Abstract
Background
Examine lymphatic malformation lymphoid aggregates for the expression of tertiary lymphoid organ markers. Determine how lymphoid aggregate density relates to lymphatic malformation clinical features.
Methods and Results
Retrospective cohort study. Tissue and clinical data were reviewed from 29 patients in the Vascular Anomaly Database who represented the spectrum of head and neck lymphatic malformations and had >5 years of follow-up. Archived formalin-fixed, paraffin-embedded lymphatic malformation tissue was immunohistochemically stained with antibodies for tertiary lymphoid organ markers, which included follicular and mature myeloid dendritic cells, high endothelial venules, segregated B and T-cells, lymphatic endothelial cells, and lymphoid homing chemokines (CXCL13, CCL21). Lymphoid aggregate density (count/mm2) was quantified by 2 independent, blinded reviewers. Lymphoid aggregate density and lymphatic malformation clinical features were characterized using analysis of variance. Larger lymphatic malformation tissue lymphoid aggregates stained consistently for tertiary lymphoid organ markers. In oral cavity and neck specimens from the same patients (n = 9), there were more tertiary lymphoid organ in oral cavity than in neck specimens (p = 0.0235). In lymphatic malformation neck tissue, de Serres stage 4 lymphatic malformations displayed the highest tertiary lymphoid organ density. No significant association was seen between tertiary lymphoid organ density and other clinical features.
Conclusion
This study demonstrates that some lymphoid aggregates within lymphatic malformations represent tertiary lymphoid organs. There was an association between tertiary lymphoid organ density and lymphatic malformation location. Further study is required to define the role of lymphoid neogenesis and tertiary lymphoid organ formation in lymphatic malformation pathogenesis.
Introduction
Lymphatic malformations (LM) are uncommon but often debilitating congenital malformations that frequently occur in the cervicofacial region.1 The overall incidence of LM is reported in the range of 1.2 to 2.8 per 1000.2 While these lesions are thought to result from the disordered development of lymphatic channels, progress in understanding their underlying pathophysiology has been slow since their initial description in 1843 by Wernher.3 Current therapies (i.e., surgery, sclerotherapy, and corticosteroids) are often insufficient, as many LMs remain symptomatic despite multimodality treatment. LM are characterized radiographically into micro- versus macrocystic lesions. Clinically, this distinction is relevant, as microcystic lesions are associated with poorer prognostic factors, such as mucosal disease, involvement of multiple anatomic sites, and location above the hyoid. It is also well understood and documented that microcystic lesions are much more difficult to treat, regardless of modality, and are more likely to recur compared with macrocystic lesions.1,4–6 The clinical variability seen in LM extends to their natural history and response to treatment, with some LMs treated successfully with a single intervention and others requiring staged and multimodality therapy. Spontaneous regression of LM can also occur,7 but it is not known what incites regression.
To date, no cellular correlate has been found to explain the obvious clinical differences between lesions that are easily treated and those that display a recurrent and unremitting course. Despite the difference in clinical characteristics, micro- and macrocystic lesions in any location are histologically and immunohistochemically indistinguishable, with LM stroma always containing multiple dilated lymphatic channels, lymphoid aggregates, and plasmacytoid dendritic cells.6,8,9 Lymphoid aggregates, including follicles within the cyst walls of LM, were first documented by Dowd in 1913.10 Similar aggregates have been noted in a variety of inflammatory, autoimmune, infectious, and neoplastic conditions and are termed tertiary lymphoid organs (TLO) as they resemble normal secondary lymphoid organs such as lymph nodes in their major cellular constituents and organization.11 TLOs can be distinguished from lymph nodes as they are unencapsulated and embedded in nonlymphoid organs at sites of chronic inflammation or infection. The current study aims are twofold: 1) to examine the lymphoid aggregates seen in LM for the presence of TLO markers, and 2) to attempt to determine the relationship between the density of lymphoid aggregates and clinical characteristics, behavior, and outcomes in LM.
Materials and Methods
Patients
Tissue specimens and clinical data from 29 patients (age range: 2 months to 21 years; mean age at surgery: 4.6 years) with LM of the head and neck, seen from 1991 to 2009 in the Vascular Anomaly Clinic, who had undergone surgical management and had a known clinical course with follow-up of greater than 5 years and were representative of the spectrum of head and neck LM, were retrospectively reviewed from a prospectively accrued vascular anomaly clinical database and tissue bank. Diagnosis of LM was based on clinical, radiologic, and pathologic features. The specimens were grouped into two groups: oral cavity lesions and neck lesions. Some patients (n = 9) had specimens from both neck and oral cavity, while others had LM limited to either the neck (n = 14) or the oral cavity (n = 6). Clinical outcome measures of de Serres LM stage4, age at surgery, adjuvant steroid treatment, persistent macroglossia/oral bleeding, sclerotherapy, spontaneous LM regression, and lymphocytopenia were obtained from the database and medical records and retrospectively reviewed. This study was approved by the Seattle Children's Hospital Institutional Review Board (IRB#11458, 11925).
Immunohistochemical analysis
Sections of formalin-fixed, paraffin-embedded LM tissue from 9 patients, selected at random by the senior author (JAP), within the 29 subjects, were dewaxed and rehydrated, treated with 0.3% H2O2 to block endogenous peroxidase, then subjected to antigen retrieval, blocked, and probed with antibody, as described in Table 1. The following biotinylated secondary antibodies were used: horse anti-mouse IgG, goat anti-mouse IgM, goat anti-rabbit IgG, goat anti-rat IgG, horse anti-goat IgG (all from Vector Laboratories, Burlingame, CA); and goat anti-rat IgM (Jackson ImmunoResearch, West Grove, PA). Streptavidin/biotin blocking reagents, horseradish peroxidase (HRP)-conjugated streptavidin, and diaminobenzidine substrate reagents were all from Vector Laboratories. All primary antibodies were used in conjunction with biotinylated secondary antibodies and HRP-streptavidin, except when anti-Bcl6 and anti-CD3 were used in double labeling, in which case HRP-conjugated secondary antibodies (donkey anti-mouse and donkey anti-rabbit; Jackson ImmunoResearch) and diaminobenzidine plus or minus nickel were used. Tonsil tissue, obtained from unrelated patients, served as the positive control tissue. Negative controls using unrelated isotypic primary antibody and/or omitting the primary antibody were performed. With the exception of those double stained for Bcl6 and CD3, sections were stained with hematoxylin before final dehydration and mounting. Not all samples were stained for all antigens, as depths of some paraffin blocks were encountered that lacked lymphoid aggregates.
Table 1.
Antibodies Used for Immunohistochemical Analysis
| Antigen | Host and Isotype | Source | Antigen Retrieval | Blocking | Dilution |
|---|---|---|---|---|---|
| AID | Rat IgG2b | EK2-5G9; Cell Signaling Technology (Beverly, MA) | 10 mM citrate pH 61 | Strpt/biotin/DAKO serum-free protein block 30 min | 1:450 |
| Bcl6 | Mouse IgG1 | ab17249; Abcam (Cambridge, MA) | 1 mM EDTA pH 8.12 | Standard blocking3 | 1:10 |
| CCL21 | Goat IgG | AF366; R&D Systems, (Minneapolis, Mn) | 10 mM citrate pH 62 | Standard blocking3 | 1:200 |
| CD3 | Rabbit IgG | ab16669; Abcam | 10 mM citrate pH 62 | Standard blocking3 | 1:500 |
| CD20 | Mouse IgG2a | CD20cy; DAKO(Carpinteria, CA) | 10 mM citrate pH 62 | Standard blocking3 | 1:1000 |
| CD83 | Mouse IgG2b | HB15a; Beckman-Coulter (Brea, CA) | 0.1% trypsin in PBS 37°C 30 min | Standard, but O/N at 4°C | 1:500 |
| CXCL13 | Goat IgG | AF801 R&D Systems | 10 mM citrate pH 62 | Standard blocking3 | 1:300 |
| CNA.42 FDC antigen | Mouse IgM | CNA.42; Cell Marque, (Rocklin, CA) | 10 mM citrate pH 62 | Standard blocking3 | 1:200 |
| PNAd | Rat IgM | MECA-79; Novus Biologicals (Littleton, CO) | 10 mM citrate pH 62 | Standard blocking3 | 1:1000 |
| Podoplanin | Mouse IgG1 | D2-40; Vector Labs (Burlingame, CA) | 10 mM citrate pH 62 | Standard blocking3 | 1:100 |
95°C bath 45 min, followed by cooling at room temperature for 15 min.
Boiling water bath for 20 min, followed by cooling at room temperature for 30 min.
1% BSA/PBS briefly, then 5% normal serum/1% BSA/PBS for 1 h, followed by 15 min streptavidin and biotin blocking steps.
Histologic analysis and tlo quantification
Quantification of lymphoid aggregates per unit area in histologic sections of LM involving neck and/or oral cavity was performed by 2 independent, blinded reviewers (JAP, SLC). The total tissue area of the histologic section was calculated using the area tool on the NDP Nanozoomer viewer version 1.1.27 (Olympus, Center Valley, PA). This resulted in a calculated density of lymphoid aggregates (count/mm2) for each patient specimen. For patients who had multiple histologic slides from a given anatomic location, total area and count across all slides were calculated to normalize the TLO density determination. Histologic sections examined in this study were not sequential, thereby reducing the risk of ‘double counting’ a 3-dimensional lymphoid aggregate that would span sequential sections.
Statistical analysis
Clinical data were analyzed with descriptive statistics. The relationship between clinically relevant outcome measures and lymphoid aggregate/TLO density was examined using analysis of variance (ANOVA) for both oral cavity and neck specimens. A paired analysis was used to examine the density of lymphoid aggregates/TLOs in neck and oral cavity specimens obtained from the same patient (n = 9). Multivariate linear regression analysis was not performed given small sample size. Inter-rater reliability was calculated using Pearson's correlation coefficient. All statistical analysis was performed using SAS version 9.1 (SAS Institute Inc., Cary, NC).
Results
Mean follow-up in the patient cohort was 9.8 years. Clinical characteristics and demographics of the study patients are outlined (Table 2). All patients were staged using the de Serres staging scheme.4
Table 2.
Patient Demographics and Clinical Characteristics
| Study Demographics (n = 29) | |
|---|---|
| Age at surgery (mean ± standard deviation) | 4.6 ± 5 years |
| Gender M:F | 17:12 |
| Site of lymphatic malformation (n) | |
| Neck only | 14 |
| Oral cavity only | 6 |
| Neck and oral cavity | 9 |
| Duration of follow-up (mean ± standard deviation) | 9.8 ± 5.5 years |
| Stage (n)*de Serres | |
| I. Unilateral infrahyoid | 8 |
| II. Unilateral suprahyoid | 3 |
| III. Unilateral suprahyoid and infrahyoid | 6 |
| IV. Bilateral suprahyoid | 4 |
| V. Bilateral suprahyoid and infrahyoid | 5 |
| Previous steroid administration (n) | |
| Yes | 14 |
| No | 15 |
| Prior sclerotherapy (n) | |
| Yes | 2 |
| No | 27 |
| Spontaneous regression (n) | |
| Yes | 3 |
| No | 17 |
Intraoral lesions not accounted for by the de Serres classification.4
Immunohistochemical analysis
Common criteria for categorizing a lymphoid aggregate as a TLO are: (1) the presence of follicular dendritic cells (FDCs), antigen-presenting cells involved in B-cell selection; (2) the presence of high endothelial venules (HEVs), specialized blood vessels that serve as points of entry into lymphoid organs for immune cells leaving the bloodstream in response to chemokines; and (3) the presence and physical segregation of B and T-cells. Many LM lymphoid aggregates were positive for the FDC markers podoplanin12 (Fig. 1A) and CNA.4213 (Figs. 1B–1D). Podoplanin was also present on lymphatic endothelial cells in and around the aggregates. Peripheral node addressin (PNAd)-positive HEVs were common and restricted to lymphoid aggregates (Figs. 2A–2C). The B-cell attractant chemokine CXCL13 and the T-cell and myeloid dendritic cell attractant chemokine CCL21 were both detected in many lymphoid aggregates (Figs. 2E and 2F), with the latter seen in stromal cells (in which it is synthesized14) closely apposed to HEVs, and in HEVs (to which, in humans, it is thought to be transcytosed) (Fig. 2D), and in lymphatic endothelial cells throughout the sections. Staining with the pan B-cell marker CD20 and pan T-cell marker CD3 revealed varying degrees of segregation of these cell types in aggregates (Figs. 3A, 3B, 3D, and 3E). Typically, FDCs and CXCL13 expression were detected in a zone comprising mostly B-cells, with T-cells concentrated in the periphery of the B-cell zone and among the B-cells in the light zone of organized lymphoid aggregates. Among the T-cells were scattered CD83-positive mature myeloid dendritic cells, which normally contribute to T-cell activation through antigen presentation (Figs. 3C and 3F), but CD83-positive cells in LM were not concentrated in the lymphoid aggregates. Bcl6, a transcription factor that represses the DNA damage response in centroblasts undergoing Ig gene modifications15 was detected in cells in the B-cell zones of some organized lymphoid aggregates (Fig. 4A). Germinal center cells of some organized lymphoid aggregates expressed activation-induced cytidine deaminase (Fig. 4B). In general, the larger and more organized aggregates were positive for multiple TLO markers, and LM that contained only small or loose aggregates were positive for few or no markers (Table 3).
FIG. 1.
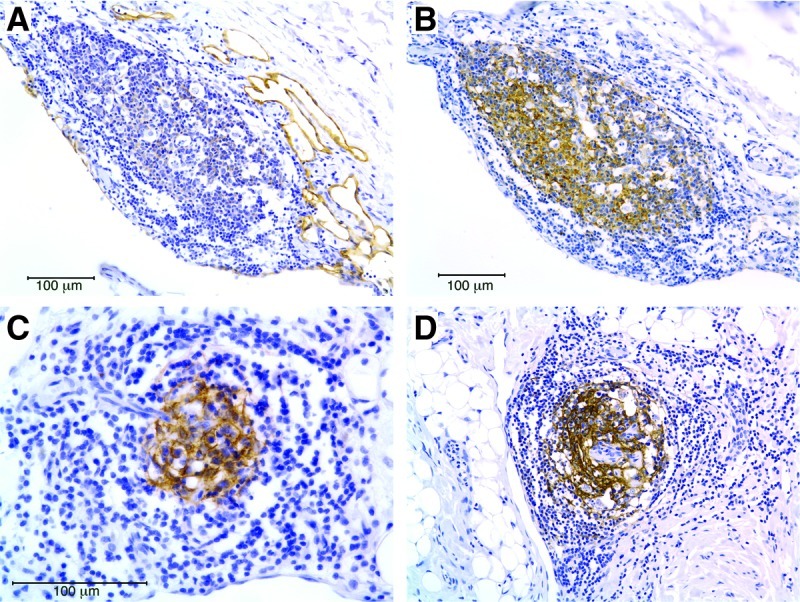
Follicular dendritic cells in LM tertiary lymphoid organs. (A) A lymphoid aggregate within a macrocystic LM in the neck is positive for podoplanin, a marker of follicular dendritic cells (FDCs) and lymphatic endothelial cells. (B) Cells in the same aggregate are positive for the FDC-specific marker CNA.42, which is not present on lymphatic vessels. (C) and (D) FDCs detected by CNA.42 staining in lymphoid aggregates from microcystic LM of the neck from two different patients. Scale bar = 100 microns. This figure is available in color in the online version of this article at www.liebertpub.com/lrb
FIG. 2.
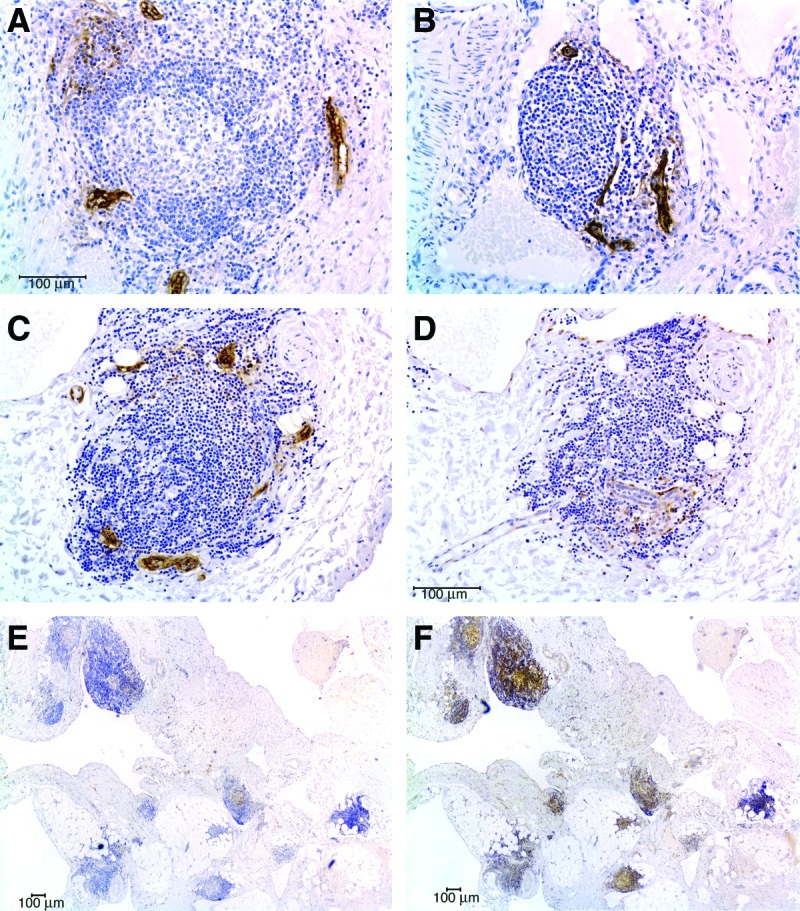
High endothelial venules, and CCL21 and CXCL13 expression in LM tertiary lymphoid organs. (A) and (B) PNAd-positive high endothelial venules (HEV) in lymphoid aggregates from two microcystic LM of the tongue. (C) A lymphoid aggregate in a microcystic LM of the neck with PNAd-positive HEVs. (D) The T-cell homing chemokine CCL21 is detected in the same lymphoid aggregate, in stromal cells closely associated with HEV. (E) CCL21 expression is widespread in lymphoid aggregates in this patient's LM. (F) The B-cell homing chemokine CXCL13 also can be detected in multiple lymphoid aggregates in the same LM from this patient. Scale bar = 100 microns. This figure is available in color in the online version of this article at www.liebertpub.com/lrb
FIG. 3.
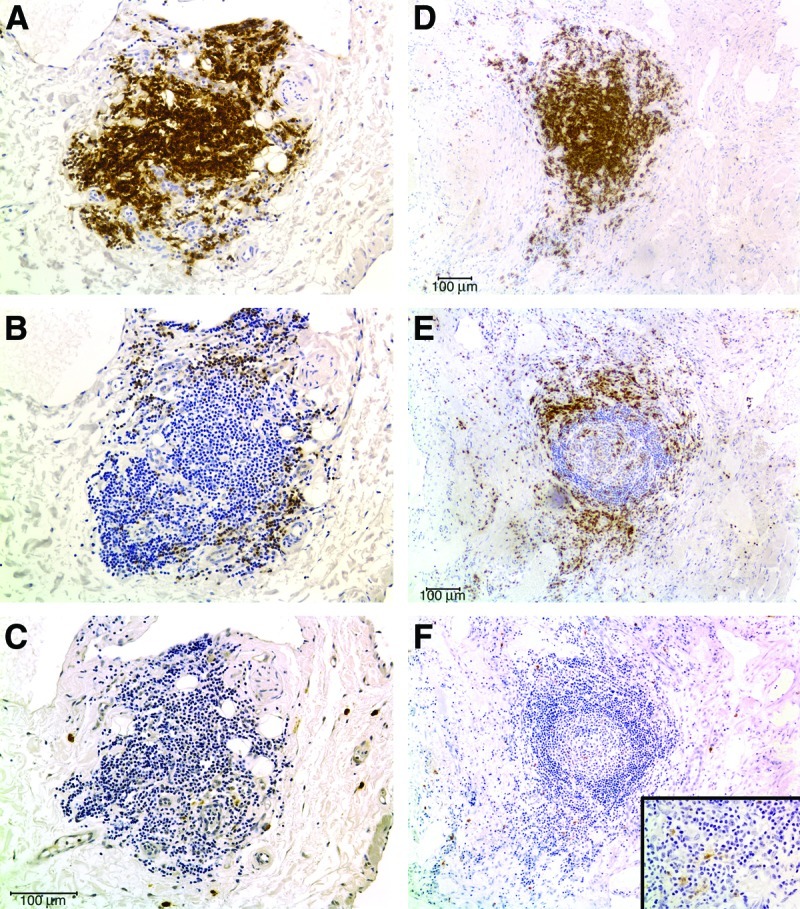
B and T-cell segregation in LM lymphoid aggregates. (A–C) The lymphoid aggregate shown in Figures 2C and 2D, stained for the B-cell marker CD20 (A), the T-cell marker CD3 (B), and the mature myeloid dendritic cell marker CD83 (C), demonstrating segregation of T and mature myeloid dendritic cells to the periphery of a B-cell zone. (D–F) CD20, CD3, and CD83 staining, respectively, of a large, organized lymphoid aggregate in a persistent microcystic LM of the tongue, showing similar segregation of B-cells (D), T-cells (E), and mature myeloid dendritic cells (F). This figure is available in color in the online version of this article at www.liebertpub.com/lrb
FIG. 4.
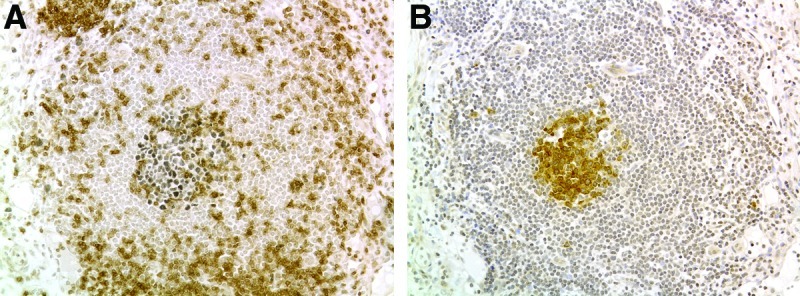
Bcl6 and AID expression in organized lymphoid aggregates. (A) A central zone of Bcl6 expression (black) in a second large lymphoid aggregate from the persistent tongue LM pictured in Figures 3D–3F. CD3 positive T-cells are brown. (B) Cells in the germinal center of this lymphoid aggregate also express activation-induced cytidine deaminase (brown cells). Scale bar = 100 microns. This figure is available in color in the online version of this article at www.liebertpub.com/lrb
Table 3.
Number of LM with Lymphoid Aggregates Positive for Indicated Marker, of Total Number of LM Stained for That Marker
| Marker | LM positive/LM stained |
|---|---|
| CCL21 | 4/7 |
| CXCL13 | 4/9 |
| PNAd | 5/7 |
| CD3/CD20 segregation | 5/7 |
| Podoplanin | 5/7 |
| CNA.42 | 6/9 |
| CD83 | 7/8 |
| Bcl6 | 2/4 |
Lymphoid aggregate density
Overall, a significantly higher density of lymphoid aggregates was seen in oral cavity than in neck specimens (paired analysis, p = 0.0235; Table 4). ANOVA followed by the Newman-Keuls test revealed no significant differences in mean total lymphoid aggregate density from lesions of different de Serres stages; a significantly greater density of lymphoid aggregates in lesions of de Serres stage IV than of stages I, III, and V; but no correlation between lymphoid aggregate density and de Serres stage (Tables 5 and 6). For oral cavity LMs, no significant association was found between lymphoid aggregate density and the de Serres disease stage.4 No correlation was seen between lymphoid aggregate density and other clinical outcome measures (Table 4). However, only 2 patients underwent sclerotherapy, 2 patients had lymphocytopenia, and 3 patients underwent spontaneous regression. Inter-rater reliability was high for the detection of lymphoid aggregates across histologic slides (Pearson correlation coefficient >0.9 in all cases).
Table 4.
Comparison of Lymphoid Aggregate Density in Paired Oral Cavity and Neck Specimens
| Site | N | Mean density (count/mm2) ± SD | P-value |
|---|---|---|---|
| Lymphoid Aggregate Density | |||
| Oral cavity | 8 | 0.053 ± 0.028 | 0.024* |
| Neck | 8 | 0.026 ± 0.019 | |
p < 0.05.
Table 5.
ANOVA Testing for Differences in Lymphoid Aggregate Numbers among Patients Divided According to Various Clinical Parameters
| |
Lymphoid Aggregate Density |
||
|---|---|---|---|
| Effect | F-value | DF (model, residual) | P-value |
| Neck | |||
| LM stage (de Serres)4 | 3.23 | 4,17 | 0.039* |
| Age at surgery | 2.39 | 1,20 | 0.137 |
| Previous sclerotherapy | 2.74 | 1,20 | 0.113 |
| Previous steroids | 0.01 | 1,20 | 0.928 |
| Lymphocytopenia | 2.37 | 1,20 | 0.139 |
| Recurrence | 0.00 | 1,20 | 0.9599 |
| Spontaneous regression | 0.17 | 1,20 | 0.6808 |
| Oral Cavity | |||
| LM stage (de Serres)4 | 0.74 | 3,11 | 0.551 |
| Age at surgery | 0.24 | 1,12 | 0.63 |
| Previous sclerotherapy | 0.01 | 1,12 | 0.906 |
| Previous steroids | 0.03 | 1,12 | 0.868 |
| Lymphocytopenia | 0.00 | 1,12 | 0.953 |
| Recurrence | 0.67 | 1,12 | 0.4295 |
| Persistent macroglossia/oral bleeding | 0.01 | 1,12 | 0.9395 |
p < 0.05.
Table 6.
ANOVA for Differences in Lymphoid Aggregate Density among Stages for Neck (de Serres staging system4)
| |
|
Lymphoid Aggregate Density |
|
|---|---|---|---|
| Stage | N | Mean density (count/mm2) ± SD | P-value |
| I | 8 | 0.020 ± 0.012 | 0.039* |
| II | 6 | 0.036 ± 0.019 | |
| III | 2 | 0.014 ± 0.003 | |
| IV | 3 | 0.048 ± 0.011 | |
| V | 3 | 0.018 ± 0.015 | |
p < 0.05.
Discussion
Our immunohistochemical study of LA in LM revealed that a subset of these aggregates (generally those that are relatively large, and organized by hematoxylin staining) contain markers of TLOs, including follicular dendritic cells, high endothelial venules, segregated B and T cells, and the chemokines CCL21 and CXCL13. CD83-positive mature dendritic cells were detected in T cell areas of some aggregates but were generally scattered throughout the LM. Bcl-6 was detected in large cells of some TLO light zones, suggesting that B-cell activation and immunoglobulin gene rearrangement can occur in these structures. These findings parallel the seminal clinicopathologic study of LM by Goetsch, in which he described accumulations of lymphoid cells in organized follicles and large, unorganized dense zones of lymphocytes,16 while for the first time demonstrating that these aggregates comprise the various cell types and two of the chemokines now considered typical of TLO.
Lymphoid neogenesis occurs in a number of conditions associated with chronic inflammation, including autoimmunity, transplant rejection, chronic inflammatory diseases, infectious diseases) and tumors.11 It is thought that TLOs can worsen or ameliorate disease, producing pathogenic antibodies in autoimmune conditions17 or a local anti-tumor18 or tolerizing response19 in cancer. Defective lymphatic drainage has also been suggested to contribute to lymphoneogenesis,20 and it is possible that low lymph flow within LM encourages immune cell retention and TLO formation. Whether invasive treatment for LM, which produces significant inflammation and lymphatic channel destruction, contributes to local lymphoid neogenesis in LM is unknown.
During normal secondary lymphoid organ formation, a positive feedback loop of stromal cell chemokine expression initiated by lymphotoxin (LT) from lymphoid tissue inducer cells is largely responsible for recruitment, organization, and, in some cases, differentiation (FDCs, HEVs) and maintenance (FDCs), of the cells involved.11,21 B cells are a possible source of LT once the TLO is formed.21 While either CCL21 or CXCL13 overexpression causes TLO formation in the mouse, neither acts independent of LT.22–25 The presence of these chemokines in LM TLO suggests that LT signaling is occurring. LT signaling can be lymphangiogenic,26 and B-cell-dependent lymphangiogenesis occurs in mice.27 It is possible that TLOs contribute to LM growth by producing LT and other lymphangiogenic factors. If true, TLO ablation through LT signal inhibition21 may improve LM treatment.
The larger numbers of lymphoid aggregates seen in oral cavity compared with neck specimens, from the same patient, may reflect the increased inflammation often associated with microcystic LM involving mucosal surfaces. LM involving the neck and oral cavity are all higher stage LM in the deSerres staging system, predominately microcystic and associated with persistent disease. The small sample of isolated oral cavity lesions (n = 5) without neck involvement may account for the lack of a significant relationship between lymphoid aggregate density and disease stage in oral cavity LM. This finding highlights the fact that the de Serres staging system risks overstaging a small but midline oral cavity LM as stage IV disease.4
This study has its limitations. While a large number of slides (n = 117) from a large cohort (n = 29) were reviewed to determine lymphoid aggregate density, variable clinical features and outcome measures within the group led to small sample sizes for a number of comparisons. The retrospective study design further increases data variability in each subject. Our pathologic specimens were obtained from a single location within the neck or oral cavity LM, not multiple specimens from different sites in each lesion. Single location sampling might not represent lymphoid aggregate density of the entire LM, reducing detection of a relationship between lymphoid aggregate density and disease stage. Biopsy is not routine in LM management; all subjects in this study had surgery for LM management. This may have biased selection to individuals with severe disease. All these factors limit our ability to make statements that would link the presence, density, or number of TLOs with clinically relevant prognostic factors that could be used to improve LM therapy. Despite these limitations, these results provide a platform for further examination of LM cellular and immunologic features, particularly as they relate to current treatment strategies.
The reason some lower stage LM regress or are successfully treated, whereas more extensive lesions cannot be completely eradicated, remains a mystery. LM have been regarded as areas of malformed lymphatic vessels or persistent embryonic rests of lymphatic tissue arrested in early development, which should be treated to prevent progressive functional compromise. While this strategy is successful in some LMs, it is not in others.5,28,29 Is it possible that LM arise and persist postnatally due to an abnormal local—and potentially systemic—immune response, centered in the stroma surrounding abnormal lymphatic vessels?9,28,30,31 If this speculation has any merit, then invasive LM treatment may actually exacerbate the clinical findings in extensive LM.
Conclusion
While it has long been recognized that lymphoid aggregates are frequently present in LM, this study is the first to describe these aggregates as TLOs, confirming their characteristic immunohistochemical appearance and underlining their potential role in LM formation and persistence. The density of lymphoid aggregates is greater in tongue LM tissue as compared to neck LM tissue; this finding, along with other unusual immunologic and histologic findings in LM, may suggest that persistence of LM in the oral mucosa may be related to chronic inflammation.9,30,31 Further study is required to determine the role–if any–that lymphoid aggregates play in LM pathogenesis.
Acknowledgments
Thanks to Nancy Poindexter and Michele Bombardieri for their helpful advice on immunohistochemistry. Thanks to Elizabeth Hankinson for her contribution to tissue staining. Thanks also to Babette Saltzman for her statistical assistance, to Stacy Russ for manuscript preparation, and to Eden Palmer for figure preparation.
Author Disclosure Statement
Drs. Kirsh, Cushing, Chen, Schwartz, and Perkins have no conflicts of interest or financial ties to disclose.
References
- 1.Kennedy TL. Whitaker M. Pellitteri P, et al. Cystic hygroma/lymphangioma: A rational approach to management. Laryngoscope. 2001;111:1929–1937. doi: 10.1097/00005537-200111000-00011. [DOI] [PubMed] [Google Scholar]
- 2.Filston HC. Hemangiomas, cystic hygromas, and teratomas of the head and neck. Semin Pediatr Surg. 1994;3:147–159. [PubMed] [Google Scholar]
- 3.Wernher A. Angeborenen Kysten-Hydrome und die Ihnen verwandten. Giessen, Germany: G.F. Heyer, Vater; 1843. pp. 79–91. [Google Scholar]
- 4.de Serres LM. Sie KC. Richardson MA. Lymphatic malformations of the head and neck. A proposal for staging. Arch Otolaryngol Head Neck Surg. 1995;121:577–582. doi: 10.1001/archotol.1995.01890050065012. [DOI] [PubMed] [Google Scholar]
- 5.Raveh E. de Jong AL. Taylor GP, et al. Prognostic factors in the treatment of lymphatic malformations. Arch Otolaryngol Head Neck Surg. 1997;123:1061–1065. doi: 10.1001/archotol.1997.01900100035004. [DOI] [PubMed] [Google Scholar]
- 6.Chen EY. Hostikka SL. Oliaei S, et al. Similar histologic features and immunohistochemical staining in microcystic and macrocystic lymphatic malformations. Lymphat Res Biol. 2009;7:75–80. doi: 10.1089/lrb.2009.0003. [DOI] [PubMed] [Google Scholar]
- 7.Perkins JA. Maniglia C. Magit A, et al. Clinical and radiographic findings in children with spontaneous lymphatic malformation regression. Otolaryngol Head Neck Surg. 2008;138:772–777. doi: 10.1016/j.otohns.2008.02.016. [DOI] [PubMed] [Google Scholar]
- 8.Yaita T. Onodera K. Xu H, et al. Histomorphometrical study in cavernous lymphangioma of the tongue. Oral Dis. 2007;13:99–104. doi: 10.1111/j.1601-0825.2006.01256.x. [DOI] [PubMed] [Google Scholar]
- 9.Fleming JN. Hostikka SL. Chen EY, et al. Plasmacytoid dendritic cells and interferon levels are increased in lymphatic malformations. Otolaryngol Head Neck Surg. 2008;139:671–676. doi: 10.1016/j.otohns.2008.07.032. [DOI] [PubMed] [Google Scholar]
- 10.Dowd CN. XI. Hygroma cysticum colli: Its structure and etiology. Ann Surg. 1913;58:112–132. doi: 10.1097/00000658-191307000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aloisi F. Pujol-Borrell R. Lymphoid neogenesis in chronic inflammatory diseases. Nature Rev. 2006;6:205–217. doi: 10.1038/nri1786. [DOI] [PubMed] [Google Scholar]
- 12.Xie Q. Chen L. Fu K, et al. Podoplanin (d2-40): A new immunohistochemical marker for reactive follicular dendritic cells and follicular dendritic cell sarcomas. Int J Clin Exp Pathol. 2008;1:276–284. [PMC free article] [PubMed] [Google Scholar]
- 13.Raymond I. Al Saati T. Tkaczuk J, et al. CNA.42, a new monoclonal antibody directed against a fixative-resistant antigen of follicular dendritic reticulum cells. Am J Pathol. 1997;151:1577–1585. [PMC free article] [PubMed] [Google Scholar]
- 14.Carlsen HS. Haraldsen G. Brandtzaeg P, et al. Disparate lymphoid chemokine expression in mice and men: No evidence of CCL21 synthesis by human high endothelial venules. Blood. 2005;106:444–446. doi: 10.1182/blood-2004-11-4353. [DOI] [PubMed] [Google Scholar]
- 15.Ranuncolo SM. Polo JM. Dierov J, et al. Bcl-6 mediates the germinal center B cell phenotype and lymphomagenesis through transcriptional repression of the DNA-damage sensor ATR. Nature Immunol. 2007;8:705–714. doi: 10.1038/ni1478. [DOI] [PubMed] [Google Scholar]
- 16.Goestch E. Hygroma colli cysticum and hygroma axillare: Pathologic and clinical study and report of twelve cases. Arch Surg. 1938;36:394–479. [Google Scholar]
- 17.Nacionales DC. Weinstein JS. Yan XJ, et al. B cell proliferation, somatic hypermutation, class switch recombination, and autoantibody production in ectopic lymphoid tissue in murine lupus. J Immunol. 2009;182:4226–4236. doi: 10.4049/jimmunol.0800771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dieu-Nosjean MC. Antoine M. Danel C, et al. Long-term survival for patients with non-small-cell lung cancer with intratumoral lymphoid structures. J Clin Oncol. 2008;26:4410–4417. doi: 10.1200/JCO.2007.15.0284. [DOI] [PubMed] [Google Scholar]
- 19.Shields JD. Kourtis IC. Tomei AA, et al. Induction of lymphoidlike stroma and immune escape by tumors that express the chemokine CCL21. Science (New York) 2010;328:749–752. doi: 10.1126/science.1185837. [DOI] [PubMed] [Google Scholar]
- 20.Thaunat O. Kerjaschki D. Nicoletti A. Is defective lymphatic drainage a trigger for lymphoid neogenesis? Trends Immunol. 2006;27:441–445. doi: 10.1016/j.it.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 21.Gommerman JL. Browning JL. Lymphotoxin/light, lymphoid microenvironments and autoimmune disease. Nature Rev. 2003;3:642–655. doi: 10.1038/nri1151. [DOI] [PubMed] [Google Scholar]
- 22.Chen SC. Vassileva G. Kinsley D, et al. Ectopic expression of the murine chemokines CCL21a and CCL21b induces the formation of lymph node-like structures in pancreas, but not skin, of transgenic mice. J Immunol. 2002;168:1001–1008. doi: 10.4049/jimmunol.168.3.1001. [DOI] [PubMed] [Google Scholar]
- 23.Fan L. Reilly CR. Luo Y, et al. Cutting edge: Ectopic expression of the chemokine TCA4/SLC is sufficient to trigger lymphoid neogenesis. J Immunol. 2000;164:3955–3959. doi: 10.4049/jimmunol.164.8.3955. [DOI] [PubMed] [Google Scholar]
- 24.Luther SA. Bidgol A. Hargreaves DC, et al. Differing activities of homeostatic chemokines CCL19, CCL21, and CXCL12 in lymphocyte and dendritic cell recruitment and lymphoid neogenesis. J Immunol. 2002;169:424–433. doi: 10.4049/jimmunol.169.1.424. [DOI] [PubMed] [Google Scholar]
- 25.Luther SA. Lopez T. Bai W, et al. BLC expression in pancreatic islets causes B cell recruitment and lymphotoxin-dependent lymphoid neogenesis. Immunity. 2000;12:471–481. doi: 10.1016/s1074-7613(00)80199-5. [DOI] [PubMed] [Google Scholar]
- 26.Mounzer RH. Svendsen OS. Baluk P, et al. Lymphotoxin-alpha contributes to lymphangiogenesis. Blood. 2010;116:2173–2182. doi: 10.1182/blood-2009-12-256065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ruddell A. Mezquita P. Brandvold KA, et al. B lymphocyte-specific c-Myc expression stimulates early and functional expansion of the vasculature and lymphatics during lymphomagenesis. Am J Pathol. 2003;163:2233–2245. doi: 10.1016/S0002-9440(10)63581-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Padwa BL. Hayward PG. Ferraro NF, et al. Cervicofacial lymphatic malformation: Clinical course, surgical intervention, and pathogenesis of skeletal hypertrophy. Plastic Reconstr Surg. 1995;95:951–960. [PubMed] [Google Scholar]
- 29.Hartl DM. Roger G. Denoyelle F, et al. Extensive lymphangioma presenting with upper airway obstruction. Arch Otolaryngol Head Neck Surg. 2000;126:1378–1382. doi: 10.1001/archotol.126.11.1378. [DOI] [PubMed] [Google Scholar]
- 30.Perkins JA. Tempero RM. Hannibal MC, et al. Clinical outcomes in lymphocytopenic lymphatic malformation patients. Lymphat Res Biol. 2007;5:169–174. doi: 10.1089/lrb.2007.5304. [DOI] [PubMed] [Google Scholar]
- 31.Tempero RM. Hannibal M. Finn LS, et al. Lymphocytopenia in children with lymphatic malformation. Arch Otolaryngol Head Neck Surg. 2006;132:93–97. doi: 10.1001/archotol.132.1.93. [DOI] [PubMed] [Google Scholar]


