Abstract
Sleep fragmentation, a symptom in many clinical disorders, leads to cognitive impairments. To investigate the mechanisms by which sleep fragmentation results in memory impairments, rats were awakened once every 2 min via 30 s of slow movement on an automated treadmill. Within 1 h of this sleep interruption (SI) schedule, rats began to sleep in the 90-s periods without treadmill movement. Total non-rapid eye movement sleep (NREM) sleep time did not change over the 24 h of SI, although there was a significant decline in rapid eye movement sleep (REM) sleep and a corresponding increase in time spent awake. In the SI group, the mean duration of sleep episodes decreased and delta activity during periods of wake increased. Control rats either lived in the treadmill without movement (cage controls, CC), or had 10-min periods of movement followed by 30 min of non-movement allowing deep / continuous sleep (exercise controls, EC). EC did not differ from baseline in the total time spent in each vigilance state. Hippocampal long-term potentiation (LTP), a long-lasting change in synaptic efficacy thought to underlie declarative memory formation, was absent in rats exposed to 24 and 72 h SI. In contrast, LTP was normal in EC rats. However, long-term depression and paired-pulse facilitation were unaltered by 24 h SI. Twenty-four hour SI also impaired acquisition of spatial learning in the hippocampus-dependent water maze test. Twenty-four hour SI elevated plasma corticosterone (CORT) to levels previously shown to enhance LTP (125 ng / mL). The results suggest that sleep fragmentation negatively impacts spatial learning. Loss of N-methyl-D-aspartate (NMDA) receptor-dependent LTP in the hippocampal CA1 region may be one mechanism involved in this deficit.
Keywords: EEG, LTD, LTP, sleep disorders, water maze
Introduction
Sleep fragmentation is a common symptom in several clinical disorders including restless leg syndrome (Rosenthal et al., 1984; Bastuji & Garcia-Larrea, 1999; Sforza et al., 1999; Saletu et al., 2000), depression (Jones et al., 1987; Perlis et al., 1997), post-traumatic stress disorder (Mellman et al., 1995; Ohayon & Shapiro, 2000), narcolepsy (Mamelak et al., 1979; Zorick et al., 1986; Tafti et al., 1992), and obstructive sleep apnea (Roehrs et al., 1985; Kimoff, 1996; Moore et al., 2001). Sleep fragmentation interferes with the architecture of normal sleep, reduces deep sleep, and impairs the restorative / cognitive benefits of sleep via, as yet, unidentified alterations in neural processing (Bonnet, 2005).
Excessive daytime sleepiness induced by experimental sleep fragmentation in normal humans has been shown to result in significant cognitive impairments even though total sleep time may not be greatly diminished (Bonnet, 1987; Stepanski, 2002). In clinical populations with severe sleep fragmentation, such as sleep apnea, total sleep time also typically diminishes only slightly (Coleman et al., 1982). Hence, unlike total sleep deprivation, it is the frequent arousals and restructuring of sleep caused by sleep fragmentation that are thought to underlie the excessive daytime sleepiness and neurocognitive impairments, rather than loss of sleep time per se. Accordingly, it has been proposed that sleep must continue uninterrupted for a minimum length of time in order for sleep to produce its full restorative effects that lead to optimal daytime vigilance and neurocognitive function (Franken, 2002; Stepanski, 2002; Bonnet, 2005).
Although many previous studies have examined the effects of total sleep deprivation in animals (Everson, 1995; Rechtschaffen & Bergmann, 1995), few animal studies have used an experimental sleep interruption (SI) paradigm to model sleep fragmentation. Here we used the gentle movement of a treadmill device to awaken rats 30 times per h for 24 or 72 h, a frequency of sleep fragmentation typically observed in sleep apnea (Wiegand & Zwillich, 1994) to generate a novel animal model of the sleep fragmentation occurring in sleep disorders.
The hippocampus is a critical structure for many cognitive / memory processes and has been reported to be especially sensitive to sleep loss (Blissitt, 2001; Guan et al., 2004; Ruskin et al., 2004). In the present experiments, we assessed the effects of sleep fragmentation on both behavioural and electrophysiological measures of hippocampal function. For the electrophysiological studies we looked for changes in hippocampal long-term potentiation (LTP) and its counterpart long-term depression (LTD). These exogenously induced forms of synaptic plasticity are commonly accepted as a model of hippocampal memory processes (Shapiro & Eichenbaum, 1999; Martin & Shapiro, 2000; Braunewell & Manahan-Vaughan, 2001). For the behavioural assessment of hippocampal-dependent memory we used the water maze task, a spatial memory task that is dependent on hippocampal function (Morris et al., 1982; Sutherland et al., 1983; Ferbinteanu et al., 1999). Hippocampal synaptic plasticity has been shown to be sensitive to changes in stress as defined by elevations in circulating corticosterone (CORT). Accordingly, we also measured plasma CORT after SI, in order to determine the potential effects of CORT on our findings.
Materials and methods
Animals
Young (30–50-days old) male Sprague–Dawley (Charles River Breeding Laboratories, Wilmington, MA) rats were used for electroencephalography (n = 8) and electrophysiology (n = 23) procedures. Adult (225–275 g) male Sprague–Dawley (Charles River Breeding Laboratories, Wilmington, MA) rats were used for the water maze task (n = 32). To control for non-specific influences that the EEG / EMG cables might have had on sleep parameters, four rats [(2 SI and 2 exercise controls (EC)] that underwent the polysomnographic recordings were used to replicate findings in the LTP experiment. Rats were housed under constant temperature (23 °C) and 12-h light : 12-h dark cycle (light-on period from 10:00 hours to 22.00 hours) with food and water available ad libitum. All animals were treated in accordance with the American Association for Accreditation of Laboratory Animal Care’s policy on care and use of laboratory animals. All procedures were approved by the institutional animal care and use committee (IACUC) of the Boston VA Healthcare System.
Sleep interruption procedure
Rats lived in a treadmill cage (l × w × h; 50.8 cm × 16.51 cm × 30.48 cm) in which the floor is a horizontal belt automatically programmed to move slowly at a rate of 0.02 m/ s, which is a speed we have determined to reliably produce awakenings. The treadmill ran at this slow speed for 30 s, followed by no treadmill movement for 90 s. This 30 s on / 90 s off schedule produced 30 interruptions of sleep per hour continuously for the 24 or 72 h of SI exposure. In order to habituate the rats to the treadmill movement the treadmills were turned on (5 min treadmill on followed by 5 min off) for 1 h on each of the 2 days prior to the experiment. As a control for the non-specific effects of locomotor activity, an EC group was included in this study. In this group, rats obtained an equivalent amount of treadmill movement / exercise, but with a treadmill on / off schedule of 10 min on / 30 min off, allowing for longer periods of undisturbed sleep.
Polysomnographic recordings
Electroencephalograph (EEG) and electromyograph (EMG) surgery was carried out under general anaesthesia (sodium pentobarbital, 65 mg / kg, i.p.). The rats were also given ketofen (5 mg / kg, s.c.) as an analgesic following surgery. Bilateral screw electrodes (Plastics One Inc. Roanoke, VA) were fixed onto the skull above the temporal cortex (2 mm caudal to bregma and 4 mm lateral to the mid sagittal suture) for recording EEG. EMG electrodes, which consisted of flexible stainless steel wires insulated with nylon except for a 1.62 mm suture pad ending (Plastics One Inc.) were fixed onto the superior nuchal muscles. After at least 7 days of recovery from surgery, the rats were placed in the treadmill apparatus and hooked up to the EEG cables (Plastics One, Inc.). Rats received 5 days of ‘cable training’, where they habituated to living on the treadmills with the electrode cables and the movement of the cables with an overhead swivel. On days 4 and 5, the rats received the treadmill movement habituation described above. On day 6, the baseline EEG / EMG was recorded for 24 h. On day 7, EEG and EMG activity was recorded for 24 h of SI or EC treatment. The treadmills were turned on at lights on and turned off 24 h later. A Grass Model, 15 polygraph with model 15A4 amplifiers (Grass-Telefactor, West Warwick, RI) was used for all EEG and EMG data collection. Behaviour was classified into three different states by means of EEG and EMG analysis: wakefulness (W), non-REM sleep (NREMS, also referred to as slow wave sleep), and rapid eye movement sleep (REMS). Grass Rodent Sleep Stager (RSS) V3.0 (Grass-Telefactor) was used for off-line EEG and EMG analysis. The full 24 h of recordings were visually scored in 10 s epochs as previously described (Thakkar et al., 1999; Thakkar et al., 2001).
Hippocampal slice electrophysiology
For the electrophysiology experiments, the treadmills were turned on at lights on and turned off 24 or 72 h later. Immediately after SI or EC treatment, rats were deeply anaesthetized with isoflurane and decapitated. After decapitation, the brains were removed and cut into a block containing both hippocampi. Horizontal slices (400-μm thickness) were cut using a vibroslicer (Campden Instruments, LTD, Lafayette, Indiana) in ice cold artificial cerebrospinal fluid (ACSF) that contained (in mM): sucrose, 209; KCl, 1.8; KH2PO4, 1.2, MgSO4, 3.3; CaCl2, 0.6; NaHCO3, 25.6; D-glucose, 10 (previously bubbled with 95% O2, 5% CO2). Slices were then placed in ACSF that contained (in mM): NaCl, 124; KCl, 1.8; KH2PO4, 1.2; MgSO4·7H2O, 1.3; CaCl2·2H2O, 2; NaHCO3, 25.6 and D-glucose, 10, and bubbled with 95% O2 and 5% CO2 at room temperature for at least 1 h. Slices were tested in a recording chamber that was constantly perfused with ACSF continuously oxygenated with 95% O2 and 5% CO2 at 33 °C. Constant voltage, rectangular, biphasic, stimulus pulses (0.2 ms) were delivered by an isolated pulse stimulator (Model 2100, A–M systems, Carlsborg, WA) through a glass electrode filled with ACSF and placed on the stratum radiatum of the CA1 region of the hippocampus to stimulate Schaffer collateral fibers. Field excitatory postsynaptic potentials (fEPSPs) were recorded with a glass micropipette filled with ACSF and placed in stratum radiatum. Signals were amplified by a microelectrode amplifier (Axoclamp 2-B, Axon Instruments, Union City, CA), digitized (Digidata 1322A, Axon Instruments), filtered at 10 kHz, and sampled at 20 kHz. The initial slope of the fEPSP was used as a measure of synaptic strength. An input–output (I–O) voltage curve was obtained by recording averaged responses at 10-V increments, starting at threshold and ending at saturation. Saturation was reached when two sequential stimulus intensities no longer produced increases in fEPSP slopes (did not vary by more than 5%). Each I–O pulse was separated by 30 s.
Stimulus strength was adjusted to evoke potentials with a slope equal to 50% of the maximum response obtained in the I–O curve. Prior to each experimental paired-pulse facilitation (PPF), long-term potentiation (LTP), or long-term depression (LTD) manipulation baseline test pulses were recorded for 15 min to ensure that the fEPSPs were stable. PPF experiments were conducted in slices that were afterward used for LTP experiments. fEPSP responses to paired pulses at a 50 ms and 100 ms interpulse interval (IPI) were recorded. LTP was induced by administering three trains of high frequency (100 Hz) pulses with a 30-s intertrain interval. In slices from a separate group of rats, LTD was induced by administering 900 low frequency (1 Hz) paired-pulses (200 ms IPI). Both LTP and LTD stimulation induction parameters have been demonstrated to be N-methyl-D-aspartate (NMDA) receptor dependent (Herron et al., 1986; Kemp & Bashir, 1999). One hour of test pulses were then recorded to observe any long-term changes in fEPSP after LTP / LTD stimulation. PCLAMP 9.0 software (Axon instruments) was used for data acquisition and off-line analyses. fEPSP slopes in all groups were normalized prior to statistical analysis.
Water maze
In the water maze (WM) protocol each rat underwent three blocks of four trials separated by a 30-min period. This version of the WM task allows rats to be fully trained in approximately 3 h (Frick et al., 2000), which is necessary for manipulations that cannot be given on multiple days, such as 24-h sleep fragmentation. Rats were tested in the water maze during the last two hours of the 12 h lights-on period. On each trial, rats were placed in the WM facing the wall in one of three quadrants that did not contain the hidden platform. The starting position was in a semi-random order so that no start point was repeated and no point was used more than three times. The location of the hidden platform remained constant. If the animal did not find the hidden platform within 60 s, the rat was placed on the platform by the experimenter for approximately 10 s before being placed in a holding cage for an additional 60 s. A video tracking system (EzVideo Multi Track System, AccuScan, Columbus, OH) was utilized to record rodent behaviour in the water maze. To test if rats used a spatial strategy to learn the task, a probe trial was given 30 min after the last swim in which the platform was removed and each rat had a 30-s free swim in the water maze. Following the probe trial, rats completed a visible platform version of the water maze, in which a flag was placed on the platform and rats were allowed four, 60-s trials to find the location of the platform. In each trial, the location of the platform and start position was moved to a new position in the pool. Between trial blocks rats were group housed in a dry cage. SI rats were observed during the 30 min inter-trial intervals in order to prevent sleep with gentle handling and sensory stimulation; however, the SI rats engaged primarily in grooming and social interaction during this period.
Coritcosterone analysis
For the CORT analyses, the treadmills were turned on at lights on and off at lights on either the following day (24 h) or 72 h later. All rats were killed within 1 h after lights on. Immediately after the treadmills were turned off for SI or EC (or at the equivalent time of day for naïve control groups) each rat was rapidly decapitated. Trunk blood was collected from each rat (~6 mL per rat) into polyethylene tubes on ice containing 600 μL (Na2-EDTA) at 20 μg / mL. Blood samples were centrifuged at 4 °C for 7 min at 1000 × g. The plasma fraction was isolated, aliquotted, and frozen at −80 °C. Plasma corticosterone was shipped on dry ice to the Cornell University Animal Health Diagnostic Laboratory for quantification of plasma corticosterone levels.
Results
Sleep interruption decreased sleep episode duration and increased delta activity
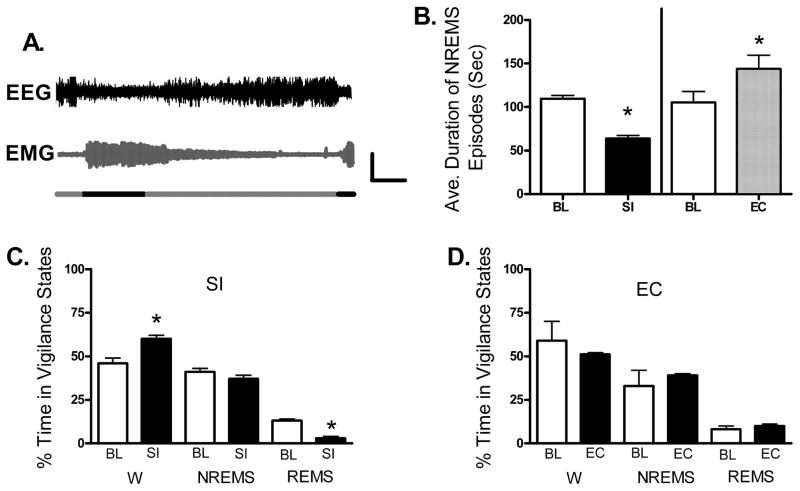
The purpose of the SI protocol utilized here, wherein the rats are awakened 30 times / h, was to mimic the frequency of sleep fragmentation observed in human clinical disorders, such as sleep apnea. Potential differences in the polysomnographic measures between the baseline, SI, or EC treatments were evaluated with paired sample t-tests. Here, in both SI and EC treatments, the rats began to sleep in the undisturbed periods within 1 h of SI onset (10:00 h), as demonstrated by high-amplitude EEG activity and reduced muscle tone in the EMG. Figure 1A shows 2 min of compressed EEG and EMG data from a representative rat illustrating that the rats learn to sleep when the treadmill is off (as shown by reduced EMG activity and increased amplitude, slow EEG activity) and are awake when the treadmill is on (as shown by increased EMG activity and reduced amplitude, fast EEG activity). The average duration of individual NREM sleep episodes was greatly reduced compared to baseline for the 24 h of SI (from 109 s to 63 s; t4 = 5.94, P < 0.05, Fig. 1B), indicating that SI rats were unable to have long durations of consolidated sleep. In contrast, the EC group actually showed the opposite, i.e. an increase in sleep episode duration compared to baseline (from 105 s to 143 s; (t2 = −4.64, P < 0.05, Fig. 1B), presumably because the EC rats were forced to concentrate their sleep in the 30 min the treadmill was off. Figure 1C shows that compared to their own baseline, rats in the SI group had no statistically significant change in total NREMS (from 41% to 37%; t4 = 0.93, P > 0.05) although they had a significant increase in W (from 46% to 60%; t4 = 2.99, P < 0.05) and decreased REMS (from 13% to 3%; t4 = −10.13, P < 0.05). In the EC rats, compared to their own baseline, there was no change in per cent of time spent in any of the three vigilance states (Fig. 1D).
Fig. 1.
SI decreases sleep episode duration and increases the time spent awake, producing a sleep pattern resembling that of sleep fragmentation observed clinically. Sleep was interrupted (fragmented) for 24 h in the sleep interrupted (SI) group by automated treadmills (TM) operating on a continuous schedule of 30 s on and 90 s off (n = 5). Rats in the exercise control (EC) group were exposed to 10 min of TM on with 30 min of TM off (n = 3). (A) EEG and EMG activity from a rat on a SI schedule. When the TM is on (black horizontal bars), there is increased amplitude EMG activity and decreased amplitude, fast EEG activity. When the TM is off (grey horizontal bars) there is reduced EMG activity and high amplitude, slow EEG activity indicative of NREM sleep. Vertical and horizontal calibration bars indicate 100 μV and 15 s, respectively. (B) Compared to baseline (BL) recordings, SI decreases the average duration of individual sleep episodes, while the EC treatment increases the average duration of sleep episodes. In C and D, graphs show the percentage of time spent in wakefulness (W), non-rapid eye movement sleep (NREMS), and rapid eye movement sleep (REMS) in the SI, EC, and CC conditions. (C) During SI the percentage of time awake increases, the percentage of time in REMS decreases and NREMS time does not change. (D) EC does not affect the percentage of time spent in any vigilance state. In all graphs asterisks indicate P < 0.05 (t-test vs. BL).
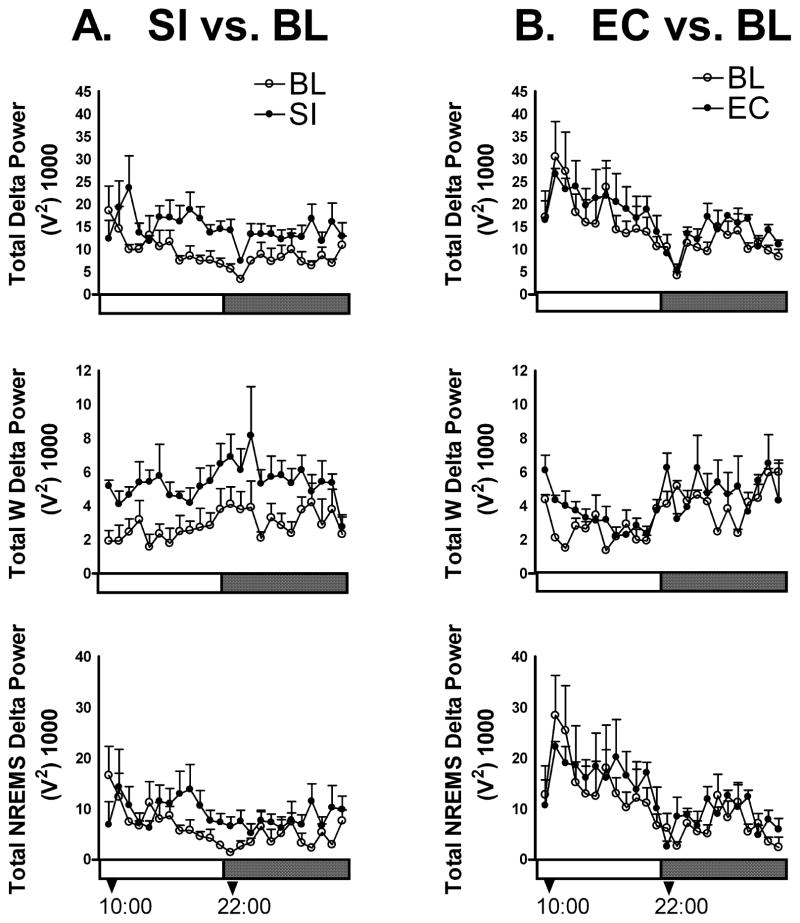
Figure 2 shows the change in delta power (1–4 Hz), a measure of homeostatic sleep pressure during 24-h SI (left column). A robust increase in the EEG delta power during SI exposure was clearly visible at all time points when the animals were awake (Fig. 2A, middle). Repeated measures ANOVA revealed a main effect of treatment (F1,8 = 7.022, P < 0.05). During periods of NREM sleep (Fig. 2A, bottom) the mean EEG delta power was not uniformly elevated throughout the 24-h period of SI exposure (main effect of treatment F1,8 = 1.30, NS). However, the treatment–time interaction for NREM delta was significant (F23, 184 = 2.30; P < 0.05 with sphericity assumed, and P = 0.054 using Huynh-Feldt correction). An analysis of the individual data revealed that this interaction was primarily due to the NREM delta elevation in two of the five rats that can be seen in the second half of the light period (Fig. 2A, bottom). During EC exposure, there was no change in the waking or NREM EEG delta power (Fig. 2B).
Fig. 2.
Delta power is increased during SI. (A) Sleep interruption (SI) (n = 5) results in an increase in total delta power (the amplitude of the electroencephalographic signal in the delta frequency range of 1–4 Hz). Graphs show average sum (± SEM) of delta power each hour during the lights on (clear horizontal bars) and lights off (dark horizontal bars) SI periods. There was a significant increase in total wakefulness (W) delta power (middle left), and a significant interaction of treatment × time for the NREM delta power (bottom left). (B) Exercise control (EC) (n = 3) did not produce any change in delta power during W (middle left) or NREMS (bottom right).
Sleep interruption impaired hippocampal LTP but not LTD, PPF, or fibre excitability
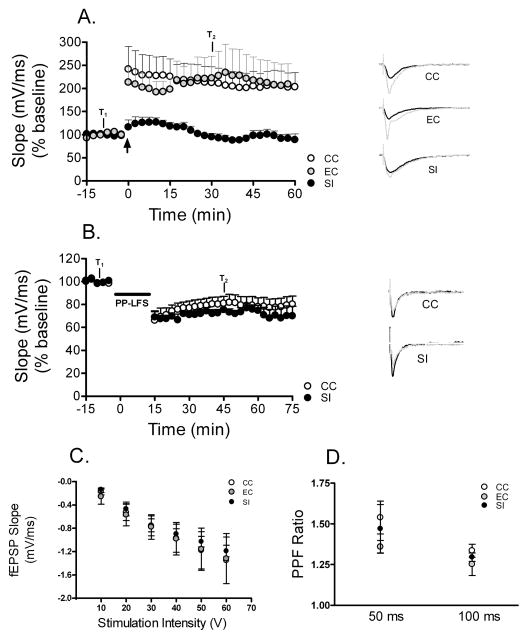
As can be seen in Fig. 3A, compared to CC rats, rats that underwent 24 h SI, but not 24 h EC, had impaired hippocampal LTP. Similarly, after 72 h of SI (n = 3), LTP was almost completely absent, whereas LTP was not altered by 72 h of EC (n = 4; 72 h SI / EC data not shown). For LTP analysis, the fEPSP slope of the middle time point (7 min) in the baseline recording (T1) was compared to the slope of the time point 30 min post-tetanus (T2) in the CC, 24 h and 72 h SI groups. Potential changes in evoked responses were analysed in a 5 × 2 repeated measures ANOVA with group as the between measures factor and time (T1 and T2) as the within measures factor. The repeated measures ANOVA was followed by Bonferroni pairwise comparisons comparing each group at T1 and at T2. Repeated measures ANOVA revealed a significant overall between groups difference (F4,22 = 3.47, P < 0.05) as well as a significant group–time interaction (F4,22 = 3.64, P < 0.05). Bonferroni pairwise comparisons showed that while the five groups did not differ from each other at T1, both the 24-h and 72-h SI rats had significantly lower fEPSP slopes than either the naïve control (P < 0.05 for 24-h and 72-h SI) or the EC (P < 0.05 for 24-h and 72-h SI) rats at T2. Moreover, in the SI groups there was no significant difference between T1 and T2 (P > 0.05 for 24-h and 72-h SI). The CC and EC rats did not differ from each another at either T1 or T2. Although LTP was blocked in the SI rats, there was no apparent effect of 24-h SI on LTD, as these rats responded similarly to CC rats at T2 (P > 0.05; Fig. 3B). Plotting I–O curves for 24-h SI and control (EC and CC) rats revealed no differences in the amount of voltage necessary to evoke fEPSP responses at any point on the I–O curve (Fig. 3C). Thus, compared to control rats, 24 h of SI did not produce a change in the stimulus intensity necessary to elicit an initial, threshold response, or a change in the stimulus intensity necessary to reach saturation, indicating that SI does not produce changes in fibre excitability.
Fig. 3.
LTP is impaired after 24 h SI. Hippocampal synaptic plasticity in rats was examined after SI as compared to EC and cage CC conditions. In A and B data shown are in two 1 / 2 min bins. (A) 24 h SI blocks LTP (n = 6) compared to 24 h EC (n = 6) and CC (n = 8) groups (P < 0.05). Top left graph shows the average responses across time for all groups. Example fEPSP traces from individual rats at timepoints T1 (black) and T2 (grey) are shown on the right. The arrow represents the timepoint of tetanic stimulation. (B) LTD induced by paired-pulse low-frequency stimulation (PP–LFS) is not significantly different between the 24-h SI (n = 6) and the CC (n = 6) groups. Left graph shows the average responses across time for all groups. Horizontal bar represent timepoints of PP-LFS. Examples of fEPSP traces from individual rats at timepoints T1 (black) and T2 (grey) are shown on the right. (C) Plot of input–output (I–O) voltage curves for the SI and control (EC and CC) rats shows no differences between the groups in the amount of voltage necessary to evoke fEPSP responses at any point on the I–O curve. The plot shows fEPSP response up to lowest obtained saturation. (D) PPF is not significantly different between groups.
We were also interested in determining whether presynaptic or postsynaptic mechanisms were involved in the SI-induced block of LTP. One physiological mechanism commonly used to test the idea that a change in synaptic efficacy is occurring through presynaptic mechanisms is paired-pulse facilitation (PPF), which is a measure of the probability of neurotransmitter release and is an index of presynaptic plasticity. Specifically, the degree to which the second (test) pulse increases compared to the first (conditioning) pulse in PPF, is the measured index of presynaptic activity and is inversely related to the probability of neurotransmitter release (Zucker., 1993). Two one-way ANOVAs, with group as the between measures factor and PPF ratios as the within measures factor, revealed that there were no differences in PPF ratios (second pulse : first pulse) between 24-h SI, 24-h EC, or CC groups at 50 ms (F2,13 = 0.93, P > 0.05, Fig. 3D) or 100 ms (F2,13 = 1.23, P > 0.05, Fig. 3D) suggesting that SI does not produce a change in presynaptic mechanisms.
Sleep interruption impaired water maze performance
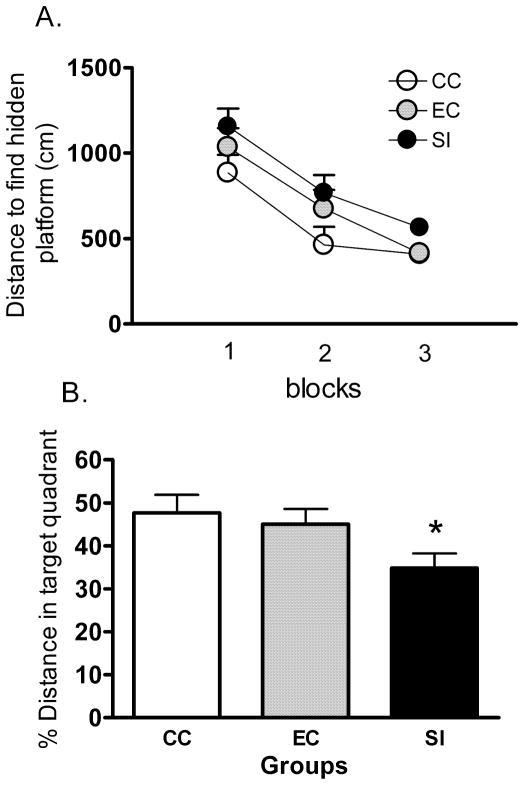
To explore further the effects of 24-h SI on hippocampal function, rats were tested in a spatial version of the water maze. The length of the path rats took to reach the hidden platform during the acquisition trials is shown in Fig. 4A. The main effect of trials was significant (repeated measures ANOVA, F11,319 = 12.302, P < 0.05) indicating that in all conditions, the animals learned the location of the platform across the 12 trials. There was a significant difference in main effect of path length to reach the platform among the three groups tested (F2,29 = 4.346, P < 0.05) indicating that SI rats swam a significantly longer distance to reach the platform than did CC rats (P < 0.05, Tukey’s), although the EC was not significantly different from either the SI or CC groups. The trial–group interaction effect was not significant (F22,319 = 0.745, P > 0.05). Differences in performance cannot be attributed to motor impairments as swim velocity was not significantly altered between groups (F2,29 = 1.317, P > 0.05) nor was there a trial–group interaction (F22,319 = 1.187, P > 0.05).
Fig. 4.
Spatial learning is impaired in the water maze after 24 h of SI. (A) Mean (± SEM) distance to reach the hidden platform for three blocks of four acquisition trials (SI, sleep interruption; n = 11; EC, n = 10; CC, n = 11). Overall, SI rats took a significantly longer path to reach the hidden platform than did CC (P < 0.05), while EC rats were not significantly different from either group (P > 0.05). A probe trial was given 30 min after the last acquisition trial (see text for details). (B) The mean percentage (+ SEM) of total distance travelled in the target quadrant that formerly contained the hidden platform in a single 30-s probe trial given 30 min after the last acquisition trial. SI rats swam significantly less distance in the target quadrant than did CC (P < 0.05), but EC rats were not significantly different from either group (P > 0.05). Asterisk indicates P < 0.05.
Thirty minutes after acquisition trials, rats were tested in a 30-s probe trial. The percentage of total distance that the animals spent searching in the quadrant that formerly contained the hidden platform was calculated (see Fig. 4B). There was a significant difference between conditions when analysing the percentage of total swim distance spent in the target quadrant (F2,29 = 3.389, P < 0.05). Tukey’s test revealed that SI rats swam less distance in the target quadrant than cage controls (P < 0.05), and no significant differences were observed between the CC and EC rats. There was no significant difference between the SI and EC group (P > 0.05). Once again, swim velocity was not significantly altered among groups (F2,22 = 2.990, P > 0.05). After the probe trial, rats were tested in a visible platform version of the water maze (data not shown). There were no significant differences among the three groups in distance travelled to find the visible platform (F2,29 = 0.453, P > 0.05) nor was there a trial–group interaction (F6,87 = 0.128, P > 0.05).
Plasma corticosterone is elevated by SI
Plasma CORT levels in rats exposed to the 24-h and 72-h SI, and EC conditions were highly variable and the raw data did not pass tests for normality (Shapiro–Wilk test). Following square root transformation, the raw CORT data were normally distributed and all subsequent analysis used the transformed data (see Table 1). CORT levels in time-matched CC rats (all rats were killed at the circadian nadir for CORT) were normally distributed and showed very low levels of CORT (2.87 ng / mL), indicating that neither the procedures used to kill the rats, nor the room the rats were tested in, caused a CORT elevation. ANOVA revealed the transformed data failed a homogeneity of variance test (Levene Statistic = 7.27, P < 0.05) hence non-parametric analysis was used. The Brown–Forsythe test for equality of means showed a significance in group mean differences (F4,22.710 = 4.57, P < 0.05). The Tamhane posthoc test (which is not dependent on equality of variances) showed that compared to CC rats, 24-h SI resulted in significantly higher CORT levels (P < 0.05), but there was not a significant difference between the 24-h EC and 24-h SI group (P > 0.05), nor between the CC and the 24-h EC group (P > 0.05). In the 72-h condition, CORT elevations induced by SI were not significantly greater than 72-h EC.
Table 1.
Corticosterone levels after 24 and 72 h of SI
| Group | n | Raw corticosterone ± SEM (ng / mL) | SQRT transformed ± SEM |
|---|---|---|---|
| CC | 8 | 2.9 ± 0.9 | 1.5 ± 0.3 |
| 24 h EC | 7 | 38.8 ± 20.9 | 5.1 ± 1.5 |
| 24 h SI | 10 | 124.7 ± 39.4 | 9.5 ± 1.9* |
| 72 h EC | 4 | 8.6 ± 4.3 | 2.7 ± 0.67 |
| 72 h SI | 8 | 74.6 ± 32.0 | 7.0 ± 1.9 |
Compared to CC rats, plasma CORT levels appear elevated after both 24-h EC, 24-h SI, and 72-h SI conditions. Only the 24-h SI condition has significantly elevated CORT levels (see text for details). Asterisk indicates significantly different than CC group (P < 0.05).
Discussion
Previous animal and human studies have documented the deleterious effects of total sleep deprivation on memory processes (Blissitt, 2001; Durmer & Dinges, 2005). However, the effects of sleep fragmentation on mnemonic mechanisms are less well understood. Here we found that hippocampal LTP, an experimentally induced model of the synaptic changes associated with memory formation, was absent after 24 and 72 h of a novel animal model of sleep fragmentation (sleep interruption, SI; 30 awakenings / h). Furthermore, 24 h of SI impairs spatial learning in the water maze, a well-studied hippocampus-mediated mnemonic task. We emphasize that our SI model was not designed to produce effects similar to total sleep deprivation but rather, to mimic the disturbances of continuity, deep sleep, and REM sleep seen in obstructive sleep apnea and other clinical disorders with sleep fragmentation.
The analysis of vigilance states, comparing the total sleep and wake times during SI to baseline conditions, showed that SI produced no change in total NREMS time. This finding parallels the human condition as the sleep fragmentation of sleep apnea does not interfere with total NREMS time, but does reduce the depth and quality of NREMS (Roehrs et al., 1985). During the 24 h of SI treatment, there was a significant increase (30%) in total time awake and a significant decrease in REMS (from 13% to 3%). This reduction of REMS during SI is less severe than that observed with selective REMS deprivation methods, which have lower REMS percentages (Mendelson et al., 1974; Thakkar et al., 1999). It is currently unclear whether or not REMS is decreased in clinical disorders with sleep fragmentation, as there are conflicting reports within and between disorders. For example, REMS has been reported to decrease (Kass et al., 1996; O’Connor et al., 2000) or remain unchanged in sleep apnea (Loadsman & Wilcox, 2000), and has been reported to increase to a greater extent in major depression than in post-traumatic stress disorder (Mellman et al., 1997). Although total NREMS time was unchanged in the SI rats, there was a reduction in the average duration of each individual sleep episode. SI also flattened the normal diurnal variation of EEG delta power and sleep (see Fig. 2). Total EEG delta power was elevated during SI, as was delta power during periods of wakefulness and NREMS, suggesting an increase in delta sleep pressure despite the normal amount of NREM sleep time. EEG delta power has been used as a marker of the homeostatic sleep drive, as delta power increases during periods of prolonged wakefulness (Tobler & Borbely, 1986; Franken et al., 2001) and during the first few hours of sleep after sleep deprivation (Pappenheimer et al., 1975). Conversely, EEG delta power is reduced after prolonged sleep (Werth et al., 1997). Furthermore, in our laboratory, rats released from 24 h of SI at lights out show an increase in sleep time and NREM delta power during the recovery sleep period, also indicative of an increase in sleep pressure (unpublished observations). These data all suggest that SI reduced the ‘quality’ of sleep and led to a buildup of delta sleep pressure. The variable effect of SI on the NREM delta of individual rats is consistent with individual ‘trait-like’ susceptibility to the effects of sleep deprivation on neurobehavioural performance that has been described in man (Van Dongen et al., 2004).
Rats in the EC group had a similar amount of treadmill exercise time as the SI group (360 min / 24 h). This exercise did not alter the distribution of total time spent in sleep and wakefulness, or delta power across the 24 h, indicating that EC does not result in a buildup of delta sleep pressure. Based on these data, we believe that the EC is in fact a good control for non-specific locomotor effects of the treadmill-induced SI. Furthermore, there was no movement artifact in these recordings; the possibility of subthreshold movement artifact influencing the power analysis is very unlikely because the EC rats had more active locomotion than did the SI rats and the waking delta power of EC was not elevated. It remains possible that the effects of SI on hippocampal functioning may be due to some aspect of the SI procedure that is not controlled for by the EC group. However, as discussed below, it is unlikely that a CORT mediated stress response can fully explain the findings. Interestingly, the average duration of individual NREMS episodes in the EC group was increased compared to baseline conditions, which presumably reflects the fact that these rats were forced to consolidate their sleep time into the 30 min when the treadmill was off.
Here, we were interested if the SI and consequent buildup of delta sleep pressure altered hippocampal synaptic plasticity. The ability of 24 h of SI to block LTP is extremely robust, whereas the EC condition had no effect on LTP. The finding that SI impaired spatial learning further suggests that disruptions of sleep impacts hippocampal function. Specifically, SI rats had decreased performance in spatial acquisition of the task relative to controls, whereas EC rats were not different from CC or SI rats in acquisition. The water maze data indicate that some spatial learning can exist even without CA1 hippocampal LTP in the SI rats, and this is supported by previous studies, which show that NMDA receptor antagonists block LTP, but rats are still able to learn in the water maze task, albeit more slowly (Ylinen et al., 1995; Kesslak et al., 2003). Thus, we conclude that while both LTP and water maze performance are decreased after SI, NMDA receptor dependent LTP in the hippocampal CA1 region by itself does not fully account for spatial learning.
It is currently generally accepted that long-lasting changes in hippocampal synaptic efficacy, examined experimentally in LTP or LTD paradigms, underlie declarative memory formation (Malenka & Bear, 2004; Lynch, 2004). For example, superior escape and spatial preference water maze learning performance correlates positively with the amount of LTP that can be induced in aged rats (Schulz et al., 2002). It has been demonstrated in the water maze task, that a pharmacological block (D-AP5) of the NMDA subtype of glutamate receptors impairs consolidation of spatial memory information and LTP (Morris et al., 1982; Morris & Frey, 1997). Moreover, it has been shown that deletion of the NMDAR1 subunit of the NMDA receptors, selectively in hippocampal CA1 pyramidal cells, impairs both LTP and spatial memory (McHugh et al., 1996; Tsien et al., 1996a; Tsien et al., 1996b).
It has been demonstrated previously that REMS deprivation (Davis et al., 2003; McDermott et al., 2003) and total sleep deprivation (Campbell et al., 2002) result in impairments in hippocampal LTP. Accordingly, we examined NMDA-receptor dependent hippocampal LTP and LTD as well as PPF to provide insight into the changes in synaptic plasticity, which may underlie the deficit in spatial memory caused by SI.
Although SI led to a deficit in LTP in the CA1 region, there did not appear to be an overall (metaplastic) shift from potentiation to depression, as SI did not alter LTD. In accord with this finding, LTP but not LTD was affected after REM sleep deprivation in a previous study (McDermott et al., 2003). While it is possible that the effects of SI on LTP are due to selective REMS reduction, some of our findings suggest that it is unlikely. The amount of LTP impairment observed here after 24 h of SI is much greater than that observed after 24 h of REMS deprivation alone (Davis et al., 2003), indicating that different, or additional, mechanisms are at work in our SI model. Although 72 h of selective REMS deprivation does produce an LTP reduction approaching the magnitude observed after 72 h of SI, the published studies do not document the actual changes in sleep making specific comparisons to our SI model difficult (Campbell et al., 2002; Davis et al., 2003; McDermott et al., 2003). Of note, REMS deprivation does not result in impairment of LTP in all brain regions. Specifically, in the visual cortex of young rats, selective REMS deprivation results in a preservation of the ability to induce LTP (Shaffery et al., 2002). In another study investigating LTP in the hippocampus, 12 h of total sleep deprivation during the light period only produced a 32% reduction in LTP, also suggesting that 24 h of SI has a greater effect on LTP than does total sleep deprivation (Campbell et al., 2002). The decrease in LTP observed after SI, was not due to changes in fibre excitability, as there were no differences in the input–output curves between the SI and EC groups. Additionally, PPF ratios were unaffected by SI, indicating that presynaptic mechanisms are probably not involved in the changes in LTP after SI.
The present findings indicate that SI prior to learning trials interfered with the acquisition of a spatial memory task. This is indicated by both increased swim distance and poorer performance in the probe trial, without decreases in motor performance or motivation, as shown by the visible platform trial (see Fig. 4). In contrast, the water maze performance of rats in the EC group more closely resembled that of cage controls, particularly on the probe trial and the third block of acquisition trials. Prior research has shown that the consolidation of hippocampal-dependent memories are sensitive to disruptions of sleep. Selective REM deprivation, especially 4 h after learning, impairs place acquisition but not cued learning of the water maze task (Smith & Rose, 1996). Total sleep deprivation for the first 5 h after acquisition also impaired performance on a hippocampus-dependent contextual fear task (Graves et al., 2003) and, in general, retention in the water maze task has been shown to be dependent on sleep post-training (Graves et al., 2001). However, little research has been conducted looking at the effects of sleep deprivation prior to learning acquisition. Recently (Guan et al., 2004) found that 6 h of total sleep deprivation (using exposure to novel objects) prior to water maze training impaired 24 h retention of the task, but not acquisition. These results are compatible with the present findings that sleep perturbation interferes with hippocamal tasks, although it appears that 6 h of total sleep deprivation produces a different pattern of cognitive effects than does 24 h of SI.
Elevations in peripheral CORT have been shown to reduce hippocampal LTP (Kim & Yoon, 1998). However, for several reasons, we hypothesize that the SI-induced increase in plasma corticosterone cannot, by itself, explain the complete absence of LTP in SI rats that is described herein. For example, although the elevation of CORT in EC rats was similar to that of the SI rats, the EC rats had virtually no reduction in hippocampal LTP. This observation was true for both the 24-h and the 72-h exposure groups. However, we note that the elevations in plasma CORT levels described were highly variable, making statistical analysis with relatively small group sizes difficult, and increasing the probability of a type 2 error (e.g. the 72 h SI group was not significantly elevated relative to cage control group, despite the marked elevation of CORT in four of eight 72-h SI rats). Interestingly, despite the variable CORT response, the SI-induced blockade of LTP was not variable (all SI rats had a complete blockade of LTP), again suggesting that CORT levels alone cannot explain the present LTP findings. Like the CORT levels, behavioural performance in the water maze was highly variable, although a correlation between water maze performance and CORT levels was not attempted in the present study. However, a previous study has shown that the deficits in spatial task acquisition after sleep deprivation are not due to the adrenal stress response to sleep deprivation (Ruskin et al., 2005). Additionally, a study that quantitatively compared the effect of plasma CORT elevations on hippocampal LTP also supports our hypothesis that the SI-induced CORT elevation cannot fully explain the SI blockade of LTP (Diamond et al., 1992). Thus, the size of the average CORT elevation observed in the 24-h SI group (125 ng / mL) has been shown to produce increases in the amount of hippocampal LTP, the opposite effect of the LTP blockade that we describe. Specifically, these authors described a U-shaped curve in which low (0–100 ng / mL) to intermediate (110–200 ng / mL) levels of CORT resulted in increased LTP in CA1 EPSP slope and population spike after primed-burst potentiation induction, whereas high levels of CORT (210– 930 ng / mL) reduced LTP (Diamond et al., 1992). These data are consistent with the idea that there is a biphasic relationship between glucocorticoid binding in the hippocampus and synaptic plasticity, wherein low levels of glucocorticoids increase the magnitude of LTP that can be induced, while high levels of glucocorticoids attenuate LTP (Kim & Yoon, 1998). The literature indicates that CORT elevations above 200 ng / mL that have been shown to reduce LTP require more severe stressors, such as physical restraint (Pavlides et al., 1993; Ylinen et al., 1995). In conclusion, additional studies are needed to fully elucidate the causal relationship between SI, stress / CORT, hippocampal LTP and spatial memory.
Together, the present results suggest that SI produces postsynaptic changes in LTP mechanisms, and not changes in LTD or short-term plasticity. Impariment of hippocampal function is further supported by observed deficits in the water maze task after SI. The SI paradigm used herein mimicked clinical sleep fragmentation (NREMS episode duration was significantly decreased but total NREMS time was unchanged). Parallel to the findings here, sleep episode duration appears to be an important predictor of cognitive functioning in man. Specifically, overnight experimental arousal of humans once every minute or every two minutes resulted in increased daytime sleepiness and decreased performance on psychometric tests (Bonnet, 1986; Martin et al., 1996) while arousals once every 10 min did not (Bonnet, 1986). Indeed, the deleterious effects of sleep fragmentation are similar in severity to those exhibited in humans after long-term sleep deprivation (Stepanski, 2002). Furthermore, the sleep fragmentation and restructuring of sleep due to arousals is thought to mediate the symptoms observed in clinical disorders (Coleman et al., 1982). Clinically, disorders involving sleep fragmentation have been shown to have deleterious effects on cognitive functioning (Stepanski, 2002). In general, our findings are also compatible with recent studies in man showing that sleep is important for learning and memory processes (Walker & Stickgold, 2004).
Acknowledgments
We thank Lynda Dauphin, Alaina Bebis, and Amy Blanchette for technical assistance. This research was supported by the Department of Veterans Affairs Medical Research Service Awards to RES and RWM, NHLBI – P50 HL060292 (RES, EA, & RWM), NHLBI – T32 HL07901 (JLT & JTM), NIMH – F32 MH070156 (JTM), K01 MH01798 (MMT), NIMH – R37 MH039683 (RWM), and NIMH – R01 MH062522 (RWM).
Abbreviations
- ACSF
artificial cerebrospinal fluid
- CC
cage controls
- CORT
corticosterone
- EC
exercise controls
- EEG
electroencephalograph
- EMG
electromyograph
- fEPSPs
field excitatory postsynaptic potentials
- LTD
long-term depression
- LTP
long-term potentiation
- PPF
paired-pulse facilitation
- REMS
rapid eye movement sleep
- SI
sleep interruption
References
- Bastuji H, Garcia-Larrea L. Sleep / wake abnormalities in patients with periodic leg movements during sleep: factor analysis on data from 24-h ambulatory polygraphy. J Sleep Res. 1999;8:217–223. doi: 10.1046/j.1365-2869.1999.00157.x. [DOI] [PubMed] [Google Scholar]
- Blissitt PA. Sleep, memory, and learning. J Neurosci Nurs. 2001;33:208– 215. doi: 10.1097/01376517-200108000-00007. [DOI] [PubMed] [Google Scholar]
- Bonnet MH. Performance and sleepiness as a function of frequency and placement of sleep disruption. Psychophysiology. 1986;23:263–271. doi: 10.1111/j.1469-8986.1986.tb00630.x. [DOI] [PubMed] [Google Scholar]
- Bonnet MH. Sleep restoration as a function of periodic awakening, movement, or electroencephalographic change. Sleep. 1987;10:364–373. doi: 10.1093/sleep/10.4.364. [DOI] [PubMed] [Google Scholar]
- Bonnet MH. Sleep Fragmentation. In: Lenfant C, editor. Sleep Deprivation. Marcel Dekker; New York: 2005. pp. 103–117. [Google Scholar]
- Braunewell KH, Manahan-Vaughan D. Long-term depression: a cellular basis for learning? Rev Neurosci. 2001;12:121–140. doi: 10.1515/revneuro.2001.12.2.121. [DOI] [PubMed] [Google Scholar]
- Campbell IG, Guinan MJ, Horowitz JM. Sleep deprivation impairs long-term potentiation in rat hippocampal slices. J Neurophysiol. 2002;88:1073–1076. doi: 10.1152/jn.2002.88.2.1073. [DOI] [PubMed] [Google Scholar]
- Coleman RM, Roffwarg HP, Kennedy SJ, Guilleminault C, Cinque J, Cohn MA, Karacan I, Kupfer DJ, Lemmi H, Miles LE, Orr WC, Phillips ER, Roth T, Sassin JF, Schmidt HS, Weitzman ED, Dement WC. Sleep-wake disorders based on a polysomnographic diagnosis. A national cooperative study. JAMA. 1982;247:997–1003. [PubMed] [Google Scholar]
- Davis CJ, Harding JW, Wright JW. REM sleep deprivation-induced deficits in the latency-to-peak induction and maintenance of long-term potentiation within the CA1 region of the hippocampus. Brain Res. 2003;973:293–297. doi: 10.1016/s0006-8993(03)02508-3. [DOI] [PubMed] [Google Scholar]
- Diamond DM, Bennett MC, Fleshner M, Rose GM. Inverted-U relationship between the level of peripheral corticosterone and the magnitude of hippocampal primed burst potentiation. Hippocampus. 1992;2:421–430. doi: 10.1002/hipo.450020409. [DOI] [PubMed] [Google Scholar]
- Durmer JS, Dinges DF. Neurocognitive consequences of sleep deprivation. Semin Neurol. 2005;25:117–129. doi: 10.1055/s-2005-867080. [DOI] [PubMed] [Google Scholar]
- Everson CA. Functional consequences of sustained sleep deprivation in the rat. Behav Brain Res. 1995;69:43–54. doi: 10.1016/0166-4328(95)00009-i. [DOI] [PubMed] [Google Scholar]
- Ferbinteanu J, Holsinger RM, McDonald RJ. Lesions of the medial or lateral perforant path have different effects on hippocampal contributions to place learning and on fear conditioning to context. Behav Brain Res. 1999;101:65–84. doi: 10.1016/s0166-4328(98)00144-2. [DOI] [PubMed] [Google Scholar]
- Franken P. Long-term vs. short-term processes regulating REM sleep. J Sleep Res. 2002;11:17–28. doi: 10.1046/j.1365-2869.2002.00275.x. [DOI] [PubMed] [Google Scholar]
- Franken P, Chollet D, Tafti M. The homeostatic regulation of sleep need is under genetic control. J Neurosci. 2001;21:2610–2621. doi: 10.1523/JNEUROSCI.21-08-02610.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frick KM, Stillner ET, Berger-Sweeney J. Mice are not little rats: species differences in a one-day water maze task. Neuroreport. 2000;11:3461– 3465. doi: 10.1097/00001756-200011090-00013. [DOI] [PubMed] [Google Scholar]
- Graves LA, Heller EA, Pack AI, Abel T. Sleep deprivation selectively impairs memory consolidation for contextual fear conditioning. Learn Mem. 2003;10:168–176. doi: 10.1101/lm.48803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graves L, Pack A, Abel T. Sleep and memory: a molecular perspective. TINS. 2001;24:237–243. doi: 10.1016/s0166-2236(00)01744-6. [DOI] [PubMed] [Google Scholar]
- Guan Z, Peng X, Fang J. Sleep deprivation impairs spatial memory and decreases extracellular signal-regulated kinase phosphorylation in the hippocampus. Brain Res. 2004;1018:38–47. doi: 10.1016/j.brainres.2004.05.032. [DOI] [PubMed] [Google Scholar]
- Herron CE, Lester RA, Coan EJ, Collingridge GL. Frequency-dependent involvement of NMDA receptors in the hippocampus: a novel synaptic mechanism. Nature. 1986;322:265–268. doi: 10.1038/322265a0. [DOI] [PubMed] [Google Scholar]
- Jones CT, Lafeber HN, Price DA, Parer JT. Studies on the growth of the fetal guinea pig. Effects of reduction in uterine blood flow on the plasma sulphation-promoting activity and on the concentration of insulin-like growth factors-I and -II. J Dev Physiol. 1987;9:181–201. [PubMed] [Google Scholar]
- Kass JE, Akers SM, Bartter TC, Pratter MR. Rapid-eye-movement- specific sleep-disordered breathing: a possible cause of excessive daytime sleepiness. Am J Respir Crit Care Med. 1996;154:167–169. doi: 10.1164/ajrccm.154.1.8680674. [DOI] [PubMed] [Google Scholar]
- Kemp N, Bashir ZI. Induction of LTD in the adult hippocampus by the synaptic activation of AMPA / kainate and metabotropic glutamate receptors. Neuropharmacology. 1999;38:495–504. doi: 10.1016/s0028-3908(98)00222-6. [DOI] [PubMed] [Google Scholar]
- Kesslak JP, Chuang KR, Berchtold NC. Spatial learning is delayed and brain-derived neurotrophic factor mRNA expression inhibited by administration of MK-801 in rats. Neurosci Lett. 2003;353:95–98. doi: 10.1016/j.neulet.2003.08.078. [DOI] [PubMed] [Google Scholar]
- Kim JJ, Yoon KS. Stress: metaplastic effects in the hippocampus. TINS. 1998;21:505–509. doi: 10.1016/s0166-2236(98)01322-8. [DOI] [PubMed] [Google Scholar]
- Kimoff RJ. Sleep fragmentation in obstructive sleep apnea. Sleep. 1996;19:S61–S66. doi: 10.1093/sleep/19.suppl_9.s61. [DOI] [PubMed] [Google Scholar]
- Loadsman JA, Wilcox I. Is obstructive sleep apnoea a rapid eye movement-predominant phenomenon? Br J Anaesth. 2000;85:354–358. doi: 10.1093/bja/85.3.354. [DOI] [PubMed] [Google Scholar]
- Lynch MA. Long-term potentiation and memory. Physiol Rev. 2004;84:87– 136. doi: 10.1152/physrev.00014.2003. [DOI] [PubMed] [Google Scholar]
- Malenka RC, Bear MF. LTP and LTD: an embarrassment of riches. Neuron. 2004;44:5–21. doi: 10.1016/j.neuron.2004.09.012. [DOI] [PubMed] [Google Scholar]
- Mamelak M, Caruso VJ, Stewart K. Narcolepsy: a family study. Biol Psychiatry. 1979;14:821–834. [PubMed] [Google Scholar]
- Martin SE, Engleman HM, Deary IJ, Douglas NJ. The effect of sleep fragmentation on daytime function. Am J Respir Crit Care Med. 1996;153:1328–1332. doi: 10.1164/ajrccm.153.4.8616562. [DOI] [PubMed] [Google Scholar]
- Martin PD, Shapiro ML. Disparate effects of long-term potentiation on evoked potentials and single CA1 neurons in the hippocampus of anesthetized rats. Hippocampus. 2000;10:207–212. doi: 10.1002/1098-1063(2000)10:3<207::AID-HIPO1>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- McDermott CM, LaHoste GJ, Chen C, Musto A, Bazan NG, Magee JC. Sleep deprivation causes behavioral, synaptic, and membrane excitability alterations in hippocampal neurons. J Neurosci. 2003;23:9687–9695. doi: 10.1523/JNEUROSCI.23-29-09687.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHugh TJ, Blum KI, Tsien JZ, Tonegawa S, Wilson MA. Impaired hippocampal representation of space in CA1-specific NMDAR1 knockout mice. Cell. 1996;87:1339–1349. doi: 10.1016/s0092-8674(00)81828-0. [DOI] [PubMed] [Google Scholar]
- Mellman TA, Kulick-Bell R, Ashlock LE, Nolan B. Sleep events among veterans with combat-related post-traumatic stress disorder. Am J Psychiatry. 1995;152:110–115. doi: 10.1176/ajp.152.1.110. [DOI] [PubMed] [Google Scholar]
- Mellman TA, Nolan B, Hebding J, Kulick-Bell R, Dominguez R. A polysomnographic comparison of veterans with combat-related PTSD, depressed men, and non-ill controls. Sleep. 1997;20:46–51. doi: 10.1093/sleep/20.1.46. [DOI] [PubMed] [Google Scholar]
- Mendelson WB, Guthrie RD, Frederick G, Wyatt RJ. The flower pot technique of rapid eye movement (REM) sleep deprivation. Pharmacol Biochem Behav. 1974;2:553–556. doi: 10.1016/0091-3057(74)90018-5. [DOI] [PubMed] [Google Scholar]
- Moore P, Bardwell WA, Ancoli-Israel S, Dimsdale JE. Association between polysomnographic sleep measures and health-related quality of life in obstructive sleep apnea. J Sleep Res. 2001;10:303–308. doi: 10.1046/j.1365-2869.2001.00264.x. [DOI] [PubMed] [Google Scholar]
- Morris RG, Frey U. Hippocampal synaptic plasticity: role in spatial learning or the automatic recording of attended experience? Philos Trans R Soc Lond B Biol Sci. 1997;352:1489–1503. doi: 10.1098/rstb.1997.0136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris RG, Garrud P, Rawlins JN, O’Keefe J. Place navigation impaired in rats with hippocampal lesions. Nature. 1982;297:681–683. doi: 10.1038/297681a0. [DOI] [PubMed] [Google Scholar]
- O’Connor C, Thornley KS, Hanly PJ. Gender differences in the polysomnographic features of obstructive sleep apnea. Am J Respir Crit Care Med. 2000;161:1465–1472. doi: 10.1164/ajrccm.161.5.9904121. [DOI] [PubMed] [Google Scholar]
- Ohayon MM, Shapiro CM. Sleep disturbances and psychiatric disorders associated with post-traumatic stress disorder in the general population. Compr Psychiatry. 2000;41:469–478. doi: 10.1053/comp.2000.16568. [DOI] [PubMed] [Google Scholar]
- Pappenheimer JR, Koski G, Fencl V, Karnovsky ML, Krueger J. Extraction of sleep-promoting factor S from cerebrospinal fluid and from brains of sleep-deprived animals. J Neurophysiol. 1975;38:1299–1311. doi: 10.1152/jn.1975.38.6.1299. [DOI] [PubMed] [Google Scholar]
- Pavlides C, Watanabe Y, McEwen BS. Effects of glucocorticoids on hippocampal long-term potentiation. Hippocampus. 1993;3:183–192. doi: 10.1002/hipo.450030210. [DOI] [PubMed] [Google Scholar]
- Perlis ML, Giles DE, Buysse DJ, Thase ME, Tu X, Kupfer DJ. Which depressive symptoms are related to which sleep electroencephalographic variables? Biol Psychiatry. 1997;42:904–913. doi: 10.1016/S0006-3223(96)00439-8. [DOI] [PubMed] [Google Scholar]
- Rechtschaffen A, Bergmann BM. Sleep deprivation in the rat by the disk-over-water method. Behav Brain Res. 1995;69:55–63. doi: 10.1016/0166-4328(95)00020-t. [DOI] [PubMed] [Google Scholar]
- Roehrs T, Conway W, Wittig R, Zorick F, Sicklesteel J, Roth T. Sleep-wake complaints in patients with sleep-related respiratory disturbances. Am Rev Respir Dis. 1985;132:520–523. doi: 10.1164/arrd.1985.132.3.520. [DOI] [PubMed] [Google Scholar]
- Rosenthal L, Roehrs T, Sicklesteel J, Zorick F, Wittig R, Roth T. Periodic movements during sleep, sleep fragmentation, and sleep-wake complaints. Sleep. 1984;7:326–330. doi: 10.1093/sleep/7.4.326. [DOI] [PubMed] [Google Scholar]
- Ruskin DN, Dunn KE, Billiot I, Bazan NG, LaHoste GJ. Eliminating the adrenal stress response does not affect sleep deprivation-induced acquisition deficits in the water maze. Life Sci. 2005;78:2833–2838. doi: 10.1016/j.lfs.2005.11.003. [DOI] [PubMed] [Google Scholar]
- Ruskin DN, Liu C, Dunn KE, Bazan NG, LaHoste GJ. Sleep deprivation impairs hippocampus-mediated contextual learning but not amygdala-mediated cued learning in rats. Eur J Neurosci. 2004;19:3121–3124. doi: 10.1111/j.0953-816X.2004.03426.x. [DOI] [PubMed] [Google Scholar]
- Saletu M, Anderer P, Saletu B, Hauer C, Mandl M, Oberndorfer S, Zoghlami A, Saletu-Zyhlarz G. Sleep laboratory studies in restless legs syndrome patients as compared with normals and acute effects of ropinirole. 2 Findings on periodic leg movements, arousals and respiratory variables. Neuropsychobiology. 2000;41:190–199. doi: 10.1159/000026659. [DOI] [PubMed] [Google Scholar]
- Schulz D, Huston JP, Jezek K, Haas HL, Roth-Harer A, Selbach O, Luhmann HJ. Water maze performance, exploratory activity, inhibitory avoidance and hippocampal plasticity in aged superior and inferior learners. Eur J Neurosci. 2002;16:2175–2185. doi: 10.1046/j.1460-9568.2002.02282.x. [DOI] [PubMed] [Google Scholar]
- Sforza E, Nicolas A, Lavigne G, Gosselin A, Petit D, Montplaisir J. EEG and cardiac activation during periodic leg movements in sleep: support for a hierarchy of arousal responses. Neurology. 1999;52:786–791. doi: 10.1212/wnl.52.4.786. [DOI] [PubMed] [Google Scholar]
- Shaffery JP, Sinton CM, Bissette G, Roffwarg HP, Marks GA. Rapid eye movement sleep deprivation modifies expression of long-term potentiation in visual cortex of immature rats. Neuroscience. 2002;110:431–443. doi: 10.1016/s0306-4522(01)00589-9. [DOI] [PubMed] [Google Scholar]
- Shapiro ML, Eichenbaum H. Hippocampus as a memory map: synaptic plasticity and memory encoding by hippocampal neurons. Hippocampus. 1999;9:365–384. doi: 10.1002/(SICI)1098-1063(1999)9:4<365::AID-HIPO4>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Smith C, Rose GM. Evidence for a paradoxical sleep window for place learning in the Morris water maze. Physiol Behav. 1996;59:93–97. doi: 10.1016/0031-9384(95)02054-3. [DOI] [PubMed] [Google Scholar]
- Stepanski EJ. The effect of sleep fragmentation on daytime function. Sleep. 2002;25:268–276. doi: 10.1093/sleep/25.3.268. [DOI] [PubMed] [Google Scholar]
- Sutherland RJ, Whishaw IQ, Kolb B. A behavioural analysis of spatial localization following electrolytic, kainate- or colchicine-induced damage to the hippocampal formation in the rat. Behav Brain Res. 1983;7:133–153. doi: 10.1016/0166-4328(83)90188-2. [DOI] [PubMed] [Google Scholar]
- Tafti M, Villemin E, Carlander B, Besset A, Billiard M. Sleep in human narcolepsy revisited with special reference to prior wakefulness duration. Sleep. 1992;15:344–351. doi: 10.1093/sleep/15.4.344. [DOI] [PubMed] [Google Scholar]
- Thakkar MM, Ramesh V, Cape EG, Winston S, Strecker RE, McCarley RW. REM sleep enhancement and behavioral cataplexy following orexin (hypocretin)-II receptor antisense perfusion in the pontine reticular formation. Sleep Res Online. 1999;2:112–120. [PubMed] [Google Scholar]
- Thakkar MM, Ramesh V, Strecker RE, McCarley RW. Microdialysis perfusion of orexin-A in the basal forebrain increases wakefulness in freely behaving rats. Arch Ital Biol. 2001;139:313–328. [PubMed] [Google Scholar]
- Tobler I, Borbely AA. Sleep EEG in the rat as a function of prior waking. Electroencephalogr Clin Neurophysiol. 1986;64:74–76. doi: 10.1016/0013-4694(86)90044-1. [DOI] [PubMed] [Google Scholar]
- Tsien JZ, Chen DF, Gerber D, Tom C, Mercer EH, Anderson DJ, Mayford M, Kandel ER, Tonegawa S. Subregion- and cell type-restricted gene knockout in mouse brain. Cell. 1996a;87:1317–1326. doi: 10.1016/s0092-8674(00)81826-7. [DOI] [PubMed] [Google Scholar]
- Tsien JZ, Huerta PT, Tonegawa S. The essential role of hippocampal CA1 NMDA receptor-dependent synaptic plasticity in spatial memory. Cell. 1996b;87:1327–1338. doi: 10.1016/s0092-8674(00)81827-9. [DOI] [PubMed] [Google Scholar]
- Van Dongen HP, Baynard MD, Maislin G, Dinges DF. Systematic interindividual differences in neurobehavioral impairment from sleep loss: evidence of trait-like differential vulnerability. Sleep. 2004;27:423–433. [PubMed] [Google Scholar]
- Walker MP, Stickgold R. Sleep-dependent learning and memory consolidation. Neuron. 2004;44:121–133. doi: 10.1016/j.neuron.2004.08.031. [DOI] [PubMed] [Google Scholar]
- Werth E, Achermann P, Borbely AA. Fronto-occipital EEG power gradients in human sleep. J Sleep Res. 1997;6:102–112. doi: 10.1046/j.1365-2869.1997.d01-36.x. [DOI] [PubMed] [Google Scholar]
- Wiegand L, Zwillich CW. Obstructive sleep apnea. Dis Mon. 1994;40:197–252. doi: 10.1016/0011-5029(94)90013-2. [DOI] [PubMed] [Google Scholar]
- Ylinen A, Pitkanen M, Sirvio J, Hartikainen T, Sivenius J, Koivisto E, Riekkinen PJ., Sr The effects of NMDA receptor antagonists at anticonvulsive doses on the performance of rats in the water maze task. Eur J Pharmacol. 1995;274:159–165. doi: 10.1016/0014-2999(94)00729-q. [DOI] [PubMed] [Google Scholar]
- Zorick F, Roehrs T, Wittig R, Lamphere J, Sicklesteel J, Roth T. Sleep-wake abnormalities in narcolepsy. Sleep. 1986;9:189–193. doi: 10.1093/sleep/9.1.189. [DOI] [PubMed] [Google Scholar]
- Zucker RS. Calcium and transmitter release. J Physiol Paris. 1993;87:25–36. doi: 10.1016/0928-4257(93)90021-k. [DOI] [PubMed] [Google Scholar]