Abstract
Cannabinoid receptor type 1 (CB1)-induced suppression of transient receptor potential vanilloid type 1 (TRPV1) activation provides a therapeutic option to reduce inflammation and pain in different animal disease models through mechanisms involving dampening of TRPV1 activation and signaling events. As we found in both mouse corneal epithelium and human corneal epithelial cells (HCEC) that there is CB1 and TRPV1 expression colocalization based on overlap of coimmunostaining, we determined in mouse corneal wound healing models and in human corneal epithelial cells (HCEC) if they interact with one another to reduce TRPV1-induced inflammatory and scarring responses. Corneal epithelial debridement elicited in vivo a more rapid wound healing response in wildtype (WT) than in CB1−/− mice suggesting functional interaction between CB1 and TRPV1. CB1 activation by injury is tenable based on the identification in mouse corneas of 2-arachidonylglycerol (2-AG) with tandem LC–MS/MS, a selective endocannabinoid CB1 ligand. Suppression of corneal TRPV1 activation by CB1 is indicated since following alkali burning, CB1 activation with WIN55,212-2 (WIN) reduced immune cell stromal infiltration and scarring. Western blot analysis of coimmunoprecipitates identified protein–protein interaction between CB1 and TRPV1. Other immunocomplexes were also identified containing transforming growth factor kinase 1 (TAK1), TRPV1 and CB1. CB1 siRNA gene silencing prevented suppression by WIN of TRPV1-induced TAK1–JNK1 signaling. WIN reduced TRPV1-induced Ca2+ transients in fura2-loaded HCEC whereas pertussis toxin (PTX) preincubation obviated suppression by WIN of such rises caused by capsaicin (CAP). Whole cell patch clamp analysis of HCEC showed that WIN blocked subsequent CAP-induced increases in nonselective outward currents. Taken together, CB1 activation by injury-induced release of endocannabinoids such as 2-AG downregulates TRPV1 mediated inflammation and corneal opacification. Such suppression occurs through protein–protein interaction between TRPV1 and CB1 leading to declines in TRPV1 phosphorylation status. CB1 activation of the GTP binding protein, Gi/o contributes to CB1 mediated TRPV1 dephosphorylation leading to TRPV1 desensitization, declines in TRPV1-induced increases in currents and pro-inflammatory signaling events.
Keywords: Human corneal epithelial cells (HCEC), Currents, Cannabinoid receptor subtype 1 (CB1), Transient receptor potential vanilloid type 1 (TRPV1), Inflammation, Small interfering RNA (siRNA) gene silencing
1. Introduction
Complex receptor interactions within the corneal epithelial layer contribute to the maintenance of tissue transparency and normal vision. Cytokines released by the epithelial cells and orbital glands modulate through activation of their cognate receptors a myriad of cell signaling pathways. This milieu promotes epithelial turnover and sustains the epithelial protective barrier function against infection and injury. Transient receptor potential vanilloid 1 (TRPV1) is expressed in the cornea, on the ophthalmic branch of trigeminal nerve endings as well as epithelial and endothelial cells regulating corneal homeostasis [1–5]. They are activated by injury to epithelial cells resulting in endogenous ligand release, environmental stresses and/or infection leading to increases in proinflammatory cytokine and chemoattractant expression [5]. Their rises in turn induce immune cell infiltration, which mitigate bacterial and viral infection. This response has adaptive value provided that it is self-limiting rather than sustained. If it instead becomes dysregulated and prolonged, wound healing is accompanied by opacification and declines in visual acuity instead of restoration of corneal transparency. Another consequence of corneal injury can be severe pain. As TRPV1 activation in sensory neurons induces pain, animal model studies showed that TRPV1 downregulation mitigates corneal pain resulting from injury [6].
TRPV1 channels and cannabinoid receptor subtype 1 (CB1) are two potential drug targets to also reduce corneal inflammation and loss of transparency caused by injury. A role for TRPV1 in determining corneal wound healing outcome following an alkali burn is indicated since only in TRPV1−/− knockouts was transparency restored whereas in a wildtype (WT) counterpart the corneas remained inflamed and became scarred. Another indication of a role for TRPV1 in inducing inflammation is that TRPV1 inhibition blocked in HCEC IL-6 and IL-8 release induced by exposure to a hypertonic challenge [1]. CB1 activation could also counter TRPV1-induced increases in IL-8 release. These inhibitory effects caused by either TRPV1 inhibition or CB1 activation hastened epithelial cell proliferation and migration. Therefore, developing strategies to either block injury-induced TRPV1 activation or promote CB1 activation may provide novel approaches to improve corneal wound healing outcome [2,7,8].
The downstream-signaling events in HCEC linked to TRPV1 activation by capsaicin (CAP), a selective TRPV1 agonist include Ca2+ transients leading sequentially to transforming growth factor β-activated kinase 1 (TAK1), c-jun N-terminal protein kinase 1 (JNK1), nuclear factor (NF)-κB phosphorylation and increases in pro-inflammatory and chemoattractant interleukin (IL)-6 and IL-8 release [2]. Drug and genetic approaches demonstrated that blocking their activation by CAP blunted increases in IL-6 and IL-8 release. Therefore, strategies to target TRPV1-linked signaling are relevant for controlling inflammatory responses and improving healing outcome after corneal injury.
CB1 is a G-protein-coupled receptor, activated by endocannabinoids such as anandamide (AEA), and 2-arachidonoylglycerol (2-AG) as well as synthetic agonists such as aminoalkylindole, WIN55,212-2 (WIN) [9]. Even though in some other tissues cannabinoids elicit immune suppressive and analgesic effects against TRPV1 activation, their mode of action remains controversial [10,11]. Canonically, CB1 inhibits cyclic adenosine monophosphate (cAMP) sensitive pathways through activating GTP-binding Gi/o proteins. In a CB1–TRPV1–HEK over-expression system, CB1 activation desensitized TRPV1 through decreases in PKA-dependent TRPV1 phosphorylation [12]. However, in trigeminal neurons, the antinociceptive effects of CB1 stem from dephosphorylating and desensitizing TRPV1 via activation of the Ca2+-dependent protein phosphatase, calcineurin [13,14].
In the present study, we demonstrate that CB1 activation in mouse corneas accelerates wound healing and reduces tissue fibrosis through suppression of innate immune responses to corneal injuries. Such declines result from TRPV1 desensitization by CB1 through Gi/o activation. This effect on TRPV1 is accompanied by dampened increases in TRPV1-induced currents and declines in TAK1–JNK1 activation. Therefore, drug-induced CB1 activation could be a novel approach to improve corneal wound healing outcome by dampening the ability of long term TRPV1 activation to induce dysregulated inflammation.
2. Materials and methods
2.1. Materials
The following chemicals were purchased from Sigma-Aldrich (St. Louis, MO): WIN55,212-2 (WIN), capsaicin (CAP), capsazepine (CPZ), pertussis toxin (PTX), epidermal growth factor (EGF), and bovine insulin. Gentamicin, TrypLE™ Express Stable Trypsin-Like Enzyme, Dulbecco's modified Eagle's medium (DMEM)/F12 medium, fetal bovine serum (FBS) and fura2-AM were purchased from Life Technologies, Invitrogen (Carlsbad, CA). FK-506, 2-AG and 2-AG-d8 were purchased from Cayman Chemical (Ann Arbor, Michigan). Anti-TAK1, phospho-TAK1, anti-JNK1, phospho-JNK1/2, goat anti-mouse IgG-HRP, goat anti-rabbit IgG-HRP antibody, and β-actin antibodies were purchased from Cell Signaling Technology (Boston, MA). Anti-CD11b and anti-phosphothreonine TRPV1 antibodies were purchased from EMD Millipore (Billerica, MA). Anti-CB1, anti-TRPV1 antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Sep-Pak Vac 1 cm3 Silica solid phase extraction (SPE) cartridges were obtained from Waters Corp. (Milford, MA, USA). All solvents were of HPLC grade.
2.2. Cell culture and siRNA transfection
SV40-adenovirus immortalized human corneal epithelial cells (HCEC) were obtained as a generous gift from Dr. Kaoru Araki-Sasaki (Ideta Eye Hospital, Kumamoto, 8600027, Japan). The cells were cultured at 37 °C in an incubator with 5% CO2 and 95% ambient air in DMEM/F12 medium, supplemented with 6% FBS, 5 ng/ml EGF and 5 μg/ml insulin. Cell cycle arrest was achieved by using DMEM/F12 medium without serum and EGF for 24 h before experimentation. Small interfering RNA (siRNA) transfection was performed as described [15]. Briefly, cells were grown in six-well plates to 50–60% confluence and following washes with PBS, they were exposed to 75 nM CB1 siRNAs (GenBank: BC100968.1; the sequences specifically targeting human CB1 genes were 3 different duplexes in 5′ → 3′ orientation: sense 1: GGAUGUGGCUUAUGAGAUAtt, antisense 1: UAUCUCAUAAGCCACAUC Ctt; sense 2: CUCACACUCUAACUGUAUAtt, antisense 2: UAUACAGUUAGAGUGUGAGtt and sense 3: GGAUCAGAGUUCCCAAGAAtt, anti-sense 3: UUCUUGGGAACUCUGAUCCtt (Life Technologies, Ambion, Grand Island, NY) was mixed with 3.6 μl of Lipofectamine RNAiMAX in 500 μl of Opti-MEM I-reduced serum medium (Life Technologies, Invitrogen). Mixtures were incubated for 20 min at room temperature and then added to each of the culture wells. After 24 h transfection, the culture medium was refreshed with 10% FBS-containing DMEM/F12 medium. All experimental measurements were performed 48 h following transfection. Western blot analyses were performed to evaluate the extent of knockdown of CB1 protein expression. Mismatched siRNAs (siControl, Thermo Fisher Scientific, Lafayette, CO) were used as a control for monitoring non-sequence-specific effects.
2.3. Single cell Ca2+ fluorescence imaging
HCEC grown on 40-mm circular coverslips (Bioptechs Inc., Butler, PA) were loaded with 2 μM fura2-AM at 37 °C for 30 min and then washed with NaCl Ringer's solution containing (in mM): NaCl (144.6), KCl (4.5), CaCl2 (0.8), KH2PO4 (1), MgCl2 (0.8), glucose (5.5), and HEPES (10) with osmolarity 300 mosM and pH 7.4. Cells were continuously superfused at 34 °C in a Focht Closed System 2 (FCS2) perfusion chamber with temperature control and placed on the stage of an inverted microscope (Nikon Diaphot 200). Relative changes in intracellular calcium levels were measured with ISEE 5.5.9 analytical imaging software in conjunction with a single-cell fluorescence imaging system (Inovision Corp., Raleigh, NC). Briefly, cells were then alternately illuminated at 340 and 380 nm, and emission was monitored every 5 s at 510 nm using a Roper Scientific CCD camera. A field of interest contained 15–20 cells. Mean running ratios were calculated for each region. The n values provided indicate the number of experiments per data point [16].
2.4. Western blot and co-immunoprecipitation analysis
As described, HCEC were gently washed with PBS, harvested in cell lysis buffer [2]. The supernatants were collected by centrifuging the cell lysates at 4 °C. BCA assay kit (Thermo Fisher Scientific, Chicago, IL) was used for measuring protein content, and 200 μg protein diluted with an equal volume of 2× Laemmli buffer. 20–50 μg of denatured protein was electrophoresed using 7.5% or 10% SDS minigels and blocking PVDF membranes with nonfat dry milk. The blots were exposed to the appropriate primary antibody overnight at 4 °C. Then they were exposed to a 1:2000 dilution of secondary antibodies for 1 h at room temperature. The immune reactivity was detected with a Western blot analysis kit (Amersham ECL Plus; GE Healthcare Life Sciences, Piscataway, NJ). Films were scanned and band density was quantified using image-conversion software (SigmaScan Pro 5.0; Systat Software, Inc., Mountain View, CA). Monoclonal anti-JNK2 and β-actin antibodies were used to test for protein loading equivalence.
An aliquot of supernatant containing 500 μg protein was exposed to either an antibody to detect either TAK1, CB1 or TRPV1, and gently agitated overnight at 4 °C. Subsequently, protein A/G beads (Cell Signaling Technology, Danvers, MA) were added to cell lysates and then exposed for another 2 h at 4 °C with gentle agitation. Beads were washed five times with cold immunoprecipitate (IP) buffer and cell lysis buffer. After a final wash, the buffer was aspirated and discarded and re-spun in 40 μl of electrophoresis sample buffer, vortexed, then centrifuged for 30 s. The mixture was boiled for 5 min, and then subjected to Western blot analysis with the appropriate antibody.
2.5. Immunocytochemistry
CB1 and TRPV1 immunocytochemical localization was determined in HCEC as described [16]. Briefly, cells were seeded onto a Lab-Tek chamber slide system (Nunc, Naperville, IL) and after reaching confluence; they were washed twice with HEPES-buffered Ringer's solution, fixed on ice for 30 min in 4% paraformaldehyde, washed three times with HEPES Ringer's solution, and then rendered permeable using 0.1% Triton X-100 solution. After blotting with 10% normal goat serum, cells were exposed to anti-CB1 and/or TRPV1 antibody (Santa Cruz Biotechnology,) plus 1% bovine serum albumin (BSA) overnight at 4 °C. After three washes with HEPES Ringer's solution, cells were incubated with goat anti-rabbit IgG FITC and/or mouse anti-goat IgG-TR (Santa Cruz Biotechnology) for 1 h and 4′,6-diamidino-2-phenylindole (DAPI) nucleic acid stain for 30 min at room temperature. Fluorescence was visualized using a Nikon fluorescence microscope with a 60× oil objective lens. Images were processed using Adobe® Photoshop 5.5 software (Adobe Systems, Inc. San Diego, CA).
2.6. Immunohistochemistry
Intact or alkali-burned mouse corneas were placed onto Tissue-Tek (SLEE, Mainz, Germany). Paraffin sections (5 μm) were prepared and processed for H&E staining and immunohistochemistry (IHC). The following antibodies were diluted in PBS: mouse monoclonal anti-α smooth muscle actin (α-SMA) antibody (Neomarker, Fremont, CA). The presence of monocytes/macrophages was examined by using rat monoclonal F4/80 antimacrophage antigen antibody. Neutrophil presence was examined by using anti-rabbit polyclonal myeloperoxidase (MPO) antibody (Neomarker) [8]. After blocking the sections with 10% fetal calf serum in PBS for 30 min at room temperature, the first primary antibody was incubated overnight at 4 °C and exposed for 1 h at room temperature to the secondary antibody. Optimized Z-sectional digital images were acquired and processed using Zeiss AxioVision software Version 5. For negative controls, the sections were incubated with the secondary antibodies and cross-reactions between different primary and secondary antibodies were tested.
2.7. Mouse corneal wound healing models
All protocols for use of mice were approved by Institutional Animal Care and Use Committees of SUNY State College of Optometry, SUNY Stony Brook Medical School and Wakayama Medical School. They were conducted in accordance with the Association for Research in Vision and Ophthalmology Statement for the Use of Animals in Ophthalmic and Vision Research.
2.7.1. Alkali burn
Three μL of 1 N NaOH solution was applied to the right eye of 6–8 week old CB1-null mice (University of Cincinnati Medical Center, Cincinnati, Ohio and State University of New York, Stony Brook Medical School, Stony Brook, NY); or age-matched wild-type mice (The Jackson Laboratory, Bar Harbor, Maine) under general anesthesia (xylazine 13 μg/g and ketamine 87–100 μg/g; I.P.) and topical anesthetic to produce an ocular surface alkali burn. The ocular surface wound was then rinsed with 10 ml PBS. Ofloxacin ointment was administered topically twice a week to reduce the risk of bacterial infection. The eyes with obvious bacterial infection were excluded from the study. Corneal tissue was processed for histology 1, 5, 10, and 20 days after alkali burn.
2.7.2. Epithelial debridement
Seven to eight week old mice of either sex were anesthetized and topical anesthetic was applied (proparacaine ophthalmic solution) until blink sensation was absent. A 2.0 mm diameter area of the epithelium was removed using an Algerbrush II corneal rust ring remover (Ambler Surgical, Exton, PA). Wounded eyes were treated with erythromycin ophthalmic ointment to minimize inflammation. The corneas were allowed to heal for up to 72 h. Wound size was determined by staining with 1% fluorescein and photographing.
2.8. Planar patch-clamp recording
Electrophysiological recordings were obtained using the whole-cell mode of the planar patch-clamp technique (Nanion, Munich, Germany) [17]. HCEC suspensions were pipetted onto a microchip with a resistance corresponding to a pipette resistance of 2–5 MΩ. Suction was applied to obtain the whole cell configuration. The external bath solution contained (in mM): NaCl (150), CsCl (6), MgCl2 (1), CaCl2 (1.5), glucose (10), and HEPES (10) at pH 7.4. The intracellular solution for whole-cell measurements contained (in mM): NaCl (150), MgCl2 (3), EGTA (5), and HEPES (10) adjusted to 7.2 with NaOH as described. Membrane capacitance and access resistance of HCEC were calculated with Patchmaster software. The series resistances (7–24 MΩ) were compensated (≈70–90%) after breaking into the whole-cell configuration. Leak subtraction was applied only on cells with leak currents below 200 pA. The holding potential (HP) was set to 0 mV to eliminate any possible contributions by voltage-dependent Ca2+ channel activity. Whole-cell currents were recorded for 400 ms using voltage steps ranging between −60 and +130 mV (10 mV increments). The resulting currents were normalized to cell membrane capacitance resulting in current density ([pA/pF]). All plots were generated with SigmaPlot software and an electrophysiology module. Bar charts were plotted with GraphPad Prism version 5 for Windows (GraphPad Software, San Diego CA) [18].
2.9. Liquid Chromatography–Mass Spectrometric (LC–MS/MS) analysis of endocannabinoids
Frozen murine corneal tissue samples were quickly homogenized (less than 60 s) using a Tenbroeck tissue grinder along with 10 ρmol of 2-AG-d8, and AEA-d8 in 3 ml ethyl acetate:methanol (2:1 v:v) and the samples were stored at −20 °C until further processing. Briefly, a portion of 2-AG and AEA was purified from the corneal homogenate [19,20] and reconstituted in 9:1 methanol:water immediately prior to LC–MS/MS analysis. The analysis used a Thermo Quantum Triple Quadrupole Mass Spectrometer (Thermo Fisher Scientific, Waltham, MA). Chromatographic separation of the endocannabinoids was accomplished in reverse-phase mode on a C18 column (10×0.2 cm, 3 m) (Phenomenex, Torrance, CA) using the following gradient. 85% of component B for 0.5 min, 85% of component B increased to 99% in 5 min, and held at 99% of component B for an additional 2 min. Component A was water and B was methanol, and each component contained 80 M silver acetate and 0.2% formic or acetic acid. The flow rate was 0.3 ml/min. The column was equilibrated at initial conditions for 3 min prior to each injection. The analysates were detected via selected reaction monitoring (SRM) as [19]+ complexes in the positive ion mode. The transitions used for each analysis are as follows: 2-AG (m/z 485 → 411); 2-AG-d8 (m/z 493 → 419); AEA (m/z 454 → 436); and AEA-d8 (m/z 462 → 446). These transitions are based on the [M+107Ag]+ complex, the [M+109Ag]+ complex may also be used. Quantification was accomplished via stable isotope dilution, based on the octadeuterated analogs.
2.10. Data analysis
Data were analyzed using independent Student's paired t-test. The results were considered significant if p<0.05. They are reported as mean±SEM for at least three independent experiments unless otherwise indicated.
3. Results
3.1. Co-localization of CB1 and TRPV1 expression
CB1 colocalization with TRPV1 was determined in the intact mouse corneal epithelium and in HCEC [7]. Fig. 1A shows in a transverse section through the mouse corneal epithelium that TRPV1 and CB1 immunostaining essentially colocalizes within the basal layer. To ascertain if their colocalization is plasma membrane delimited, HCEC coimmunostaining was performed. Fig. 1B clearly indicates immunohistochemical overlap between TRPV1 and CB1 along the cell periphery, which is consistent with the finding that increases in Ca2+ influx by selective agonists are dependent on Ca2+ in the extracellular medium [7].
Fig. 1.
Corneal CB1 and TRPV1 protein expression colocalization. (A) Immunohistochemical staining of transverse section of whole mouse cornea. CB1 and TRPV1 expression is evident in the basal epithelial layer (arrowheads). Texas Red and FITC fluorescence staining represent TRPV1 and CB1 localization. Yellow staining is indicative of their overlap because of close proximity to one another. DAPI blue staining identifies cell nuclei. Scale bar=100 μm. (B) Colocalization of CB1 and TRPV1 protein expression in HCEC monolayer. Left and right panels show CB1 FITC (green) and TRPV1 Texas Red (red) distribution along cell margins. Merged overlap of images is shown in yellow indicating their individual expression to be in close proximity, and DAPI blue staining identifies cell nuclei. Scale bar=50 μm.
3.2. CB1 activation reduces corneal fibrosis through suppression of innate immune responses
To determine if in vitro CB1 interaction with TRPV1 has in vivo physiological relevance, a mouse corneal wound healing model was used to assess the role of CB1 activation in modulating immune responses and reepithelialization subsequent to an alkali burn. This was done by comparing in WT and CB1−/− mouse corneas monocyte and neutrophil stromal infiltration by evaluating CD11b immunostaining. Fig. 2A shows that after 15 days large increases in CD11b staining positive cells were only evident in the CB1−/− mice. On the other hand, in the WT mice such staining faded suggesting injury-induced CB1 activation by endogenous mediators suppressed immune cell infiltration. Another approach to assess the possible immunosuppressive role of CB1 activation in modulating the wound healing response to injury entailed determining if body-wide activation of CB1 with a selective CB1 agonist, WIN55,212-2 (WIN) improved the wound healing outcome. Fig. 2B shows that 5 days after injury, wound closure occurred only in the WIN injected group. In contrast, a corneal ulcer was apparent in the untreated group. In addition, after 20 days, although corneal ulceration was no longer present in the untreated mice, scarification and neovascularization were more severe compared to that in the treated group.
Fig. 2.
CB1 gene ablation promotes immune cell infiltration following mouse cornea alkali burning. (A) CD11b staining is compared 15 days after injury between WT mice (left panel) and CB1−/− knockout mice (right panel). Scale bar=100 μm. (B) WT mice eyes are shown up to 20 days after alkali burn injury. Control mice (upper row) received subperitoneal saline injection immediately following injury. Bottom row was injected with the CB1 agonist, WIN55,212-2 (1 mg/kg/day). In the WIN-treated mouse, no ulceration was seen at 5 days post-injury (whereas the control eye had an epithelial defect and more opacification). (C) H.E. mouse corneal staining shows at ten days post injury a more organized and thinner stroma in the WIN-treated group than in the control cornea. Myeloperoxidase (MPO), F4/80 and α-smooth muscle actin (SMA) immunoreactivity levels were much less in the treated corneas compared with control group. This difference is reflective of less immune cell infiltration in the WIN treated group and fewer keratocytes undergoing myofibroblast transdifferentiation. Scale bar=100 μm.
To evaluate if WIN treatment improved the wound healing outcome, globe diameters were measured [8] in the two groups at 5, 10 and 20 days following injury. This was done because changes in diameter are indicative of tissue contracture caused by TRPV1-induced myofibroblast transdifferentiation and increases in contractile protein expression of α-smooth muscle actin (α-SMA). After 20 days, the mean diameter of four eyes isolated from four controls was 2.65±0.14 mm (SEM) whereas in the WIN treated group it was instead 2.88±0.05 mm (p<0.05). This increase in diameter is consistent with declines in TRPV1-induced myofibroblast transdifferentiation and SMA expression that lead to contracture and scarring.
Another assessment entailed evaluating if WIN injection reduced injury-induced increases in stromal thickness resulting from increases in inflammation and stromal fluid accumulation. After 10 days, stromal thickness was 2.6±0.5-fold larger than in freshly isolated uninjured control eyes (n=4) corneas whereas in WIN treated mice swelling was reduced to a 1.68±0.15-fold increase (n=4) (p<0.05).
These effects of WIN on globe diameter and stromal thickness were validated based on histological and immunohistochemical changes. Fig. 2C shows at 10 days post-injury that in WIN treated mice the stromal collagen lamellar parallel architecture was better restored during wound healing than in the control. This difference is consistent with more myeloperoxidase (MPO) and F4/80 positive staining cells and α-SMA expression in the control. Taken together, these results indicate that CB1 activation by injury mediated release of endogenous ligands and exogenous WIN administration promoted wound closure through inhibition of immune cell infiltration, neovascularization and fibrosis.
3.3. Corneal epithelial CB1 activation affects wound healing outcome
Epithelial debridement was performed to assess if epithelial CB1 activation by less severe injury hastens reepithelialization. Accordingly, we compared in WT and CB1−/− mice the time dependence of epithelial wound closure. Fluoresce in staining monitored remaining exposed stroma during healing. In the WT, wound closure occurred within 32 h following debridement whereas in CB1−/− mice healing required 40 h (Fig. 3A). This 8 h delay suggests that epithelial CB1 activation promotes wound closure through suppression of inflammation [21]. This possibility was considered by comparing immune cell infiltration in WT mice with that in CB1−/− mice. Fig. 3B shows a transverse full thickness view of WT and CB1−/− corneas at 10 h. This was the only time at which it was possible to identify CD11b positive green staining cells only in the CB1−/− mice. This difference suggests that CB1 activation by injury may contribute to the self-limiting innate immune response and thereby shorten the time required for wound closure. Such an inflammation control role for CB1 activation is consistent with the finding that corneal wound closure in CB1+/− heterozygous mice was 4 h more rapid than that in the CB1−/−mice (data not shown). Therefore, CB1 activation hastens wound closure through regulating innate immune responses. This finding is consistent with results showing in HCEC that CB1 activation by WIN accelerated cell migration [4].
Fig. 3.
CB1 activation promotes mouse corneal reepithelialization. (A) Time dependent epithelial wound healing was evaluated following epithelial debridement with an Algerbrush in control (WT) and CB1−/−mice. Wound area was identified based on extent of fluorescein staining. Corneal reepithelialization in the WT group required 30 h whereas it was delayed by 10 h in the CB1−/− groups since closure occurred at 40 h. (B) Immune cell infiltration was assessed at 10 h in WT (left panel) and CB1−/− (right panel). CD 11b green immunostaining in the remaining deepithelialized area (DE) is only evident in the CB1−/− (KO) cornea. Scale bar=50 μm.
3.4. Identification of a CB1 endogenous agonist in cornea
CB1-induced wound closure hastening prompted us to probe for endogenous corneal endocannabinoid receptor ligands described in the nervous system [22,23]. In a preparation consisting of ten mouse corneas, 2-arachidonylglycerol (2-AG) a relatively selective CB1 agonist was detected at a level of 4.8±3.5 pmol/per cornea. On the other hand, the nonselective ligands activating both CB1 and TRPV1 channels, arachidonoyl ethanolamide and anandamide, were undetectable. Nevertheless, 2-AG presence substantiates the notion that an endogenous endocannabinoid ligand can be released by injury to induce CB1 activation.
3.5. CB1 activation suppresses TRPV1-mediated currents
In rat trigeminal ganglion neurons, WIN elicited antihyperalgesia and antinociception through desensitizing TRPV1 [13]. To determine in HCEC if the immunosuppressive effects of CB1 in the cornea are also accounted for by TRPV1 desensitization, planar patch clamping in the whole-cell recording mode evaluated CAP-induced increases in TRPV1 channel conductance with or without WIN pre-incubation (Fig. 4A). Fig. 4B shows the protocol used to stimulate non-selective cation channel currents whereas Fig. 4C/D illustrates the CAP-induced control current increases. The same responses were determined after WIN pre-treatment (Fig. 4E/F). CAP evidently increased outward currents at voltage steps above +60 mV (Fig. 4G). In contrast, with WIN pre-incubation such increases were diminished (Fig. 4H). With leak correction, 10 μM CAP increased outwardly rectifying currents at +130 mV from 37.1±16.8 pA/pF (control, n=4) to 132.1±15.5 pA/pF (CAP, n=4; *p<0.05) (Fig. 5D, left columns). In contrast, after WIN pre-incubation, CAP did not increase outwardly rectifying currents. The maximal outward current density only slightly increased from 18.9±5.5 pA/pF (control, n=5) to 19.8±3.0 pA/pF (n=5; p>0.05) (Fig. 5D, right columns). This insignificant change suggests that WIN inhibited CAP-induced currents through TRPV1 desensitization.
Fig. 4.
CB1 activation suppresses CAP-induced non-selective cation channel currents in HCEC. (A) Experimental design: whole-cell configuration for the planar patch-clamp. (B) Voltage pulse protocol: holding potential was set to 0 mV to avoid any voltage-dependent ion channel currents. (C) Nonselective cation channel currents induced by depolarization from −60 mV to 130 mV in the whole-cell configuration (with leak current compensation, n=7) are compared to those induced by this voltage protocol. (D) CAP (10 μM) induced increases in nonselective cation channel currents (n=7). (E/F) The effects of 10 μM WIN alone are compared to those induced by CAP following WIN exposure. (G) CAP increased outward currents were summarized in a current/voltage plot (n=7). The upper trace (filled circles) was obtained after exposure to CAP and the lower trace (open circles) is indicated in the absence of CAP. CAP-induced outward currents at potentials above +60 mV were discernable. (H) During WIN (10 μM) exposure, CAP-induced current increases occurring in the absence of WIN (open circles) were reduced to control levels (filled circles).
Fig. 5.
WIN suppressed CAP-induced cation channel outward currents in HCEC. (A) CAP-induced cation outward currents to voltage ramps from −60 mV up to +130 mV (with leak current compensation). They were normalized to capacitance to obtain current density (pA/pF). The upper (red) and lower traces (black) show the current responses in the presence and absence of CAP (10 μM), respectively. (B) HCEC were pretreated with 10 μM WIN for 30 min followed by conditions described in (A). (C) Summary of the individual effects of 10 μM WIN (n=4; p<0.05) preincubation on increases in current induced by 20 μM CAP (n=7; p<0.05) at −60 mV and 130 mV (without leak current compensation). (D) Summary of the effects of CAP (10 μM) (with leak current compensation) shows the outward current density in the presence and absence of WIN (10 μM). CAP alone increased currents from 37.08±16.75 pA/pF (control, n=4) to 132.1±15.54 pA/pF (CAP, n=4; p<0.005; left columns). The right columns show WIN pretreatment reduced CAP effects. Maximal outward current density was unchanged from the untreated control. Such suppression suggests that WIN inhibited CAP-induced currents through TRPV1 desensitization.
No increases in inward currents could be detected in most recordings because software leak subtraction markedly suppressed these currents (>20 nS) (Fig. 4C–D). To enhance sensitivity for detecting WIN inhibition of CAP-induced currents, the same experiments were repeated with 20 μM CAP without leak correction. Similar results were obtained at this higher CAP concentration; namely, increases in outwardly rectifying currents occurred with CAP at voltage steps above +80 mV (data not shown). The outward current density increased from 174±43 pA/pF to 245±54 pA/pF (n=7; p<0.05). Recovery occurred to 191±39 pA/pF (n=7; p<0.05) (Fig. 5C, left columns). On the contrary, with WIN pre-treatment, neither outward rectification nor any increase in nonselective outward currents could be detected (139±12 pA/pF vs. 142±11 pA/pF; n=4; p>0.05) (Fig. 5C, right columns). Taken together, irrespective of leak current subtraction, CB1 activation by WIN suppressed CAP-induced increases in nonselective outward currents. These results suggest that WIN activation of CB1 desensitizes TRPV1-induced currents caused by exposure to CAP.
3.6. CB1 inhibits TRPV1-induced Ca2+ transients through Gi/o activation
As CB1 activation can mediate responses through Gi/o activation, we determined if Gi/o inhibition with pertussis toxin preexposure altered an effect by WIN on CAP-induced Ca2+ transients. Fig. 6A shows that the fluorescence F340/380 nm ratio increased by about 65% during exposure to CAP whereas WIN preincubation reduced this rise by 60%. Fig. 6B shows that overnight pre-incubation with pertussis toxin (PTX), fully reversed WIN-induced suppression of CAP-induced Ca2+ transients whereas preincubation with a selective calcineurin (PP2B) antagonist, FK506, did not block WIN inhibition of this response. This negative effect suggests that changes in PP2B (i.e. a Ca2+ dependent phosphatase) activation caused by modulation of TRPV1-induced Ca2+ influx by CB1 do not account for how CB1 suppresses TRPV1 activation. Such a possibility was considered since changes in TRPV1 phosphorylation status modulate TRPV1 activation in capsaicin-sensitive nociceptive neurons. In these neurons, this channel is phosphorylated at serine and threonine residues whereas increases in Ca2+ influx activate PP2B resulting in TRPV1 activation dephosphorylation [13].
Fig. 6.
CB1 activation suppressed CAP-induced calcium transient. (A) Fura2-AM loaded HCEC were exposed to CAP (10 μM), which maximally induced a nearly two-fold [Ca2+]i transient (upward directed triangle) whereas preincubation with WIN (10 μM, downward directed triangle symbol) suppressed this rise by 65%. (B) Summary from five different measurements under each of the four indicated conditions: a) CAP-induced calcium transient (angular hatched bar); b) pre-incubation for 30 min with WIN inhibited CAP transient (horizontal hatched bar); c) following exposure to a Gi/o inhibitor, pertussis toxin (PTX, 500 ng/ml) WIN suppression of CAP-induced Ca2+ transients was fully reversed (hatched bar); and d) preincubation with a selective calcineurin antagonist, FK506 (100 μM), did not block WIN inhibition of this response (downward directed hatched bar). Results are expressed as means±SEM (n=5), p<0.05, the difference was considered to be significant.
We validated that the decline in CAP-induced Ca2+ transients by WIN is a result of a Gi/o mediated declineinTRPV1 phosphorylation status [22]. This was done by evaluating with an anti-phospho-threonine antibody if changes occur in p-TRPV1 expression levels in immunoprecipitates obtained with an anti-TRPV1 antibody. The anti-TRPV1 antibody selectivity for detecting TRPV1 expression was documented by showing that the anticipated 100 kD band was selectively diminished in TRPV1 siRNA gene silenced cells by 74% from the level in cells transfected instead with irrelevant scrambled siRNAs (c.f. Fig. 7A). Fig. 7B shows that WIN preincubation decreased by 55% the TRPV1 phosphorylation status detected by Western blot analysis of TRPV1 immunopreciptiates. The PTX-induced reversal of the inhibitory effect by WIN shown in Fig. 6B effect suggests that CB1 activation leads to TRPV1 desensitization through decreases in cAMP-dependent TRPV1 phosphorylation by PKA [11–13].
Fig. 7.
CB1 and TRPV1 protein–protein interaction. (A) Anti-TRPV1 antibody selectivity validation. Western blot detects TRPV1 protein expression with apparent molecular weight in agreement with previous reports. It was selectively suppressed by 74% decline following TRPV1 siRNA transfection of HCEC. (B) CB1-induces decline in TRPV1 phosphorylation status. Western blot analysis of TRPV1 immunopreciptiates using an anti-phospho-threonine antibody revealed that 30 min 10 μM WIN preincubation dampened TRPV1 phosphorylation. Anti-phosphothreonine antibody specificity was validated based on blot with the positive control provided by the commercial source. (C) Anti-CB1 antibody selectivity validation. Western blot detects CB1 protein expression with apparent molecular weight in agreement with previous reports. It was selectively suppressed by 76% decline following CB1 siRNA transfection. (D) TRPV1 complexation with CB1 and TAK1. Co-immunoprecipitation of whole cell lysates obtained with either an anti-TAK1, CB1 or TRPV1 antibody following CAP (10 μM) exposure. Immunocomplexes were probed for TRPV1 content with Western blot analysis using an anti-TRPV1 antibody. (E) CB1 complexation with TRPV1 and TAK1. Co-immunoprecipitation of whole cell lysates obtained with either an anti-TAK1, CB1 or TRPV1 antibody following CAP (10 μM) exposure. Immunocomplexes were probed for CB1 content with Western blot analysis using an anti-CB1 antibody. Antibody selectivity was validated by showing that the IgG immunoprecipitated complexes yielded no immunoreactive bands. Results are expressed as means±SEM (n=3). Differences of p<0.05 were considered as significant.
3.7. CB1 and TRPV1 protein–protein interaction
Although it is apparent that CB1 activation desensitizes TRPV1, it is unclear if this effect is dependent on CB1 complexation with TRPV1. To make this assessment, HCEC lysates were immunoprecipitated with a selective anti-CB1 antibody and the immunocomplexes were probed with Western blot analysis for TRPV1 and vice versa. The results shown in Fig. 7C validate CB1 antibody selectivity since in CB1 siRNA gene silenced cells CB1 expression declined by 76%. The results shown in Fig. 7D revealed that there was a significant amount of TRPV1 expression in a CB1 immune complex. This result agrees with the identification of CB1 in another immunocomplex formed by TRPV1 coimmunoprecipitation (Fig. 7E). Both results suggest that there is a direct protein–protein interaction between these two receptors. Furthermore, in these complexes a downstream TRPV1 signaling mediator, TAK1, was also identified. Antibody selectivity was validated by showing that the IgG immunoprecipitated complexes yielded no immunoreactive bands.
3.8. CB1 activation obviates TRPV1-linked pro-inflammatory signaling pathways
TAK1 stimulation of JNK1 MAPK phosphorylation contributes to inducing increases in IL-6/8 release [3]. We determined if TRPV1 desensitization by CB1 dampened TAK1 and JNK1 activation. Fig. 8A shows CAP (20 μM) induced JNK1/2 phosphorylation at 5 min. After 30 min pre-incubation with WIN, AM251, or 5Z-7-oxozeaenol (5z-OX, a highly potent inhibitor of TAK1), these increases were abolished. However, pre-incubation for 30 min with AM251 (10 μM) prior to exposure to WIN (10 μM), significantly increased CAP-induced increases in JNK1/2 phosphorylation status (n=3, p<0.01). Fig. 8B shows CB1 gene silencing negated WIN-induced TRPV1 suppression since in CB1 siRNA transduced cells CAP-induced p-TAK1 formation was not affected by WIN whereas it was obviated in the scrambled cells. These downstream inhibitory effects of WIN on TRPV1-induced signaling activation suggest that they may be a consequence of CB1-induced desensitization of TRPV1.
Fig. 8.
Blunting by CB1 activation of TRPV1-induced JNK1 and TAK1 phosphorylation. (A) TRPV1 and CB1 mediated JNK1/2 phosphorylation. Western blot analysis with anti-phospho JNK1/2 antibody reveals increases in JNK1/2 phosphorylation induced by 5 min exposure to CAP (20 μM). Such rises were inhibited by 30 min preexposure to either WIN (10 μM), or CPZ (10 μM). Pre-incubation with AM251 (10 μM), for 30 min prior to WIN (10 μM), exposure suppressed declines in CAP (20 μM)-induced JNK1/2 phosphorylation induced by WIN (10 μM). (B) Dependence of WIN blunting of TRPV1-induced TAK1 phosphorylation on CB1 protein expression. CAP (10 μM)-induced increases in TAK1 phosphorylation at 5 min in cells transduced with scrambled siRNA (left panel) are compared with those in CB1 siRNA transduced cells (right panel). WIN-induced suppression of TRPV1-induced TAK1 phosphorylation was eliminated in CB1 siRNA transduced cells (last lane on the right). The results are shown as means±SEM (n=3, p<0.05).
4. Discussion
TRPV1 induction of an innate immune response to severe injury or stresses encountered in some types of dry eye disease has adaptive value in suppressing corneal pathogenic infiltration provided it is self-limiting. On the other hand, a sustained response can instead lead to dysregulated inflammation and fibrosis [5]. As the therapeutic options are limited to control inflammation, it is relevant to identify novel strategies to prevent this response from becoming chronic. This study suggests that CB1 is a potential drug target to regulate TRPV1-induced inflammation since in two different mouse corneal wound healing models CB1 activation reduced TRPV1-induced inflammatory responses and opacification as well as hastened epithelial barrier restoration. These CB1 mediated effects stemmed from Gi/o activation and protein–protein interaction between CB1 and TRPV1. Associated with these changes, declines occurred in TRPV1 phosphorylation status resulting in suppression of TRPV1-induced Ca2+ transients and declines in underlying nonselective outward directed currents. Such suppression may directly account for declines in TRPV1-linked downstream signaling events inducing increases in IL-6 and IL-8 expression.
CB1 receptor expression was first identified in the human corneal epithelium based on receptor labeling [23]. Such expression is functional since its activation by WIN induced Ca2+ transients and blunted TRPV1-induced increases in IL-8 expression [4]. This immune regulatory role is in accord with what has been described in multiple tissue types [10]. In the current study, the functional importance of CB1 was demonstrated in reducing TRPV1-induced inflammation and retardation of wound healing. CB1 has an immunosuppressive role in the cornea since stromal neutrophil recruitment was augmented in the CB1−/−mice compared to their WT counterpart following epithelial debridement. Furthermore, following alkali burning mouse corneas, systemic WIN injection also reduced stromal neutrophil, macrophage infiltration, prevented perforation and reduced scarring (Fig. 2C). As a consequence of these mitigating effects by CB1 activation, corneal transparency restoration was better than that in the untreated mice. Taken together, these results indicate that CB1 is a potential drug target to improve the outcome of corneal wound healing subsequent to injury.
CB1 activation following injury by an endogenous ligand is tenable since a selective endocannabinoid CB1 ligand, 2-AG, was detected in mouse corneal extracts. 2-AG identification is in agreement with the reported endocannabinoid measurements made in human cadaver corneas except that AEA and PEA were also identified in human corneas [24,25]. This difference may reflect species difference and/or possibly fewer corneas used in our assays.
CB1 interaction with TRPV1 resulted in desensitization of this channel to activation by CAP as well as blunting of downstream signaling events leading to increases in a proinflammatory cytokine and chemoattractant expression. However, it is uncertain if signaling blunting by CB1 occurred irrespective of TRPV1 desensitization. Suppression by CB1 of TRPV1 activation is consistent with their close proximity to one another as well as direct protein–protein interaction (Figs. 1A/B and 7D/E). Such colocalization may be requisite for WIN to completely abolish non-selective cation channel currents induced by TRPV1-activation as well as reduce CAP-induced Ca2+ transients by 60% (Figs. 4/5/6A). Our results are consistent with reports in keratinocytes and urothelium showing that CB1 is co-expressed with TRPV1 and controls TRPV1 sensitivity [26,27].
A variety of different mechanisms have been described that account for TRPV1 sensitivity modulation. The different modes of regulation may be cell type specific. One of them associates increases in TRPV1 phosphorylation status with activation [28]. Our results are consistent with this notion based on our finding shown in Fig. 7B that decreases in TRPV1 sensitization by WIN were associated with a decline in TRPV1 phosphorylation status of 55%. Such changes can be elicited through changes in either PKC or PKA activity [29,30,31]. In different cell types and settings, stimulation of either of these kinases can increase channel open time leading to increases in plasma membrane Ca2+ influx and outward directed currents. With regard to a cAMP dependent pathway inducing through PKA activation TRPV1 phosphorylation at serine/threonine residues, our results suggest that WIN reduced TRPV1 activation through a decline in PKA activity as a consequence of adenylate cyclase inhibition since suppression by WIN of TRPV1-mediated Ca2+ transients was completely reversed by prior inhibition of Gi/o activation with PTX (Fig. 6B). An alternative possibility that WIN activates instead calcineurinto decrease the TRPV1 phosphorylation status was excluded since preincubation with a selective PP2B inhibitor, FK506, did not affect the inhibitory effects of WIN on CAP-induced Ca2+ transients (Fig. 6B) [13].
CB1 activation also blunted TRPV1-linked signaling events activated by CAP since WIN abrogated CAP-induced TAK1 and JNK1 phosphorylation (Fig. 8A/B). These declines are attributable to CB1 activation since in CB1 siRNA gene silenced cells WIN failed to reduce CAP-induced TAK1 phosphorylation (Fig. 8B). This assessment was warranted since WIN has also been reported to activate TRPA1 in sensory neurons resulting in TRPV1 desensitization [32] as well as activate peroxisomal proliferator activated receptor α (PPARα) [33].
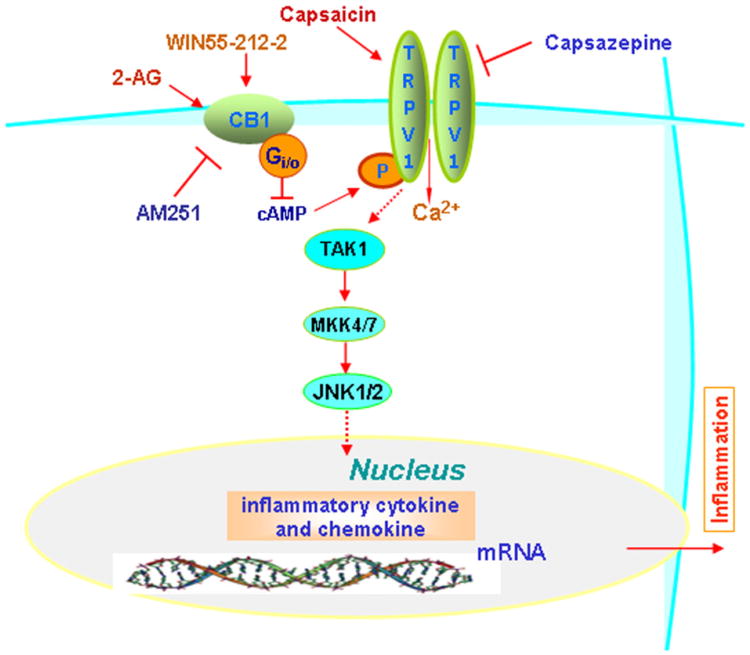
In conclusion, in mice corneas, CB1 activation by injury dampens inflammatory responses to TRPV1 stimulation. Such suppression accelerates wound healing rates and reduces scar formation. Fig. 9 provides a summary model describing the different types of signaling interactions between CB1 and TRPV1 that account for inflammatory responses to their activation. According to the model, declines in TRPV1-induced signaling and responses stem from protein–protein interaction between TRPV1 and CB1. Their complexation may be needed for CB1 to reduce TRPV1 activity through declines in TRPV1 phosphorylation status and Ca2+ transients. Therefore, CB1 could be a novel drug target to improve corneal wound healing outcome.
Fig. 9.
Working model of signaling events mediating CB1 blunting of TRPV1-induced increases in proinflammatory cytokine expression. WIN-induced CB1 activation leads to CB1-TRPV1 interaction and TRPV1 desensitization as a consequence of inhibition of PKA-mediated increases in TRPV1 phosphorylation status. Such suppression by CB1 is a consequence of its activation of Gi/o leading to a decline in cAMP formation by adenylate cyclase. Dampening of TRPV1 activation by CAP decreases CAP mediated downstream-linked signaling events leading to declines in TRPV1-induced pro-inflammatory cytokine expression. Dashed arrows indicate possible linked events.
Acknowledgments
This work was supported by grants from the NEI EY04795 (P.S.R.); EY20506 (K.V); and the Ministry of Education, Science, Sports and Culture of Japan (C21592241 to Y.O. and C19592036 to S.S.); and the Mitsui Life Social Welfare Foundation, Mochida Memorial Foundation, Takeda Science Foundation, and Uehara Foundation (S.S.). The authors greatly appreciate the technical assistance by Nefeli Slavi from the Graduate School of Master's Program in Charité, Berlin (Germany) during her lab rotation studies. The planar patch-clamp setup was supported in part by Berliner Sonnenfeld-Stiftung.
Abbreviations
- WIN
WIN55,212-2
- 2-AG
2-arachidonylglycerol
- CAP
capsaicin
- TAK1
transforming growth factor-β-activated kinase 1
- CPZ
capsazepine
- PTX
pertussis toxin
- 5z-OX
5Z-7-oxozeaenol
- Gi/G0 or Gi protein
Gi alpha subunit
- JNK
c-Jun N-amino terminal kinases
References
- 1.Bates BD, Mitchell K, Keller JM, Chan CC, Swaim WD, Yaskovich R, Mannes AJ, Iadarola MJ. Pain. 2010;149(3):522–528. doi: 10.1016/j.pain.2010.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pan Z, Wang Z, Yang H, Zhang F, Reinach PS. Investigative Ophthalmology & Visual Science. 2010;52(1):485–493. doi: 10.1167/iovs.10-5801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang Z, Yang Y, Yang H, Capo-Aponte JE, Tachado SD, Wolosin JM, Reinach PS. Molecular Vision. 2011;17:3137–3146. [PMC free article] [PubMed] [Google Scholar]
- 4.Yang H, Wang Z, Capo-Aponte JE, Zhang F, Pan Z, Reinach PS. Experimental Eye Research. 2010;91(3):462–471. doi: 10.1016/j.exer.2010.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Okada Y, Reinach PS, Shirai K, Kitano A, Kao WW, Flanders KC, Miyajima M, Liu H, Zhang J, Saika S. American Journal of Pathology. 2011;178(6):2654–2664. doi: 10.1016/j.ajpath.2011.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Murata Y, Masuko S. Brain Research. 2006;1085(1):87–94. doi: 10.1016/j.brainres.2006.02.035. [DOI] [PubMed] [Google Scholar]
- 7.Mergler S, Valtink M, Coulson-Thomas VJ, Lindemann D, Reinach PS, Engelmann K, Pleyer U. Experimental Eye Research. 2010;90(6):758–770. doi: 10.1016/j.exer.2010.03.010. [DOI] [PubMed] [Google Scholar]
- 8.Zhang F, Yang H, Wang Z, Mergler S, Liu H, Kawakita T, Tachado SD, Pan Z, Capo-Aponte JE, Pleyer U, Koziel H, Kao WW, Reinach PS. Journal of Cellular Physiology. 2007;213(3):730–739. doi: 10.1002/jcp.21141. [DOI] [PubMed] [Google Scholar]
- 9.Turu G, Hunyady L. Journal of Molecular Endocrinology. 2009;44(2):75–85. doi: 10.1677/JME-08-0190. [DOI] [PubMed] [Google Scholar]
- 10.Pandey R, Mousawy K, Nagarkatti M, Nagarkatti P. Pharmacological Research. 2009;60(2):85–92. doi: 10.1016/j.phrs.2009.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang W, Cao X, Liu C, Liu L. Neurological Sciences. 2012;33(1):79–85. doi: 10.1007/s10072-011-0620-6. [DOI] [PubMed] [Google Scholar]
- 12.Hermann H, De Petrocellis L, Bisogno T, Schiano Moriello A, Lutz B, Di Marzo V. Cellular and Molecular Life Sciences. 2003;60(3):607–616. doi: 10.1007/s000180300052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Patwardhan AM, Jeske NA, Price TJ, Gamper N, Akopian AN, Hargreaves KM. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(30):11393–11398. doi: 10.1073/pnas.0603861103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nagarkatti P, Pandey R, Rieder SA, Hegde VL, Nagarkatti M. Future Medicinal Chemistry. 2009;1(7):1333–1349. doi: 10.4155/fmc.09.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang Z, Yang H, Tachado SD, Capo-Aponte JE, Bildin VN, Koziel H, Reinach PS. Investigative Ophthalmology & Visual Science. 2006;47(12):5267–5275. doi: 10.1167/iovs.06-0642. [DOI] [PubMed] [Google Scholar]
- 16.Yang H, Mergler S, Sun X, Wang Z, Lu L, Bonanno JA, Pleyer U, Reinach PS. Journal of Biological Chemistry. 2005;280(37):32230–32237. doi: 10.1074/jbc.M504553200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fertig N, Blick RH, Behrends JC. Biophysical Journal. 2002;82(6):3056–3062. doi: 10.1016/S0006-3495(02)75646-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mergler S, Valtink M, Taetz K, Sahlmuller M, Fels G, Reinach PS, Engelmann K, Pleyer U. Experimental Eye Research. 2011;93(5):710–719. doi: 10.1016/j.exer.2011.09.021. [DOI] [PubMed] [Google Scholar]
- 19.Kingsley PJ, Marnett LJ. Analytical Biochemistry. 2003;314(1):8–15. doi: 10.1016/s0003-2697(02)00643-7. [DOI] [PubMed] [Google Scholar]
- 20.Matias I, Wang JW, Moriello AS, Nieves A, Woodward DF, Di Marzo V. Prosta-glandins, Leukotrienes, and Essential Fatty Acids. 2006;75(6):413–418. doi: 10.1016/j.plefa.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 21.Ma C, Martins-Green M. Wound Repair and Regeneration. 2009;17(3):387–396. doi: 10.1111/j.1524-475X.2009.00478.x. [DOI] [PubMed] [Google Scholar]
- 22.Stella N, Schweitzer P, Piomelli D. Nature. 1997;388(6644):773–778. doi: 10.1038/42015. [DOI] [PubMed] [Google Scholar]
- 23.Sugiura T, Kodaka T, Nakane S, Miyashita T, Kondo S, Suhara Y, Takayama H, Waku K, Seki C, Baba N, Ishima Y. Journal of Biological Chemistry. 1999;274(5):2794–2801. doi: 10.1074/jbc.274.5.2794. [DOI] [PubMed] [Google Scholar]
- 24.Lopshire JC, Nicol GD. Journal of Neuroscience. 1998;18(16):6081–6092. doi: 10.1523/JNEUROSCI.18-16-06081.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Straiker AJ, Maguire G, Mackie K, Lindsey J. Investigative Ophthalmology & Visual Science. 1999;40(10):2442–2448. [PubMed] [Google Scholar]
- 26.Chen J, Matias I, Dinh T, Lu T, Venezia S, Nieves A, Woodward DF, Di Marzo V. Biochemical and Biophysical Research Communications. 2005;330(4):1062–1067. doi: 10.1016/j.bbrc.2005.03.095. [DOI] [PubMed] [Google Scholar]
- 27.Walczak JS, Cervero F. Molecular Pain. 2011;7:31. doi: 10.1186/1744-8069-7-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Toth BI, Dobrosi N, Dajnoki A, Czifra G, Olah A, Szollosi AG, Juhasz I, Sugawara K, Paus R, Biro T. The Journal of Investigative Dermatology. 2011;131(5):1095–1104. doi: 10.1038/jid.2010.421. [DOI] [PubMed] [Google Scholar]
- 29.Studer M, McNaughton PA. The Journal of Physiology. 2010;588(Pt 19):3743–3756. doi: 10.1113/jphysiol.2010.190611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mandadi S, Tominaga T, Numazaki M, Murayama N, Saito N, Armati PJ, Roufogalis BD, Tominaga M. Pain. 2006;123(1–2):106–116. doi: 10.1016/j.pain.2006.02.016. [DOI] [PubMed] [Google Scholar]
- 31.Mohapatra DP, Nau C. Journal of Biological Chemistry. 2005;280(14):13424–13432. doi: 10.1074/jbc.M410917200. [DOI] [PubMed] [Google Scholar]
- 32.Akopian AN, Ruparel NB, Patwardhan A, Hargreaves KM. Journal of Neuroscience. 2008;28(5):1064–1075. doi: 10.1523/JNEUROSCI.1565-06.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Downer EJ, Clifford E, Amu S, Fallon PG, Moynagh PN. Journal of Biological Chemistry. 2012;287(30):25440–25453. doi: 10.1074/jbc.M112.371757. [DOI] [PMC free article] [PubMed] [Google Scholar]