Abstract
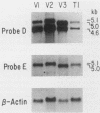
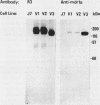
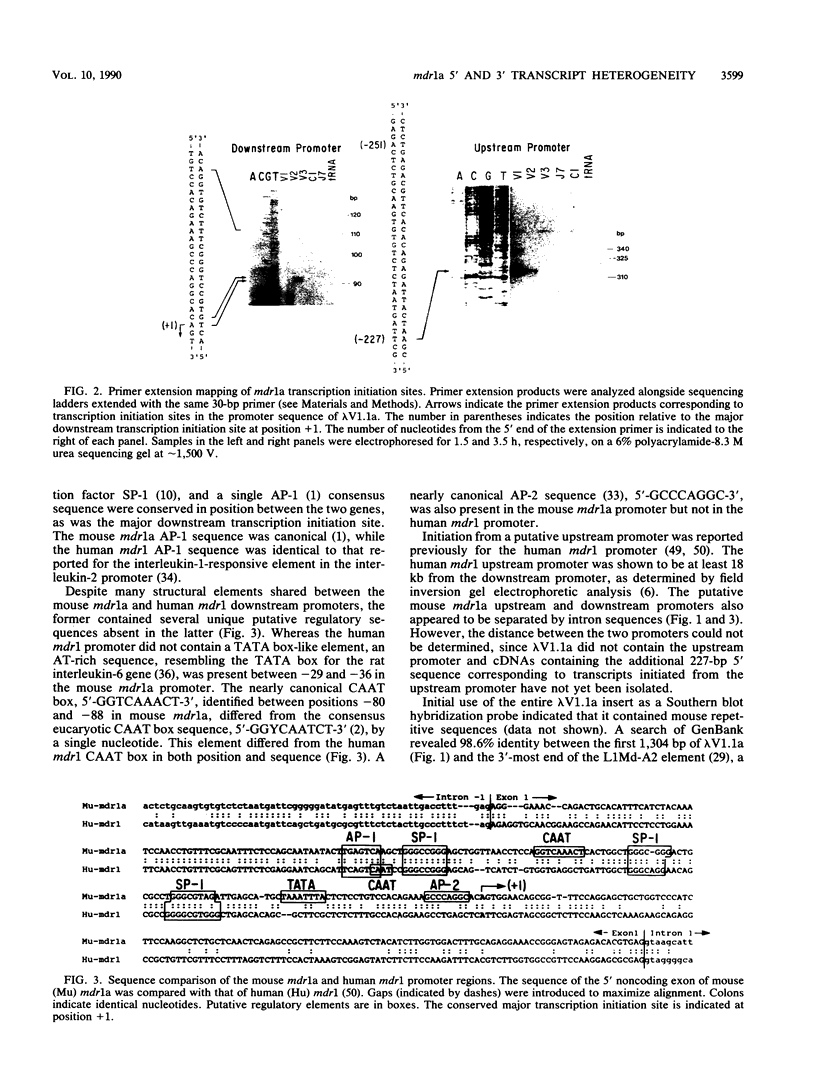
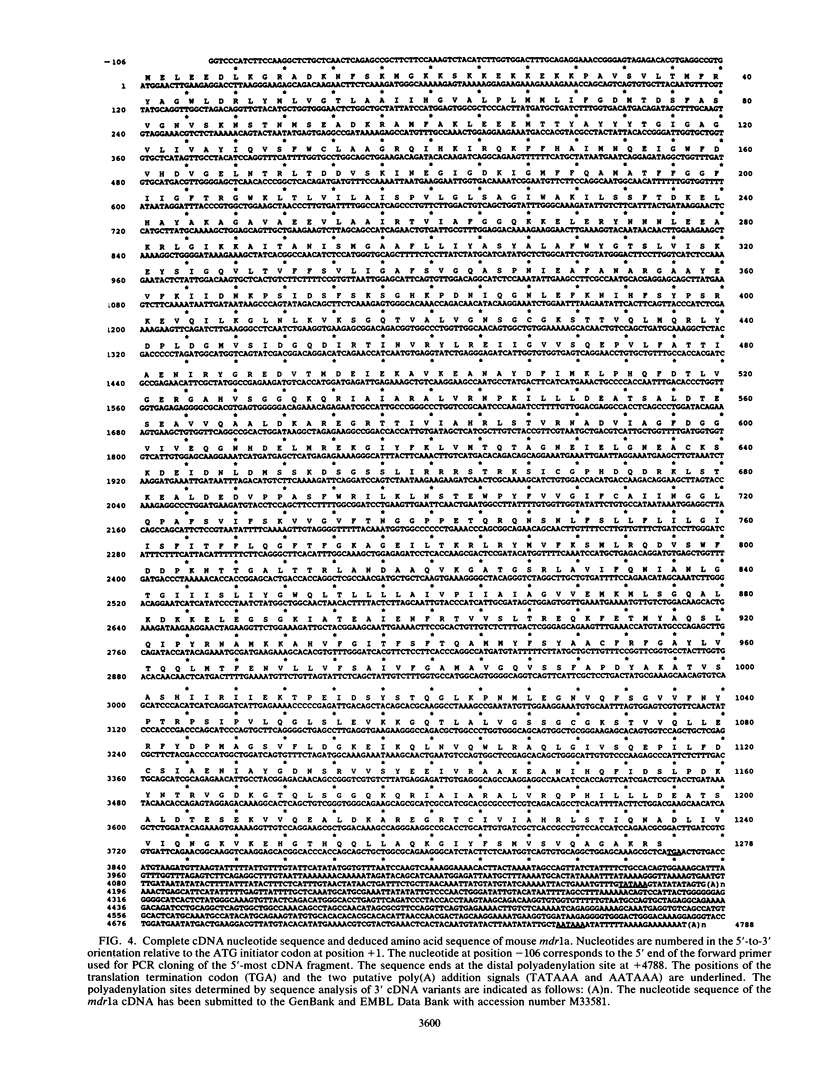
In multidrug-resistant mouse J774.2 cells, the differential overproduction of functionally distinct phosphoglycoprotein isoforms reflects the amplification or transcriptional activation or both of two mdr gene family members, mdr1a and mdr1b. The mdr1a gene is a complex transcriptional unit whose expression is associated with multiple transcript sizes. Independently selected multidrug-resistant J774.2 cell lines differentially overexpress either 4.6- and 5.0-kilobase (kb) or 4.7- and 5.1-kb mdr1a transcripts. However, abundant overproduction of the mdr1a gene product was observed only in cell lines which overexpressed the 4.6- and 5.0-kb mRNAs. In order to determine the basis for mdr1a transcript heterogeneity and the relationship between transcript size and steady-state mdr1a protein levels, genomic and cDNA sequence analyses of the 5' and 3' ends of the mdr1a gene were carried out. Promoter sequence analysis and primer extension mapping indicated that mdr1a transcripts were differentially initiated from two putative promoters to generate either 5.1- and 4.7-kb or 5.0- and 4.6-kb transcripts in four multidrug-resistant J774.2 cell lines. Sequence analysis of 3' cDNA variants and a 3' genomic fragment revealed that the 5.1- and 5.0-kb mRNAs had identical 3'-untranslated regions which differed from those of the 4.7- and 4.6-kb mRNAs as a result of the utilization of a more downstream alternative poly(A) addition signal. Transcript initiation from the putative upstream promoter correlated with a 70 to 85% decrease in steady-state mdr1a protein levels relative to transcript levels. In addition, the identification of putative AP-1 and AP-2 promoter elements suggests a possible role for protein kinase A and protein kinase C in the regulation of mdr1a. The implications of these findings for mdr gene expression and regulation are discussed.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Angel P., Imagawa M., Chiu R., Stein B., Imbra R. J., Rahmsdorf H. J., Jonat C., Herrlich P., Karin M. Phorbol ester-inducible genes contain a common cis element recognized by a TPA-modulated trans-acting factor. Cell. 1987 Jun 19;49(6):729–739. doi: 10.1016/0092-8674(87)90611-8. [DOI] [PubMed] [Google Scholar]
- Benoist C., O'Hare K., Breathnach R., Chambon P. The ovalbumin gene-sequence of putative control regions. Nucleic Acids Res. 1980 Jan 11;8(1):127–142. doi: 10.1093/nar/8.1.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burt R. K., Thorgeirsson S. S. Coinduction of MDR-1 multidrug-resistance and cytochrome P-450 genes in rat liver by xenobiotics. J Natl Cancer Inst. 1988 Nov 2;80(17):1383–1386. doi: 10.1093/jnci/80.17.1383. [DOI] [PubMed] [Google Scholar]
- Carr B. I. Pleiotropic drug resistance in hepatocytes induced by carcinogens administered to rats. Cancer Res. 1987 Nov 1;47(21):5577–5583. [PubMed] [Google Scholar]
- Chen C. J., Chin J. E., Ueda K., Clark D. P., Pastan I., Gottesman M. M., Roninson I. B. Internal duplication and homology with bacterial transport proteins in the mdr1 (P-glycoprotein) gene from multidrug-resistant human cells. Cell. 1986 Nov 7;47(3):381–389. doi: 10.1016/0092-8674(86)90595-7. [DOI] [PubMed] [Google Scholar]
- Chin J. E., Soffir R., Noonan K. E., Choi K., Roninson I. B. Structure and expression of the human MDR (P-glycoprotein) gene family. Mol Cell Biol. 1989 Sep;9(9):3808–3820. doi: 10.1128/mcb.9.9.3808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu R., Boyle W. J., Meek J., Smeal T., Hunter T., Karin M. The c-Fos protein interacts with c-Jun/AP-1 to stimulate transcription of AP-1 responsive genes. Cell. 1988 Aug 12;54(4):541–552. doi: 10.1016/0092-8674(88)90076-1. [DOI] [PubMed] [Google Scholar]
- Croop J. M., Raymond M., Haber D., Devault A., Arceci R. J., Gros P., Housman D. E. The three mouse multidrug resistance (mdr) genes are expressed in a tissue-specific manner in normal mouse tissues. Mol Cell Biol. 1989 Mar;9(3):1346–1350. doi: 10.1128/mcb.9.3.1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dano K. Active outward transport of daunomycin in resistant Ehrlich ascites tumor cells. Biochim Biophys Acta. 1973 Oct 25;323(3):466–483. doi: 10.1016/0005-2736(73)90191-0. [DOI] [PubMed] [Google Scholar]
- Dynan W. S., Tjian R. The promoter-specific transcription factor Sp1 binds to upstream sequences in the SV40 early promoter. Cell. 1983 Nov;35(1):79–87. doi: 10.1016/0092-8674(83)90210-6. [DOI] [PubMed] [Google Scholar]
- Endicott J. A., Ling V. The biochemistry of P-glycoprotein-mediated multidrug resistance. Annu Rev Biochem. 1989;58:137–171. doi: 10.1146/annurev.bi.58.070189.001033. [DOI] [PubMed] [Google Scholar]
- Fairchild C. R., Ivy S. P., Rushmore T., Lee G., Koo P., Goldsmith M. E., Myers C. E., Farber E., Cowan K. H. Carcinogen-induced mdr overexpression is associated with xenobiotic resistance in rat preneoplastic liver nodules and hepatocellular carcinomas. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7701–7705. doi: 10.1073/pnas.84.21.7701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein L. J., Galski H., Fojo A., Willingham M., Lai S. L., Gazdar A., Pirker R., Green A., Crist W., Brodeur G. M. Expression of a multidrug resistance gene in human cancers. J Natl Cancer Inst. 1989 Jan 18;81(2):116–124. doi: 10.1093/jnci/81.2.116. [DOI] [PubMed] [Google Scholar]
- Greenberger L. M., Lothstein L., Williams S. S., Horwitz S. B. Distinct P-glycoprotein precursors are overproduced in independently isolated drug-resistant cell lines. Proc Natl Acad Sci U S A. 1988 Jun;85(11):3762–3766. doi: 10.1073/pnas.85.11.3762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberger L. M., Williams S. S., Georges E., Ling V., Horwitz S. B. Electrophoretic analysis of P-glycoproteins produced by mouse J774.2 and Chinese hamster ovary multidrug-resistant cells. J Natl Cancer Inst. 1988 Jun 1;80(7):506–510. doi: 10.1093/jnci/80.7.506. [DOI] [PubMed] [Google Scholar]
- Greenberger L. M., Williams S. S., Horwitz S. B. Biosynthesis of heterogeneous forms of multidrug resistance-associated glycoproteins. J Biol Chem. 1987 Oct 5;262(28):13685–13689. [PubMed] [Google Scholar]
- Gros P., Ben Neriah Y. B., Croop J. M., Housman D. E. Isolation and expression of a complementary DNA that confers multidrug resistance. Nature. 1986 Oct 23;323(6090):728–731. doi: 10.1038/323728a0. [DOI] [PubMed] [Google Scholar]
- Gros P., Croop J., Housman D. Mammalian multidrug resistance gene: complete cDNA sequence indicates strong homology to bacterial transport proteins. Cell. 1986 Nov 7;47(3):371–380. doi: 10.1016/0092-8674(86)90594-5. [DOI] [PubMed] [Google Scholar]
- Gros P., Raymond M., Bell J., Housman D. Cloning and characterization of a second member of the mouse mdr gene family. Mol Cell Biol. 1988 Jul;8(7):2770–2778. doi: 10.1128/mcb.8.7.2770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan K. L., Weiner H. Influence of the 5'-end region of aldehyde dehydrogenase mRNA on translational efficiency. Potential secondary structure inhibition of translation in vitro. J Biol Chem. 1989 Oct 25;264(30):17764–17769. [PubMed] [Google Scholar]
- Henikoff S. Unidirectional digestion with exonuclease III creates targeted breakpoints for DNA sequencing. Gene. 1984 Jun;28(3):351–359. doi: 10.1016/0378-1119(84)90153-7. [DOI] [PubMed] [Google Scholar]
- Hsu S. I., Lothstein L., Horwitz S. B. Differential overexpression of three mdr gene family members in multidrug-resistant J774.2 mouse cells. Evidence that distinct P-glycoprotein precursors are encoded by unique mdr genes. J Biol Chem. 1989 Jul 15;264(20):12053–12062. [PubMed] [Google Scholar]
- Imagawa M., Chiu R., Karin M. Transcription factor AP-2 mediates induction by two different signal-transduction pathways: protein kinase C and cAMP. Cell. 1987 Oct 23;51(2):251–260. doi: 10.1016/0092-8674(87)90152-8. [DOI] [PubMed] [Google Scholar]
- Inaba M., Fujikura R., Sakurai Y. Active efflux common to vincristine and daunorubicin in vincristine-resistant P388 leukemia. Biochem Pharmacol. 1981 Jul 1;30(13):1863–1865. doi: 10.1016/0006-2952(81)90027-7. [DOI] [PubMed] [Google Scholar]
- Juliano R. L., Ling V. A surface glycoprotein modulating drug permeability in Chinese hamster ovary cell mutants. Biochim Biophys Acta. 1976 Nov 11;455(1):152–162. doi: 10.1016/0005-2736(76)90160-7. [DOI] [PubMed] [Google Scholar]
- Kozak M. Circumstances and mechanisms of inhibition of translation by secondary structure in eucaryotic mRNAs. Mol Cell Biol. 1989 Nov;9(11):5134–5142. doi: 10.1128/mcb.9.11.5134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loeb D. D., Padgett R. W., Hardies S. C., Shehee W. R., Comer M. B., Edgell M. H., Hutchison C. A., 3rd The sequence of a large L1Md element reveals a tandemly repeated 5' end and several features found in retrotransposons. Mol Cell Biol. 1986 Jan;6(1):168–182. doi: 10.1128/mcb.6.1.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lothstein L., Hsu S. I., Horwitz S. B., Greenberger L. M. Alternate overexpression of two P-glycoprotein [corrected] genes is associated with changes in multidrug resistance in a J774.2 cell line. J Biol Chem. 1989 Sep 25;264(27):16054–16058. [PubMed] [Google Scholar]
- Mellado W., Horwitz S. B. Phosphorylation of the multidrug resistance associated glycoprotein. Biochemistry. 1987 Nov 3;26(22):6900–6904. doi: 10.1021/bi00396a005. [DOI] [PubMed] [Google Scholar]
- Mellon P. L., Clegg C. H., Correll L. A., McKnight G. S. Regulation of transcription by cyclic AMP-dependent protein kinase. Proc Natl Acad Sci U S A. 1989 Jul;86(13):4887–4891. doi: 10.1073/pnas.86.13.4887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell P. J., Wang C., Tjian R. Positive and negative regulation of transcription in vitro: enhancer-binding protein AP-2 is inhibited by SV40 T antigen. Cell. 1987 Sep 11;50(6):847–861. doi: 10.1016/0092-8674(87)90512-5. [DOI] [PubMed] [Google Scholar]
- Muegge K., Williams T. M., Kant J., Karin M., Chiu R., Schmidt A., Siebenlist U., Young H. A., Durum S. K. Interleukin-1 costimulatory activity on the interleukin-2 promoter via AP-1. Science. 1989 Oct 13;246(4927):249–251. doi: 10.1126/science.2799385. [DOI] [PubMed] [Google Scholar]
- Ng W. F., Sarangi F., Zastawny R. L., Veinot-Drebot L., Ling V. Identification of members of the P-glycoprotein multigene family. Mol Cell Biol. 1989 Mar;9(3):1224–1232. doi: 10.1128/mcb.9.3.1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Northemann W., Braciak T. A., Hattori M., Lee F., Fey G. H. Structure of the rat interleukin 6 gene and its expression in macrophage-derived cells. J Biol Chem. 1989 Sep 25;264(27):16072–16082. [PubMed] [Google Scholar]
- Okuda A., Sakai M., Muramatsu M. The structure of the rat glutathione S-transferase P gene and related pseudogenes. J Biol Chem. 1987 Mar 15;262(8):3858–3863. [PubMed] [Google Scholar]
- Parkin N., Darveau A., Nicholson R., Sonenberg N. cis-acting translational effects of the 5' noncoding region of c-myc mRNA. Mol Cell Biol. 1988 Jul;8(7):2875–2883. doi: 10.1128/mcb.8.7.2875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riordan J. R., Deuchars K., Kartner N., Alon N., Trent J., Ling V. Amplification of P-glycoprotein genes in multidrug-resistant mammalian cell lines. 1985 Aug 29-Sep 4Nature. 316(6031):817–819. doi: 10.1038/316817a0. [DOI] [PubMed] [Google Scholar]
- Sakai M., Okuda A., Hatayama I., Sato K., Nishi S., Muramatsu M. Structure and expression of the rat c-jun messenger RNA: tissue distribution and increase during chemical hepatocarcinogenesis. Cancer Res. 1989 Oct 15;49(20):5633–5637. [PubMed] [Google Scholar]
- Sakai M., Okuda A., Muramatsu M. Multiple regulatory elements and phorbol 12-O-tetradecanoate 13-acetate responsiveness of the rat placental glutathione transferase gene. Proc Natl Acad Sci U S A. 1988 Dec;85(24):9456–9460. doi: 10.1073/pnas.85.24.9456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar D., Erlichman J., Rubin C. S. Identification of a calmodulin-binding protein that co-purifies with the regulatory subunit of brain protein kinase II. J Biol Chem. 1984 Aug 10;259(15):9840–9846. [PubMed] [Google Scholar]
- Shen D. W., Fojo A., Chin J. E., Roninson I. B., Richert N., Pastan I., Gottesman M. M. Human multidrug-resistant cell lines: increased mdr1 expression can precede gene amplification. Science. 1986 May 2;232(4750):643–645. doi: 10.1126/science.3457471. [DOI] [PubMed] [Google Scholar]
- Skovsgaard T. Mechanism of cross-resistance between vincristine and daunorubicin in Ehrlich ascites tumor cells. Cancer Res. 1978 Dec;38(12):4722–4727. [PubMed] [Google Scholar]
- Thorgeirsson S. S., Huber B. E., Sorrell S., Fojo A., Pastan I., Gottesman M. M. Expression of the multidrug-resistant gene in hepatocarcinogenesis and regenerating rat liver. Science. 1987 May 29;236(4805):1120–1122. doi: 10.1126/science.3576227. [DOI] [PubMed] [Google Scholar]
- Toda K., Terashima M., Mitsuuchi Y., Yamasaki Y., Yokoyama Y., Nojima S., Ushiro H., Maeda T., Yamamoto Y., Sagara Y. Alternative usage of different poly(A) addition signals for two major species of mRNA encoding human aromatase P-450. FEBS Lett. 1989 Apr 24;247(2):371–376. doi: 10.1016/0014-5793(89)81373-0. [DOI] [PubMed] [Google Scholar]
- Ueda K., Cardarelli C., Gottesman M. M., Pastan I. Expression of a full-length cDNA for the human "MDR1" gene confers resistance to colchicine, doxorubicin, and vinblastine. Proc Natl Acad Sci U S A. 1987 May;84(9):3004–3008. doi: 10.1073/pnas.84.9.3004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda K., Clark D. P., Chen C. J., Roninson I. B., Gottesman M. M., Pastan I. The human multidrug resistance (mdr1) gene. cDNA cloning and transcription initiation. J Biol Chem. 1987 Jan 15;262(2):505–508. [PubMed] [Google Scholar]
- Ueda K., Pastan I., Gottesman M. M. Isolation and sequence of the promoter region of the human multidrug-resistance (P-glycoprotein) gene. J Biol Chem. 1987 Dec 25;262(36):17432–17436. [PubMed] [Google Scholar]
- Van der Bliek A. M., Baas F., Van der Velde-Koerts T., Biedler J. L., Meyers M. B., Ozols R. F., Hamilton T. C., Joenje H., Borst P. Genes amplified and overexpressed in human multidrug-resistant cell lines. Cancer Res. 1988 Nov 1;48(21):5927–5932. [PubMed] [Google Scholar]
- Zeheb R., Beittenmiller H. F., Horwitz S. B. Use of antibodies to probe membrane glycoproteins associated with drug-resistant J774.2 cells. Biochem Biophys Res Commun. 1987 Mar 13;143(2):732–739. doi: 10.1016/0006-291x(87)91415-x. [DOI] [PubMed] [Google Scholar]
- van der Bliek A. M., Borst P. Multidrug resistance. Adv Cancer Res. 1989;52:165–203. doi: 10.1016/s0065-230x(08)60213-4. [DOI] [PubMed] [Google Scholar]
- van der Bliek A. M., Kooiman P. M., Schneider C., Borst P. Sequence of mdr3 cDNA encoding a human P-glycoprotein. Gene. 1988 Nov 30;71(2):401–411. doi: 10.1016/0378-1119(88)90057-1. [DOI] [PubMed] [Google Scholar]