Abstract
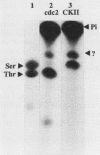
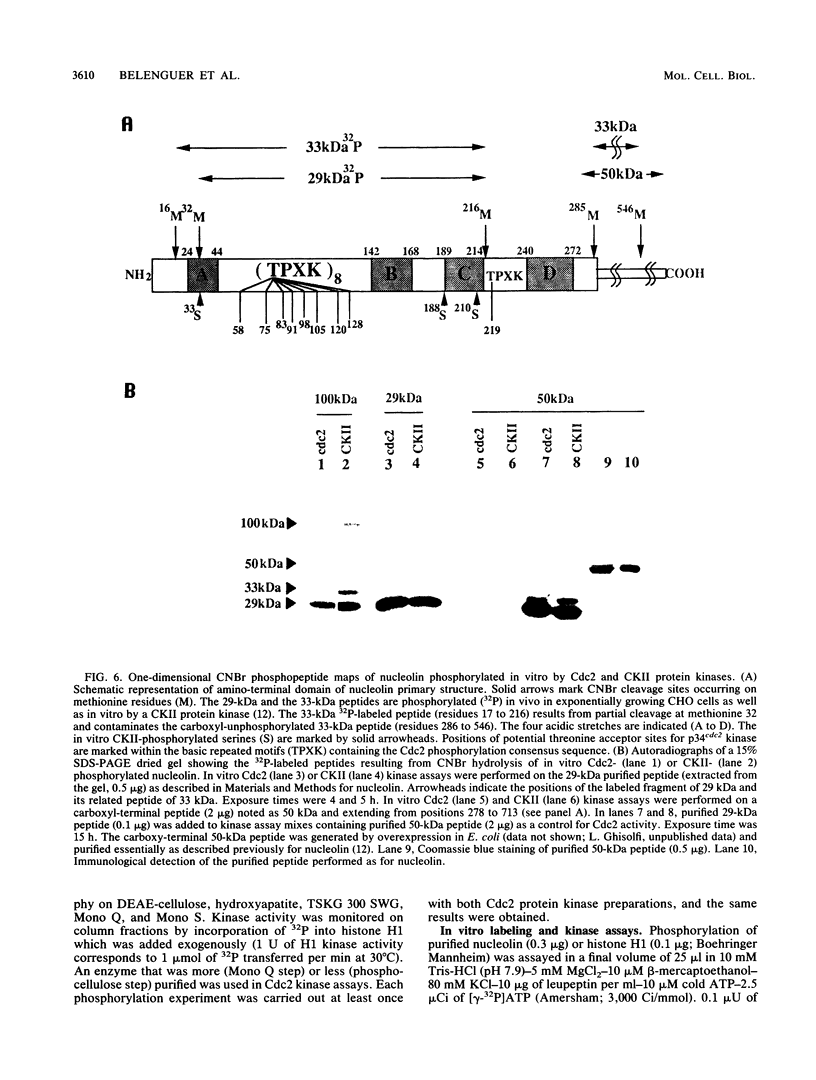
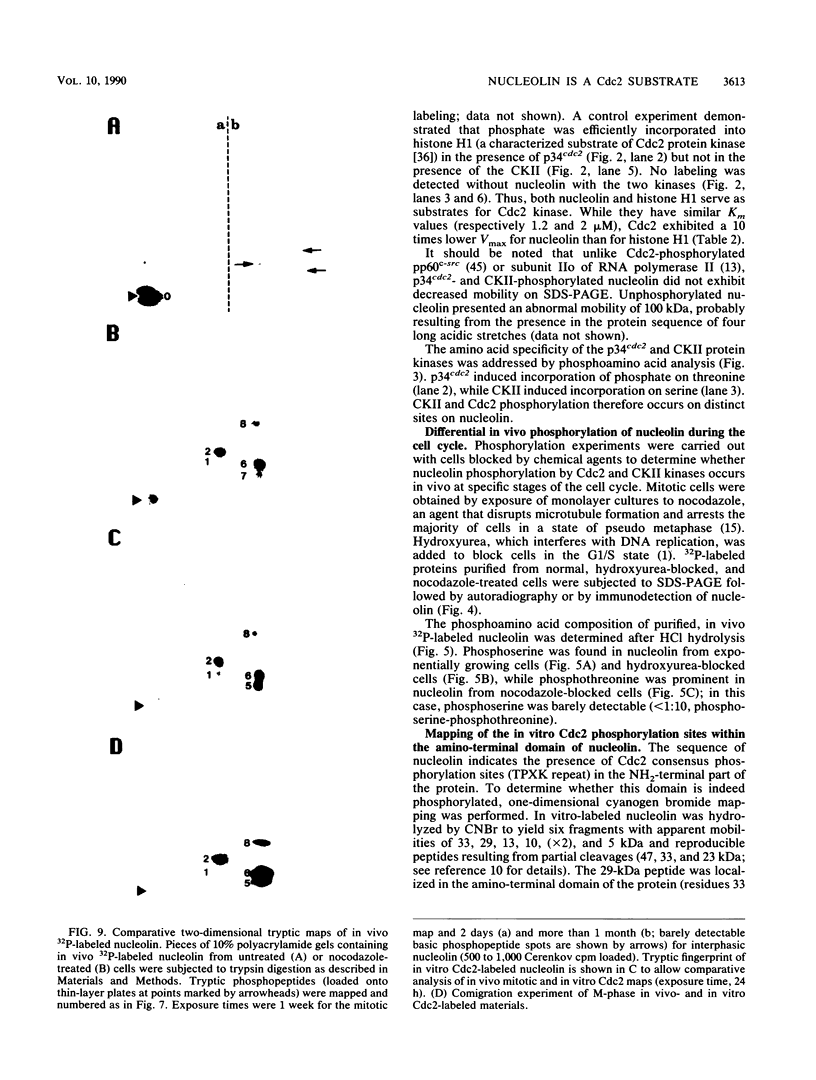
Nucleolin is a ubiquitous multifunctional protein involved in preribosome assembly and associated with both nucleolar chromatin in interphase and nucleolar organizer regions on metaphasic chromosomes in mitosis. Extensive nucleolin phosphorylation by a casein kinase (CKII) occurs on serine in growing cells. Here we report that while CKII phosphorylation is achieved in interphase, threonine phosphorylation occurs during mitosis. We provide evidence that this type of in vivo phosphorylation involves a mammalian homolog of the cell cycle control Cdc2 kinase. In vitro M-phase H1 kinase from starfish oocytes phosphorylated threonines in a TPXK motif present nine times in the amino-terminal part of the protein. The same sites which matched the p34cdc2 consensus phosphorylation sequence were used in vivo during mitosis. We propose that successive Cdc2 and CKII phosphorylation could modulate nucleolin function in controlling cell cycle-dependent nucleolar function and organization. Our results, along with previous studies, suggest that while serine phosphorylation is related to nucleolin function in the control of rDNA transcription, threonine phosphorylation is linked to mitotic reorganization of nucleolar chromatin.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams R. L., Lindsay J. G. Hydroxyurea reversal of inhibition and use as a cell-synchronizing agent. J Biol Chem. 1967 Mar 25;242(6):1314–1317. [PubMed] [Google Scholar]
- Allan J., Hartman P. G., Crane-Robinson C., Aviles F. X. The structure of histone H1 and its location in chromatin. Nature. 1980 Dec 25;288(5792):675–679. doi: 10.1038/288675a0. [DOI] [PubMed] [Google Scholar]
- Arion D., Meijer L., Brizuela L., Beach D. cdc2 is a component of the M phase-specific histone H1 kinase: evidence for identity with MPF. Cell. 1988 Oct 21;55(2):371–378. doi: 10.1016/0092-8674(88)90060-8. [DOI] [PubMed] [Google Scholar]
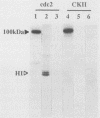
- Belenguer P., Baldin V., Mathieu C., Prats H., Bensaid M., Bouche G., Amalric F. Protein kinase NII and the regulation of rDNA transcription in mammalian cells. Nucleic Acids Res. 1989 Aug 25;17(16):6625–6636. doi: 10.1093/nar/17.16.6625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourbon H. M., Lapeyre B., Amalric F. Structure of the mouse nucleolin gene. The complete sequence reveals that each RNA binding domain is encoded by two independent exons. J Mol Biol. 1988 Apr 20;200(4):627–638. doi: 10.1016/0022-2836(88)90476-7. [DOI] [PubMed] [Google Scholar]
- Bradbury E. M., Inglis R. J., Matthews H. R., Sarner N. Phosphorylation of very-lysine-rich histone in Physarum polycephalum. Correlation with chromosome condensation. Eur J Biochem. 1973 Feb 15;33(1):131–139. doi: 10.1111/j.1432-1033.1973.tb02664.x. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brizuela L., Draetta G., Beach D. Activation of human CDC2 protein as a histone H1 kinase is associated with complex formation with the p62 subunit. Proc Natl Acad Sci U S A. 1989 Jun;86(12):4362–4366. doi: 10.1073/pnas.86.12.4362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bugler B., Bourbon H., Lapeyre B., Wallace M. O., Chang J. H., Amalric F., Olson M. O. RNA binding fragments from nucleolin contain the ribonucleoprotein consensus sequence. J Biol Chem. 1987 Aug 15;262(23):10922–10925. [PubMed] [Google Scholar]
- Bugler B., Caizergues-Ferrer M., Bouche G., Bourbon H., Amalric F. Detection and localization of a class of proteins immunologically related to a 100-kDa nucleolar protein. Eur J Biochem. 1982 Nov 15;128(2-3):475–480. doi: 10.1111/j.1432-1033.1982.tb06989.x. [DOI] [PubMed] [Google Scholar]
- Caboche M., Bachellerie J. P. RNA methylation and control of eukaryotic RNA biosynthesis. Effects of cycloleucine, a specific inhibitor of methylation, on ribosomal RNA maturation. Eur J Biochem. 1977 Mar 15;74(1):19–29. doi: 10.1111/j.1432-1033.1977.tb11362.x. [DOI] [PubMed] [Google Scholar]
- Caizergues-Ferrer M., Belenguer P., Lapeyre B., Amalric F., Wallace M. O., Olson M. O. Phosphorylation of nucleolin by a nucleolar type NII protein kinase. Biochemistry. 1987 Dec 1;26(24):7876–7883. doi: 10.1021/bi00398a051. [DOI] [PubMed] [Google Scholar]
- Cisek L. J., Corden J. L. Phosphorylation of RNA polymerase by the murine homologue of the cell-cycle control protein cdc2. Nature. 1989 Jun 29;339(6227):679–684. doi: 10.1038/339679a0. [DOI] [PubMed] [Google Scholar]
- Cooper J. A., Sefton B. M., Hunter T. Detection and quantification of phosphotyrosine in proteins. Methods Enzymol. 1983;99:387–402. doi: 10.1016/0076-6879(83)99075-4. [DOI] [PubMed] [Google Scholar]
- De Brabander M. J., Van de Veire R. M., Aerts F. E., Borgers M., Janssen P. A. The effects of methyl (5-(2-thienylcarbonyl)-1H-benzimidazol-2-yl) carbamate, (R 17934; NSC 238159), a new synthetic antitumoral drug interfering with microtubules, on mammalian cells cultured in vitro. Cancer Res. 1976 Mar;36(3):905–916. [PubMed] [Google Scholar]
- Dunphy W. G., Brizuela L., Beach D., Newport J. The Xenopus cdc2 protein is a component of MPF, a cytoplasmic regulator of mitosis. Cell. 1988 Jul 29;54(3):423–431. doi: 10.1016/0092-8674(88)90205-x. [DOI] [PubMed] [Google Scholar]
- Earnshaw W. C. Anionic regions in nuclear proteins. J Cell Biol. 1987 Oct;105(4):1479–1482. doi: 10.1083/jcb.105.4.1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elder J. H., Pickett R. A., 2nd, Hampton J., Lerner R. A. Radioiodination of proteins in single polyacrylamide gel slices. Tryptic peptide analysis of all the major members of complex multicomponent systems using microgram quantities of total protein. J Biol Chem. 1977 Sep 25;252(18):6510–6515. [PubMed] [Google Scholar]
- Erard M. S., Belenguer P., Caizergues-Ferrer M., Pantaloni A., Amalric F. A major nucleolar protein, nucleolin, induces chromatin decondensation by binding to histone H1. Eur J Biochem. 1988 Aug 15;175(3):525–530. doi: 10.1111/j.1432-1033.1988.tb14224.x. [DOI] [PubMed] [Google Scholar]
- Gas N., Escande M. L., Stevens B. J. Immunolocalization of the 100 kDa nucleolar protein during the mitotic cycle in CHO cells. Biol Cell. 1985;53(3):209–218. doi: 10.1111/j.1768-322x.1985.tb00369.x. [DOI] [PubMed] [Google Scholar]
- Gautier J., Norbury C., Lohka M., Nurse P., Maller J. Purified maturation-promoting factor contains the product of a Xenopus homolog of the fission yeast cell cycle control gene cdc2+. Cell. 1988 Jul 29;54(3):433–439. doi: 10.1016/0092-8674(88)90206-1. [DOI] [PubMed] [Google Scholar]
- Geahlen R. L., Harrison M. L. Induction of a substrate for casein kinase II during lymphocyte mitogenesis. Biochim Biophys Acta. 1984 Jun 19;804(2):169–175. doi: 10.1016/0167-4889(84)90146-0. [DOI] [PubMed] [Google Scholar]
- Giordano A., Whyte P., Harlow E., Franza B. R., Jr, Beach D., Draetta G. A 60 kd cdc2-associated polypeptide complexes with the E1A proteins in adenovirus-infected cells. Cell. 1989 Sep 8;58(5):981–990. doi: 10.1016/0092-8674(89)90949-5. [DOI] [PubMed] [Google Scholar]
- Hayles J., Nurse P. A review of mitosis in the fission yeast Schizosaccharomyces pombe. Exp Cell Res. 1989 Oct;184(2):273–286. doi: 10.1016/0014-4827(89)90327-3. [DOI] [PubMed] [Google Scholar]
- Heck M. M., Hittelman W. N., Earnshaw W. C. In vivo phosphorylation of the 170-kDa form of eukaryotic DNA topoisomerase II. Cell cycle analysis. J Biol Chem. 1989 Sep 15;264(26):15161–15164. [PubMed] [Google Scholar]
- Herrera A. H., Olson M. O. Association of protein C23 with rapidly labeled nucleolar RNA. Biochemistry. 1986 Oct 7;25(20):6258–6264. doi: 10.1021/bi00368a063. [DOI] [PubMed] [Google Scholar]
- Hohmann P. Phosphorylation of H1 histones. Mol Cell Biochem. 1983;57(1):81–92. doi: 10.1007/BF00223526. [DOI] [PubMed] [Google Scholar]
- Hopp T. P., Woods K. R. Prediction of protein antigenic determinants from amino acid sequences. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3824–3828. doi: 10.1073/pnas.78.6.3824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter T., Sefton B. M. Transforming gene product of Rous sarcoma virus phosphorylates tyrosine. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1311–1315. doi: 10.1073/pnas.77.3.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huttner W. B., DeGennaro L. J., Greengard P. Differential phosphorylation of multiple sites in purified protein I by cyclic AMP-dependent and calcium-dependent protein kinases. J Biol Chem. 1981 Feb 10;256(3):1482–1488. [PubMed] [Google Scholar]
- Kuenzel E. A., Mulligan J. A., Sommercorn J., Krebs E. G. Substrate specificity determinants for casein kinase II as deduced from studies with synthetic peptides. J Biol Chem. 1987 Jul 5;262(19):9136–9140. [PubMed] [Google Scholar]
- Labbe J. C., Lee M. G., Nurse P., Picard A., Doree M. Activation at M-phase of a protein kinase encoded by a starfish homologue of the cell cycle control gene cdc2+. Nature. 1988 Sep 15;335(6187):251–254. doi: 10.1038/335251a0. [DOI] [PubMed] [Google Scholar]
- Labbe J. C., Picard A., Peaucellier G., Cavadore J. C., Nurse P., Doree M. Purification of MPF from starfish: identification as the H1 histone kinase p34cdc2 and a possible mechanism for its periodic activation. Cell. 1989 Apr 21;57(2):253–263. doi: 10.1016/0092-8674(89)90963-x. [DOI] [PubMed] [Google Scholar]
- Labbé J. C., Capony J. P., Caput D., Cavadore J. C., Derancourt J., Kaghad M., Lelias J. M., Picard A., Dorée M. MPF from starfish oocytes at first meiotic metaphase is a heterodimer containing one molecule of cdc2 and one molecule of cyclin B. EMBO J. 1989 Oct;8(10):3053–3058. doi: 10.1002/j.1460-2075.1989.tb08456.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langan T. A., Gautier J., Lohka M., Hollingsworth R., Moreno S., Nurse P., Maller J., Sclafani R. A. Mammalian growth-associated H1 histone kinase: a homolog of cdc2+/CDC28 protein kinases controlling mitotic entry in yeast and frog cells. Mol Cell Biol. 1989 Sep;9(9):3860–3868. doi: 10.1128/mcb.9.9.3860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapeyre B., Bourbon H., Amalric F. Nucleolin, the major nucleolar protein of growing eukaryotic cells: an unusual protein structure revealed by the nucleotide sequence. Proc Natl Acad Sci U S A. 1987 Mar;84(6):1472–1476. doi: 10.1073/pnas.84.6.1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews H. R., Huebner V. D. Nuclear protein kinases. Mol Cell Biochem. 1984;59(1-2):81–99. doi: 10.1007/BF00231306. [DOI] [PubMed] [Google Scholar]
- McVey D., Brizuela L., Mohr I., Marshak D. R., Gluzman Y., Beach D. Phosphorylation of large tumour antigen by cdc2 stimulates SV40 DNA replication. Nature. 1989 Oct 12;341(6242):503–507. doi: 10.1038/341503a0. [DOI] [PubMed] [Google Scholar]
- Olson M. O., Thompson B. A. Distribution of proteins among chromatin components of nucleoli. Biochemistry. 1983 Jun 21;22(13):3187–3193. doi: 10.1021/bi00282a023. [DOI] [PubMed] [Google Scholar]
- Pelech S. L., Meijer L., Krebs E. G. Characterization of maturation-activated histone H1 and ribosomal S6 kinases in sea star oocytes. Biochemistry. 1987 Dec 1;26(24):7960–7968. doi: 10.1021/bi00398a062. [DOI] [PubMed] [Google Scholar]
- Peter M., Nakagawa J., Dorée M., Labbé J. C., Nigg E. A. Identification of major nucleolar proteins as candidate mitotic substrates of cdc2 kinase. Cell. 1990 Mar 9;60(5):791–801. doi: 10.1016/0092-8674(90)90093-t. [DOI] [PubMed] [Google Scholar]
- Pines J., Hunter T. Isolation of a human cyclin cDNA: evidence for cyclin mRNA and protein regulation in the cell cycle and for interaction with p34cdc2. Cell. 1989 Sep 8;58(5):833–846. doi: 10.1016/0092-8674(89)90936-7. [DOI] [PubMed] [Google Scholar]
- Schneider H. R., Reichert G. H., Issinger O. G. Enhanced casein kinase II activity during mouse embryogenesis. Identification of a 110-kDa phosphoprotein as the major phosphorylation product in mouse embryos and Krebs II mouse ascites tumor cells. Eur J Biochem. 1986 Dec 15;161(3):733–738. doi: 10.1111/j.1432-1033.1986.tb10501.x. [DOI] [PubMed] [Google Scholar]
- Shenoy S., Choi J. K., Bagrodia S., Copeland T. D., Maller J. L., Shalloway D. Purified maturation promoting factor phosphorylates pp60c-src at the sites phosphorylated during fibroblast mitosis. Cell. 1989 Jun 2;57(5):763–774. doi: 10.1016/0092-8674(89)90791-5. [DOI] [PubMed] [Google Scholar]
- Srivastava M., Fleming P. J., Pollard H. B., Burns A. L. Cloning and sequencing of the human nucleolin cDNA. FEBS Lett. 1989 Jun 19;250(1):99–105. doi: 10.1016/0014-5793(89)80692-1. [DOI] [PubMed] [Google Scholar]
- Suzuki N., Matsui H., Hosoya T. Effects of androgen and polyamines on the phosphorylation of nucleolar proteins from rat ventral prostates with particular reference to 110-kDa phosphoprotein. J Biol Chem. 1985 Jul 5;260(13):8050–8055. [PubMed] [Google Scholar]
- Uemura T., Morikawa K., Yanagida M. The nucleotide sequence of the fission yeast DNA topoisomerase II gene: structural and functional relationships to other DNA topoisomerases. EMBO J. 1986 Sep;5(9):2355–2361. doi: 10.1002/j.1460-2075.1986.tb04504.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uemura T., Yanagida M. Isolation of type I and II DNA topoisomerase mutants from fission yeast: single and double mutants show different phenotypes in cell growth and chromatin organization. EMBO J. 1984 Aug;3(8):1737–1744. doi: 10.1002/j.1460-2075.1984.tb02040.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westendorf J. M., Swenson K. I., Ruderman J. V. The role of cyclin B in meiosis I. J Cell Biol. 1989 Apr;108(4):1431–1444. doi: 10.1083/jcb.108.4.1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodford T. A., Pardee A. B. Histone H1 kinase in exponential and synchronous populations of Chinese hamster fibroblasts. J Biol Chem. 1986 Apr 5;261(10):4669–4676. [PubMed] [Google Scholar]
- Zalta J., Zalta J. P., Simard R. Isolation of nucleoli. A method that combines high yield, structural integrity, and biochemical preservation. J Cell Biol. 1971 Nov;51(21):563–568. doi: 10.1083/jcb.51.2.563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zandomeni R., Zandomeni M. C., Weinmann R. A rapid purification method for calf thymus casein kinase II. FEBS Lett. 1988 Aug 1;235(1-2):247–251. doi: 10.1016/0014-5793(88)81272-9. [DOI] [PubMed] [Google Scholar]
- Zingde S. M., Shirsat N. V., Gothoskar B. P. Peptide mapping of proteins in gel bands after partial cleavage with acidic cyanogen bromide vapors. Anal Biochem. 1986 May 15;155(1):10–13. doi: 10.1016/0003-2697(86)90216-2. [DOI] [PubMed] [Google Scholar]