Abstract
6-Bromo-7-hydroxycoumarin (Bhc)-caged ceramide (Cer) analogs were incorporated into supported lipid bilayers containing a mixture of coexisting liquid-ordered (Lo) and liquid-disordered (Ld) phases. The release of N-palmitoyl and N-butanoyl-D-erythro-sphingosine (C16- and C4-Cer) by photolysis of the caged Cers using long wavelength UV light was studied using a combination of atomic force microscopy and fluorescence microscopy. This approach demonstrated the ability to generate Cer with spatial and temporal control, providing an alternative method to the enzymatic generation of Cer. The generation of C16-Cer from Bhc-C16-Cer disrupted the Lo domains, with the incorporation of small fluid phase regions and the disappearance of some smaller domains. Cer-rich gel-phase domains were not observed, in contrast to results reported by either direct Cer incorporation or enzymatic Cer generation. Photorelease of C4-Cer from Bhc-C4-Cer resulted in qualitatively similar changes in bilayer morphology, with disappearance of some Lo domains and no evidence for Cer-rich gel domains, but with a smaller height difference between the ordered and disordered phases.
INTRODUCTION
Ceramide (Cer) is a raft-associated sphingolipid that has been implicated in diverse cellular processes, including differentiation, senescence, apoptosis, and immune response.1–5 Although Cer is present in relatively small amounts in resting cells, its concentration can reach levels of up to 10 mol% of the total lipid content in apoptotic cells.1 It is produced by de novo synthesis, enzymatic hydrolysis of the phosphorylcholine head group of sphingomyelin (SM), and several other enzymatic routes. Cer is one of the most hydrophobic natural lipids and has significant effects on the biophysical properties of membranes, including its propensity to promote phase separation and the formation of gel-phase domains and to induce negative membrane curvature and either membrane permeabilization or membrane fusion.6–9 Enzymatic generation of Cer in cells has also been shown to induce coalescence of small raft domains into larger signaling platforms, providing a mechanism for amplifying signaling by membrane receptors.3, 4 Membrane rafts are small transient domains that are enriched in SM, cholesterol (Chol), and certain proteins; they are thought to exist in a liquid-ordered (Lo) phase that is distinct from the surrounding liquid-disordered (Ld) membrane and to play an important role in signal transduction.10–13
A number of studies have examined the effects of direct incorporation of Cer in supported bilayers that exhibit Ld/Lo phase separation, demonstrating the formation of a third Cer-enriched gel phase for some lipid mixtures.14–17 Generation of Cer by sphingomyelinase (SMase) in similar phase-separated model membranes leads to extensive membrane restructuring that is considerably more complex to interpret than the results obtained by direct Cer incorporation.15, 18–25 Using atomic force microscopy (AFM) combined with fluorescence, our previous work has identified a range of structural changes in SMase-treated supported lipid bilayers.19, 22, 23, 26 These include the formation of Cer-rich regions in the original Lo domains and the complete disappearance of some domains with the concomitant formation of highly ordered dye-excluding and Chol-depleted membrane regions. The heterogeneity of model membranes containing SMase-generated Cer reflects the inherent difficulties in targeting and controlling the extent of enzyme activity in vitro.18, 20, 23 Recently, the enzymatic generation of Cer in bilayers prepared in microfluidic channels has been investigated in an attempt to address these issues.20 However, so far approaches using enzymatic Cer generation in model membranes have given variable results and do not reproduce the precise regulation of SMase activity and localized hydrolysis of SM that occur in cells. Thus, an approach for producing a known, controlled concentration of Cer within localized regions of bilayer membranes would be a significant advantage, both for model membrane studies and for probing biological pathways that are modulated by Cer.
Photolabile groups have been widely used to release bioactive molecules with both spatial and temporal control.27–29 The covalent attachment of a photoremovable protecting group (cage) to the biomolecule of interest renders it biologically inactive until photolysis releases the active form and triggers specific biological activity. Although photodeprotection has been widely applied to release small molecule neurotransmitters and to study peptide and protein function, there are only a few examples of photocaged lipids, notably for sphingosine 1-phosphate, ceramide-1-phosphate, ceramide and phosphatidylinositol-3,4,5-triphosphate.30–33 In each case, the caged lipid is designed to be membrane permeable for delivery to cells and to release a lipid second messenger involved in a specific biological pathway. Several studies have also shown that photoswitchable lipids can be employed to modulate the shape and phase separation behavior of vesicle bilayer membranes.34–36
We have recently reported the development of a photocaged dihydro-Cer that can be delivered into and released in cells.30 Herein we describe the incorporation of similarly caged N-palmitoyl-D-erythro-sphingosine (C16-Cer, 1) and N-butanoyl-D-erythro-sphingosine (C4-Cer, 2) into phase-separated supported bilayers and their photolysis with long wavelength UV light to generate the corresponding Cers with a high degree of spatial and temporal control. Using correlated fluorescence-AFM, we have examined the structural reorganization that occurs when controlled quantities of C4- or C16-Cer are produced in bilayers with coexisting Lo and Ld phases. Despite the documented differences in biological activity between long- and short-chain Cers, photorelease of Cer from the two caged molecules has similar effects on membrane morphology for the ternary lipid mixture examined herein.

MATERIALS AND METHODS
Chemicals
1,2-Dioleoyl-sn-glycero-3-phosphocholine (dioleoylphosphocholine, DOPC), egg sphingomyelin (ESM; ESM is comprised of ~85% N-C16:0-SM), N-palmitoyl-D-erythro-sphingosine (C16:0-ceramide, C16-Cer), and cholesterol were purchased from Avanti Polar Lipids (Alabaster, AL) and used without further purification. 1,1′-Dieicosanyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate (DiI-C20) was purchased from Molecular Targeting Technologies (Westchester, PA). (2S,3R,4E)-2-Palmitoylamido-3-hydroxyoctadec-4-enyl (6-bromo-7-hydroxycoumarin-4-yl)methyl carbonate (caged C16-Cer, 1) and (2S,3R,4E)-2-butyramido-3-hydroxyoctadec-4-enyl (6-bromo-7-hydroxycoumarin-4-yl)methyl carbonate (caged C4-Cer, 2) were synthesized as described previously and purified by HPLC prior to use, where necessary. Optical adhesive 88 was obtained from Norland Products (Cranbury, NJ) and used to glue mica onto cover glass (Fisher Scientific, Hampton, NH). All aqueous solutions were prepared using 18.3 MΩ cm Milli-Q water. KMops buffer (100 mM KCl, 10 mM 3-(N-morpholino)-propanesulfonate (Mops), pH 7.4) was used for some imaging and photolysis experiments. Buffered solutions were passed through a 0.22 μm filter (Millipore, Billerica, MA) before use.
Supported lipid bilayers
Small unilamellar vesicles (SUVs) were prepared as previously described subject to some minor modifications.26 Lipids, caged Cer, and DiI-C20 were dissolved in chloroform, methanol, ethanol, or mixtures thereof as required. After these solutions were mixed in appropriate amounts, the solvents were evaporated and the dry lipid films were hydrated in Milli-Q water and sonicated at 60 °C in a bath sonicator to clarity to form SUVs with a final lipid concentration of 0.5 mg/mL. Lipid films were stored for up to 1 week at −20 °C prior to use; however, fresh vesicles were prepared for each imaging experiment.
Planar supported bilayers were formed on mica via vesicle fusion. Freshly cleaved mica disks (15–25 μm thick for fluorescence imaging) were glued onto circular cover glasses. To prepare bilayers of DOPC/ESM/Chol/caged Cer, 950 μL of 8 mM CaCl2 was first added to a mica/glass slide clamped in a liquid cell, and warmed to 45 °C. Aliquots (20–50 μL) of vesicle suspension were introduced at the same temperature, and the samples were incubated for 15 min before being gradually cooled to 22 °C over a period of 2 h. Bilayers were gently washed with Milli-Q water or KMops buffer to remove unattached vesicles before imaging.
Fluorescence microscopy and correlated fluorescence-atomic force microscopy
Fluorescence imaging and correlated fluorescence-atomic force microscopy were performed on the same microscope platform at room temperature (~22 °C). The imaging system consisted of a NanoWizard II BioAFM (JPK Instruments, Berlin, Germany) integrated with an IX81 inverted optical microscope (Olympus Corporation, Tokyo, Japan). Epifluorescence images were obtained using lamp excitation with an Olympus UPlanSAPO 100x, NA = 1.4 oil immersion objective, Cy3 and DAPI filter sets (Chroma Technology, Bellows Falls, VT), and a high resolution CoolSNAP CCD camera (Semrock, AZ). Fluorescence images were scaled, cropped, and correlated with the corresponding AFM scans using Image J freeware (NIH, Bethesda, MD).
Caged Cer photolysis experiments were carried out on the same setup using the lamp and DAPI filter set. The aperture built into the microscope illuminator was used to confine UV irradiation of the sample to a hexagonal area ~25 μm in diameter. To assess the uncaging ability, we irradiated an aqueous dispersion of 1 for several minutes on the fluorescence microscope. Post-UV HPLC analysis of the solution confirmed that photolysis of the caged lipid had taken place to afford C16-Cer and 6-bromo-7-hydroxycoumarin (data not shown).
AFM images were captured using uncoated silicon nitride DNP-S-10 (Veeco, Camarillo, CA) AFM cantilevers with a typical spring constant of 0.12 N/m. Contact mode topographic images were collected at scan rates of 0.7–1 Hz, and continuous adjustments to the set point kept the force exerted on the sample at a minimum. Scans were collected at 512 × 512 pixel resolution and were line fitted with first- to third-order polynomials when necessary.
Solid-supported bilayers of the desired composition were prepared, and fluorescence and AFM images of the same sample area were acquired sequentially after first assessing sample homogeneity over several areas by fluorescence. The sample was photolyzed for the specified period of time and fluorescence or correlated images were recorded to monitor changes in the bilayer morphology. Typically fluorescence images were acquired before and after AFM scans of the same area to check for changes in membrane morphology occurring during or due to scanning.
RESULTS
Photo-uncaging of 1 in phase-separated bilayers
Caged Cer 1 was incorporated in DOPC/ESM/Chol mixtures, which form bilayers with coexisting Ld and Lo phases. These mixtures have been extensively used by several groups, including our own, to study enzymatic Cer generation in model membranes that mimic the phase separation behavior of rafts in cellular membranes.18, 20, 22, 23, 37 Figure 1 shows a representative example of a planar supported bilayer formed in the dark from a quaternary mixture of DOPC/ESM/Chol/1 in 8/7/4/1 molar ratios and containing 0.5 mol % DiI-C20 in water. The DiI-C20 fluorescence image (Figure 1A) reveals dark, micrometer-scale Lo domains in a bright Ld bulk phase, indicating that this lipophilic probe preferentially partitions into the disordered environment.38 Although caged Cer analogs have low fluorescence quantum yields,30 the emission of 1 can be used to visualize its relative distribution in the membrane (Figure 1B). The inverted fluorescence contrast for the coumarin channel with respect to the DiI-C20 image (bright Lo domains) suggests that the caged lipid is enriched in the ordered phase. Correlated DiI-C20 fluorescence and AFM imaging confirmed the assignment of Lo and Ld phases in the optical images (Figure 2A). Height measurements from the AFM scans showed that the ordered domains were 0.8–1 nm taller than the surrounding fluid phase. This height difference is consistent with reported values for ternary lipid mixtures of DOPC/ESM/Chol, as well as with those incorporating similar molar fractions of C16-Cer.19, 26
Figure 1.

Epifluorescence images of a planar supported lipid bilayer composed of DOPC/ESM/Chol/1 (8/7/4/1 molar ratio, labeled with 0.5 mol % DiI-C20). (A) Lo domains appear dark in the DiI channel, indicating that DiI-C20 is enriched in the Ld phase; (B) inverted contrast for the coumarin fluorescence image of the same area suggests that the caged ceramide preferentially partitions into the ordered phase. Scale bar = 4 μm.
Figure 2.

Caged C16-Cer photolysis in a DOPC/ESM/Chol/1 supported bilayer (8/7/4/1 molar ratio, 0.5 mol% DiI-C20). The correlated fluorescence and AFM images reveal the progressive morphological changes that occur in the Lo domains as Cer is generated. (A) T0 min before UV irradiation; (B) T15 min after 5 min UV irradiation; (C) T30 min after a second 5 min UV irradiation; (D) T50 min after an additional 20 min dark equilibration. Blue arrows mark domains where regions of a lower phase grow in during and after photolysis. Scale bar = 3 μm.
AFM and fluorescence were also used to detect and follow membrane restructuring when C16-Cer was generated photochemically in these supported bilayers. First, HPLC analysis (Figure S1, Supporting Information) confirmed the time-dependent disappearance of 1 in SUVs prepared from DOPC/ESM/Chol/1 (8/7/4/1 molar ratio with 0.5 mol % DiI-C20) on irradiation at 350 nm. Figure 2 illustrates a photolysis experiment in a supported bilayer formed from SUVs of the same quaternary lipid mixture where a small region of the bilayer was irradiated using the microscope lamp and aperture (λ 377 ± 25 nm) for a total of 10 min. During this time the sample was periodically monitored using both imaging techniques. Structural changes first became apparent by AFM (Figure 2B) as small pockets of lower height or slight indentations within the Lo domains. As more Cer was generated with an extended irradiation time, the size and frequency of these features increased, and the largest pockets were detectable by fluorescence (Figure 2C, arrows) with intensities that were similar to the fluid phase. Closer examination of the AFM scans showed that these pockets were at approximately the same height as the Ld phase. The bilayer continued to evolve immediately after photolysis (Figure 2D), but its morphology remained unchanged after approximately 20 min. This suggests that the short-term reorganization of membrane lipids to accommodate the de novo Cer was complete. The fluorescence intensity in the coumarin channel decreased rapidly during photolysis of 1 and was not useful for assessing changes in the bilayer morphology. This is consistent with loss of the bromohydroxy coumarin moiety to the bulk solution.
Repeated AFM scanning of a bilayer after photochemical Cer generation appeared to accelerate the dissolution of Lo domains, particularly in sample regions where smaller sized domains predominated (Figure S2). Therefore, further uncaging experiments were conducted in which the area of interest was monitored exclusively by fluorescence in order to assess the extent of bilayer restructuring in the absence of any mechanical perturbation caused by the scanning AFM tip. Figure 3 illustrates the progressive disappearance of ordered domains in a typical sample containing 5 mol % 1. After imaging an area of the initial bilayer (Figure 3A), we closed the aperture on the microscope illuminator to give a ~25-μm diameter illumination area for generation of C16-Cer (Figure 3B). The region of interest was irradiated for 30 min, and the sample was then monitored for an additional 20 min. By this point the bilayer had attained a stable morphology with a significantly decreased surface coverage of the Lo domains and the complete disappearance of some smaller domains. Fully opening the aperture identified adjacent areas of the bilayer where no Cer had been generated, providing a clear in situ comparison of pre- and post-UV membrane morphology (Figure 3A, C).
Figure 3.

Caged C16-Cer photolysis in a DOPC/ESM/Chol supported bilayer (8/7/4/1 molar ratio, 0.5 mol % DiI-C20 in water). As increasing amounts of Cer are generated with longer irradiation times, Lo domains decrease in size and gradually disappear. (A) T0 min before photolysis; (B) closed aperture indicating the region to be irradiated; (C) T50 min 20 min after a 30 min UV-irradiation with the aperture opened to reveal adjacent areas of the bilayer not exposed to UV light. Scale bar = 5 μm.
A control experiment in which a DOPC/ESM/Chol (molar ratio, 2/2/1) bilayer containing 0.5 mol % DiI-C20 was irradiated for a comparable period of time showed no changes in membrane morphology in the absence of caged Cer (Figure S3). Bilayers containing synthetic C16-Cer (5 mol %) were also prepared from DOPC/ESM/Chol/C16-Cer in 8/7/4/1 molar ratios and stained with 0.5 mol % DiI-C20. AFM images of these samples showed Lo/Ld phase separation with domains that were of similar size, shape, and height above the fluid phase to those formed from mixtures with 5 mol % 1 or no Cer (Figure S4).26 We typically observed some variability in domain shape and size within and between individual samples for phase-separated supported bilayers that contain Cer. This variability may be attributed to local sample-substrate effects and/or subtle differences in the sample’s thermal history.39
We then examined the effects of generating higher mole fractions of C16-Cer. Bilayers of DOPC/ESM/Chol/1 in an 8/6/4/2 molar ratio and containing 0.5 mol % DiI-C20 present coexisting Lo/Ld phases with domains comparable to those observed at 5 mol % 1 (Figure 4). After 30 min of UV irradiation, similar indentations in the ordered domains were observed, with some fragmentation of large domains and disappearance of small domains. The AFM and fluorescence images of these samples were qualitatively similar to those obtained for bilayers with a lower mol fraction of 1 and provided no evidence for formation of the Cer-rich subdomains that have been observed previously upon both direct and enzymatic Cer generation in similar ternary lipid mixtures.15, 16, 19, 23, 26 Several attempts were made to incorporate a larger mole fraction of 1 in the supported bilayers. Supported bilayers prepared from DOPC/ESM/Chol vesicles with up to 20 mol % of 1 were densely covered with vesicles that could not be removed by extensive washing. After UV irradiation of these samples, most of the adsorbed vesicles had disappeared; Cer generation in the vesicles may have promoted vesicle rupturing or desorption. Only modest restructuring of the underlying bilayers took place, on a scale comparable to that observed in the samples incorporating 5 or 10 mol % 1. This suggests that a significant fraction of 1 may be in the adsorbed vesicles, leading to lower than expected concentrations of 1 in the supported bilayer. Attempts to incorporate 1 in a preformed bilayer by incubation with a 50 μM aqueous suspension (with 0.5 % ethanol) were not successful, as judged by lack of change in the bilayer following UV irradiation.
Figure 4.
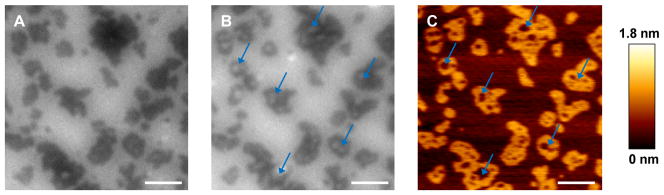
Photolysis of bilayers with 10 mol % 1 gives similar results to those obtained with 5 mol % 1. A DOPC/ESM/Chol/1 bilayer (8/6/4/2 molar ratio, 0.5 mol % DiI-C20) has Lo domains of similar size and height to those obtained with 5 mol % 1. Photochemical generation of C16-Cer induces time-dependent lipid reorganization that can be observed by both fluorescence and AFM. (A) T0 min before UV; (B) T60 min 30 min after a 30 min UV irradiation; (C) AFM of the same area acquired immediately after the fluorescence image in (B). Blue arrows mark domains where regions of a lower phase grow in during and after photolysis. Scale bar = 2 μm.
Photo-uncaging of 2 in phase-separated bilayers
Photolysis of caged C4 Cer (2) in DOPC/ESM/Chol lipid bilayers gave qualitatively similar results to those obtained for 1. A bilayer prepared from DOPC/ESM/Chol/2 (8/7/4/1 molar ratio, 0.5 mol % DiI-C20) showed a similar pattern of dark Lo domains surrounded by a bright fluid phase in the DiI channel (Figure 5A). The reversal in fluorescence contrast in the coumarin channel is consistent with enrichment of 2 in the Lo domains (Figure 5B). The fluorescence contrast in the coumarin channel was slightly higher than that obtained for bilayers containing 1. Changes in bilayer fluorescence after UV irradiation were followed by fluorescence (Figure 5C–E); parallel to the results for 1, small regions of fluid phase appeared in the initial Lo domains. Interestingly, in this case the improved contrast in the coumarin channel allowed the changes in bilayer morphology to be followed in both fluorescence channels (Figure 5E). The fluorescence intensity for the coumarin channel decreased after irradiation. The image measured after 5 min photolyis (Figure 5E) required a longer exposure time to achieve a similar intensity to Figure 5B. An estimate of the decrease in fluorescence intensity induced by photolysis was obtained in a different experiment; after 5 min UV photolysis, the intensity decreased by a factor of 10, after background subtraction. The fluorescence quantum yield for the product 6-bromo-7-hydroxy-4-hydroxymethylcoumarin is ~25 times lower than that for caged Cers (0.5 vs 0.02).30 Therefore, the 10-fold decrease in coumarin fluorescence intensity after photolysis indicates that most of the photolysed coumarin is released into the aqueous solution. The difference between irradiated and unirradiated areas of the bilayer were evident when the bilayer was imaged with the microscope aperture opened, as shown in the larger scale images of the same bilayer measured before and after UV irradiation (Figure S5).
Figure 5.
Caged C4-Cer photolysis in a DOPC/ESM/Chol/2 supported bilayer (8/7/4/1 molar ratio, 0.5 mol % DiI-C20). (A, B) T0 min before UV irradiation for DiI and coumarin channels; (C) T5 min immediately after a 5 min UV irradiation, DiI channel; (D, E) T20min 15 min after UV irradiation for DiI and coumarin channels; (F) T35min 30 min after UV, DiI channel. The exposure time for (E) was increased by a factor of 2, due to weaker fluorescence caused by release of the coumarin fluorophore into the aqueous solution as 2 is photolyzed. The blue arrows indicate that the modifications to the domains are detectable in this channel. Red arrows in (D) and (F) mark a domain that has continued to evolve after photolysis. Scale bar = v 3 μm.
A correlated AFM-fluorescence experiment was also carried out for a DOPC/ESM/Chol/2 bilayer, as presented in Figure 6. Fluorescence images showed morphology changes similar to those in Figure 5, and the corresponding before and after UV AFM images confirmed the appearance of many small regions of lower phase in the initial Lo domains. The height of the Lo domains was lower (0.4 – 0.6 nm) than that of Lo domains in bilayers containing the C16-Cer analog (1), both before and after photolysis. An experiment in which a 20 μM aqueous solution of C4-Cer was added to an intact DOPC/SM/Chol bilayer did not show changes in bilayer morphology associated with incorporation of Cer.
Figure 6.
Caged C4-Cer photolysis in a DOPC/ESM/Chol/2 bilayer (8/7/4/1 molar ratio, 0.5 mol % DiI-C20). (A) T0 min before UV irradiation; (B) T10 min immediately after UV irradiation; (C) T40 min 30 min after UV irradiation; (D, E) AFM images before UV irradiation and after image C. The morphological changes are evident in both fluorescence (blue arrows) and AFM images but are clearer with the higher resolution available with AFM. Line scans for the regions marked with blue arrows in the AFM images illustrate the appearance of a new region of lower height in one domain. Scale bar = 3 μm.
DISCUSSION
Incorporation of caged Cer 1 did not modify the morphology of the ternary lipid bilayers that we have used to model membrane rafts, nor did it lead to a significant change in the height difference between the ordered domains and the surrounding fluid phase. The coumarin fluorescence is slightly more intense in the ordered domains, consistent with similar amounts of 1 in both ordered and disordered phases. Note that one can only draw qualitative conclusions from the fluorescence intensity data since the probe partition coefficient may be affected by differences in probe brightness and orientation of the fluorophore dipole in the two phases.38 The partitioning of the caged Cer is in contrast to that for SM and Cer, both of which show a strong preference for localization in ordered membrane phases.14 However, the distribution of 1 in bilayers prepared from this ternary lipid mixture is consistent with our observation that 1 (10 mol %) is uniformly distributed in fluid POPC bilayers,40 whereas C16-Cer forms gel phase domains at >4 mol% Cer in POPC bilayers.14 It is likely that conjugation of the bulky coumarin substituent (with a partially deprotonated phenol) to the lipid headgroup is responsible for modifying the partitioning behavior of 1. Addition of bulky dyes to lipid headgroups also frequently modifies the partitioning of the lipid between ordered and disordered phases. 38
Photolysis of 1 leads to changes in the bilayer morphology, most notably a progressive decrease in domain size and in some cases the complete disappearance of the domains. Correlated AFM and fluorescence imaging indicate that small fluorescent regions appear in some Lo domains; both the height and fluorescence intensity of these features are similar to those of the bulk Ld phase. Similar changes are observed when bilayers are prepared with either 5 or 10 mol % 1. The domain reorganization is consistent with significant partitioning of the caged Cer into the ordered domains, as also suggested by the coumarin fluorescence images. We hypothesize that the reduction in domain size reflects loss of Chol in response to conversion of 1 to Cer; the area occupied by Cer will be smaller than that occupied by the more bulky caged Cer, which may also contribute to reduced domain area. Irradiations of small bilayer regions clearly demonstrate that the photo-uncaging strategy can be used to spatially restrict the areas in which the Cer-induced changes occur (eg. Figure 3).
Several AFM studies have demonstrated that bilayers formed from vesicles in which long-chain Cer (C16- or C18-Cer) has been premixed with ternary lipid mixtures display Cer-enriched domains in addition to Lo domains and a fluid phase.15–17, 19, 26 These studies indicate that ~8 mol % Cer is required for the formation of detectable domains in SM/DOPC/Chol membranes. Enzymatic, in situ Cer generation from SM by SMase treatment of preformed SM-containing bilayers resulted in the formation of new Cer-enriched regions; since the overall morphology is much more complex in enzyme-treated bilayers, it is likely that regions with high concentrations of Cer are formed by enzymatic catalysis.15, 18, 20, 23 By contrast to these results, photolysis of 1 provides no evidence for the formation of a new Cer-enriched phase, although there are clear changes in bilayer morphology when Cer is released in the supported bilayer. There are several possible explanations for the absence of Cer domains. Although the amount of Cer generated will be below the threshold for domain formation when 5 mol % 1 is incorporated in the initial vesicles, this is unlikely to be the case for bilayers containing 10 mol % 1, assuming efficient photolysis of the caged compound. This assumption is supported by experiments demonstrating that a 5 min irradiation is sufficient for >95% photodecomposition of 1 and other caged Cers using several different lamps for both aqueous dispersions and SUVs (as demonstrated in Figure S1 and in our earlier study30). This is further corroborated by the observation that longer irradiation periods do not induce additional changes in bilayer morphology, consistent with photolysis of all the caged Cer. It should also be noted that decreases in the fluorescence intensity in the coumarin channel confirm that the photolysed coumarin is released into the aqueous solution, rather than being retained in the bilayer. The presence of the hydrophilic hydroxyl group and the anionic phenolate at neutral pH make the product coumarin much less likely to localize in the bilayer, compared to other less hydrophilic coumarins.41, 42
A sub-threshold level of Cer for domain formation could also occur if the initial concentration of 1 in the supported bilayers is less than that in the initial vesicle suspension. Although it is generally assumed that the lipid ratios in supported bilayers are comparable to those in the bulk vesicle suspension, several recent studies demonstrate significant heterogeneity in composition for individual vesicles within a single sample.43–46 For example, Stamou and coworkers used a single vesicle fluorescence assay to demonstrate variations of up to an order of magnitude in the relative composition of individual liposomes.43, 44 The heterogeneity depended on the vesicle preparation method and size, with small vesicles (e.g., SUVs) exhibiting a greater degree of heterogeneity than large vesicles (GUVs). Heterogeneity in the initial vesicle population may lead to selection of a subset of vesicles during bilayer formation via vesicle fusion, since the kinetics for vesicle adsorption and rupture are strongly affected by lipid composition as well as the surface and additives in the aqueous solution.47 Recently, we have concluded that selection for a subset of vesicles from a heterogeneous vesicle population during bilayer formation may contribute to unexpected trends in morphology for bolalipid/POPC mixtures.48 In the present study, the presence of the bromohydroxycoumarin moiety, which is partially deprotonated at the pH used to form SUVs and supported bilayers in our experiments, may be an important factor. Variations in the net negative charge on the SUVs due to different fractions of 1 coupled with electrostatic repulsion between the vesicles and the negatively charged surface could lead to formation of a supported bilayer with a lower mol fraction of 1 than in the bulk vesicle suspension. Consistent with this explanation, attempts to incorporate >10 mol % 1 provided no evidence of increased Cer content in the supported lipid bilayers.
Although we believe that vesicle heterogeneity and selection for a subset of vesicles with less than the bulk concentration of 1 contribute to our results, there are alternate explanations for the absence of Cer-enriched domains. Comparison of results for Cer incorporation by premixing and enzymatic generation highlights the importance of non-equilibrium effects in determining the morphology for enzyme treated bilayers and monolayers.18, 22, 26, 49 It is possible that a similar effect (i.e., non-equilibrium lipid organization) occurs when Cer is generated by photo-uncaging. Diffusion of both 1 and Cer will be considerably slower in the Lo domains (where a significant fraction of 1 resides, based on coumarin fluorescence intensities) than in a fluid DOPC bilayer. For example, diffusion coefficients of 1.5 – 3.4 μM2/s and 0.11 – 0.16 μM2/s have been measured for Ld and Lo phases, respectively, by fluorescence correlation spectroscopy.50 It is also possible that lipid equilibration/membrane restructuring of SMase-treated bilayers occurs more rapidly than in the absence of enzyme since insertion of hydrophobic domains and translocation of the bound enzyme may perturb lipid packing and induce local disorder. Finally, the ability of Cer to form gel-phase domains is strongly modulated by the presence of Chol; at high Chol content gel domains are hypothesized to be replaced by a Chol-enriched Lo phase.14
The incorporation of caged C4-Cer (2) in DOPC/SM/Chol bilayers shows a similar behavior to that obtained for the C16-Cer analog. One important difference is the observation of a smaller height difference between the domains and fluid phase, both before and after photolysis (Figure 6). This is consistent with incorporation of 2 in the Lo domains, in agreement with the stronger coumarin fluorescence intensity in this phase. A previous study of the effects of Cers with various N-acyl chain lengths on bilayers with Lo domains containing C18-SM concluded that short-chain Cers differed from C16- and C18-Cer analogs in two respects.16 First, they did not promote the formation of Cer gel-phase domains. Second, they showed a strong preference for localization in Lo domains and led to a reduction in the fractional surface coverage of the domains and in the height difference between the domains and the surrounding Ld phase. Fluorescence correlation spectroscopy indicated that the diffusion coefficient for dye-labeled lipids in the Lo domains increased by a factor of 2–3 times when C6- and C2-Cers were incorporated in Lo domains, providing evidence for strong perturbation of lipid packing. By contrast, direct addition of C16- and C18-Cer had no effect on lipid diffusion within the Lo domains, although they did promote the formation of Cer-enriched gel-phase domains. These results are consistent with several other studies demonstrating that short-chain Cers are unable to stabilize rafts, have reduced capacity to displace Chol from ordered domains, and do not readily form gel-phase domains.9, 51, 52 It has been suggested that perturbation of lipid packing by C4- and C6-Cer may reflect wobbling of the N-acyl chain between the aqueous phase and the hydrophobic core of the bilayer.16 Finally, the addition of Cers with asymmetric chain lengths can lead to formation of mixed or partially interdigitated phases, which would reduce the bilayer thickness.53, 54 Overall, we conclude that the lack of Cer-enriched domains upon photolysis of 2 is consistent with the expected behavior of short-chain Cer.
Attempts to incorporate a higher mol fraction of 1 led to samples with many adherent vesicles. Interestingly, we observed that vesicles were readily removed by photolysis, which may indicate that loss of the bromohydroxycoumarin moiety renders the individual vesicles less “sticky”. It is also possible that generation of Cer promotes vesicle permeability and/or rupture. In this context, a number of recent examples have demonstrated that incorporation of photoswitchable surfactants or amphiphiles can be used to trigger membrane permeability for applications such as drug delivery.55–57 Chemical generation of Cer by coupling of sphingosine to fatty acid salts in lipid bilayers has been shown to alter membrane properties and promote vesicle fusion.58 Experiments aimed at testing the suitability of caged Cer photolysis for triggering vesicle permeability are in progress in our laboratory.
It is relevant to compare the present results on the photochemical generation of ceramide to two recent studies that have employed the photoisomerization of azobenzene to control lipid organization in bilayer membranes. In one example, cis-trans isomerization of an azobenzene moiety attached to the hydroxyl group of Chol induced shape changes in GUVs and disrupted liquid-ordered domains, leading to their disappearance or the incorporation of fluid phase regions.59 A second example used photoisomerization of an azobenzene –substituted amphiphile to promote phase separation for lipid mixtures with compositions close to a phase boundary.34 Changes in line tension due to conversion of trans-azobenzene localized in the bilayer headgroup region to the more bulky cis isomer were postulated to account for the observed effects.
CONCLUSIONS
In summary, the photorelease of Cer from caged molecules provides a method to modulate lipid organization with both spatial and temporal control. This provides a potentially useful alternative to the enzymatic generation of ceramide in model membranes. The generation of C16-Cer via photo-uncaging of 1 in DOPC/SM/Chol bilayers leads to changes in Lo domains that are distinct from both the direct incorporation of Cer and enzymatic Cer generation, most notably by the lack of Cer-rich gel-phase domains. The differences may reflect low incorporation of 1 in supported bilayers due to a heterogeneous population of vesicles and selective adsorption and rupture of vesicles containing low concentrations of caged Cer during bilayer formation; non-equilibrium lipid mixing may also contribute. Photorelease of a short-chain Cer results in qualitatively similar results, with disappearance of some Lo domains, but notably with a smaller height difference between the ordered and disordered phases.
Supplementary Material
Acknowledgments
DMCR and LJJ thank the Natural Sciences and Engineering Research Council for support in the form of a postgraduate scholarship and a Discovery Grant. RB acknowledges support from NIH Grant HL-083187. SPP acknowledges support from the Reactive Intermediates Student Exchange program.
Footnotes
The authors declare no competing financial interests
Five figures with data for the photolysis of 1 in vesicle solution and additional AFM and fluorescence images documenting the effects of incorporation and photolysis of 1 and 2 in supported lipid bilayers.
References
- 1.Hannun YA. Functions of ceramide in coordinating cellular responses to stress. Science. 1996;274:1855–1859. doi: 10.1126/science.274.5294.1855. [DOI] [PubMed] [Google Scholar]
- 2.Hannun YA, Obeid LM. Many ceramides. J Biol Chem. 2011;286:27855–27862. doi: 10.1074/jbc.R111.254359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stancevic B, Kolesnick R. Ceramide-rich platforms in transmembrane signaling. FEBS Lett. 2010;584:1728–1740. doi: 10.1016/j.febslet.2010.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang Y, Li X, Becker KA, Gulbins E. Ceramide-enriched membrane domains-structure and function. Biochim Biophys Acta. 2009;1788:178–183. doi: 10.1016/j.bbamem.2008.07.030. [DOI] [PubMed] [Google Scholar]
- 5.Perera MN, Ganesan V, Siskind LJ, Szulc ZM, Bielawski J, Bielawska A, Bittman R, Colombini M. Ceramide channels: Influence of molecular structure on channel formation in membranes. Biochim Biophys Acta. 2012;1818:1291–1301. doi: 10.1016/j.bbamem.2012.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fanani ML, Hartel S, Maggio B, De Tullio L, Jara J, Olmos F, Oliveira RG. The action of sphingomyelinase in lipid monolayers as revealed by microscopic image analysis. Biochim Biophys Acta. 2010;1798:1309–1323. doi: 10.1016/j.bbamem.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 7.Lopez-Montero I, Monroy F, Velez M, Devaux PF. Ceramide: From lateral segregation to mechanical stress. Biochim Biophys Acta. 2010;1798:1348–1356. doi: 10.1016/j.bbamem.2009.12.007. [DOI] [PubMed] [Google Scholar]
- 8.Zou S, Johnston LJ. Ceramide-enriched microdomains in planar membranes. Curr Opin Coll Inter Sci. 2010;15:489–498. [Google Scholar]
- 9.Goni FM, Alonso A. Effects of ceramide and other simple sphingolipids on membrane lateral structure. Biochim Biophys Acta. 2009;1788:169–177. doi: 10.1016/j.bbamem.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 10.Simons K, Ikonen E. Functional rafts in cell membranes. Nature. 1997;387:569–572. doi: 10.1038/42408. [DOI] [PubMed] [Google Scholar]
- 11.Edidin M. The state of lipid rafts: From model membranes to cells. Annu Rev Biophys Biomol Struct. 2003;32:257–283. doi: 10.1146/annurev.biophys.32.110601.142439. [DOI] [PubMed] [Google Scholar]
- 12.Pike LJ. The challenge of lipid rafts. J Lipid Res. 2009;50:S323–S328. doi: 10.1194/jlr.R800040-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Simons K, Gerl MJ. Revitalizing membrane rafts: New tools and insights. Nat Rev Mol Cell Biol. 2010;11:688–699. doi: 10.1038/nrm2977. [DOI] [PubMed] [Google Scholar]
- 14.Castro BM, Silva LC, Fedorov A, de Almeida RFM, Prieto M. Cholesterol-rich fluid membranes solubilize ceramide domains. Implications for the structure and dynamics of mammalian intracellular and plasma membranes. J Biol Chem. 2009;284:22978–22987. doi: 10.1074/jbc.M109.026567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chiantia S, Kahya N, Ries J, Schwille P. Effects of ceramide on liquid-ordered domains investigated by simultaneous afm and fcs. Biophys J. 2006;90:4500–4508. doi: 10.1529/biophysj.106.081026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chiantia S, Kahya N, Schwille P. Raft domain reorganization driven by short- and long-chain ceramide: A combined afm and fcs study. Langmuir. 2007;23:7659–7665. doi: 10.1021/la7010919. [DOI] [PubMed] [Google Scholar]
- 17.Sullan RMA, Li JK, Zou S. Quantification of the nanomechanical stability of ceramide-enriched domains. Langmuir. 2009;25:12874–12877. doi: 10.1021/la903442s. [DOI] [PubMed] [Google Scholar]
- 18.Carrer DC, Kummer E, Chwastek G, Chiantia S, Schwille P. Asymmetry determines the effects of natural ceramides on model membranes. Soft Matter. 2009;5:3279–3286. [Google Scholar]
- 19.Carter Ramirez DM, Ogilvie WW, Johnston LJ. Nbd cholesterol probes to track cholesterol distribution in model membranes. Biochim Biophys Acta. 2010;1798:558–568. doi: 10.1016/j.bbamem.2009.12.005. [DOI] [PubMed] [Google Scholar]
- 20.Chao L, Gast AP, Hatton TA, Jensen KF. Sphingomyelinase-induced phase transformations: Causing morphology switches and multiple time-domain ceramide generation in model raft membranes. Langmuir. 2010;26:344–356. doi: 10.1021/la902084u. [DOI] [PubMed] [Google Scholar]
- 21.Lopez-Montero I, Velez M, Devaux PF. Surface tension induced by sphingomyelin to ceramide conversion in lipid membranes. Biochim Biophys Acta. 2007;1768:553–561. doi: 10.1016/j.bbamem.2007.01.001. [DOI] [PubMed] [Google Scholar]
- 22.Ira, Johnston LJ. Sphingomyelinase generation of ceramide promotes clustering of nanoscale domains in supported bilayer membranes. Biochim Biophys Acta. 2008;1778:185–197. doi: 10.1016/j.bbamem.2007.09.021. [DOI] [PubMed] [Google Scholar]
- 23.Ira, Zou S, Carter Ramirez DM, Vanderlip S, Ogilvie W, Jakubek Z, Johnston LJ. Enzymatic generation of ceramide induces membrane restructuring: Correlated afm and fluorescence imaging of supported bilayers. J Struct Biol. 2009;168:78–79. doi: 10.1016/j.jsb.2009.03.014. [DOI] [PubMed] [Google Scholar]
- 24.Sot J, Ibarguren M, Busto JV, Montes LR, Goni FM, Alonso A. Cholesterol displacement by ceramide in sphingomyelin-containing liquid-ordered domains, and generation of gel regions in giant lipidic vesicles. FEBS Lett. 2008;582:3230–3236. doi: 10.1016/j.febslet.2008.08.016. [DOI] [PubMed] [Google Scholar]
- 25.Silva LC, de Almeida RFM, Castro BM, Fedorov A, Prieto M. Ceramide-domain formation and collapse in lipid rafts: Membrane reorganization by an apoptotic lipid. Biophys J. 2007;92:502–516. doi: 10.1529/biophysj.106.091876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ira, Johnston LJ. Ceramide promotes restructuring of model raft membranes. Langmuir. 2006;22:11284–11289. doi: 10.1021/la061636s. [DOI] [PubMed] [Google Scholar]
- 27.Brieke C, Rohrbach F, Gottschalk A, Mayer G, Heckel A. Light-controlled tools. Angew Chem, Int Ed Engl. 2012;51:8446–8476. doi: 10.1002/anie.201202134. [DOI] [PubMed] [Google Scholar]
- 28.Lee HM, Larson DR, Lawrence DS. Illuminating the chemistry of life: Design, synthesis and applications of “caged’ and related photoresponsive compounds. ACS Chem Biol. 2009;4:409–427. doi: 10.1021/cb900036s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yu H, Li J, Wu D, Qiu Z, Zhang Y. Chemistry and biological applications of photo-labile organic molecules. Chem Soc Rev. 2010;39:464–473. doi: 10.1039/b901255a. [DOI] [PubMed] [Google Scholar]
- 30.Kim YA, Carter Ramirez DM, Costain WJ, Johnston LJ, Bittman R. A new tool to assess ceramide bioactivity: 6-bromo-7-hydroxycoumarinyl-caged ceramide. Chem Commun. 2011;47:9236–9238. doi: 10.1039/c1cc12345a. [DOI] [PubMed] [Google Scholar]
- 31.Lankalapalli RS, Ouro A, Arana L, Gomez-Munoz A, Bittman R. Caged ceramide 1-phosphate analogues: Synthesis and properties. J Org Chem. 2009;74:8844–8847. doi: 10.1021/jo902076w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mentel M, Laketa V, Subramanian D, Gillandt H, Schultz C. Photoactivatable and cell-membrane-permeable phosphatidylinositol 3.4.5-triphosphate. Angew Chem, Int Ed. 2011;50:3811–3814. doi: 10.1002/anie.201007796. [DOI] [PubMed] [Google Scholar]
- 33.Shigenaga A, Hirakawa H, Yamamoto J, Ogura K, Denda M, Yamaguchi K, Tsuji D, Itoh K, Otaka A. Design and synthesis of caged ceramide: Uv-responsive ceramide releasing system based on uv-induced amide bond cleavage followed by o-n acyl transfer. Tetrahedron. 2011;67:3984–3990. [Google Scholar]
- 34.Hamada T, Sugimoto R, Nagasaki T, Takagi M. Photochemical control of membrane raft organization. Soft Matter. 2011;7:220–224. [Google Scholar]
- 35.Hamada T, Sugimoto R, Vestergaard MC, Nagasaki T, Takagi M. Membrane disk and sphere: Controllable mesoscopic structures for the capture and release of a targeted object. J Am Chem Soc. 2010;132:10528–10532. doi: 10.1021/ja103895b. [DOI] [PubMed] [Google Scholar]
- 36.Liu XM, Yang B, Wang YL, Wang JY. Photoisomerisable cholesterol derivatives as photo-trigger of liposomes: Effect of polarity, temperature, incorporation ratio and cholesterol. Biochim Biophys Acta. 2005;1720:28–34. doi: 10.1016/j.bbamem.2005.10.016. [DOI] [PubMed] [Google Scholar]
- 37.Silva LC, Futerman AH, Prieto M. Lipid raft composition modulates sphingomyelinase activity and ceramide-induced membrane physical alterations. Biophys J. 2009;96:3210–3222. doi: 10.1016/j.bpj.2008.12.3923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Baumgart T, Hunt G, Farkas ER, Webb WW, Feigenson GW. Fluorescence probe partitioning between lo/ld phases in lipid membranes. Biochim Biophys Acta. 2007;1768:2182–2194. doi: 10.1016/j.bbamem.2007.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Blanchette CD, Lin WC, Ratto TV, Longo ML. Galactosylceramide domain microstructure: Impact of cholesterol and nucleation/growth conditions. Biophys J. 2006;90:4466–4478. doi: 10.1529/biophysj.105.072744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Carter Ramirez DM. PhD Thesis. University of Ottawa; 2013. Fluorescent and photocaged lipids to probe the ceramide-mediated reorganization of biological membranes. [Google Scholar]
- 41.Paloncyova M, Berka K, Otyepka M. Convergence of free energy profile of coumarin in lipid bilayer. J Chem Theory Comput. 2012;8:1200–1211. doi: 10.1021/ct2009208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wojtowicz K. Comparison of the effect of 4-hydroxycoumarin and umbelliferone on the phase transition of dipalmitoylphosphatidylcholine (dppc) bilayers. Pharm Rep. 2008;60:555–560. [PubMed] [Google Scholar]
- 43.Elizondo E, Larsen J, Hatzakis NS, Cabrera I, Bjornholm T, Veciana J, Stamou D, Ventosa N. Influence of the preparation route on the supramolecular organization of lipids in a vesicular system. J Am Chem Soc. 2012;134:1918–1921. doi: 10.1021/ja2086678. [DOI] [PubMed] [Google Scholar]
- 44.Larsen J, Hatzakis NS, Stamou D. Observation of inhomogeneity in the lipid composition of individual nanoscale liposomes. J Am Chem Soc. 2011;133:10685–10687. doi: 10.1021/ja203984j. [DOI] [PubMed] [Google Scholar]
- 45.Buboltz JT, Bwalya C, Williams K, Schutzer M. High resolution mapping of phase behavior in a ternary lipid mixture: Do lipid-raft phase boundaries depend on the sample preparation procedure? Langmuir. 2007;23:11968–11971. doi: 10.1021/la702490r. [DOI] [PubMed] [Google Scholar]
- 46.Veatch SL, Keller SL. Seeing spots: Complex phase behavior in simple membranes. Biochim Biophys Acta. 2005;1746:172–185. doi: 10.1016/j.bbamcr.2005.06.010. [DOI] [PubMed] [Google Scholar]
- 47.Richter RP, Berat R, Brisson AR. Formation of solid-supported lipid bilayers: An integrated view. Langmuir. 2006;22:3497–3505. doi: 10.1021/la052687c. [DOI] [PubMed] [Google Scholar]
- 48.Mulligan K, Brownholland D, Carnini A, Thompson DH, Johnston LJ. Afm investigations of phase separation in supported membranes of binary mixtures of popc and an eicosanyl-based bisphosphocholine bolalipid. Langmuir. 2010;26:8525–8533. doi: 10.1021/la904532s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fanani ML, De Tullio L, Hartel S, Jara J, Maggio B. Sphingomyelinase-induced domain shape relaxation driven by out-of-equilibrium changes of composition. Biophys J. 2009;96:67–76. doi: 10.1529/biophysj.108.141499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Machan R, Hof M. Lipid diffusion in planar membranes investigated by fluorescence correlation spectroscopy. Biochim Biophys Acta. 2010;1798:1377–1391. doi: 10.1016/j.bbamem.2010.02.014. [DOI] [PubMed] [Google Scholar]
- 51.Megha, Sawatzki P, Kolter T, Bittman R, London E. Effect of ceramide n-acyl chain and polar headgroup structure on the properties of ordered lipid domains (lipid rafts) Biochim Biophys Acta. 2007;1768:2205–2212. doi: 10.1016/j.bbamem.2007.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nyholm TKM, Grandell PM, Westerlund B, Slotte JP. Sterol affinity for bilayer membranes is affected by their ceramide content and the ceramide chain length. Biochim Biophys Acta. 2010;1798:1008–1013. doi: 10.1016/j.bbamem.2009.12.025. [DOI] [PubMed] [Google Scholar]
- 53.Carrer DC, Schreier S, Patrito M, Maggio B. Effects of a short-chain ceramide on bilayer domain formation, thickness, and chain mobililty: Dmpc and asymmetric ceramide mixtures. Biophys J. 2006;90:2394–2403. doi: 10.1529/biophysj.105.074252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pinto SN, Silva LC, de Almeida RFM, Prieto M. Membrane domain formation, interdigitation, and morphological alterations induced by the very long chain asymmetric c24:1 ceramide. Biophys J. 2008;95:2867–2879. doi: 10.1529/biophysj.108.129858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Diguet A, Yanagisawa M, Liu YJ, Brun E, Abadie S, Rudiuk S, Baigl D. Uv-induced bursting of cell-sized multicomponent lipid vesicles in a photosensitive surfactant solution. J Am Chem Soc. 2012;134:4898–4904. doi: 10.1021/ja211664f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sebai SC, Milioni D, Walrant A, Alves ID, Sagan S, Huin C, Auvray L, Massotte D, Cribier S, Tribet C. Photocontrol of the translocation of molecules, peptides and quantum dots through cell and lipid membranes doped with azobenzene copolymers. Angew Chem, Int Ed Engl. 2012;51:2132–2136. doi: 10.1002/anie.201106777. [DOI] [PubMed] [Google Scholar]
- 57.Uda RM, Hiraishi E, Ohnishi R, Nakahara Y, Kimura K. Morphological changes in vesicles and release of an encapsulated compound triggered by a photoresponsive malachite green leuconitrile derivative. Langmuir. 2010;26:5444–5450. doi: 10.1021/la904190c. [DOI] [PubMed] [Google Scholar]
- 58.Kunishima M, Tokaji M, Matsuoka K, Nishida J, Kanamori M, Hoki K, Tani S. Spontaneous membrane fusion induced by chemical formation of ceramides in a lipid bilayer. J Am Chem Soc. 2006;128:14452–14453. doi: 10.1021/ja0652969. [DOI] [PubMed] [Google Scholar]
- 59.Yasuhara K, Sasaki Y, Kikuchi J. A photo-responsive cholesterol capable of inducing a morphological transformation of the liquid-ordered microdomain in lipid bilayers. Colloid Polym Sci. 2008;286:1675–1680. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.




