Abstract
Behavioral studies have demonstrated that time perception in adults, children, and nonhuman animals is subject to Weber’s Law. More specifically, as with discriminations of other features, it has been found that it is the ratio between two durations rather than their absolute difference that controls the ability of an animal to discriminate them. Here, we show that scalp-recorded event-related electrical brain potentials (ERPs) in both adults and 10-month-old human infants, in response to changes in interstimulus interval (ISI), appear to obey the scalar property found in time perception in adults, children, and nonhuman animals. Using a timing-interval oddball paradigm, we tested adults and infants in conditions where the ratio between the standard and deviant interval in a train of homogeneous auditory stimuli varied such that there was a 1:4 (only for the infants), 1:3, 1:2, and 2:3 ratio between the standard and deviant intervals. We found that the amplitude of the deviant-triggered mismatch negativity ERP component (deviant-ISI ERP minus standard-ISI ERP) varied as a function of the ratio of the standard to deviant interval. Moreover, when absolute values were varied and ratio was held constant, the mismatch negativity did not vary.
INTRODUCTION
Space, time, and number are three abstract stimulus attributes that are used by animals and humans to represent their world (Gallistel, 1990). A large literature documents the interval timing abilities of nonhuman animals and adult humans (for reviews, see Meck, 2003; Matell & Merck, 2000; Gibbon, Malapani, Dale, & Gallistel, 1997; Gibbon & Allan, 1984). A ubiquitous finding in this literature is that temporal discrimination obeys Weber’s Law in that discrimination depends on the ratio between two values not on their absolute difference. More specifically, timing behavior shows scalar variability. The scalar property has been interpreted as evidence that the variability (standard deviation) in temporal representations increases linearly with the mean of the interval being represented (e.g., Melgire et al., 2005; Dale et al., 2001; Wearden & Bray, 2001; Gibbon et al., 1984).
Very little research has addressed whether the hallmarks of interval timing in adults and nonhuman animals are present early in human development. Young children have been shown to discriminate temporal intervals in both temporal generalization tasks and temporal bisection tasks (e.g., Droit-Volet, Meck, & Penney, 2007; Droit-Volet, 2002; Droit-Volet, Clement, & Wearden, 2001; Droit-Volet & Wearden, 2001). Children have been studied in the bisection paradigm, in which individuals are trained to differentiate two standard durations (one short, one long) and then are tested with intermediate durations where they are asked to categorize each value as being closer to the short or the long standard value. Five and 8-year-old children showed orderly psychophysical functions whereby the probability of making a “long” response increased with duration (Rattat & Droit-Volet, 2001).
Importantly, children, like adults and nonhuman animals, exhibit scalar variability whereby the standard deviation in the remembered interval increases in proportion to the mean of the interval. For example, Droit-Volet and colleagues tested 3-, 5-, and 8-year-old children in a temporal generalization task. One group of children was shown a circle that stayed on for 4 sec and another group was shown a circle that stayed on for 8 sec. Children in both groups were told “Look it’s your circle. It stays on for a certain amount of time.” Children in both groups were then shown circles that stayed on for the standard amount of time (4 or 8 sec) or one of six different nonstandard durations (three durations below and three durations above the standard value). Children were simply asked to respond “yes” or “no” as to whether the circle was their circle or not. Children of all three ages showed temporal generalization gradients whereby the probability of categorizing a circle as the standard value increased with the similarity to the standard value. In addition, when the probability of categorizing a circle as the standard value was plotted as a function of each test duration expressed as a fraction of the standard value, the generalization gradients superimposed for the two groups of children, implying variability scaled as a function of the standard value.
Collectively, these studies suggest many similarities between timing processes in children, adults, and animals. In all cases, temporal intervals seem to be remembered with scalar variability, whereby the standard deviation of the remembered intervals increases in proportion to the mean intervals. An important set of questions then is at what point in development the ability to represent temporal intervals first appears, how this ability changes with development, and how this processing is accomplished in the brain.
It has also been demonstrated that infants’ heart rate responses are sensitive to temporal structure. For example, Clifton (1974) conditioned newborns with a conditioned stimulus (CS) followed after 2 sec by an unconditioned stimulus (US). She found that when the US was omitted after 30 trials, infants showed heart rate deceleration at the time the US should have appeared. Similarly, Colombo and Richman (2002) measured 4-month-old infants’ heart rate responses to an on–off stimulus pattern with an interstimulus interval (ISI) of 3 sec for one group of infants and 5 sec for another group. On the ninth trial, the off period was set at 15 sec rather than 3 or 5 sec so that infants’ heart rate responses to an omitted stimulus could be measured. Infants’ heart rates decelerated within 0.5 sec of the time at which the omitted stimulus should have appeared.
Recently, vanMarle and Wynn (2006) demonstrated that 6-month-old infants discriminate durations that differ by a 1:2 ratio but fail to discriminate durations that differ by a 2:3 ratio. Successful discrimination was found at two different sets of absolute values that differed by a 1:2 ratio and failure to discriminate was shown at two sets of absolute values that differed by a 2:3 ratio. Similar ratio dependence in temporal discrimination was shown in 6-month-old infants by Brannon, Suanda, and Libertus (in press). In addition, Brannon et al. (in press) found that the precision with which infants make temporal discriminations increased from 6 to 10 months of age, such that 10-month-old infants successfully discriminated a 2:3 ratio.
Our goal here was to test whether neural activations associated with temporal discrimination in infants also show ratio dependence. In a previous study, we used the ERP component known as the mismatch negativity (MMN) to study the brain responses of 10-month-old infants to deviations in temporal intervals (Brannon, Wolfe Roussel, Meck, & Woldorff, 2004). The MMN is a negative polarity waveform that usually peaks between 120 and 200 msec after the onset of the deviance (Näätänen & Winkler, 1999) and is often presented as the difference wave resulting from subtraction of the ERP elicited by the standard stimulus from the ERP response elicited by the deviant stimulus. In passive deviance detection paradigms (oddball paradigms), the MMN is elicited when a stream of regularly occurring stimuli (the standard stimulus) is interrupted by a stimulus (the deviant stimulus) that differs in a physical feature such as frequency or intensity. However, the MMN is also elicited in response to shortening in the temporal interval between stimuli, indicating that the MMN is not merely a result of new afferent neurons being activated (Näätänen, Jiang, Lavikainen, Reinikainen, & Paavilainen, 1993). In our previous study, we demonstrated that 10-month-old infants exhibited an MMN in response to a threefold deviation in a temporal interval between tones that was similar to that of adults (Brannon et al., 2004; see also Trainor, Samuel, Desjardins, & Sonnadara, 2001; Nordby, Roth, & Pfefferbaum, 1988 with adults).
Interestingly, larger deviant–standard differences are known to elicit earlier and larger amplitude MMNs in adults as compared to relatively smaller differences (Näätänen & Winkler, 1999; see similar demonstration by Alho, 1995 for pitch deviants). Furthermore, the MMN is thought to depend on a comparison in sensory memory between the standard and the deviant. Longer ISIs between standards and deviants are known to attenuate the MMN, and this is thought to result from a decay in a sensory template over time (Mäntysalo & Näätänen, 1987; but see Schroger, 1996, for an exception).
The assessment of the neural correlates of timing has been accomplished both by examining ERP responses to variations in the duration of stimuli and to variations in the intervals between stimuli. A hallmark of interval timing is that duration discrimination is ratio dependent. Accordingly, ERP studies using temporal oddball paradigms indicate that duration deviants that have the same percentage change from the standard duration, but different absolute changes, elicit similar amplitude MMNs in adults (e.g., Tse & Penney, 2006; Jaramillo, Paavilainen, & Näätänen, 2000). Moreover, Sable, Gratton, and Fabiani (2003) recently demonstrated that the magnitude of the MMN response is proportional to the logarithm of the deviant/standard interval ratio. These authors interpreted this result as indicating that time intervals are represented with a ratio scale. However, to our knowledge, there are no systematic examinations of the effects of standard/deviant duration ratio in infants reported in the literature.1
Examining whether neural correlates of timing show ratio dependence in infancy could be important for at least two reasons. First, it would be of great interest to gain insight into the neural circuitry responsible for interval timing in early human development. However, this is quite difficult given that source analysis is a challenging enterprise in infant ERP data especially with small numbers of electrodes (e.g., Reynolds & Richards, 2005). A second reason is that ERP components could potentially provide a dependent measure in infants that can show parametric modulation as a function of stimulus factors. In looking-time behavioral datasets with human infants, scalar variance has been inferred from a pattern of successes and failures in samples of infants tested at different ratios (e.g., Lipton & Spelke, 2003; Xu & Spelke, 2000). ERP datasets could potentially provide neural activity measures, which could enable a psychophysical analysis whereby the amplitude of a component could vary as a function of the ratio of two temporal intervals, and might additionally provide insight into the level of brain processing by which such analyses might occur.
Accordingly, we predicted that the amplitude of the MMN would be modulated by the ratio between the standard and the deviant ISI in a sequence of tones. Experiment 1 varied the ratio of deviant-to-standard ISI duration and Experiment 2 held the ratio between the standard and the deviant ISI duration constant but varied the absolute value of the standard and deviant intervals.
EXPERIMENT 1
The goal of Experiment 1 was to test how the magnitude of the MMN in an auditory oddball-timing stimulus sequence would be modulated by the degree to which the standard and deviant ISI differed. To do this, we tested four groups of 10-month-old infants in four distinct conditions using a between-subject design, and, in parallel, tested a group of adults in three of these four conditions in a within-subject design.
Methods
Participants
The participants were 92 infants (23 in each of four conditions) with a mean age of 10 months 1 day (range = 9 months 6 days to 11 months 7 days) and 15 adults with a mean age of 22 years (SD = 4.9 years). Forty-four of the infants and 6 of the adults were female. Data from 22 additional infants were discarded due to fussiness (n = 4), premature removal of the cap (n = 10), equipment difficulties (n = 4), or excessive artifacts2 (n = 4). Data from nine additional adults were discarded due to either equipment difficulties (n = 2), excessive artifacts (n = 6), or external interference (n = 1). All infants and adults were healthy with normal vision and hearing. Adult participants and parents of the infant participants gave written informed consent, and all procedures were approved by the Duke University Institutional Review Board.
Stimuli
Infants and adults heard a stream of stimuli which were 50 msec 1000-Hz tones presented at approximately 60 dbSL. Tones were considered “standards” when they followed the more common 1500-msec ISI and “deviants” if they followed an infrequent ISI of either 375-, 500-, 750-, or 1000-msec in different blocks. The actual ISI for deviants and standards in all conditions and for both age groups was jittered randomly plus and minus 50 msec around each respective mean in order to reduce triggered alpha and to facilitate our use of the Adjacent Response (Adjar) technique (Woldorff, 1993) to eliminate overlap (see below). Fourteen percent of the tones in each condition occurred at the deviant ISI. Deviants ISIs were always preceded and followed by standard ISIs. It is important to note that, throughout our study, the deviants were always shorter than the standard ISIs. This decision was made because lengthening the ISI for the deviants would result in a larger N1 due to decreased refractoriness of the neurons, which would make it more difficult to disentangle the enhanced negativity derived from the larger N1 from the enhanced negativity derived from a larger MMN. Thus, we thought it more conservative to show the enhanced MMN in a situation (i.e., shorter intervals for the deviant) where the N1 would be expected to be smaller compared to the standard rather than larger.
Procedure
The experiment was conducted in a sound-attenuated, electrically shielded room in the Center for Cognitive Neuroscience at Duke University. For the infant studies, infants sat on the lap of a parent approximately 60 cm away from a puppet stage. One experimenter conducted a silent visual puppet show to entertain the baby and keep the baby as still as possible. The speakers through which the sounds were presented were located approximately 120 cm from the infants’ heads. Infants were tested until they were unable to sit calmly on their parent’s lap any longer (average 18 min). Separate groups of infants were tested in four different conditions. In all conditions, the standard ISI was 1500 msec, with the deviant ISI being either 375, 500, 750, or 1000 msec.
For the adult studies, the participants sat approximately 60 cm away from a computer screen and engaged in a visual target detection task with visual stimuli that were timed randomly relative to the auditory stimuli. Adults touched a button with their left hand when they observed a visual target stimulus. Adults were tested for 45 min, during which 58 auditory deviants were presented in each ISI condition via headphones. Adults were instructed to attend to the visual target detection task (analogous to the infants attending to the visual puppet show) and to ignore the auditory stimuli.
Using a blocked within-subjects design, a single sample of 15 adults was tested in three of the four conditions (500 vs. 1500, 750 vs. 1500, 1000 vs. 1500) in which infants were tested. The 375 versus 1500 condition was not included in order to keep the test time under 1 hr. The blocked conditions were counterbalanced for order.
EEG/ERP Acquisition and Analysis Methods
Brain electrical activity was recorded using tin electrodes placed in a custom-made elastic cap (Electro-Cap, Eaton, OH). Infants and adults wore an elastic chest band, which was attached to the cap to help secure it in place. In addition, adhesive foam disks were used to secure the front of the cap on to the infant’s forehead. Nineteen scalp sites were used for infants and 64 sites for adults. For the infants, one additional electrode was placed on the right cheek in order to help detect eye blinks and thereby aid in artifact rejection. For similar purposes, the 64 channels for the adults included four electrooculogram electrodes below and lateral to the eyes. Impedances were maintained as low as possible, aiming for under 5 kΩ for adults and under 10 kΩ for infants. Simply filling each electrode with gel and eliminating air bubbles generally reduced impedance levels sufficiently for infants, whereas some additional light abrading was necessary with adults to enable the gel to make a good connection with the scalp.
Recordings were referenced to the right mastoid during acquisition and were later algebraically re-referenced to an average of the right and left mastoids. The electroencephalogram (EEG) was amplified with a gain of 1000 for adults and 150 for infants; the gain was reduced for infants to accommodate the larger EEG signals that infants have compared with adults, thought to be due largely to reduced resistance and thinner skulls for the infants (DeBoer, Scott, & Nelson, 2005). A recording bandpass of 0.01–100 Hz was used, and the EEG was digitized continuously onto disk at a rate of 500 samples per second per channel.
The recorded EEG was examined off-line (both visually and with computer algorithms) to reject those epochs with eye movements, blinks, motion, or other artifacts in any of the channels. After artifact rejection, individual infants had an average of 66 deviant ISI trials (range = 17–153) and 402 standard ISI trials (range = 81–893), whereas adults had, on average, 51 deviant ISI trials (range = 41–57) and 312 standard ISI trials (range = 245–346) in each condition. The data were selectively averaged for standards and deviants for each individual. Data were normalized using a calibration pulse of the system and filtered for activity above 20 Hz using a low-pass filter. EEG was high-pass filtered (>1 Hz) during averaging to remove low-frequency noise and drift. A baseline correction of −100 to 0 was used, applied after the high-pass filtering.
Adjar
The Adjar technique (Woldorff, 1993) was used to remove the overlapping neural activity from the previous standard-tone stimulus that was distorting the ERP to the shortened-ISI deviant tone. In particular, this previous-response overlap was estimated by convolving the standard-tone ERP waveform with the temporal distribution of the previous-event occurrences for the deviant-ISI stimuli. The result of that convolution, which is the estimate of the overlapping activity from the previous stimulus in the sequence, was then subtracted from the ERP responses to the deviant-ISI tones. Note that the actual ISIs from only the included trials were used in these computations.
We focused the analyses on the data at the Fz lead due to the MMN generally being maximal at frontal and fronto-central sites (Brannon et al., 2004; Näätänen, 1990). To determine the time window for which the standard and deviant ERP waveforms differed significantly from each other, t tests were conducted in consecutive 6-msec windows comparing deviant and standard waveforms (minimum of 3 consecutive windows with p < .05). All analyses were conducted after the Adjar technique had removed overlap distortion.
Results and Discussion
The main finding was that the amplitude of the MMN was modulated by the difference between the standard and the deviant ISI duration. Figure 1 displays the grand-average difference waves derived by subtracting the standard ISI waveforms from the deviant-ISI waveforms at electrode site Fz, shown for all conditions for both adults and infants. To illustrate how the difference waves were constructed, we show the standard and deviant waveforms separately for one condition in infants in Figure 2.
Figure 1.
Deviant-minus-standard ERP difference waveforms (after Adjar overlap correction) from Experiment 1, showing four conditions for infants (left) and three conditions for adults (right) in which the ratio between standard and deviant varied. Data are from electrode site Fz. Negative potentials are plotted upward. Each tick mark ref lects 100 msec. The MMN is visible between 100 and 200 msec for both infants and adults.
Figure 2.
Sample ERPs for standard and deviant ISIs in one condition (375 vs. 1500 msec) for infants to illustrate the waveforms used to construct difference waves.
Statistics on the MMN were conducted using a time window for which all four conditions showed a significant MMN. Table 1 shows the time windows for each condition that yielded significant differences between the deviant and standard intervals for adults and infants. In general, the time windows for infants started slightly earlier and extended somewhat later than those for adults. The 1000 versus 1500 condition yielded the smallest significant MMN time window for both infants and adults, and the time windows of MMN significance for all the other conditions overlapped with this condition. We therefore use the smallest common time window to test for MMN differences, while also testing the full time window to see whether it yielded a different result. The pattern of results was the same regardless of whether the full MMN time windows for each condition or the smallest common MMN time window was used.
Table 1.
Mismatch Negativity (MMN) and Late Positivity (LP) Time Windows (msec) Poststimulus for Experiment 1
| Infants | Adults | |||
|---|---|---|---|---|
| Condition | MMN | LP | MMN | LP |
| 375 vs. 1500 | 108–240 | 282–612 | N/A | N/A |
| 500 vs. 1500 | 102–246 | 324–522 | 120–204 | 588–708 |
| 750 vs. 1500 | 102–240 | 468–612 | 126–198 | N/A |
| 1000 vs. 1500 | 144–240 | N/A | 162–180 | N/A |
Time windows were derived by conducting t tests in 6-msec windows comparing deviant and standard waveforms (minimum of 3 consecutive windows with p < .05, thus p < .000125 for 3 bins).
Separate repeated measures analyses of variance (ANOVAs) were conducted comparing the response to the standard-ISI and the deviant-ISI stimuli at the Fz electrode across the smallest common MMN time window. In each of the ISI conditions, for both the infants and the adults, there was a significant difference between the ERP elicited by the standard and the deviant (Table 2).
Table 2.
Statistical Results for Experiment 1
| Condition | Time Window | ANOVA Results | |
|---|---|---|---|
| Mismatch Negativity | |||
| Infants | 375 vs. 1500 | 144–240 |
F(1, 22) = 18.07 p < .001 |
| 500 vs. 1500 | 144–240 |
F(1, 22) = 28.94 p < .001 |
|
| 750 vs. 1500 | 144–240 |
F(1, 22) = 12.15 p < .01 |
|
| 1000 vs. 1500 | 144–240 |
F(1, 22) = 6.46 p = .02 |
|
| Adults | 500 vs. 1500 | 162–180 |
F(1, 14) = 29.46 p < .001 |
| 750 vs. 1500 | 162–180 |
F(1, 14) = 49.39 p < .001 |
|
| 1000 vs. 1500 | 162–180 |
F(1, 14) = 5.06 p = .04 |
|
| Late Positivity | |||
| Infants | 375 vs. 1500 | 324–522 |
F(1, 22) = 23.26 p < .001 |
| 500 vs. 1500 | 324–522 |
F(1, 22) = 18.00 p < .001 |
|
| 750 vs. 1500 | 324–522 |
F(1, 22) = 2.95 p = .1 |
|
| 750 vs. 1500 | 468–612 |
F(1, 22) = 6.44 p = .02 |
|
| Adults | 500 vs. 1500 | 588–708 |
F(1, 14) = 22.75 p < .001 |
Repeated measures ANOVAs indicate results for the indicated time windows.
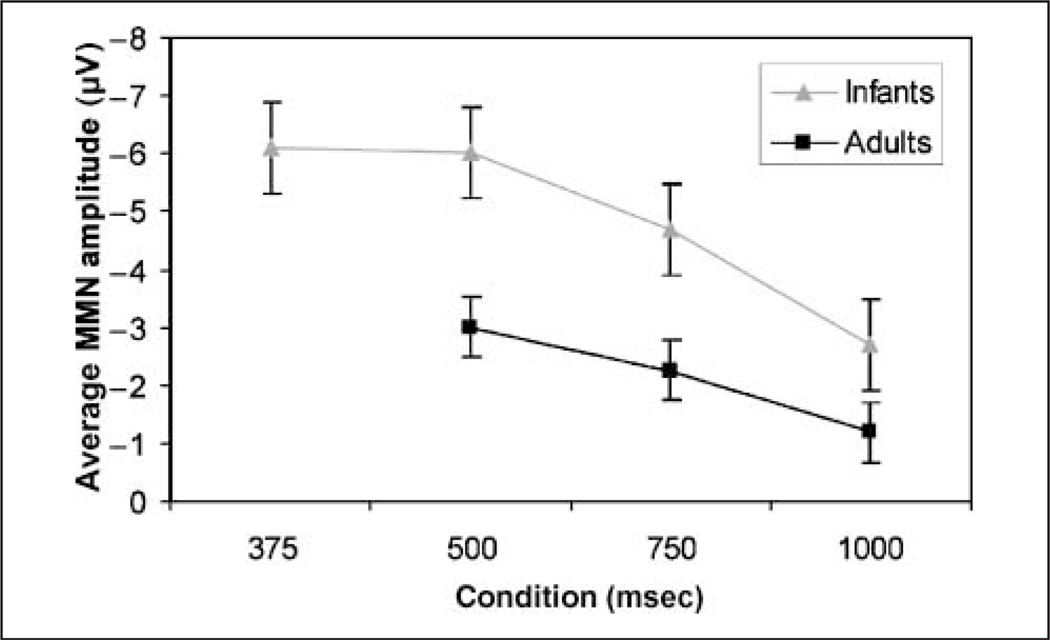
Figure 3 shows the MMN amplitude for each condition for infants and adults. For infants, the amplitude of the MMN was maximal when the deviant and standard differed most (375 and 500 vs. 1500) and was smallest for the 1000-msec condition (vs. 1500) where the standard and deviant differed by only a 2:3 ratio. A between-group t test comparing the amplitudes of the MMN for infants in the 375-msec and those in the 500-msec condition showed that these amplitudes did not differ significantly between these two conditions [t(44) = 0.18, p = .9]. However, the MMN amplitudes in both the 375 and the 500 conditions were statistically different from those in the 1000 condition [375 vs. 1000: t(44) = 2.08, p = .04; 500 vs. 1000: t(44) = 2.7, p < .01]. The amplitude of the MMN for the 750 condition did not differ significantly from the 375 and the 500 condition [375 vs. 750: t(44) = 0.40, p = .69; 500 vs. 750: t(44) = 0.66, p = .51]. There was a trend toward a significant difference between the 750 and the 1000 condition [750 vs. 1000: t(44) = 1.8, p = .07]. However, a linear regression analysis with deviant-ISI conditions of 500, 750, and 1000 revealed a small but significant positive correlation [r2 = .08, t(68) = 2.95, p < .01], demonstrating that the MMN amplitude increased as the deviation between standard and deviant increased.3
Figure 3.
The mean amplitude for the MMN for infants and adults in each condition. Mean amplitudes were extracted across the full time windows for each condition. Error bars ref lect standard errors.
Because the adults were run on all the ISI conditions, MMN differences in amplitude were tested with within-subject t tests. These analyses showed that the 500 condition exhibited an MMN with a significantly different amplitude than in the 1000 condition and the 750 condition showed a marginally significant difference compared to the 1000 condition [500 vs. 1000: t(14) = 2.65, p = .02; 750 vs. 1000: t(14) = 1.83, p = .09]. However, the MMN amplitude for the 500 and the 750 conditions did not differ significantly [t(14) = 1.09, p = .29]. Again, a linear regression with all three conditions (500, 750, and 1000) revealed a significant positive correlation [r2 = .14, t(14) = 2.62, p = .01], demonstrating that the MMN amplitude increased as the deviation between standard and deviant increased. In addition, separate linear regressions were performed for each adult to determine the slope of the function relating condition to maximum amplitude (i.e., 3 data points for each subject). A single-sample t test revealed that the slopes (beta values) were significantly different from zero [t(14) = 2.65, p = .02]. Note that this particular analysis could not be effectively done for infants because they were tested in a between-subjects design.
Following the MMN, infants exhibited an additional response of a late anterior deviance-related positivity, the magnitude of which varied by condition and was also modulated by the degree to which the deviant differed from the standard. The positivity in the difference wave was greatest for the 375 versus 1500 condition, and was no longer detectable in the 1000 versus 1500 condition. To determine the time window for which the standard and deviant waveforms differed significantly from each other, t tests were again conducted in 6-msec bins on the Adjar-corrected data (Table 1). As can be seen in Table 1, there was a significant positivity in the infants in conditions 375, 500, and 750 but not in 1000. A between-subject ANOVA comparing the three conditions that exhibited a positivity showed a main effect of condition over a time window that was common to two of the three conditions [325 to 525 msec poststimulus; F(2, 66) = 3.5, p = .04]. Post hoc t tests revealed that the 375 condition was significantly different from the 750 condition over the same intermediate time window [t(44) = 2.55, p = .01]. The 500 condition showed only a trend toward a significant difference from the 750 condition [t(44) = 1.73, p = .09]. The 375 and 500 conditions did not differ significantly from each other [t(44) = 0.98, p = .33]. A linear regression with conditions 375, 500, 750, and 1000 revealed a significant positive correlation [r2 = 0.14, t(91) = 3.87, p < .001], again demonstrating that the amplitude of the late positivity increased with increasing deviation between standard and deviant ISI.
In adults, the late positivity was only found in the 500 versus 1500 condition. t Tests on 6-msec bins of the Adjar-corrected data revealed significant differences between standard and deviant waveforms between 585 and 710 msec poststimulus (Table 1).
We had no specific predictions about differences in the topography of the MMN between the deviant conditions, and we were concerned about the validity of a between-subject comparison in the infants in the spatial distribution of the MMN due to great variability in head size and shape. We therefore did not attempt to compare the differences in distribution between different conditions for infants. However, it was of interest to compare the topographic distributions of the MMN that were observed for the infants and the adults. We therefore compared the topographic distribution of the difference wave for the adults and the infants collapsed across all the ISI conditions of Experiment 1. Figure 4 provides a comparison of the time periods that showed a significant difference between standard and deviant waveforms and that were therefore maximally sensitive to the timing deviation. The MMN in infants appeared to be more prolonged and its distribution somewhat more anterior compared to the more fronto-central distribution in adults. However, it is also possible that the apparent differences in topographic distributions of brain activity in infants and adults were due to the large variability in infants’ head shapes and sizes and the fit of the caps. In light of these considerations, and because the development of the precise spatial distribution of the MMN was not crucial to our question of whether the timing-related MMN was modulated by the ratio of the standard to deviant ISIs, we did not pursue the examination of these possible differences in topography with statistics.
Figure 4.
Topographic distributions of the difference wave for infants and adults for the time periods found to be maximally sensitive to the timing deviation. Note the difference in the amplitude scales in the four panels. Data are collapsed across all deviation conditions for each experiment.
EXPERIMENT 2
Experiment 1 found that the amplitude of the MMN increased with the difference between standard and deviant ISIs in an auditory oddball paradigm. This is consistent with the idea that the MMN exhibits scalar variability and is modulated by the ratio between the standard and deviant value. However, to confirm that it is the ratio and not the absolute difference of time intervals that is important for the amplitude of the MMN, it is also necessary to compare responses involving two sets of absolute values with the same ratio. Scalar timing theory predicts that the MMN amplitude will not differ for two sets of absolute values that have the same ratio. Accordingly, we tested a new group of 10-month-old infants in a within-subject design with two conditions at the same ratio (750 vs. 1500 msec and 500 vs. 1000 msec). These data are compared with a subset of the adults who participated in Experiment 1 who were tested on the same two conditions.
Methods
Participants
Participants were 23 infants with a mean age of 9 months 27 days (range = 9 months 10 days to 10 months 23 days) and 12 of the 15 adults tested in Experiment 1 (mean age = 23 years, SD = 5.3 years). Fourteen of the infants and five of the adults were female. Data from 38 additional infants were discarded due to either fussiness (n = 4), premature removal of the cap (n = 6), experimenter error (n = 1), or excessive artifacts (n = 27). All infants and adults were healthy with normal vision and hearing. Adult participants and parents of the infant participants gave written informed consent, and all research was approved by the Duke University Institutional Review Board.
Stimuli and Procedure
The stimuli and procedure were identical to Experiment 1 except that each infant was tested in two conditions across four miniblocks that each consisted of 198 trials. Half the infants were tested in an ABBA order and the other half in a BAAB order, where A refers to the condition with a standard of 1500 msec and a deviant of 750 msec and B refers to the condition in which the standard was 1000 msec and the deviant was 500 msec. Infants were tested until they were unable to sit calmly even if this required stopping in the midst of a miniblock (average 23 min). The procedure for the adults was identical to that used in Experiment 1.
EEG/ERP Acquisition and Analysis Methods
The EEG/ERP acquisition and analysis methods were identical to that used in Experiment 1. All data were Adjar-corrected as described for Experiment 1. After artifact rejection, individual infants had an average of 64 deviant (range = 38–109) and 408 standard trials (range = 250–657) in the 500 versus 1000 condition and 67 deviant trials (range = 43–105) and 410 standard trials (range = 251–644) in the 750 versus 1500 msec condition. After artifact rejection, adults had an average of 51 deviant (range = 42–60) and 309 standard trials (range = 260–353) in the 500 versus 1000 condition and 52 deviant (range = 42–60) and 315 standard trials (range = 271–338) in the 750 versus 1500 msec condition.
Results and Discussion
Figure 5 shows the grand-average ERPs at the Fz lead for adults and infants for both conditions (500 vs. 1000 and 750 vs. 1500). As in Experiment 1, we determined the time window for which the standard and deviant waveforms differed significantly from each other by conducting t tests in 6-msec windows on the data (after the Adjar filtering technique had removed the overlap distortion). Table 3 shows the time windows for each condition that yielded significant differences between the deviant and standard intervals for adults and infants for each condition. For the infants, the significant time window for the 750 versus 1500 condition started slightly later than the one for the 500 versus 1000 condition. For adults, the time window for the 500 versus 1000 condition was slightly smaller than the one for the 750 versus 1500 condition. Similar to our findings in Experiment 1, the time windows for infants extended later as compared to the adults (see Table 3).
Figure 5.
Deviant-minus-standard ERP difference waveforms (after Adjar overlap correction) from Experiment 2, showing two conditions for infants (left) and adults (right) with the same ratio between standard and deviant ISIs, but different absolute values. Data are from frontal electrode Fz. Negative potentials are plotted upward. Each tick mark reflects 100 msec. The MMN is visible between 100 and 200 msec for both infants and adults. In this case, with the same temporal intervals, there were no statistical differences between the deviation-related MMN responses for the conditions in either the infants or the adults.
Table 3.
Mismatch Negativity (MMN) and Late Positivity (LP) Time Windows (msec) Poststimulus for Experiment 2
| Infants | Adults | |||
|---|---|---|---|---|
| Condition | MMN | LP | MMN | LP |
| 500 vs. 1000 | 114–264 | N/A | 126–186 | 630–666 726–798 |
| 750 vs. 1500 | 192–270 | 384–474 | 126–198 | N/A |
Time windows were derived by conducting t tests in 6-msec windows comparing deviant and standard waveforms (minimum of 3 consecutive windows with p < .05).
Repeated measures ANOVAs comparing the response to the standard-ISI and the deviant-ISI stimuli at the Fz electrode for the smallest common MMN time window in each group revealed significant differences between the ERP elicited by the standard and deviant in each condition. Table 4 shows the F values for each condition. Statistical analysis over the larger MMN time windows revealed the same pattern of results. Thus, both conditions elicited an MMN in infants and adults. However, the key result was that there was no statistical difference, or even a trend, between the MMN amplitude for the two ISI conditions, both for the adults [t(11) = 0.10, p = .92] or for the infants [t(22) = 0.26, p = .80], in neither the smallest common nor the larger MMN time windows for each group.
Table 4.
Statistical Results for Experiment 2
| Condition | Time Window | ANOVA Results | |
|---|---|---|---|
| Mismatch Negativity | |||
| Infants | 500 vs. 1000 | 192–264 |
F(1, 22) = 9.33 p < .01 |
| 750 vs. 1500 | 192–264 |
F(1, 22) = 7.05 p = .01 |
|
| Adults | 500 vs. 1000 | 126–186 |
F(1, 11) = 18.67 p < .01 |
| 750 vs. 1500 | 126–186 |
F(1, 11) = 33.50 p < .001 |
|
| Late Positivity | |||
| Infants | 750 vs. 1500 | 384–474 |
F(1, 22) = 5.66 p = .03 |
| Adults | 500 vs. 1000 | 630–666 |
F(1, 11) = 7.87 p = .02 |
| 500 vs. 1000 | 726–798 |
F(1, 11) = 10.04 p < .01 |
|
Repeated measures ANOVAs indicate results for entire time windows.
Following the MMN, infants exhibited a late anterior deviance-related positivity for the deviants in the 750 versus 1500 condition but not in the 500 versus 1000 condition. Although the late anterior deviance-related positivity was only significant in one of the conditions, there was no significant difference between the two conditions in the deviance difference waves for this time window [t(22) = 0.72, p = .48]. In contrast, adults exhibited a late anterior deviance-related positivity in the 500 versus 1000 condition but not in the 750 versus 1500 condition (Table 3). For adults, however, the difference waves in the two conditions differed significantly in the two different late anterior deviance-related time windows [630–666: t(11) = 2.4, p = .04; 726–798: t(11) = 2.59, p = .03].
The two conditions (500 vs. 1000 and 750 vs. 1500) were collapsed to create topographical maps for the same time window used in Experiment 1. Figure 4 illustrates that the spatial distribution of the MMN was very similar for infants and adults between the two experiments.
GENERAL DISCUSSION
Collectively, the two experiments reported here demonstrate that 10-month-old infants and adults show a similar brain response to a change in a temporal interval separating auditory tones and that the amplitude of this response is governed by the ratio between the standard and deviant ISI. Specifically, the amplitude of the MMN, a well-known deviance-related ERP component, increased with increasing disparity between the standard and deviant duration, with this modulation following a monotonic, approximately linear function (except for the 375 condition) relative to the ratio of the timing deviations rather than their absolute differences. The fact that at the extreme end of the timing deviations, assessed in Experiment 1, there was an almost complete overlap in the infant MMN to the 375-msec and 500-msec condition (relative to the standard 1500 msec interval) suggests that there may be an upper boundary for the amplitude of the MMN and that increased sensitivity to changes beyond a 1:4 ratio cannot be observed with this measure (see Näätänen et al., 1993).
In Experiment 2, when two sets of timing deviations with different absolute values but the same ratio were tested, there was no significant difference in the amplitude of the MMN for infants or adults, suggesting that the MMN amplitude is dependent on ratio rather than linear difference. One might argue that this conclusion is unwarranted because it is based on a null result in Experiment 2 and because this result is compared with data from Experiment 1, which did not show statistical differences between all adjacent conditions. However, we did find a clear significant linear regression indicating that the 750 condition was, in fact, intermediate between the 500 and 1000 conditions for both infants and adults. In contrast, when the two conditions with constant ratio in Experiment 2 were compared for infants and adults with within-subjects t tests, the p values were .92 and .80, respectively, demonstrating that there was not even a trend toward these two ERPs differing.
Thus, overall, the results of Experiments 1 and 2 suggest that the MMN is modulated as a function of the ratio between the deviant to standard ISI. This effect was remarkably similar in infants and adults. However, it should be noted that there were some slight differences in the timing, amplitude, and distribution of the MMN in adults and infants. Consistent with a previous report from our group, the MMN started at approximately the same time in infants and adults but was statistically significant for a longer duration in infants. In addition, both the MMN and the later, anterior, deviance-related scalp-positive ERP wave appeared to be larger in infants than in adults.
In contrast to the MMN response, the late anterior deviance-related positivity appeared to vary as a function of the ratio of the standard to deviant durations in infants but not in adults. In Experiment 1, the amplitude of this component decreased with decreasing difference between standard and deviant ISI for infants, a pattern not observed in adults. Moreover, the late anterior deviance-related positivity only appeared in some of the adult conditions and was very small. In Experiment 2, the late anterior deviance-related positivity in infants did not differ in the two conditions that shared a common ratio but differed in absolute values. Adults, however, did show a significant difference in the two conditions, although this was mainly due to there being no detectable anterior deviance-related positivity in one of the two conditions. The variability and small size of this late positivity suggests that future work is necessary to establish the functional characteristics of this longer-latency deviance-related response.
Our electrophysiological results are consistent with recent behavioral work on the timing abilities of human infants. Using looking-time measures, vanMarle and Wynn (2006) demonstrated that at 6 months, infants require a 1:2 ratio change in the duration of an event to detect a difference. In addition, Brannon et al. (in press) have recently replicated vanMarle and Wynn’s finding that 6-month-old infants required a twofold change in duration for discrimination and further found that by 10 months of age infants were able to discriminate a 2:3 ratio change in duration but failed to discriminate a 3:4 ratio in duration. Thus, the fact that the 2:3 ratio condition in Experiment 1 elicited a significant but small MMN in 10-month-old infants is consistent with the claim that 10-month-old infants can successfully discriminate this ratio.
Although our finding that the MMN is similarly modulated by the ratio between standard and deviant durations for infants and adults suggests a common mechanism for timing-related change detection over development, some cautions must be raised. The MMN is thought to be generated by neural activity mainly in the auditory cortex, with possibly some contribution from the right frontal cortex (Tse & Penney, 2007; Tse, Tien, & Penney, 2006; Molholm, Martinez, Ritter, Javitt, & Foxe, 2005; Rinne, Degerman, & Alho, 2005; Alho, 1995; Giard, Perrin, Pernier, & Bouchet, 1990). Investigations into the neural bases of time perception suggest that the basal ganglia, the prefrontal cortex, and the posterior parietal cortex are of particular importance for interval-timing tasks and for those that require the integration of somatosensory signals or number processing (Buhusi & Meck, 2005; Meck & Benson, 2002). Thus, the observed neural response to temporal deviations may not reflect neural activity specific to timing but, instead, the subsequent cortical activity in response to the detection of an auditory deviation, which in this case is due to a temporal interval change.
Accordingly, although we can conclude that the brain electrical activity indexed by the MMN indicates that the human brain, both in adults and in 10-month-old children, rapidly detects a temporal change in its environmental stimulation, the precise relationship of this response to the brain circuits performing the timing calculations is not yet known. One interesting next step in this research would be to use larger electrode arrays and source analyses to isolate the neural generators specific to detection of temporal deviations and compare these to the generators implicated when infants or adults hear deviations in other auditory features such as pitch or amplitude.
In summary, our previous report (Brannon et al., 2004) demonstrated that by 10 months of age the infant brain could discriminate a threefold change in duration and that the MMN to duration changes is remarkably similar in adults and infants. Our current report demonstrates that by 10 months of age the infant brain can discriminate a 2:3 ratio change in duration. Moreover, our findings suggest that the amplitude of the MMN is governed by the ratio of the standard interval to deviant interval and not the absolute distance between them. Thus, this suggests that by 10 months of age the infant brain is timing intervals according to the same principles that guide adult timing. Future work should explore whether ratio dependence holds at even younger ages and whether the MMN can track increasing sensitivity in timing over development.
Acknowledgments
We thank Lauren Wolfe, Tatiana Gautier, Klaus Libertus, Laura Pruitt, Anna Nehring, Umay Suanda, and Nicole Nelson for help in collecting the data. We are also grateful to Sara Cordes, Laura Busse, Tineke Grent-’t-Jong for help in data analyses and Sara Cordes for comments on a draft of the paper. This research was supported by R03 MH64955 and a Merck Scholar Award to E.M.B. The adult studies were also supported by R01 MH60415 to M.G.W.
Footnotes
A portion of these data was presented at the annual meeting of the Society for Neuroscience, 2005, and at the annual meeting of the Cognitive Neuroscience Society, 2005.
Note that although most prior studies of the MMN elicited by duration deviants have used stimulus durations in the tens to a few hundred milliseconds range (e.g., Tse & Penney, 2006; Jacobsen & Schröger, 2003; Sable et al., 2003; Jaramillo et al., 2000), there are now several reports in the literature of MMN effects to deviations that occur in the multiple hundreds of milliseconds to seconds range (e.g., Brannon et al., 2004; Näätänen, Syssoeva, & Takegata, 2004). These findings for longer durations are important for establishing the validity of the present design, which employs ISIs that range from 375 to 1500 msec, because earlier work had suggested that when the ISI exceeds a putative temporal window of integration, an MMN to a deviant interval is not elicited (Kujala, Kallio, Tervaniemi, & Näätänen, 2001; Yabe, Tervaniemi, Reinikainen, & Näätänen, 1997).
Infants were kept in the final sample if at least 33% of trials were free of artifacts. Trials with artifacts (those in which the infant blinked or had excessive muscle movements) were excluded by a computer-based algorithm that rejected trials in which the peak-to-peak amplitude exceeded a given threshold.
The r2 is small but remains significant if the 375 condition is included [r2 = .05, t(91) = 2.24, p < .05].
REFERENCES
- Alho K. Cerebral generators of mismatch negativity (MMN) Ear and Hearing. 1995;16:38–51. doi: 10.1097/00003446-199502000-00004. [DOI] [PubMed] [Google Scholar]
- Brannon EM, Suanda S, Libertus K. Increasing precision in temporal discriminations over development parallels the development of number discrimination. Developmental Science. doi: 10.1111/j.1467-7687.2007.00635.x. (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brannon EM, Wolfe Roussel L, Meck WH, Woldorff M. Timing in the baby brain. Cognitive Brain Research. 2004;21:227–233. doi: 10.1016/j.cogbrainres.2004.04.007. [DOI] [PubMed] [Google Scholar]
- Buhusi CV, Meck WH. What makes us tick? Functional and neural mechanisms of interval timing. Nature Reviews Neuroscience. 2005;6:755–765. doi: 10.1038/nrn1764. [DOI] [PubMed] [Google Scholar]
- Clifton RK. Hear rate conditioning in the newborn infant. Journal of Experimental Child Psychology. 1974;18:9–21. doi: 10.1016/0022-0965(74)90084-8. [DOI] [PubMed] [Google Scholar]
- Colombo J, Richman WA. Infant timekeeping: Attention and temporal estimation in 4-month-olds. Psychological Science. 2002;13:475–479. doi: 10.1111/1467-9280.00484. [DOI] [PubMed] [Google Scholar]
- Dale CL, Grafton G, Gibbon J. Event-related brain potentials isolate the motor component in a tapping task. NeuroReport. 2001;12:3015–3018. doi: 10.1097/00001756-200110080-00007. [DOI] [PubMed] [Google Scholar]
- DeBoer T, Scott LS, Nelson CA. ERPs in developmental populations. In: Handy TC, editor. Event-related potentials: A methods handbook. Cambridge: MIT Press; 2005. [Google Scholar]
- Droit-Volet S. Scalar timing in temporal generalization in children with short and long stimulus durations. Quarterly Journal of Experimental Psychology: Series A. 2002;55A:1193–1209. doi: 10.1080/02724980244000161. [DOI] [PubMed] [Google Scholar]
- Droit-Volet S, Clement A, Wearden J. Temporal generalization in 3- to 8-year-old children. Journal of Experimental Child Psychology. 2001;80:271–288. doi: 10.1006/jecp.2001.2629. [DOI] [PubMed] [Google Scholar]
- Droit-Volet S, Meck WH, Penney TB. Sensory modality and time perception in children and adults. Behavioural Processes. 2007;74:244–250. doi: 10.1016/j.beproc.2006.09.012. [DOI] [PubMed] [Google Scholar]
- Droit-Volet S, Wearden J. Temporal bisection in children. Journal of Experimental Child Psychology. 2001;80:142–159. doi: 10.1006/jecp.2001.2631. [DOI] [PubMed] [Google Scholar]
- Gallistel CR. The organization of learning. Cambridge: MIT Press; 1990. [Google Scholar]
- Giard MH, Perrin F, Pernier J, Bouchet P. Brain generators implicated in processing of auditory stimulus deviance: A topographic study. Psychophysiology. 1990;27:627–640. doi: 10.1111/j.1469-8986.1990.tb03184.x. [DOI] [PubMed] [Google Scholar]
- Gibbon J, Allan L, editors. Timing and time perception. Vol. 423. New York: New York Academy of Sciences; 1984. [Google Scholar]
- Gibbon J, Church RM, Meck WH. Scalar timing in memory. Annals of the New York Academy of Sciences. 1984;423:52–77. doi: 10.1111/j.1749-6632.1984.tb23417.x. [DOI] [PubMed] [Google Scholar]
- Gibbon J, Malapani C, Dale CL, Gallistel CR. Toward a neurobiology of temporal cognition: Advances and challenges. Current Opinion in Neurobiology. 1997;7:170–184. doi: 10.1016/s0959-4388(97)80005-0. [DOI] [PubMed] [Google Scholar]
- Jacobsen T, Schröger E. Measuring duration mismatch negativity. Clinical Neurophysiology. 2003;114:1133–1143. doi: 10.1016/s1388-2457(03)00043-9. [DOI] [PubMed] [Google Scholar]
- Jaramillo M, Paavilainen P, Näätänen R. Mismatch negativity and behavioural discrimination in humans as a function of the magnitude of change in sound duration. Neuroscience Letters. 2000;290:101–104. doi: 10.1016/s0304-3940(00)01344-6. [DOI] [PubMed] [Google Scholar]
- Kujala T, Kallio J, Tervaniemi M, Näätänen R. The mismatch negativity as an index of temporal processing in audition. Clinical Neurophysiology. 2001;112:1712–1719. doi: 10.1016/s1388-2457(01)00625-3. [DOI] [PubMed] [Google Scholar]
- Lipton JS, Spelke ES. Origins of number sense. Psychological Science. 2003;14:396–401. doi: 10.1111/1467-9280.01453. [DOI] [PubMed] [Google Scholar]
- Mäntysalo S, Näätänen R. The duration of a neuronal trace of an auditory, stimulus as indicated by event-related potentials. Biological Psychology. 1987;24:183–195. doi: 10.1016/0301-0511(87)90001-9. [DOI] [PubMed] [Google Scholar]
- Matell MS, Meck WH. Neuropsychological mechanisms of interval timing behaviour. BioEssays. 2000;22:94–103. doi: 10.1002/(SICI)1521-1878(200001)22:1<94::AID-BIES14>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Meck WH. Functional and neural mechanisms of interval timing. New York: CRC Press; 2003. [DOI] [PubMed] [Google Scholar]
- Meck WH, Benson AM. Dissecting the brain’s internal clock: How frontal-striatal circuitry keeps time and shifts attention. Brain and Cognition. 2002;48:195–211. doi: 10.1006/brcg.2001.1313. [DOI] [PubMed] [Google Scholar]
- Melgire M, Ragot R, Samson S, Penney TB, Meck WH, Poulhas V. Auditory/visual duration bisection in patients with left or right medial-temporal lobe resection. Brain and Cognition. 2005;58:119–124. doi: 10.1016/j.bandc.2004.09.013. [DOI] [PubMed] [Google Scholar]
- Molholm S, Martinez A, Ritter W, Javitt DC, Foxe JJ. The neural circuitry of pre-attentive auditory change-detection: An fMRI study of pitch and duration mismatch negativity generators. Cerebral Cortex. 2005;15:545–551. doi: 10.1093/cercor/bhh155. [DOI] [PubMed] [Google Scholar]
- Näätänen R. The role of attention in auditory information processing as revealed by event-related potentials and other brain measures of cognitive function. Behavioral, and Brain Science. 1990;13:201–288. [Google Scholar]
- Näätänen R, Jiang D, Lavikainen J, Reinikainen K, Paavilainen P. Event-related potentials reveal a memory trace for temporal features. NeuroReport. 1993;5:310–312. doi: 10.1097/00001756-199312000-00033. [DOI] [PubMed] [Google Scholar]
- Näätänen R, Syssoeva O, Takegata R. Automatic time perception in the human brain for intervals ranging from milliseconds to seconds. Psychophysiology. 2004;41:660–663. doi: 10.1111/j.1469-8986.2004.00182.x. [DOI] [PubMed] [Google Scholar]
- Näätänen R, Winkler I. The concept of auditory stimulus representation in cognitive neuroscience. Psychological Bulletin. 1999;125:826–859. doi: 10.1037/0033-2909.125.6.826. [DOI] [PubMed] [Google Scholar]
- Nordby H, Roth WT, Pfefferbaum A. Event-related potentials to time-deviant and pitch-deviant tones. Psychophysiology. 1988;25:249–261. doi: 10.1111/j.1469-8986.1988.tb01238.x. [DOI] [PubMed] [Google Scholar]
- Rattat AC, Droit-Volet S. Variability in 5- and 8-year-olds’ memory for duration and interfering task in temporal bisection. Behavioral Processes. 2001;55:81–91. doi: 10.1016/s0376-6357(01)00168-1. [DOI] [PubMed] [Google Scholar]
- Reynolds GD, Richards JE. Familiarization, attention, and recognition memory in infancy: An ERP and cortical source localization study. Developmental Psychology. 2005;41:598–615. doi: 10.1037/0012-1649.41.4.598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinne T, Degerman A, Alho K. Superior temporal and inferior frontal cortices are activated by infrequent sound duration decrements: An fMRI study. Neuroimage. 2005;26:66–72. doi: 10.1016/j.neuroimage.2005.01.017. [DOI] [PubMed] [Google Scholar]
- Sable JJ, Gratton G, Fabiani M. Sound presentation rate is represented logarithmically in human cortex. European Journal of Neuroscience. 2003;17:2492–2496. doi: 10.1046/j.1460-9568.2003.02690.x. [DOI] [PubMed] [Google Scholar]
- Schroger E. The influence of stimulus intensity and inter-stimulus interval on the detection of pitch and loudness changes. Electroencephalography and Clinical Neurophysiology: Evoked Potentials. 1996;100:517–526. [PubMed] [Google Scholar]
- Trainor LJ, Samuel SS, Desjardins RN, Sonnadara RR. Measuring temporal resolution in infants using mismatch negativity. NeuroReport. 2001;12:2443–2448. doi: 10.1097/00001756-200108080-00031. [DOI] [PubMed] [Google Scholar]
- Tse CY, Penney TB. Pre-attentive timing of empty intervals is from marker offset to onset. Psychophysiology. 2006;43:172–179. doi: 10.1111/j.1469-8986.2006.389.x. [DOI] [PubMed] [Google Scholar]
- Tse C-Y, Penney TB. Preattentive change detection using the event-related optical signal—Optical imaging of cortical activity elicited by unattended temporal deviants. IEEE Engineering in Medicine and Biology Magazine. 2007;26:52–58. doi: 10.1109/memb.2007.384096. [DOI] [PubMed] [Google Scholar]
- Tse C-Y, Tien K-R, Penney TB. Event-related optical imaging reveals the temporal dynamics of right temporal and frontal cortex activation in pre-attentive change detection. Neuroimage. 2006;29:314–320. doi: 10.1016/j.neuroimage.2005.07.013. [DOI] [PubMed] [Google Scholar]
- vanMarle K, Wynn K. Six-month-old infants use analog magnitudes to represent duration. Developmental Science. 2006;9:F41–F49. doi: 10.1111/j.1467-7687.2006.00508.x. [DOI] [PubMed] [Google Scholar]
- Wearden JH, Bray S. Scalar timing without reference memory? Episodic temporal generalization and bisection in humans. Quarterly Journal of Experimental Psychology. 2001;54B:289–309. doi: 10.1080/02724990042000173. [DOI] [PubMed] [Google Scholar]
- Woldorff MG. Distortion of ERP averages due to overlap from temporally adjacent ERPs: Analysis and correction. Psychophysiology. 1993;30:98–119. doi: 10.1111/j.1469-8986.1993.tb03209.x. [DOI] [PubMed] [Google Scholar]
- Xu F, Spelke ES. Large number discrimination in 6-month-old infants. Cognition. 2000;74:B1–B11. doi: 10.1016/s0010-0277(99)00066-9. [DOI] [PubMed] [Google Scholar]
- Yabe H, Tervaniemi M, Reinikainen K, Näätänen R. Temporal window of integration revealed by MMN to sound omission. NeuroReport. 1997;8:1971–1974. doi: 10.1097/00001756-199705260-00035. [DOI] [PubMed] [Google Scholar]