Abstract
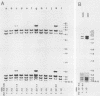
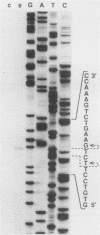
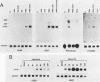
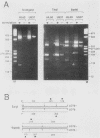
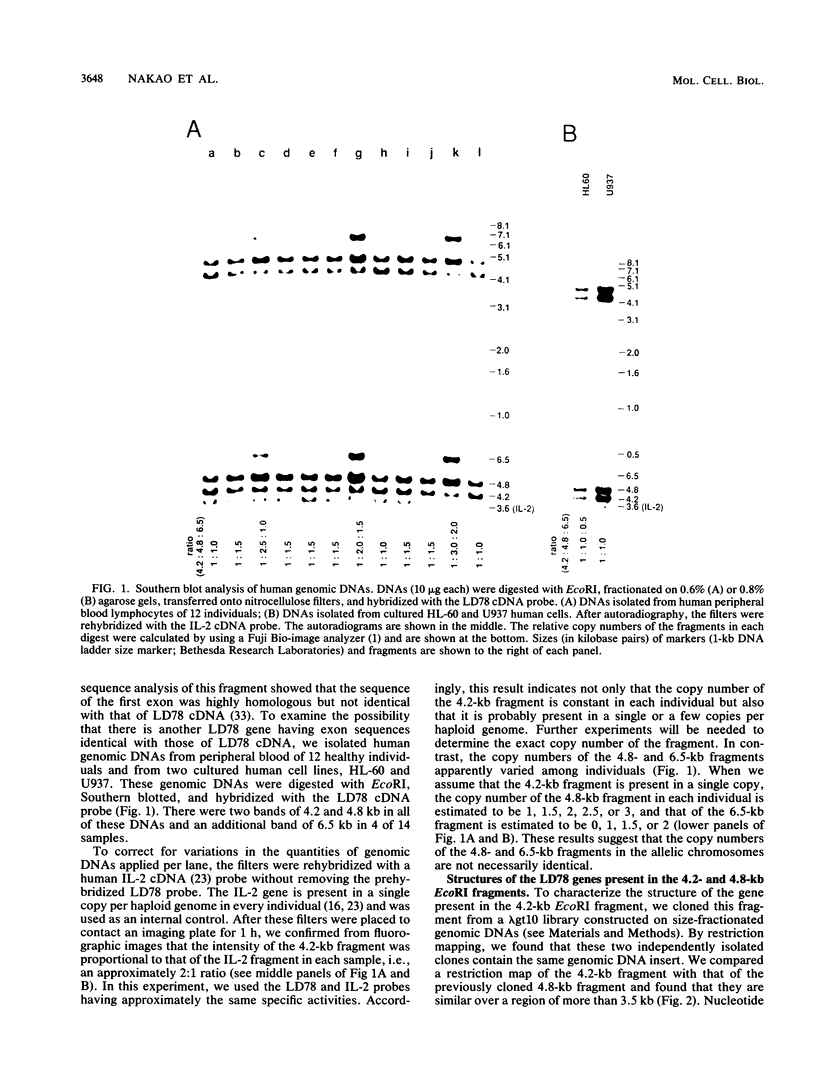
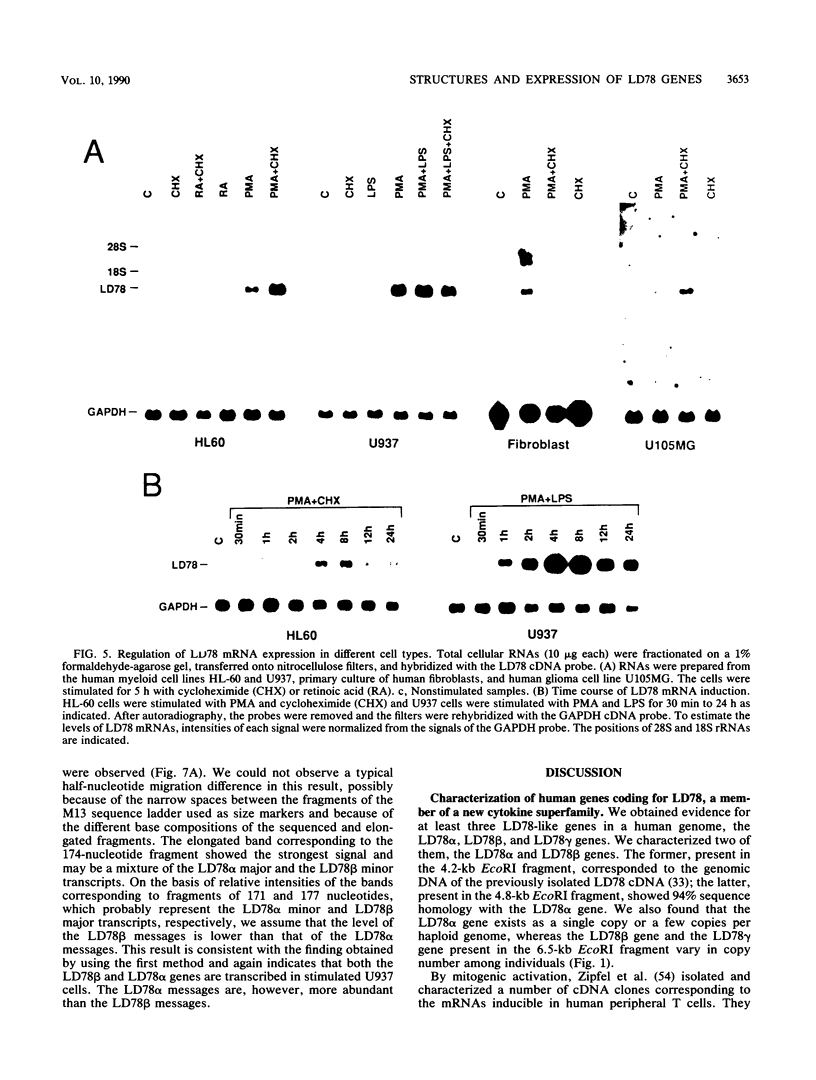
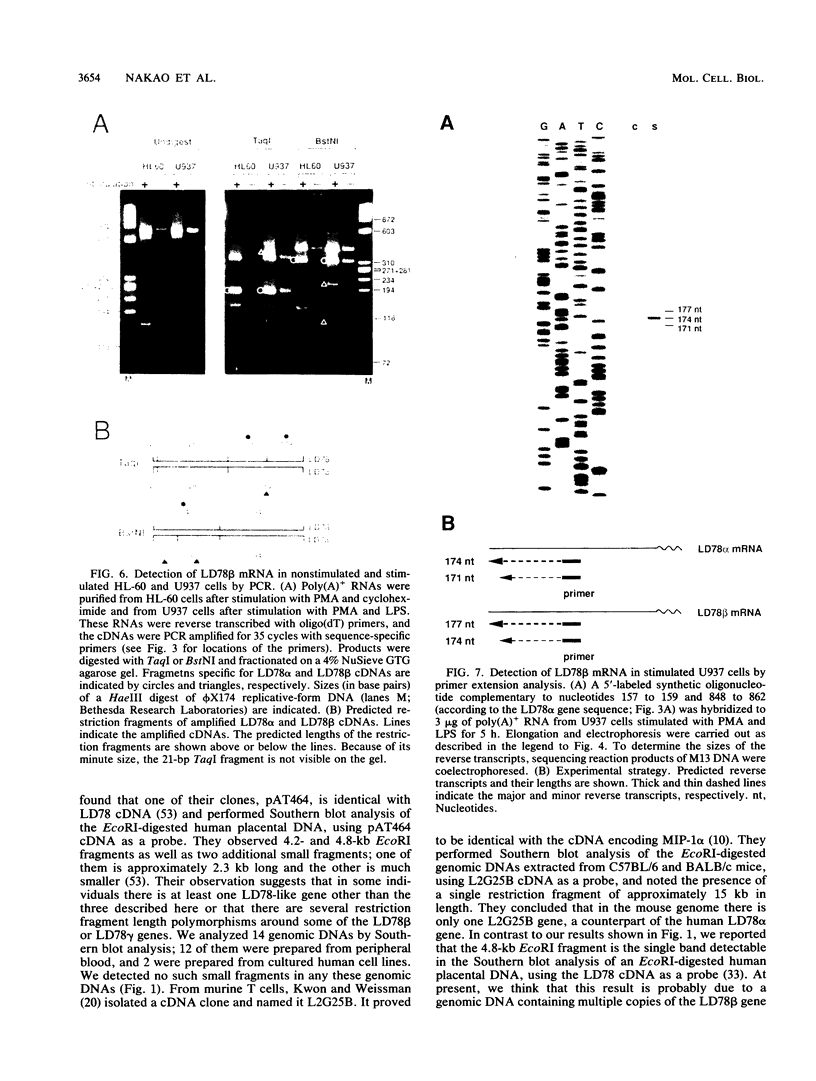
LD78 is a member of a newly identified superfamily of small inducible proteins involved in inflammatory responses, wound healing, and tumorigenesis. Southern blot analysis of the EcoRI-digested human genomic DNAs, using previously isolated LD78 cDNA as a probe, showed that in each individual there are 4.2- and 4.8-kilobase-pair (kb) fragments and that some have an additional 6.5-kb fragment. The 4.2-kb fragment contained genomic DNA sequences corresponding to the LD78 cDNA and was named the LD78 alpha gene. The 4.8-kb fragment contained similar sequences, showing 94% homology to the LD78 alpha gene, and was named the LD78 beta gene. The LD78 alpha gene was present in a single or a few copies per haploid genome, whereas the copy number of the LD78 beta gene and of the 6.5-kb fragment hybridizable to LD78 cDNA varied among the samples tested. Treatment of human myeloid cell lines HL-60 and U937 with phorbol 12-myristate 13-acetate (PMA) increased within 2 h cellular levels of the RNA hybridizable to LD78 cDNA. The human glioma cell line U105MG and primary culture of human fibroblasts also expressed the hybridizable RNA in response to PMA. Addition of cycloheximide had no apparent effect on this response in U937 cells and inhibited the response in fibroblasts, whereas it stimulated the response in HL-60 and U105MG cells. mRNA phenotyping experiments revealed that the LD78 alpha and LD78 beta genes were both transcribed in PMA-stimulated U937 cells.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amemiya Y., Miyahara J. Imaging plate illuminates many fields. Nature. 1988 Nov 3;336(6194):89–90. doi: 10.1038/336089a0. [DOI] [PubMed] [Google Scholar]
- Bigner D. D., Bigner S. H., Pontén J., Westermark B., Mahaley M. S., Ruoslahti E., Herschman H., Eng L. F., Wikstrand C. J. Heterogeneity of Genotypic and phenotypic characteristics of fifteen permanent cell lines derived from human gliomas. J Neuropathol Exp Neurol. 1981 May;40(3):201–229. doi: 10.1097/00005072-198105000-00001. [DOI] [PubMed] [Google Scholar]
- Breitman T. R., Selonick S. E., Collins S. J. Induction of differentiation of the human promyelocytic leukemia cell line (HL-60) by retinoic acid. Proc Natl Acad Sci U S A. 1980 May;77(5):2936–2940. doi: 10.1073/pnas.77.5.2936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burd P. R., Rollins B. J., Wilson S. D., Billings P. R., Stiles C. D., Dorf M. E. Comparison of fibroblast and T-cell activation genes. Cell Immunol. 1988 Sep;115(2):481–483. doi: 10.1016/0008-8749(88)90200-6. [DOI] [PubMed] [Google Scholar]
- Chan J. Y., Slamon D. J., Nimer S. D., Golde D. W., Gasson J. C. Regulation of expression of human granulocyte/macrophage colony-stimulating factor. Proc Natl Acad Sci U S A. 1986 Nov;83(22):8669–8673. doi: 10.1073/pnas.83.22.8669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang H. C., Hsu F., Freeman G. J., Griffin J. D., Reinherz E. L. Cloning and expression of a gamma-interferon-inducible gene in monocytes: a new member of a cytokine gene family. Int Immunol. 1989;1(4):388–397. doi: 10.1093/intimm/1.4.388. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Collins S. J. The HL-60 promyelocytic leukemia cell line: proliferation, differentiation, and cellular oncogene expression. Blood. 1987 Nov;70(5):1233–1244. [PubMed] [Google Scholar]
- Cross S. L., Halden N. F., Lenardo M. J., Leonard W. J. Functionally distinct NF-kappa B binding sites in the immunoglobulin kappa and IL-2 receptor alpha chain genes. Science. 1989 Apr 28;244(4903):466–469. doi: 10.1126/science.2497520. [DOI] [PubMed] [Google Scholar]
- Davatelis G., Tekamp-Olson P., Wolpe S. D., Hermsen K., Luedke C., Gallegos C., Coit D., Merryweather J., Cerami A. Cloning and characterization of a cDNA for murine macrophage inflammatory protein (MIP), a novel monokine with inflammatory and chemokinetic properties. J Exp Med. 1988 Jun 1;167(6):1939–1944. doi: 10.1084/jem.167.6.1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davatelis G., Wolpe S. D., Sherry B., Dayer J. M., Chicheportiche R., Cerami A. Macrophage inflammatory protein-1: a prostaglandin-independent endogenous pyrogen. Science. 1989 Feb 24;243(4894 Pt 1):1066–1068. doi: 10.1126/science.2646711. [DOI] [PubMed] [Google Scholar]
- Doherty P. J., Huesca-Contreras M., Dosch H. M., Pan S. Rapid amplification of complementary DNA from small amounts of unfractionated RNA. Anal Biochem. 1989 Feb 15;177(1):7–10. doi: 10.1016/0003-2697(89)90003-1. [DOI] [PubMed] [Google Scholar]
- Doi T., Greenberg S. M., Rosenberg R. D. Structure of the rat platelet factor 4 gene: a marker for megakaryocyte differentiation. Mol Cell Biol. 1987 Feb;7(2):898–904. doi: 10.1128/mcb.7.2.898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray P. W., Goeddel D. V. Structure of the human immune interferon gene. Nature. 1982 Aug 26;298(5877):859–863. doi: 10.1038/298859a0. [DOI] [PubMed] [Google Scholar]
- Green C. J., Charles R. S., Edwards B. F., Johnson P. H. Identification and characterization of PF4varl, a human gene variant of platelet factor 4. Mol Cell Biol. 1989 Apr;9(4):1445–1451. doi: 10.1128/mcb.9.4.1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holbrook N. J., Smith K. A., Fornace A. J., Jr, Comeau C. M., Wiskocil R. L., Crabtree G. R. T-cell growth factor: complete nucleotide sequence and organization of the gene in normal and malignant cells. Proc Natl Acad Sci U S A. 1984 Mar;81(6):1634–1638. doi: 10.1073/pnas.81.6.1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurka J., Smith T. A fundamental division in the Alu family of repeated sequences. Proc Natl Acad Sci U S A. 1988 Jul;85(13):4775–4778. doi: 10.1073/pnas.85.13.4775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawahara R. S., Deuel T. F. Platelet-derived growth factor-inducible gene JE is a member of a family of small inducible genes related to platelet factor 4. J Biol Chem. 1989 Jan 15;264(2):679–682. [PubMed] [Google Scholar]
- Kunkel L. M., Smith K. D., Boyer S. H., Borgaonkar D. S., Wachtel S. S., Miller O. J., Breg W. R., Jones H. W., Jr, Rary J. M. Analysis of human Y-chromosome-specific reiterated DNA in chromosome variants. Proc Natl Acad Sci U S A. 1977 Mar;74(3):1245–1249. doi: 10.1073/pnas.74.3.1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon B. S., Weissman S. M. cDNA sequences of two inducible T-cell genes. Proc Natl Acad Sci U S A. 1989 Mar;86(6):1963–1967. doi: 10.1073/pnas.86.6.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen C. G., Anderson A. O., Appella E., Oppenheim J. J., Matsushima K. The neutrophil-activating protein (NAP-1) is also chemotactic for T lymphocytes. Science. 1989 Mar 17;243(4897):1464–1466. doi: 10.1126/science.2648569. [DOI] [PubMed] [Google Scholar]
- Luster A. D., Ravetch J. V. Genomic characterization of a gamma-interferon-inducible gene (IP-10) and identification of an interferon-inducible hypersensitive site. Mol Cell Biol. 1987 Oct;7(10):3723–3731. doi: 10.1128/mcb.7.10.3723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda S., Nishino N., Obaru K., Mita S., Nomiyama H., Shimada K., Fujimoto K., Teranishi T., Hirano T., Onoue K. Cloning of interleukin 2 mRNAs from human tonsils. Biochem Biophys Res Commun. 1983 Sep 30;115(3):1040–1047. doi: 10.1016/s0006-291x(83)80040-0. [DOI] [PubMed] [Google Scholar]
- Minta J. O., Pambrun L. In vitro induction of cytologic and functional differentiation of the immature human monocytelike cell line U-937 with phorbol myristate acetate. Am J Pathol. 1985 Apr;119(1):111–126. [PMC free article] [PubMed] [Google Scholar]
- Miyatake S., Otsuka T., Yokota T., Lee F., Arai K. Structure of the chromosomal gene for granulocyte-macrophage colony stimulating factor: comparison of the mouse and human genes. EMBO J. 1985 Oct;4(10):2561–2568. doi: 10.1002/j.1460-2075.1985.tb03971.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyatake S., Seiki M., Yoshida M., Arai K. T-cell activation signals and human T-cell leukemia virus type I-encoded p40x protein activate the mouse granulocyte-macrophage colony-stimulating factor gene through a common DNA element. Mol Cell Biol. 1988 Dec;8(12):5581–5587. doi: 10.1128/mcb.8.12.5581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyatake S., Yokota T., Lee F., Arai K. Structure of the chromosomal gene for murine interleukin 3. Proc Natl Acad Sci U S A. 1985 Jan;82(2):316–320. doi: 10.1073/pnas.82.2.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukaida N., Shiroo M., Matsushima K. Genomic structure of the human monocyte-derived neutrophil chemotactic factor IL-8. J Immunol. 1989 Aug 15;143(4):1366–1371. [PubMed] [Google Scholar]
- Nagata S., Tsuchiya M., Asano S., Yamamoto O., Hirata Y., Kubota N., Oheda M., Nomura H., Yamazaki T. The chromosomal gene structure and two mRNAs for human granulocyte colony-stimulating factor. EMBO J. 1986 Mar;5(3):575–581. doi: 10.1002/j.1460-2075.1986.tb04249.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathans J., Piantanida T. P., Eddy R. L., Shows T. B., Hogness D. S. Molecular genetics of inherited variation in human color vision. Science. 1986 Apr 11;232(4747):203–210. doi: 10.1126/science.3485310. [DOI] [PubMed] [Google Scholar]
- Nathans J., Thomas D., Hogness D. S. Molecular genetics of human color vision: the genes encoding blue, green, and red pigments. Science. 1986 Apr 11;232(4747):193–202. doi: 10.1126/science.2937147. [DOI] [PubMed] [Google Scholar]
- Nimer S. D., Morita E. A., Martis M. J., Wachsman W., Gasson J. C. Characterization of the human granulocyte-macrophage colony-stimulating factor promoter region by genetic analysis: correlation with DNase I footprinting. Mol Cell Biol. 1988 May;8(5):1979–1984. doi: 10.1128/mcb.8.5.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obaru K., Fukuda M., Maeda S., Shimada K. A cDNA clone used to study mRNA inducible in human tonsillar lymphocytes by a tumor promoter. J Biochem. 1986 Mar;99(3):885–894. doi: 10.1093/oxfordjournals.jbchem.a135549. [DOI] [PubMed] [Google Scholar]
- Obaru K., Hattori T., Yamamura Y., Takatsuki K., Nomiyama H., Maeda S., Shimada K. A cDNA clone inducible in human tonsillar lymphocytes by a tumor promoter codes for a novel protein of the beta-thromboglobulin superfamily. Mol Immunol. 1989 Apr;26(4):423–426. doi: 10.1016/0161-5890(89)90131-4. [DOI] [PubMed] [Google Scholar]
- Rappolee D. A., Mark D., Banda M. J., Werb Z. Wound macrophages express TGF-alpha and other growth factors in vivo: analysis by mRNA phenotyping. Science. 1988 Aug 5;241(4866):708–712. doi: 10.1126/science.3041594. [DOI] [PubMed] [Google Scholar]
- Rollins B. J., Morrison E. D., Stiles C. D. Cloning and expression of JE, a gene inducible by platelet-derived growth factor and whose product has cytokine-like properties. Proc Natl Acad Sci U S A. 1988 Jun;85(11):3738–3742. doi: 10.1073/pnas.85.11.3738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saffer J. D., Thurston S. J. A negative regulatory element with properties similar to those of enhancers is contained within an Alu sequence. Mol Cell Biol. 1989 Feb;9(2):355–364. doi: 10.1128/mcb.9.2.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sollner-Webb B., Reeder R. H. The nucleotide sequence of the initiation and termination sites for ribosomal RNA transcription in X. laevis. Cell. 1979 Oct;18(2):485–499. doi: 10.1016/0092-8674(79)90066-7. [DOI] [PubMed] [Google Scholar]
- Stanley E., Metcalf D., Sobieszczuk P., Gough N. M., Dunn A. R. The structure and expression of the murine gene encoding granulocyte-macrophage colony stimulating factor: evidence for utilisation of alternative promoters. EMBO J. 1985 Oct;4(10):2569–2573. doi: 10.1002/j.1460-2075.1985.tb03972.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor A., Erba H. P., Muscat G. E., Kedes L. Nucleotide sequence and expression of the human skeletal alpha-actin gene: evolution of functional regulatory domains. Genomics. 1988 Nov;3(4):323–336. doi: 10.1016/0888-7543(88)90123-1. [DOI] [PubMed] [Google Scholar]
- Tokunaga K., Nakamura Y., Sakata K., Fujimori K., Ohkubo M., Sawada K., Sakiyama S. Enhanced expression of a glyceraldehyde-3-phosphate dehydrogenase gene in human lung cancers. Cancer Res. 1987 Nov 1;47(21):5616–5619. [PubMed] [Google Scholar]
- Treisman R. Identification of a protein-binding site that mediates transcriptional response of the c-fos gene to serum factors. Cell. 1986 Aug 15;46(4):567–574. doi: 10.1016/0092-8674(86)90882-2. [DOI] [PubMed] [Google Scholar]
- Treisman R. Transient accumulation of c-fos RNA following serum stimulation requires a conserved 5' element and c-fos 3' sequences. Cell. 1985 Oct;42(3):889–902. doi: 10.1016/0092-8674(85)90285-5. [DOI] [PubMed] [Google Scholar]
- Tsuchiya M., Kaziro Y., Nagata S. The chromosomal gene structure for murine granulocyte colony-stimulating factor. Eur J Biochem. 1987 May 15;165(1):7–12. doi: 10.1111/j.1432-1033.1987.tb11187.x. [DOI] [PubMed] [Google Scholar]
- Vollrath D., Nathans J., Davis R. W. Tandem array of human visual pigment genes at Xq28. Science. 1988 Jun 17;240(4859):1669–1672. doi: 10.1126/science.2837827. [DOI] [PubMed] [Google Scholar]
- Weiner A. M., Deininger P. L., Efstratiadis A. Nonviral retroposons: genes, pseudogenes, and transposable elements generated by the reverse flow of genetic information. Annu Rev Biochem. 1986;55:631–661. doi: 10.1146/annurev.bi.55.070186.003215. [DOI] [PubMed] [Google Scholar]
- Westwick J., Li S. W., Camp R. D. Novel neutrophil-stimulating peptides. Immunol Today. 1989 May;10(5):146–147. doi: 10.1016/0167-5699(89)90164-3. [DOI] [PubMed] [Google Scholar]
- Wolpe S. D., Davatelis G., Sherry B., Beutler B., Hesse D. G., Nguyen H. T., Moldawer L. L., Nathan C. F., Lowry S. F., Cerami A. Macrophages secrete a novel heparin-binding protein with inflammatory and neutrophil chemokinetic properties. J Exp Med. 1988 Feb 1;167(2):570–581. doi: 10.1084/jem.167.2.570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamura Y., Hattori T., Obaru K., Sakai K., Asou N., Takatsuki K., Ohmoto Y., Nomiyama H., Shimada K. Synthesis of a novel cytokine and its gene (LD78) expressions in hematopoietic fresh tumor cells and cell lines. J Clin Invest. 1989 Dec;84(6):1707–1712. doi: 10.1172/JCI114353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zipfel P. F., Balke J., Irving S. G., Kelly K., Siebenlist U. Mitogenic activation of human T cells induces two closely related genes which share structural similarities with a new family of secreted factors. J Immunol. 1989 Mar 1;142(5):1582–1590. [PubMed] [Google Scholar]
- Zipfel P. F., Irving S. G., Kelly K., Siebenlist U. Complexity of the primary genetic response to mitogenic activation of human T cells. Mol Cell Biol. 1989 Mar;9(3):1041–1048. doi: 10.1128/mcb.9.3.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Heijne G. Signal sequences. The limits of variation. J Mol Biol. 1985 Jul 5;184(1):99–105. doi: 10.1016/0022-2836(85)90046-4. [DOI] [PubMed] [Google Scholar]