Rajpert-De Meyts et al, 2003, Sonne et al, 2009).
In the current study, we explore the perinatal and long-term effects of in utero DBP exposure on germ cells within a p53-null environment in the mouse. The p53 gene is one of the most extensively studied tumor suppressors, as approximately 80% of all human cancers contain a defect in this signaling pathway (Meulmeester and Jochemsen, 2008, Venkatachalam et al, 2001). p53 exhibits its tumor suppressor properties by sensing and responding to different types of DNA damage, allowing for further repair or elimination of the damaged cells through apoptosis mechanisms (Donehower, 1996). In rats, it is known that MNGs disappear during the perinatal period following gestational DBP exposure (Barlow and Foster, 2003, Ferrara et al, 2006). We hypothesized that using the p53-null apoptosis-resistant mouse model would result in persistence of DBP-induced MNGs, allowing for the examination of their potential as precursor cells in the development of TGCC.
Materials and Methods
Chemicals
Di-(n-butyl) phthalate (99% purity) (CAS#: 84-74-2) and corn oil (CAS#: 8001-30-7) were obtained from Sigma Aldrich (St. Louis, MO).
Animals
Adult male homozygous (p53 −/−) and female heterozygous (p53+/−) B6.129S2-Trp53tm1Tyj/J mice were obtained from Jackson Laboratories (Bar Harbor, ME) and bred in-house. Female heterozygous mice (p53 +/−) were paired with male homozygous mice (p53 −/−) for five days. Successful copulation was determined by the detection of a vaginal plug and was considered to be gestational day (GD) 0. After plug detection, females were separated from males. Male pups were separated from dams after weaning on PND 25. Animals were maintained in a temperature and humidity controlled environment with a 12-hour alternating light-dark cycle. Mice were kept in community cages with access to both water and Purina Rodent Chow 5001 (Farmer’s Exchange, Framingham, MA) ad libitum. All experimental animal protocols were approved by the Brown University Institutional Animal Care and Use Committee, in compliance with National Institutes of Health guidelines.
DNA Isolation and Genotyping
Tail digestion and DNA isolation was performed using a Qiagen DNeasy blood and tissue kit (Cat. 69504, Qiagen, Valencia, CA) according to the manufacturer’s directions. Genotyping was performed as described previously (Embree-Ku and Boekelheide, 2002).
Exposure Paradigm
Pregnant mice were treated with 250 or 500 mg/kg/day (1 mL/kg body weight) di-(n-butyl) phthalate (DBP) in a corn oil vehicle by oral gavage daily from GD 12 until birth. The 250 and 500 mg/kg doses of DBP were chosen because they have been shown to increase the number of MNGs within the seminiferous cord, as well as the number of nuclei present in an MNG in mice (Gaido et al, 2007). Male p53-null mice treated with 250 mg/kg DBP in utero were separated from dams after weaning (PND 25), and housed until signs related to tumor development appeared, such as weight loss (10–15% of body weight) and lethargy, at which time they were euthanized by CO2 asphyxiation. Male mice treated with 500 mg/kg DBP were euthanized by CO2 asphyxiation at GD 19, PND 1, 4, 7, or 10 (Figure 1).
Figure 1.
Female B6.129S2-Trp53tm1Tyj/J mice treated with 250 or 500 mg/kg/day DBP in a corn oil vehicle (1ml/kg body weight) from gestational day (GD) 12 until birth. Mice given 500 mg/kg/day DBP were euthanized and testes were collected on GD19, post-natal day (PND) 1, 4, 7 or 10. Mice given 250 mg/kg/day DBP were euthanized after displaying signs related to tumor development (ages ranged from 87–515 days old).
Sample Collection and Processing for Histology
Testis tissue was removed at the selected time points, fixed for 24 hours in modified Davidson’s solution, and post-fixed in 10% neutral buffered formalin. For the perinatal study, the right testis of each male pup was embedded in glycol methacrylate (Technovit 7100, Heraeus Kulzer GmBH, Wehrheim, Germany) for histopathology, and the left testis was processed in paraffin for immunohistochemistry (IHC). For the long-term study, two cross-sections were taken from the middle of each formalin-fixed testis, with one half embedded in glycol methacrylate, while the other half was processed in paraffin. Glycol methacrylate embedded tissues were serial sectioned (3 µm), and stained with periodic acid-Schiff reagent followed by a hematoxylin counter-stain (PAS-H). Testis samples embedded in paraffin were sectioned (5 µm) and stained with hematoxylin and eosin (H&E).
MNG Quantification
Tissue slides were blinded and scanned into an Aperio ScanScope CS microscope (Aperio Technologies, Vista, CA) and quantified using ImageScope software. For the perinatal time-points, each seminiferous cord was evaluated for the total testis area, and the number of MNGs. MNGs were determined based on the presence of germ cells containing two or more nuclei within the same cellular membrane. The presence of MNGs was confirmed using an Olympus BH-2 microscope (Center Valley, PA). To account for intra-litter variability, the MNG counts for each pup within a litter were averaged to yield a total mean of MNGs/area per dam. The dams were used as the unit of effect within a group for statistical analysis across groups. A minimum of 3 dams were used for each treatment group and time-point.
For adult time-points, tissue sections from 87–515 days of age were blinded and scanned as previously described. Seminiferous tubules were serial sectioned, and assessed for the presence of abnormal germ cells. These abnormal germ cells were defined by their large size (>20 µm in length) along the surface of the basal lamina with excessive nuclear material, abnormal chromatin distribution, enlarged cytoplasm and their presence in at least three serial sections.
Immunohistochemical markers
Two immunohistochemical markers, octamer-binding protein 4 (Oct-3/4) and placental alkaline phosphatase (PLAP), were used to characterize the MNGs and abnormal germ cells (Hoei-Hansen, 2008, Looijenga et al, 2003). Antigen retrieval was performed on paraffin-embedded tissue sections (5 µm) using a 10 mM citrate buffer (pH 6) solution. Endogenous peroxidase activity was blocked with 3% hydrogen peroxidase solution in water, followed by avidin/biotin blocking (SP-2001, Vector Laboratories, Burlingame, CA). A mouse monoclonal antibody against the POU5F1 domain of Oct-3/4 (C-10, sc-5279, Santa Cruz Biotechnology, Santa Cruz, CA) was applied at 0.5 µg/ml following the manufacturer’s protocol from the Vector M.O.M. Peroxidase Kit (PK-2200, Vector Laboratories, Burlingame, CA). A rabbit monoclonal against PLAP (SP15, AB-16695, Abcam, Cambridge, MA) was applied at 0.5 µg/ml, and sections were incubated overnight at 4°C, after which a biotinylated goat anti-rabbit secondary antibody (2.5 µg/ml) was applied. This antibody complex was visualized using the horseradish Peroxidase Avidin-biotin complex method. Reaction was revealed using 3,3’-diaminobenzidine with nickel, and all tissue sections were counterstained with hematoxylin. Positive controls used for Oct-3/4 and PLAP staining included mouse testis at GD15, and mouse placenta, respectively.
Statistical Analysis
GraphPad Prism (La Jolla, CA) and the R software environment (R Foundation for Statistical Computing, Vienna, Austria) were used for statistical analysis and graph creation. A two-way ANOVA followed by a Bonferroni post test was performed, with a p-value <0.05/k being considered statistically significant (k is the number of comparisons). Using individual pup data (not average within a litter), a Poisson (log-linear) regression was used to model the longitudinal change of abnormal germ cells from long-term time-points, where log expected counts of abnormal germ cells was modeled with pup’s ‘age’ as a linear predictor and ‘cross-section area’ as an offset factor. The Poisson regression model was estimated using Generalized Estimating Equations (GEE) (Zeger and Liang, 1986), which produces robust standard error to account for intra-litter variability.
Results
DBP-induced MNGs decrease with time
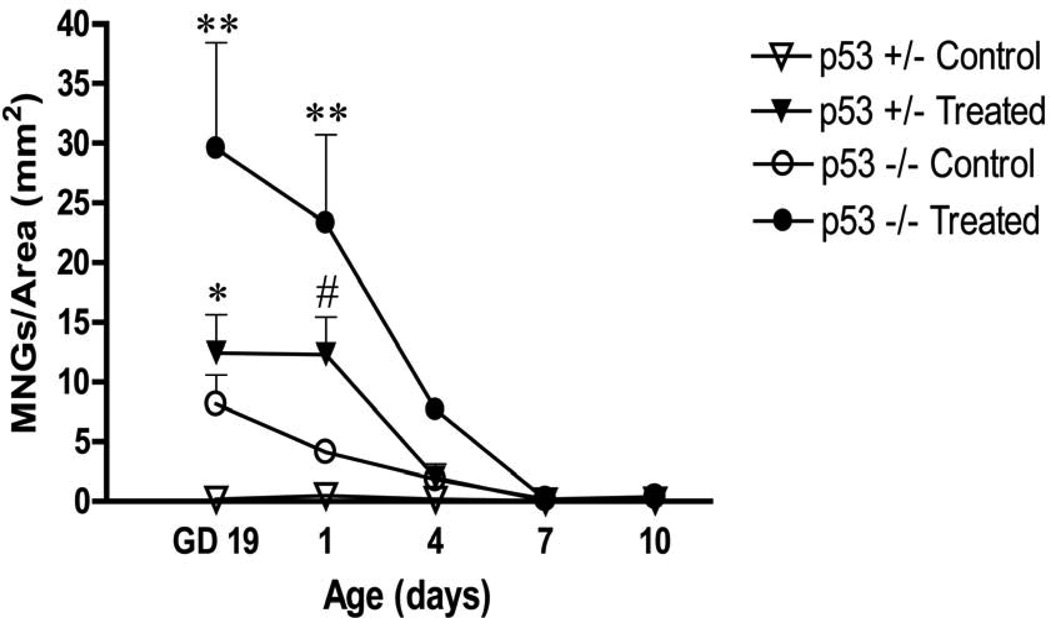
Dams were dosed with either corn oil or DBP from GD12 until birth and fetuses were examined and assessed for the presence of MNGs on GD19, PND 1, 4, 7 and 10. Histopathological analysis of GD19, PND 1, 4, 7 and 10 testes after in utero DBP exposure revealed the presence of MNGs within the centers of seminiferous cord cross-sections. At GD19 and PND1, DBP-treated p53-null pups had a significantly greater density of MNGs compared to all other groups. Both DBP-treated p53-null and heterozygous mice had a significantly greater density of MNGs compared to their respective controls at GD19 and PND1. A representative histopathological picture is shown for both untreated and DBP-treated p53-null testis at GD19 demonstrating the presence of MNGs throughout the seminiferous cords in the DBP-treated testis, including one large MNG containing multiple nuclei (Figure 2). While there was no statistical difference in maternal body weight gain and number of pups within a litter, at each day assessed there was an apparent trend toward lower maternal weight gain and fewer pups per litter in DBP-exposed treatment groups. Also, a genotype-specific difference was apparent in unexposed animals, with a higher spontaneous rate of MNG formation in the p53-null mice compared to p53-heterozygous mice at the early time-points. A trend of time-dependent loss of DBP-induced MNGs/area was also observed. Interestingly, MNGs were still present, albeit at a low frequency, at PND 10 (0.12–0.37 MNGs/mm2) in all groups (Figure 3). This persistence led to further investigation of their potential tumorigenicity at later time-points.
Figure 2.
Histology of p53-null testes from GD 19 fetuses exposed in utero to either corn oil (A) or 500 mg/kg/day DBP (B) from GD 12 until birth. Inset shows a magnification of a representative MNG. Scale bar, 50 µm; MNGs are indicated by arrows; PASH staining.
Figure 3.
Time-course for the incidence of MNGs in testes of DBP-treated p53-null and p53-heterozygous pups of dams dosed in utero with 500 mg/kg/day DBP from GD12 until birth. The MNG counts for each pup within a litter were averaged, and the dams were used as the unit of effect within a group for statistical analysis across groups. Dams were compared by a one-way ANOVA (minimum number of dams, n=3). Bars indicate the mean concentration ± SEM; ** indicates p<0.001, * p<0.01, # p<0.05 significant difference from the respective corn oil control for each genotype.
Abnormal DBP-induced germ cells in adult mice
The presence of MNGs at PND 10 in all groups raised the question of the extent to which these cells persist later in life. To address this, pregnant dams were dosed in utero with 250 mg/kg/day from GD 12 until parturition, and pups were euthanized at various adult time-points. Testis samples appeared histopathologically normal, with well-defined seminiferous tubules and active spermatogenesis. However, after careful examination of DBP-treated p53-null testis cross sections, abnormal germ cells (>20 µm in length), were occasionally observed adjacent to the basement membrane of seminiferous tubules (Figure 4). Abnormal cells were found in 52% of the DBP-exposed p53-null mice (n=25) examined between 87–515 days of age. These data were fitted using a Poisson regression, which revealed a significant decreasing temporal trend (p < 0.02) of abnormal germ cells with increasing age in exposed p53-null mice (Figure 5). The abnormal germ cells were not found in DBP-treated wild-type (n=3, age range 87–127 days), DBP-treated p53 heterozygous (n=27, age range 109–658 days), or control p53-null (n=12, age range 103–228 days) mice.
Figure 4.
Histopathology of adult testis from p53-null pups of dams that were dosed in utero with 250 mg/kg/day DBP from GD12 until birth. Cross sections of 109 (A), 127 (B), 161 (C), 164 (D), 188 (E) and 218 (F) days old testis. Scale bar, 20 µm; Arrows (A–F) indicate abnormal germ cells; Periodic acid Schiff (PASH) stain.
Figure 5.
Incidence of abnormal germ cells in DBP-treated p53-null adult offspring of dams dosed in utero with 250 mg/kg/day DBP from GD12 until birth. Abnormal germ cells were found in 52% of DBP-treated p53-null adult mice (n=25) between the ages of 108–218 days old. The data were fitted using a Poisson (log-linear) regression where log expected counts of abnormal germ cells were modeled with ‘age’ as a linear predictor and ‘area’ as an offset factor. The model fitted values were shown in the above plot as the solid line. The decreasing temporal trend is statistically significant (p<0.02).
MNGs and abnormal germ cells do not display markers of gonocytes or TGCC
The large abnormal germ cells found in the DBP-treated p53-null mice were proposed to be DBP-induced MNGs that persisted into adulthood. In an effort to characterize these interesting cells, testis sections from both acute and long term time-points were stained with two immunohistochemical markers, Oct-3/4 and PLAP, markers for primordial germ cells and CIS cells, respectively. Negative staining was found for both Oct-3/4 and PLAP in MNGs at the perinatal (Fig. 6, B and E) and abnormal germ cells at the long-term (Fig. 6, C and F) time-points. Controls for Oct-3/4 (Fig. 6A) and PLAP (Fig. 6D) were used to confirm positive staining for each marker.
Figure 6.
Immunohistochemistry for Oct 3/4 and PLAP in fetal (B and E) and adult (C and F) testis. Positive controls using GD 15 testis germ cells for Oct 3/4 (A) and placental syncytiotrophoblasts for PLAP (D) are shown. Staining is shown for Oct 3/4 (B) and PLAP (E) in GD 19 p53-null fetal testis from dams dosed in utero with 500 mg/kg/day DBP from GD 12 until birth. Abnormal germ cells were observed in adult p53-null testis and stained for Oct 3/4 in 108 days old (C) and PLAP in 127 days old (F) testis. Scale bar, 20 µm (A, C, D, and F), 50µm (B and E); Arrows indicate the presence of MNGs, and arrowheads indicate abnormal germ cells in adult mice; counterstained with hematoxylin.
Discussion
The increasing incidence of testicular cancer world-wide is a serious concern, with the United States showing a 44% rise between 1973 and 1998 (McGlynn et al, 2003). The TDS hypothesis provides a possible explanation for this trend in male reproductive tract abnormalities; particularly noteworthy is the implication that lifestyles and environmental exposures early in life may play a role (Skakkebaek et al, 2003, Skakkebaek et al, 2001). Phthalates are of special interest because they are ubiquitous EDCs to which women are exposed daily (Swan, 2008). In addition, rat models have demonstrated that in utero exposure to DBP and other phthalates can lead to testicular dysgenesis-like effects including hypospadias, cryptorchidism, impaired Leydig cell function, decrease in steroidogenic gene expression, and the induction of MNGs (Barlow et al, 2003, Ferrara et al, 2006, Mylchreest et al, 2002, Shultz et al, 2001). MNG formation is present in both fetal rat and mouse seminiferous cords after phthalate exposure (Gaido et al, 2007). In utero exposure to DBP in both Sprague-Dawley (Barlow and Foster, 2003) and Wistar rats (Ferrara et al, 2006) produced MNGs, along with unusual mitotic bodies, observed during early postnatal life (PND 3–8). By PND 16, Sprague-Dawley MNGs were no longer present in rat testes, while there was a marked decrease in MNGs by PND 15 in Wistar rats (Barlow and Foster, 2003, Ferrara et al, 2006). Thus, in wildtype rodents, DBP-induced MNGs are present from late gestation through the first couple of weeks of postnatal life before being eliminated entirely.
MNGs are hypothesized to form following nuclear division without cytokinesis, or from the improper fusion of two or more adjacent inter-connected germ cells (Kleymenova et al, 2005). Further, it has been speculated that there may be an association between these cells and the formation of CIS cells, the precursors to TGCC (Ferrara et al, 2006). TGCC is proposed to arise from germ cells that have failed to undergo successful differentiation; these developmentally abnormal germ cells are hypothesized to become CIS cells that subsequently transform into cancer (Sharpe and Skakkebaek, 2008).
Mouse models are invaluable in exploring the pathogenesis of many different diseases. Cancer studies frequently employ the p53 deficient mouse because of the critical role of this tumor suppressor gene in tumorigenesis (Lozano and Liu, 1998, Lozano and Zambetti, 2005). p53 is responsible for many important cellular functions including the regulation of apoptosis and the initiation of cell cycle repair mechanisms after cellular stress (Sinha Hikim and Swerdloff, 1999, Tripathi et al, 2009). Previous studies have shown that p53 aids in the removal of damaged germ cells during spermatogenesis, and that the p53-null mouse has a decreased rate of toxicant-induced germ cell apoptosis (Embree-Ku et al, 2002, Yin et al, 1998). Given the importance of p53 to germ cell apoptosis, and the ability of in utero DBP exposure to induce MNGs in the mouse, this study was designed to determine the life history of these abnormal germ cells in the p53-null mouse model. In particular, we were interested in determining whether these abnormal germ cells might persist into adulthood and progress into a cancerous phenotype.
The perinatal effects of in utero DBP exposure were assessed quantitatively. Evaluation of testis tissue revealed an increase in MNG frequency following exposure. Further quantitative histopathological investigation revealed that in utero DBP exposure induced MNG formation, and that these MNGs were then largely eliminated in the early postnatal period. Of particular interest is our discovery that DBP exposure acted in concert with p53 deficiency to induce a high frequency of MNGs compared to untreated p53-null mice. This gene-environment interaction, with both p53 deficiency and DBP exposure contributing to enhanced MNG formation, was most apparent at the GD19 time-point. As shown in Table 1, the absence of both p53 alleles allowed for the formation of more MNGs in control mice compared to their heterozygous counterparts at early time-points. Furthermore, DBP exposure was responsible for an approximately three-fold higher induction of MNGs in the p53-null mice and a two-fold higher induction in the heterozygous group at GD 19. These data, as well as previous studies, have demonstrated that DBP treatment alone is sufficient to induce MNGs, but clearly p53 plays a role in inhibiting their formation and/or accelerating their elimination.
Table 1.
Frequency of MNGs at GD 19 in testes of p53-null and p53-heterozygous pups of dams dosed daily in utero with either corn oil (control) or 500 mg/kg/day DBP (treated) from GD 12 until birth. The MNG counts for each pup within a litter were averaged, and the dams were used as the unit of effect within a group for statistical analysis across groups. Dams were compared by a one-way ANOVA (minimum number of dams per group, n=3).
| Frequency of MNGs at GD 19 | |
|---|---|
| MNGs/mm2 | |
| p53-null DBP-treated | 29.6 ± 8.9 * |
| p53-heterozygous DBP-treated | 12.5 ± 3.2 † |
| p53-null control | 8.2 ± 2.4 † |
| p53-heterozygous control | 0.2 ± 0.2 |
Mean concentration ± SEM;
indicates p<0.05 compared to all groups,
indicates p<0.05 compared to p53-heterozygous control.
In the mouse, there are two rounds of germ cell apoptosis that take place early in life. The first round begins on GD 13.5, while a second round of apoptosis begins after birth around PND 8, when the first wave of spermatogenesis is initiated (Peters, 1970, Rucker et al, 2000). This critical period of apoptosis is responsible for maintaining a stable number of germ cells that the Sertoli cells are able to support (Mori et al, 1997, Rucker et al, 2000). In addition, p53 protein is present at high levels in sexually immature testes, and in the testis of newborn mice (Rodriguez et al, 1997). The marked loss of MNGs postnatally, even in p53-null mice, suggests that as the testis develops, it relies upon other p53-independent pathways or mechanisms to aide in the removal of MNGs from the seminiferous cords.
To determine whether MNGs can persist later in life, we investigated the long-term effects of in utero exposure to DBP and the impact of p53 deficiency. Our fundamental question was whether the MNGs induced in late gestation could evolve into CIS cells and subsequently produce TGCC in adulthood. Histopathological examination of testis cross-sections from in utero DBP-exposed p53-null mice revealed the presence of rare large abnormal germ cells along the basement membrane of the seminiferous tubules. These cells were found only in mice with DBP exposure that lacked p53, and were characterized by an enlarged cytoplasm, and nuclei with abnormal chromatin distribution. These adult abnormal germ cells are presumably persistent perinatal MNGs, sharing similar features of large size and irregular nuclei with those MNGs observed in the perinatal time point experiments. Also, the in utero DBP-exposed adult testes appear morphologically normal, with the exception of these rare abnormal germ cells, indicating that there are no lasting consequences of exposure at the histological level. The genotype-specific presence of these cells suggests that their persistence requires both a mid-to-late gestational phthalate exposure as well as a p53-deficient environment. The fact that these abnormal germ cells were progressively lost during adulthood suggests that they are non-proliferative, end-stage cells.
Immunohistochemical staining was negative for both Oct-3/4 and PLAP markers in both perinatal and long-term time-points. A previous study examining Oct-3/4 staining following gestational DBP exposure in rats found prominent staining in early gestation that declined around GD 15.5–17.5, the period of gonocyte proliferation (Ferrara et al, 2006). DBP treatment was able to enhance Oct-3/4 staining during this period of replication compared to corn oil treated rats, although no positive staining was observed by GD 19. The earliest time-point in this study was GD 19, by which time Oct-3/4 expression would normally be lost. DBP-induced MNGs did not stain for either marker, which supports the hypothesis that MNGs are not of primordial origin, but arise and develop shortly thereafter, during the period of germ cell proliferation. Also, neither of the markers was present in the abnormal germ cells found in adults. These results support the hypothesis that the adult abnormal germ cells are derived from perinatal MNGs, and indicate that these cells are not related to CIS cells seen in men and therefore do not appear to be precursors to TGCC.
The current study demonstrates a role for p53 in the development of MNGs and their presumptive adult progeny. We observed a time-dependent decrease in MNG content at early post-natal time points, which was dependent on both treatment and p53 status. This genotype-specific effect implicates a role for p53 in eliminating, or preventing the formation of, DBP-induced MNGs in early post-natal life. Abnormal germ cells were discovered in adult mice, but required both gestational DBP exposure and the complete loss of functional p53. Future studies should be aimed at elucidating the role of p53 in DBP-induced MNG elimination in early postnatal life.
Acknowledgments
Funding: Supported by National Institute for Environmental Sciences grants R21ES013020, T32-ES7272 (Training in Environmental Pathology), and R01 ES017272.
References
- Ahlgren M, Wohlfahrt J, Olsen LW, Sorensen TI, Melbye M. Birth weight and risk of cancer. Cancer. 2007;110:412–419. doi: 10.1002/cncr.22773. [DOI] [PubMed] [Google Scholar]
- Akre O, Ekbom A, Hsieh CC, Trichopoulos D, Adami HO. Testicular nonseminoma and seminoma in relation to perinatal characteristics. J Natl Cancer Inst. 1996;88:883–889. doi: 10.1093/jnci/88.13.883. [DOI] [PubMed] [Google Scholar]
- Barlow NJ, Foster PM. Pathogenesis of male reproductive tract lesions from gestation through adulthood following in utero exposure to Di(n-butyl) phthalate. Toxicol Pathol. 2003;31:397–410. doi: 10.1080/01926230390202335. [DOI] [PubMed] [Google Scholar]
- Barlow NJ, Phillips SL, Wallace DG, Sar M, Gaido KW, Foster PM. Quantitative changes in gene expression in fetal rat testes following exposure to di(n-butyl) phthalate. Toxicol Sci. 2003;73:431–441. doi: 10.1093/toxsci/kfg087. [DOI] [PubMed] [Google Scholar]
- Brown LM, Pottern LM, Hoover RN. Prenatal and perinatal risk factors for testicular cancer. Cancer Res. 1986;46:4812–4816. [PubMed] [Google Scholar]
- Cheng L, Sung MT, Cossu-Rocca P, Jones TD, MacLennan GT, De Jong J, Lopez-Beltran A, Montironi R, Looijenga LH. OCT4: biological functions and clinical applications as a marker of germ cell neoplasia. J Pathol. 2007;211:1–9. doi: 10.1002/path.2105. [DOI] [PubMed] [Google Scholar]
- Donehower LA. The p53-deficient mouse: a model for basic and applied cancer studies. Semin Cancer Biol. 1996;7:269–278. doi: 10.1006/scbi.1996.0035. [DOI] [PubMed] [Google Scholar]
- Embree-Ku M, Boekelheide K. FasL deficiency enhances the development of tumors in p53+/− mice. Toxicol Pathol. 2002;30:705–713. doi: 10.1080/01926230290166797. [DOI] [PubMed] [Google Scholar]
- Embree-Ku M, Venturini D, Boekelheide K. Fas is involved in the p53-dependent apoptotic response to ionizing radiation in mouse testis. Biol Reprod. 2002;66:1456–1461. doi: 10.1095/biolreprod66.5.1456. [DOI] [PubMed] [Google Scholar]
- Ferrara D, Hallmark N, Scott H, Brown R, McKinnell C, Mahood IK, Sharpe RM. Acute and long-term effects of in utero exposure of rats to di(n-butyl) phthalate on testicular germ cell development and proliferation. Endocrinology. 2006;147:5352–5362. doi: 10.1210/en.2006-0527. [DOI] [PubMed] [Google Scholar]
- Fisher JS, Macpherson S, Marchetti N, Sharpe RM. Human 'testicular dysgenesis syndrome': a possible model using in-utero exposure of the rat to dibutyl phthalate. Hum Reprod. 2003;18:1383–1394. doi: 10.1093/humrep/deg273. [DOI] [PubMed] [Google Scholar]
- Foster PM. Disruption of reproductive development in male rat offspring following in utero exposure to phthalate esters. Int J Androl. 2006;29:140–147. doi: 10.1111/j.1365-2605.2005.00563.x. discussion 181-5. [DOI] [PubMed] [Google Scholar]
- Gaido KW, Hensley JB, Liu D, Wallace DG, Borghoff S, Johnson KJ, Hall SJ, Boekelheide K. Fetal mouse phthalate exposure shows that Gonocyte multinucleation is not associated with decreased testicular testosterone. Toxicol Sci. 2007;97:491–503. doi: 10.1093/toxsci/kfm049. [DOI] [PubMed] [Google Scholar]
- Heudorf U, Mersch-Sundermann V, Angerer J. Phthalates: toxicology and exposure. Int J Hyg Environ Health. 2007;210:623–634. doi: 10.1016/j.ijheh.2007.07.011. [DOI] [PubMed] [Google Scholar]
- Hoei-Hansen CE. Application of stem cell markers in search for neoplastic germ cells in dysgenetic gonads, extragonadal tumours, and in semen of infertile men. Cancer Treat Rev. 2008;34:348–367. doi: 10.1016/j.ctrv.2007.12.007. [DOI] [PubMed] [Google Scholar]
- Hoei-Hansen CE, Olesen IA, Jorgensen N, Carlsen E, Holm M, Almstrup K, Leffers H, Rajpert-De Meyts E. Current approaches for detection of carcinoma in situ testis. Int J Androl. 2007;30:398–404. doi: 10.1111/j.1365-2605.2007.00797.x. discussion 404-5. [DOI] [PubMed] [Google Scholar]
- Jones TD, Ulbright TM, Eble JN, Cheng L. OCT4: A sensitive and specific biomarker for intratubular germ cell neoplasia of the testis. Clin Cancer Res. 2004;10:8544–8547. doi: 10.1158/1078-0432.CCR-04-0688. [DOI] [PubMed] [Google Scholar]
- Kleymenova E, Swanson C, Boekelheide K, Gaido KW. Exposure in utero to di(n-butyl) phthalate alters the vimentin cytoskeleton of fetal rat Sertoli cells and disrupts Sertoli cell-gonocyte contact. Biol Reprod. 2005;73:482–490. doi: 10.1095/biolreprod.104.037184. [DOI] [PubMed] [Google Scholar]
- Lacerda HM, Akre O, Merletti F, Richiardi L. Time trends in the incidence of testicular cancer in childhood and young adulthood. Cancer Epidemiol Biomarkers Prev. 2009;18:2042–2045. doi: 10.1158/1055-9965.EPI-08-1140. [DOI] [PubMed] [Google Scholar]
- Lehmann KP, Phillips S, Sar M, Foster PM, Gaido KW. Dose-dependent alterations in gene expression and testosterone synthesis in the fetal testes of male rats exposed to di (n-butyl) phthalate. Toxicol Sci. 2004;81:60–68. doi: 10.1093/toxsci/kfh169. [DOI] [PubMed] [Google Scholar]
- Looijenga LH, Stoop H, de Leeuw HP, de Gouveia Brazao CA, Gillis AJ, van Roozendaal KE, van Zoelen EJ, Weber RF, Wolffenbuttel KP, van Dekken H, Honecker F, Bokemeyer C, Perlman EJ, Schneider DT, Kononen J, Sauter G, Oosterhuis JW. POU5F1 (OCT3/4) identifies cells with pluripotent potential in human germ cell tumors. Cancer Res. 2003;63:2244–2250. [PubMed] [Google Scholar]
- Lottrup G, Andersson AM, Leffers H, Mortensen GK, Toppari J, Skakkebaek NE, Main KM. Possible impact of phthalates on infant reproductive health. Int J Androl. 2006;29:172–180. doi: 10.1111/j.1365-2605.2005.00642.x. discussion 181-5. [DOI] [PubMed] [Google Scholar]
- Lozano G, Liu G. Mouse models dissect the role of p53 in cancer and development. Semin Cancer Biol. 1998;8:337–344. doi: 10.1006/scbi.1998.0096. [DOI] [PubMed] [Google Scholar]
- Lozano G, Zambetti GP. What have animal models taught us about the p53 pathway? J Pathol. 2005;205:206–220. doi: 10.1002/path.1704. [DOI] [PubMed] [Google Scholar]
- Mahood IK, Hallmark N, McKinnell C, Walker M, Fisher JS, Sharpe RM. Abnormal Leydig Cell aggregation in the fetal testis of rats exposed to di (n-butyl) phthalate and its possible role in testicular dysgenesis. Endocrinology. 2005;146:613–623. doi: 10.1210/en.2004-0671. [DOI] [PubMed] [Google Scholar]
- Mahood IK, Scott HM, Brown R, Hallmark N, Walker M, Sharpe RM. In utero exposure to di(n-butyl) phthalate and testicular dysgenesis: comparison of fetal and adult end points and their dose sensitivity. Environ Health Perspect. 2007;115(Suppl 1):55–61. doi: 10.1289/ehp.9366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Main KM, Skakkebaek NE, Virtanen HE, Toppari J. Genital anomalies in boys and the environment. Best Pract Res Clin Endocrinol Metab. 2010;24:279–289. doi: 10.1016/j.beem.2009.10.003. [DOI] [PubMed] [Google Scholar]
- McGlynn KA, Devesa SS, Sigurdson AJ, Brown LM, Tsao L, Tarone RE. Trends in the incidence of testicular germ cell tumors in the United States. Cancer. 2003;97:63–70. doi: 10.1002/cncr.11054. [DOI] [PubMed] [Google Scholar]
- Meulmeester E, Jochemsen AG. p53: a guide to apoptosis. Curr Cancer Drug Targets. 2008;8:87–97. doi: 10.2174/156800908783769337. [DOI] [PubMed] [Google Scholar]
- Michos A, Xue F, Michels KB. Birth weight and the risk of testicular cancer: a meta-analysis. Int J Cancer. 2007;121:1123–1131. doi: 10.1002/ijc.22771. [DOI] [PubMed] [Google Scholar]
- Mori C, Nakamura N, Dix DJ, Fujioka M, Nakagawa S, Shiota K, Eddy EM. Morphological analysis of germ cell apoptosis during postnatal testis development in normal and Hsp 70-2 knockout mice. Dev Dyn. 1997;208:125–136. doi: 10.1002/(SICI)1097-0177(199701)208:1<125::AID-AJA12>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Mylchreest E, Sar M, Wallace DG, Foster PM. Fetal testosterone insufficiency and abnormal proliferation of Leydig cells and gonocytes in rats exposed to di(n-butyl) phthalate. Reprod Toxicol. 2002;16:19–28. doi: 10.1016/s0890-6238(01)00201-5. [DOI] [PubMed] [Google Scholar]
- Neale RE, Carriere P, Murphy MF, Baade PD. Testicular cancer in twins: a meta-analysis. Br J Cancer. 2008;98:171–173. doi: 10.1038/sj.bjc.6604136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okumura-Nakanishi S, Saito M, Niwa H, Ishikawa F. Oct-3/4 and Sox2 regulate Oct-3/4 gene in embryonic stem cells. J Biol Chem. 2005;280:5307–5317. doi: 10.1074/jbc.M410015200. [DOI] [PubMed] [Google Scholar]
- Pesce M, Wang X, Wolgemuth DJ, Scholer H. Differential expression of the Oct-4 transcription factor during mouse germ cell differentiation. Mech Dev. 1998;71:89–98. doi: 10.1016/s0925-4773(98)00002-1. [DOI] [PubMed] [Google Scholar]
- Peters H. Migration of gonocytes into the mammalian gonad and their differentiation. Philos Trans R Soc Lond B Biol Sci. 1970;259:91–101. doi: 10.1098/rstb.1970.0048. [DOI] [PubMed] [Google Scholar]
- Rajpert-De Meyts E, Bartkova J, Samson M, Hoei-Hansen CE, Frydelund-Larsen L, Bartek J, Skakkebaek NE. The emerging phenotype of the testicular carcinoma in situ germ cell. APMIS. 2003;111:267–278. doi: 10.1034/j.1600-0463.2003.11101301.x. discussion 278-9. [DOI] [PubMed] [Google Scholar]
- Rajpert-De Meyts E, Hanstein R, Jorgensen N, Graem N, Vogt PH, Skakkebaek NE. Developmental expression of POU5F1 (OCT-3/4) in normal and dysgenetic human gonads. Hum Reprod. 2004;19:1338–1344. doi: 10.1093/humrep/deh265. [DOI] [PubMed] [Google Scholar]
- Rasmussen F, Gunnell D, Ekbom A, Hallqvist J, Tynelius P. Birth weight, adult height, and testicular cancer: cohort study of 337,249 Swedish young men. Cancer Causes Control. 2003;14:595–598. doi: 10.1023/a:1024860826830. [DOI] [PubMed] [Google Scholar]
- Richiardi L, Askling J, Granath F, Akre O. Body size at birth and adulthood and the risk for germ-cell testicular cancer. Cancer Epidemiol Biomarkers Prev. 2003;12:669–673. [PubMed] [Google Scholar]
- Richiardi L, Bellocco R, Adami HO, Torrang A, Barlow L, Hakulinen T, Rahu M, Stengrevics A, Storm H, Tretli S, Kurtinaitis J, Tyczynski JE, Akre O. Testicular cancer incidence in eight northern European countries: secular and recent trends. Cancer Epidemiol Biomarkers Prev. 2004;13:2157–2166. [PubMed] [Google Scholar]
- Rodriguez I, Ody C, Araki K, Garcia I, Vassalli P. An early and massive wave of germinal cell apoptosis is required for the development of functional spermatogenesis. EMBO J. 1997;16:2262–2270. doi: 10.1093/emboj/16.9.2262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rucker EB, 3rd, Dierisseau P, Wagner KU, Garrett L, Wynshaw-Boris A, Flaws JA, Hennighausen L. Bcl-x and Bax regulate mouse primordial germ cell survival and apoptosis during embryogenesis. Mol Endocrinol. 2000;14:1038–1052. doi: 10.1210/mend.14.7.0465. [DOI] [PubMed] [Google Scholar]
- Sharpe RM, Skakkebaek NE. Testicular dysgenesis syndrome: mechanistic insights and potential new downstream effects. Fertil Steril. 2008;89:e33–e38. doi: 10.1016/j.fertnstert.2007.12.026. [DOI] [PubMed] [Google Scholar]
- Shultz VD, Phillips S, Sar M, Foster PM, Gaido KW. Altered gene profiles in fetal rat testes after in utero exposure to di(n-butyl) phthalate. Toxicol Sci. 2001;64:233–242. doi: 10.1093/toxsci/64.2.233. [DOI] [PubMed] [Google Scholar]
- Sinha Hikim AP, Swerdloff RS. Hormonal and genetic control of germ cell apoptosis in the testis. Rev Reprod. 1999;4:38–47. doi: 10.1530/ror.0.0040038. [DOI] [PubMed] [Google Scholar]
- Skakkebaek NE, Holm M, Hoei-Hansen C, Jorgensen N, Rajpert-De Meyts E. Association between testicular dysgenesis syndrome (TDS) and testicular neoplasia: evidence from 20 adult patients with signs of maldevelopment of the testis. APMIS. 2003;111:1–9. doi: 10.1034/j.1600-0463.2003.11101031.x. discussion 9–11. [DOI] [PubMed] [Google Scholar]
- Skakkebaek NE, Rajpert-De Meyts E, Jorgensen N, Main KM, Leffers H, Andersson AM, Juul A, Jensen TK, Toppari J. Testicular cancer trends as 'whistle blowers' of testicular developmental problems in populations. Int J Androl. 2007;30:198–204. doi: 10.1111/j.1365-2605.2007.00776.x. discussion 204-5. [DOI] [PubMed] [Google Scholar]
- Skakkebaek NE, Rajpert-De Meyts E, Main KM. Testicular dysgenesis syndrome: an increasingly common developmental disorder with environmental aspects. Hum Reprod. 2001;16:972–978. doi: 10.1093/humrep/16.5.972. [DOI] [PubMed] [Google Scholar]
- Sonne SB, Almstrup K, Dalgaard M, Juncker AS, Edsgard D, Ruban L, Harrison NJ, Schwager C, Abdollahi A, Huber PE, Brunak S, Gjerdrum LM, Moore HD, Andrews PW, Skakkebaek NE, Rajpert-De Meyts E, Leffers H. Analysis of gene expression profiles of microdissected cell populations indicates that testicular carcinoma in situ is an arrested gonocyte. Cancer Res. 2009;69:5241–5250. doi: 10.1158/0008-5472.CAN-08-4554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swan SH. Environmental phthalate exposure in relation to reproductive outcomes and other health endpoints in humans. Environ Res. 2008;108:177–184. doi: 10.1016/j.envres.2008.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swerdlow AJ, De Stavola BL, Swanwick MA, Mangtani P, Maconochie NE. Risk factors for testicular cancer: a case-control study in twins. Br J Cancer. 1999;80:1098–1102. doi: 10.1038/sj.bjc.6690470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripathi R, Mishra DP, Shaha C. Male germ cell development: turning on the apoptotic pathways. J Reprod Immunol. 2009;83:31–35. doi: 10.1016/j.jri.2009.05.009. [DOI] [PubMed] [Google Scholar]
- Venkatachalam S, Tyner SD, Pickering CR, Boley S, Recio L, French JE, Donehower LA. Is p53 haploinsufficient for tumor suppression? Implications for the p53+/− mouse model in carcinogenicity testing. Toxicol Pathol. 2001;29(Suppl):147–154. doi: 10.1080/019262301753178555. [DOI] [PubMed] [Google Scholar]
- Witorsch RJ, Thomas JA. Personal care products and endocrine disruption: A critical review of the literature. Crit Rev Toxicol. 2010;40(Suppl 3):1–30. doi: 10.3109/10408444.2010.515563. [DOI] [PubMed] [Google Scholar]
- Yin Y, Stahl BC, DeWolf WC, Morgentaler A. p53-mediated germ cell quality control in spermatogenesis. Dev Biol. 1998;204:165–171. doi: 10.1006/dbio.1998.9074. [DOI] [PubMed] [Google Scholar]
- Zeger SL, Liang KY. Longitudinal data analysis for discrete and continuous outcomes. Biometrics. 1986;42:121–130. [PubMed] [Google Scholar]