Abstract
Increased vascular impedance in the fetoplacental circulation is associated with fetal hypoxia and growth restriction. We sought to investigate the role of hydrogen sulfide (H2S) in regulating vasomotor tone in the fetoplacental vasculature. H2S is produced endogenously by catalytic activity of cystathionine β-synthase and cystathionine γ-lyase (CSE). Immunohistochemical analysis localized CSE to smooth muscle cells encircling arteries in stem villi. Immunoreactivity was reduced in placentas from pregnancies with severe early-onset growth-restriction and preeclampsia displaying abnormal umbilical artery Doppler waveforms compared with preeclamptic placentas with normal waveforms and controls. These findings were confirmed at the protein and mRNA levels. MicroRNA-21, which negatively regulates CSE expression, was increased in placentas with abnormal Doppler waveforms. Exposure of villus explants to hypoxia-reoxygenation significantly reduced CSE protein and mRNA and increased microRNA-21 expression. No changes were observed in cystathionine β-synthase expression, immunolocalized principally to the trophoblast, in pathologic placentas or in vitro. Finally, perfusion of normal placentas with an H2S donor, after preconstriction with a thromboxane mimetic, resulted in dose-dependent vasorelaxation. Glibenclamide and NG-nitro-l-arginine methyl ester partially blocked the effect, indicating that H2S acts through ATP-sensitive K+ channels and nitric oxide synthesis. These results demonstrate that H2S is a powerful vasodilator of the placental vasculature and that expression of CSE is reduced in placentas associated with increased vascular resistance.
One of the principal functions of the placenta is to act as a gas exchanger, which it achieves by bringing the maternal and fetal circulations into close approximation over a large surface area. The flow in these circulations is now routinely assessed noninvasively in vivo in high-risk pregnancies using Doppler ultrasound.1 Umbilical artery Doppler velocimetry provides an indirect measure of downstream vascular resistance in the fetoplacental vasculature.2 Pregnancies complicated by severe early-onset forms of preeclampsia (PE)3 and/or intrauterine growth restriction (IUGR)4 typically exhibit increased resistance in the umbilical circulation, with absent or even reversed end-diastolic flow velocity. These highly abnormal Doppler flow patterns are associated with a poor perinatal prognosis,5 in part because they identify a subset of pregnancies with chronic fetal hypoxia, metabolic acidosis, and reduced circulating glucose and amino acid levels.6, 7, 8
In the human placenta, the umbilical arteries branch into a series of resistance arteries contained in the distributing stem villi. These arteries eventually supply the capillary network in the terminal villi that are the principal sites of gaseous exchange. In the absence of nerves in the placenta, vasomotor control of the resistance arteries is performed by locally produced vasoreactive molecules. To date, nitric oxide (NO)9 and carbon monoxide (CO)10 have been shown to have dilator actions in vitro. Accumulating studies suggest that hydrogen sulfide (H2S) also exerts significant effects in the adult cardiovascular system, mainly via endothelium-independent vasodilation.11 However, its potential role in the placental circulation has not been investigated. We, therefore, investigated expression of the enzymes generating H2S in pathologic placentas with and without increased vascular resistance.
Endogenous H2S production is catalyzed by cystathionine β-synthase (CBS) and cystathionine γ-lyase (CSE).12, 13 CSE is the dominant enzyme for synthesis of H2S in the cardiovascular system, and reduced expression/activity and decreased H2S expression contribute to the pathogenesis of pulmonary hypertension in rodents.14 Although CBS is not present in vascular tissues, CBS deficiency leads to secondary hypertension and endothelial dysfunction through hyperhomocysteinemia.15, 16 The most compelling evidence for the role of H2S in the cardiovascular system comes from studies using CSE single and double knockout mice, where significantly higher systolic arterial blood pressure was reported in CSE−/− and, to a lesser extent, CSE+/−- compared with CSE+/+ mice. In addition, CSE−/− mice are highly sensitive to H2S-mediated vasodilatation compared with wild-type mice.17 The mechanism of action of H2S has been best described in the nervous system, where ATP-sensitive K+ (KATP) channels18 and N-methyl-d-aspartate receptors19 have been identified as the prime targets. Similarly, KATP channels present in vascular smooth muscle cells (SMCs) were identified as the target protein of H2S, mediating its vascular effect.20 In addition, the vascular effect of H2S can be potentiated by NO.20, 21 In view of these findings, we tested the effect of the KATP channel blocker glibenclamide and the NO synthesis blocker NG-nitro-l-arginine methyl ester (l-NAME) when applied with sodium hydrosulfide (NaHS) in perfused human placentas.
In intrauterine tissues, Patel et al22 confirmed endogenous H2S placental production and release and the presence of placental CSE and CBS in rats and humans. Holwerda et al23 showed decreased placental mRNA expression of CBS in early-onset PE, and You et al24 showed down-regulation of CBS and CSE mRNA and of CBS protein expression in the myometrium during labor. However, the vasoactive effects of H2S in the fetoplacental vascular bed remain unexplored in any species.
We hypothesized that placentas from pregnancies complicated by the severe forms of PE and IUGR with abnormal umbilical artery Doppler waveforms show impaired endogenous H2S production secondary to a reduction in the H2S-producing enzymes. To test this hypothesis, the first aim was to determine the expression of CSE and CBS in cesarean-delivered human placentas from healthy pregnancies and from pregnancies complicated by IUGR or PE. To test the relationship between local H2S production and umbilical artery Doppler waveforms in high-risk pregnancies, we additionally split the PE placentas into two groups with respect to umbilical artery Doppler findings, ie, PE with abnormal umbilical artery Doppler findings (PE-AD) and PE with normal umbilical artery Doppler findings and, thus, normal resistance (PE-ND). Because CSE protein and mRNA expression were significantly reduced in IUGR and PE-AD samples, we further explored its regulation by examining the expression of microRNA-21 (miR-21). Recent evidence suggests that miR-21 overexpression participates in CSE/H2S-mediated SMC differentiation by repressing the protein expression of CSE and SP1, inhibiting H2S production, stimulating SMC proliferation, and reducing SMC differentiation.25 Second, because oxidative stress due to placental ischemia-reperfusion injury is a major component of the placental pathology associated with PE and IUGR,26, 27 we examined the effects of hypoxia-reoxygenation (H/R) on the gene and protein expression of CSE and CBS and that of miR-21 in human placental explants in vitro. The final aim was to test whether H2S plays a role in dilating the human placenta by perfusing preconstricted healthy human cesarean-delivered placentas with an H2S donor. The mechanism of action of H2S in the fetoplacental circulation was determined using the KATP channel blocker glibenclamide and the NO synthesis blocker l-NAME.
Materials and Methods
Placental Collection and Ethical Approval
IUGR was diagnosed ultrasonically when abdominal circumference measurements were below the 10th percentile28 and was confirmed at birth by a neonatal weight below the 10th percentile.29 Of the six IUGR cases, three had normal umbilical artery Doppler velocimetry findings, two had abnormal umbilical arterial Doppler velocimetry findings, and one had absent end diastolic blood flow of the umbilical artery (Table 1). PE was defined as the onset of gestational hypertension and proteinuria after 20 weeks of gestation, and the cases examined herein were early-onset PE. We additionally divided the PE category into two groups based on the results of Doppler velocimetry, ie, PE-AD (n = 7) and PE-ND (n = 7). Hypertension was defined as two or more recordings of a diastolic blood pressure of ≥90 mm Hg taken ≥4 hours apart (PE-AD: means ± SEM systolic blood pressure, 171 ± 14 mm Hg, and means ± SEM diastolic blood pressure, 109 ± 7 mm Hg; PE-ND: means ± SEM systolic blood pressure, 172 ± 15 mm Hg, and means ± SEM diastolic blood pressure, 102 ± 4 mm Hg). Proteinuria was defined as the excretion of ≥300 mg of protein over 24 hours. The PE-AD group included all the criteria for IUGR, and birth weight was significantly higher in the PE-ND group (10th to 50th percentile) (Table 1).
Table 1.
Clinical Information for Term Healthy and Pathologic Placentas
| Characteristic | Group |
|||
|---|---|---|---|---|
| Healthy | IUGR | PE-AD | PE-ND | |
| Gestational age (weeks) | 39 ± 0.6 | 32 ± 3.5∗ | 28.5 ± 1.3∗ | 29.5 ± 1.5∗ |
| Fetal weight (g) | 3615 ± 417 | 1045 ± 465∗ | 928 ± 159∗ | 1255 ± 226∗ |
| Umbilical artery Doppler | H = 6 |
NH = 3 PEDF = 2 AREDF = 1 |
PEDF = 2 AREDF = 5 |
H = 7 |
Data are presented as means ± SD.
AREDF, absent or reversed end-diastolic blood flow; H, healthy; PEDF, abnormal but present end-diastolic blood flow.
P < 0.001.
Human placentas for perfusion and explant culture were collected with Cambridge Local Ethical Committee approval and informed written patient consent on an anonymous basis. Gestational age was calculated from the last menstrual period and was confirmed by routine ultrasonography at 11 to 12 weeks of gestation. All the placentas were delivered at term by elective cesarean birth from nonlaboring normotensive healthy singleton pregnancies, with no history of cigarette smoking, diabetes, autoimmune diseases, or thrombophilic conditions.
Culture of Placental Explants
Placental tissue was collected from cesarean-delivered singleton pregnancies within 10 minutes of delivery. Villous samples were taken midway between the chorionic and basal plates from the periphery of three lobules free of visible infarction, calcification, hematoma, or tears on a random systematic basis. Samples were briefly rinsed in cold PBS and placed in ice-cold transport medium (TCS large-vessel endothelial cell basal medium; TCS Cellworks, Milton Keynes, UK) containing 2% fetal bovine serum, heparin, hydrocortisone, human epidermal growth factor, human basic fibroblast growth factor, 25 μg/mL of gentamicin and 50 ng/mL of amphotericin B, 1 mmol/L vitamin C, and 1 mmol/L Trolox that had been equilibrated with 5% O2/90% N2/5% CO2.
After transport to the laboratory on ice for approximately 1 hour, placental samples were further dissected into small pieces approximately 5 mm in diameter in ice-cold culture medium in a glove box under 10% O2/85% N2/5% CO2, as previously described.30 Briefly, 5-mm3 placental explants were cultured on individual Costar Netwell inserts (Corning Inc., Lowell, MA) (24-mm diameter, 500-μm mesh) in 4 mL of the large-vessel endothelial cell basal medium containing 2% fetal bovine serum, heparin, hydrocortisone, human epidermal growth factor, human basic fibroblast growth factor, 25 μg/mL of gentamicin, and 50 ng/mL of amphotericin B, equilibrated with 5% O2/90% N2/5% CO2 per well in 6-well plates for 24 hours. Approximately 6 to 10 pieces were added to each well, depending on the experimental requirements. Placental explants were incubated in pregassed medium under normoxic conditions (controls) (10% O2/85% N2/5% CO2) or were subjected to hypoxia (0.5% O2/94.5% N2/5% CO2) for 3 hours and subsequent reoxygenation at normoxia (10% O2/85% N2/5% CO2) for the following 20 hours (N = 4) (H/R treatment). All the inserts were transferred into pre-gassed medium after 1 hour of incubation. At the end of the 24-hour culture period, some placental explants for each condition were flash frozen in liquid nitrogen and stored at −80°C until further processing for mRNA or protein, and a few were fixed in 4% paraformaldehyde overnight for immunohistochemical analysis.
Western Blot Analysis
Frozen villous samples were homogenized in ice-cold lysis buffer (1 mL of buffer per 100 mg of tissue) containing 20 mmol/L Tris, pH 7.4, 1 mmol/L EGTA, 0.01% Triton X-100, 1 mmol/L sodium pyrophosphate, 1 mmol/L sodium orthovanadate, 10 mmol/L β-glycerol phosphate, 50 mmol/L sodium fluoride, and a complete mini protease inhibitor cocktail (Roche Diagnostics, East Sussex, UK). Tissue homogenates were centrifuged at 15,000 × g, 4°C for 20 minutes to remove tissue debris and the clear supernatants. Protein concentrations were determined on the supernatant using a bicinchoninic acid protein assay kit (Sigma-Aldrich, Poole, UK). Lysates were mixed with 3× SDS-PAGE sample buffer, boiled for 5 minutes, and allowed to return to room temperature. Equal amounts of protein (20 to 30 μg) were separated by SDS-PAGE using 10% polyacrylamide resolving gels and were transferred onto nitrocellulose membrane (Invitrogen, Paisley, UK) and subjected to immunoblot analysis. Membranes were blocked for 1 hour at 25°C in 5% milk diluted in Tris-buffered saline and 0.1% Tween 20 and incubated with the following primary antibodies overnight at 4°C: anti-CSE (diluted 1:500; Abnova, Novus Biologicals, Cambridge, UK) and anti-CBS (diluted 1:500; Sigma-Aldrich). After washing and incubating with secondary antibodies, immunoreactive proteins were visualized by the ECL Plus chemiluminescence system (Amersham Biosciences, Buckinghamshire, UK) following the manufacturer’s instructions. Protein bands were quantified using ImageJ software version 1.46r (NIH, Bethesda, MD). Protein loading was normalized against Ponceau S staining. We used Ponceau S as a loading control because it has been validated and found to be comparable with β-actin staining.31 The values are expressed as percentages of the control lysate (100%) for each experiment.
RNA Isolation and Quantitative Real-Time RT-PCR Analysis
Total RNA was isolated from human placentas using an RNeasy micro kit (Qiagen, Hilden, Germany). The RNA was quantified by spectrophotometry (Nanodrop Technologies, Wilmington, DE). In brief, 20 μg of total RNA from each sample was reverse transcribed using a master mix containing SuperScript II Reverse Transcriptase (Invitrogen). The ABI PRISM 7700 sequence detection system (ABI, Warrington, UK) was used to perform real-time PCR according to the manufacturer’s protocols. CT values for each transcript (ie, CSE, CBS) were compared with those for 18S rRNA. RNU48 was used as an endogenous control for real-time PCR quantification of miR21. All the primers and probes were obtained from Applied Biosystems Assays-on-Demand (ABI) and used a 5′ FAM reporter and a 3′ nonfluorescent minor groove binder.
Colorimetric Immunohistochemical Analysis
Paraformaldehyde-fixed tissues embedded in paraffin wax were sectioned at 7 μm. After dewaxing and blocking of endogenous peroxidases by incubation with 3% H2O2 for 30 minutes, the sections were incubated with nonimmune serum for 20 minutes. Primary antibodies [anti-CSE (diluted 1:300; Abnova) and anti-CBS (diluted 1:200; Sigma-Aldrich)] were applied overnight at 4°C, and binding was detected using Vectastain Elite ABC kits (Vector Laboratories, Burlingame, CA) and SigmaFast diaminobenzidine (Sigma-Aldrich) according to the manufacturers’ instructions. Sections were lightly counterstained with hematoxylin. When necessary, antigen retrieval was performed before blocking using 1 mmol/L Tris–0.1 mmol/L EDTA buffer, pH 9.0, in a pressure cooker for 2 minutes. Negative controls were performed by replacement of primary antibody with equal concentrations of nonimmune serum.
Placental Perfusion
Perfusion pressure and flow (Transonic Systems Inc., Ithaca, NY) were measured in eight healthy cesarean-delivered human placentas. The placental perfusion protocol was modified from that of Brownbill and Sibley.32 Briefly, a single lobe from each placenta was perfused with equilibrated (95% O2/5% CO2, pH 7.4) Earle’s bicarbonate buffer containing dextran 70 (mol. wt. 70 to 80 kDa) (31390; Sigma-Aldrich), 1% bovine serum albumin, 0.402 mmol/L l-arginine, and 10 UI/mL of heparin (Wockhardt UK Ltd., Wrexham, UK) by cannulating the chorionic artery and vein of the lobe. The basal pressure was settled at 30 to 40 mm Hg, with a constant flow for ≥20 minutes. The flow to achieve this perfusion pressure was regulated between 5 and 8 mL.min−1 (depending on the lobule size), with a peristaltic pump giving a fixed pulsatility of 160 bpm. The experiments were performed at a constant flow and pulsatility (rate). Placentas that did not stabilize pressure after 20 minutes of a constant flow and rate were thus rejected. The catheters implanted in the chorionic artery were connected to sterile pressure transducers set at the placental chorionic plate level (COBE; Argon Division, Maxxim Medical, Athens, TX), and these pressures were recorded continuously by a custom-built data acquisition system (Maastricht-Programmable AcQuisition system; Maastricht Instruments, Maastricht, The Netherlands) at a 500-Hz sample rate. The Gilson Minipuls 2 peristaltic pumps (Anachem, Luton, UK) used were appropriately set for each placenta to maintain 30- to 40-mm Hg arterial pressure (simulating the in vivo physiologic umbilical arterial pressure). To fix flow without significantly altering the pulsatility, we used different inside diameters for the tubing in the peristaltic pump.
After preconstriction with the thromboxane mimetic U46619 (10−7 mol/L; Cayman Chemical, Ann Arbor, MI), increasing doses of NaHS solution (10−12 to 10−6 mol/L) were infused. Glibenclamide (10−5 mol/L) was administered to block KATP channels and l-NAME (10−5 mol/L) to inhibit endogenous NO synthesis in a continuous manner in the perfusate before preconstriction with U46619. The placenta reached steady state constriction after 5 to 10 minutes of the U46619 infusion. After 3 minutes of steady plateau, the increasing doses of NaHS were given. The l-NAME and glibenclamide blockades were given in the infusion (perfusate), and the NaHS was administered in 5-mL boluses, replacing the perfusate infusion (therefore, the concentration in the lumen is that infused during the 40 to 60 seconds that last the bolus, depending on the flow rate). Between boluses, a waiting period of 3 to 5 minutes was introduced (with the perfusate) until reaching a plateau in the vasodilator response.
Flow and pressure were continuously recorded by a 2R flow probe connected to a T106 monitor (Transonic Systems Inc.) and the Maastricht-Programmable AcQuisition system. The flow was compared with the venous output, confirming the integrity of the vessel network at the beginning and end of the experiment. Doses of U46619, glibenclamide, and l-NAME were optimized in pilot experiments.
Statistical Analysis
Data are expressed as means ± SEM. Comparisons were made using a 2-tailed t-test or analysis of variance with a Student Newman-Keuls post hoc test or Wilcoxon nonparametric test where appropriate. Differences were considered to be significant at P ≤ 0.05.
Results
Localization and Expression of CBS and CSE in Healthy and Pathologic Human Placentas
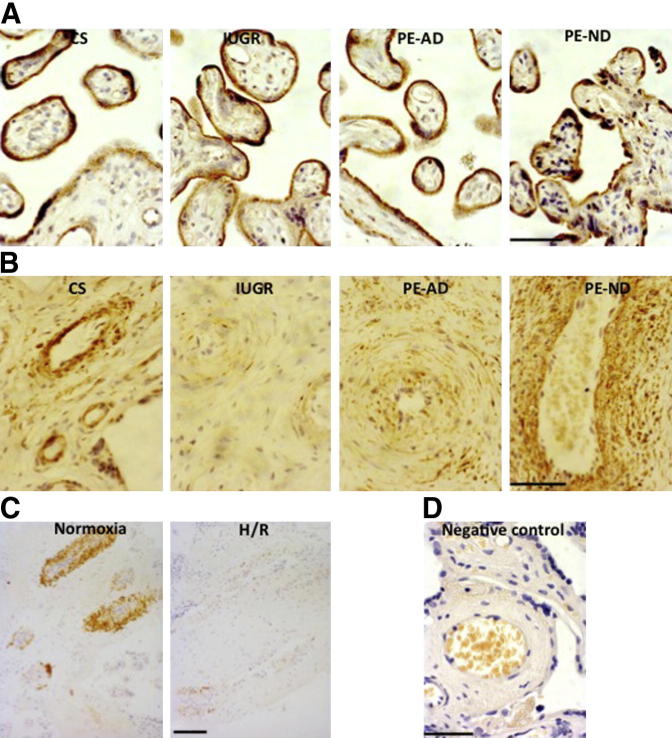
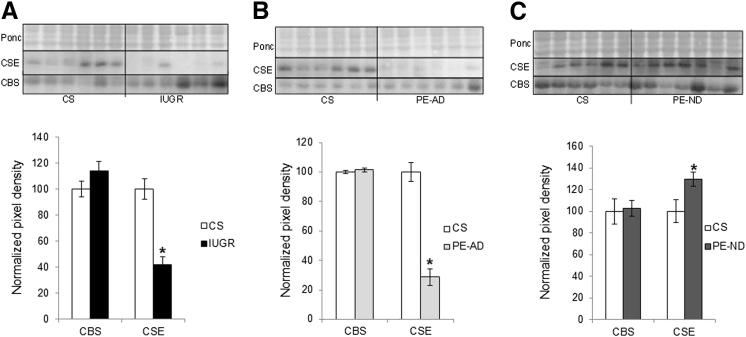
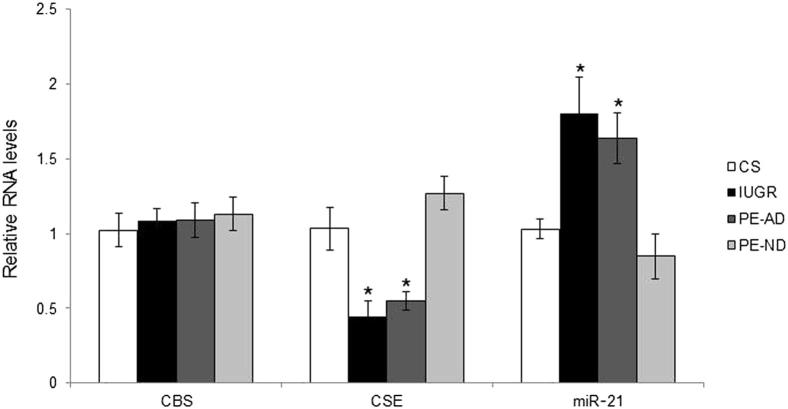
CBS was localized only to the syncytiotrophoblast (Figure 1A). In contrast, CSE was immunolocalized principally to the SMCs surrounding the large placental stem villus arteries (Figure 1B). We investigated whether the potential for synthesis of H2S was deficient in placentas from pregnancies complicated by PE and IUGR. Protein (Figure 2) and mRNA (Figure 3) expression levels of CSE were significantly decreased in IUGR and PE-AD placentas compared with healthy placentas delivered by cesarean birth. However, this was not the case in PE-ND samples, which had unaltered mRNA CSE expression (Figure 3) and even marginally increased CSE protein expression (Figure 2C). Reduced placental expression of CSE thus associated with increased placental vascular resistance, as assessed by umbilical Doppler velocimetry. There were no differences in protein (Figure 2) or mRNA (Figure 3) levels of CBS among the four different groups. The protein expression of CBS and CSE was examined by Western blot analysis, and bands of approximately 43 kDa corresponding to CSE and approximately 61 kDa corresponding to CBS were recognized. Immunohistochemical analysis results matched the protein findings (Figure 1, A and B). We used a semiquantitative scale from 0 to 3 to analyze six randomly selected areas per four slides per group at ×40 magnification: 0 = no staining, 1 = weak intensity of staining, 2 = moderate staining, and 3 = strong staining. Randomly selected slides showed that controls exhibited a staining score of 2.6; PE-AD, 1.7; and PE-ND, 2.7.
Figure 1.
Placental immunostaining. Localization of CBS (A) and CSE (B) in human placentas from healthy cesarean-delivered (CS), IUGR, PE-AD, and PE-ND pregnancies and localization of CSE in term human placental explants cultured at different oxygen concentrations [ie, normoxia (10% O2 for 20 hours) and H/R (0.5% O2/10% O2 for 20 hours)] (C). D: Negative control stained by replacing primary antibody with nonimmune serum. Brown color signifies positive staining. CBS localized in the syncytiotrophoblast, whereas CSE was predominantly expressed by SMCs of large placental arteries. Scale bars = 100 μm.
Figure 2.
Protein expression profiles of placentas. Protein expression of CBS and CSE in IUGR (A) and PE-AD (B) or PE-ND (C) placentas compared with cesarean-delivered (CS) controls. Lysates from CS control (n = 6), IUGR (n = 6), PE-AD (n = 6), and PE-ND (n = 7) placentas were immunoblotted with antibodies against CSE and CBS. Ponceau S (Ponc) expression served to normalize gel loading. Normalized results (means ± SEM) are plotted, expressing CS controls as 100%. ∗P < 0.05 versus CS controls (one-way analysis of variance and Student Newman-Keuls test).
Figure 3.
mRNA expression profiles of placentas. mRNA expression of CBS and CSE and miR-21 in cesarean-delivered (CS) control, IUGR, PE-AD, andr PE-ND placentas. RNA from CS control, IUGR, and PE placentas was isolated, and relative levels of CBS and CSE mRNA or miR-21 were detected using quantitative real-time RT-PCR. CBS and CSE mRNA levels were normalized to the 18S RNA levels and miR-21 to the RNU48 levels. Results are given as means ± SEM. ∗P < 0.05 versus CS controls (one-way analysis of variance and Student Newman-Keuls test).
We used term cesarean-delivered healthy placentas as controls for these experiments. Significant differences in means ± SEM gestational ages among control (39 ± 0.6 weeks), IUGR (32 ± 3.5 weeks), PE-AD (28.5 ± 1.3 weeks), and PE-ND (29.5 ± 1.5 weeks) samples (Table 1) reflect the inability to obtain cesarean-delivered healthy preterm controls to match the gestational age of severe IUGR and PE samples that were delivered prematurely for clinical reasons. However, no differences in CBS mRNA expression were reported between first-trimester and term human placentas.33 It is, thus, unlikely that the reduction in CSE expression in pathologic pregnancies is due to differences in gestational age. Both proteins are present in early placental samples and display the same localization as in term samples (data not shown).
miR-21 as a Potential Regulator of CSE Expression
Because recent evidence suggests that SMC CSE expression could be regulated by miR-21,25 we quantified miR-21 expression using quantitative real-time-PCR in PE and IUGR placentas compared with controls and found significant up-regulation of miR-21 expression in IUGR and PE-AD placentas. There was no change in miR-21 expression in PE-ND placentas (Figure 3). These results inversely match the changes in CSE protein and mRNA expression and are consistent with the hypothesis that miR-21 might play a regulatory role in down-regulating CSE expression.
CBS and CSE Expression in Placental Explants Challenged with H/R
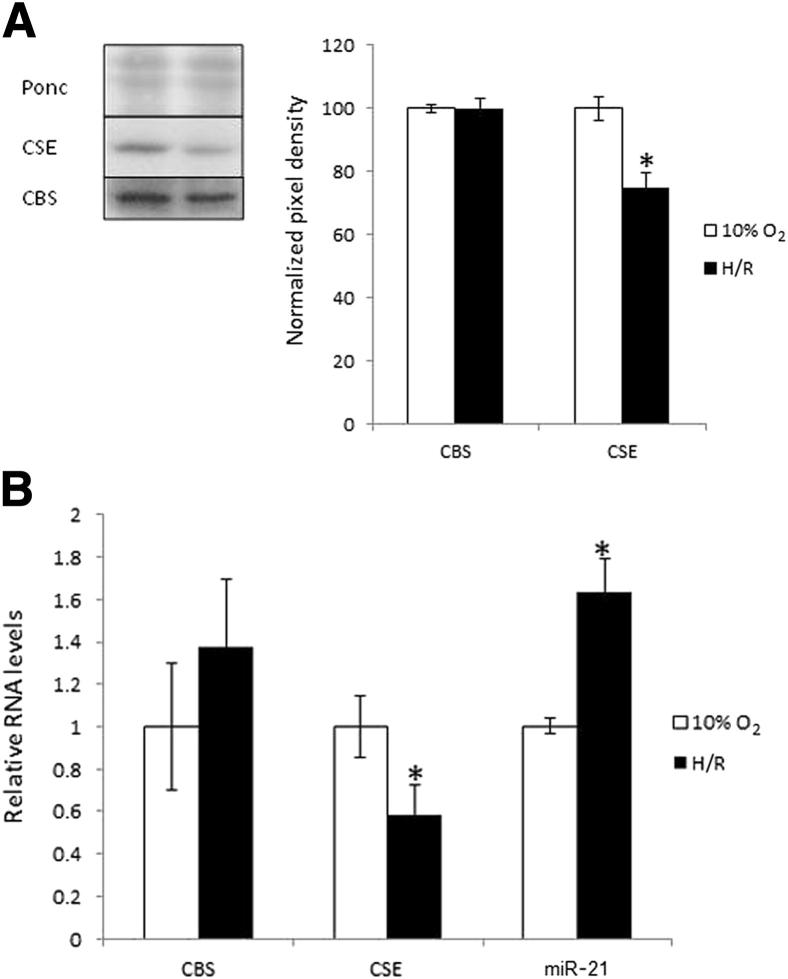
We next explored the principal mechanism that would regulate the changes in CSE expression by subjecting placental explants to either normoxia or H/R for 20 hours to mimic the conditions in severe IUGR and PE. We measured explant protein and mRNA expression levels of CSE and CBS and levels of miR-21 (Figure 4). In agreement with the in vivo findings, compared with normoxic controls, H/R reduced the protein expression of CSE; however, the expression levels of CBS remained significantly unchanged (Figure 4). The finding of reduced CSE protein expression in H/R explants was confirmed by immunohistochemical analysis, which localized the protein expression principally to SMCs (Figure 1C). In view of the previous findings in the pathologic placentas, we also examined miR-21 expression in placental explants and found that H/R up-regulated miR-21 expression, providing a mechanism for miR-21–driven reduction in CSE down-regulation (Figure 4B).
Figure 4.
Protein and mRNA expression profiles in placental explants. Protein (A) and mRNA (B) expression of CBS and CSE and miR-21 expression (B) in term placental explants cultured at different oxygen concentrations. A: Lysates from term placental explants (n = 4), which were cultured under normoxia (10% O2) or H/R (1% to 10% O2) for 20 hours, were immunoblotted with antibodies against CSE and CBS. Ponceau S (Ponc) expression served to normalize gel loading. Normalized results (means ± SEM) are plotted, expressing cesarean-delivered (CS) controls as 100%. ∗P < 0.05 versus CS controls (analysis of variance and the Protected Least Significant Difference test with P < 0.05). B: RNA was isolated and relative levels of CBS and CSE mRNA or miR-21 were detected using quantitative real-time RT-PCR. CBS and CSE mRNA levels (means ± SEM) were normalized to the 18S RNA levels and miR-21 to the RNU48 levels. ∗P < 0.05 H/R versus normoxic controls (Wilcoxon nonparametric test).
H2S Effect on Perfused Human Placentas and the Mechanism of Action
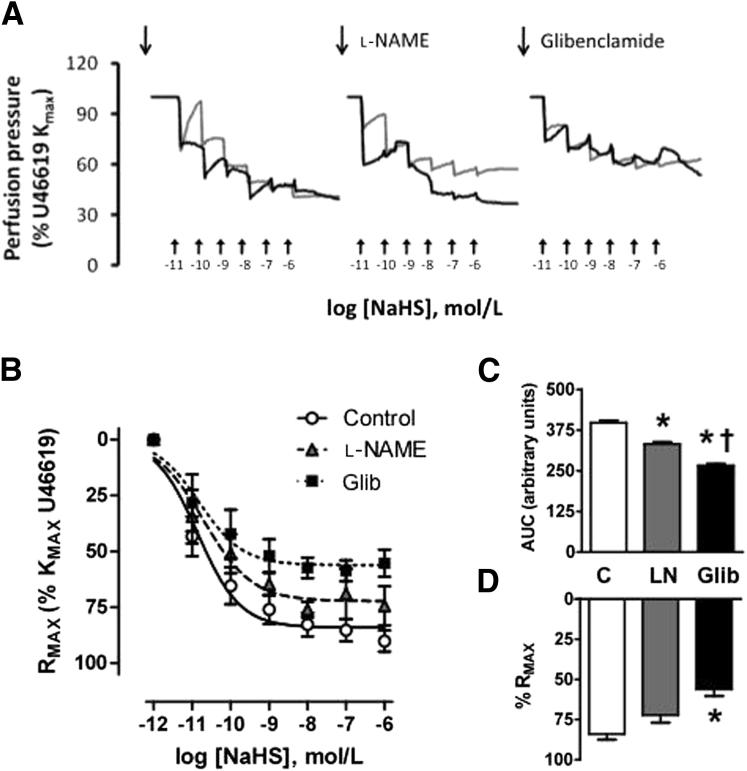
Given the evidence for reduced CSE expression and speculated reduction in H2S bioavailability associated with adverse clinical outcomes in pregnancies complicated with abnormal umbilical artery Doppler and, thus, increased vasoconstriction, we decided to test whether H2S can act as a vasodilator in the fetoplacental circulation. Perfusion of cesarean-delivered human placentas obtained from healthy term pregnancies with increasing doses of the H2S donor, NaHS, induced concentration-dependent reductions in perfusion pressure and vascular resistance after preconstriction with the thromboxane mimetic U46619 (10−7 mol/L). The effects of NaHS were significantly attenuated in the presence of the KATP channel blocker glibenclamide (10−5 mol/L) in terms of perfusion pressure (Figure 5A) and maximal relaxation (Figure 5, B–D). In addition, treatment with 10−5 mol/L NO synthesis blocker l-NAME reduced the vasodilator effect of NaHS, and this effect was intermediate between the effects of treatment with glibenclamide and treatment with NaHS alone (Figure 5, B–D).
Figure 5.
Perfusion pressure and vasodilator response in human placentas. A: Perfusion pressure (percentage of U46619 Michaelis constant Km) during the experimental protocol for two healthy human placentas. Top arrows show preconstriction with the thromboxane mimetic U46619. Bottom arrows show logarithmic scale of NaHS concentration. Different line colors represent different placentas. Set of curves: basal response (untreated) (left panel); responses in the presence of the NO synthase blocker l-NAME (middle panel); and responses in the presence of the KATP blocker glibenclamide (right panel). B: Vasodilator response to NaHS in human placentas. Values are means ± SEM for the concentration-response curves (B), the area above the curve (C), and the maximal relaxation (%Rmax) (D) to NaHS. Groups are untreated placentas (C; n = 6), placentas treated with the NO synthase blocker l-NAME (LN; n = 6), and placentas treated with the KATP blocker glibenclamide (Glib; n = 6). ∗P < 0.05 versus the untreated group; †P < 0.05 versus the l-NAME group (one-way analysis of variance and Student Newman-Keuls test).
Discussion
These studies demonstrate that reduced placental CSE expression at the mRNA and protein levels is associated with abnormal umbilical artery Doppler waveforms in high-risk pregnancies. These are novel observations that need to be further confirmed. They allude to the potential role of decreased H2S availability in the pathogenesis of increased umbilical arterial resistance in cases of severe preterm IUGR. We suggest that these changes are epigenetically regulated because the expression of miR-21, which has been reported to play a role in CSE down-regulation,25 is elevated in these abnormal placentas. We mimicked the changes in CSE and miR-21 expression in normal placental explants subjected to H/R, providing a mechanism to integrate these findings with the known underlying pathology of the uteroplacental circulation.34 CSE protein and mRNA expression were unaffected in placentas from PE-ND pregnancies, implying that H2S acts as a local fetoplacental vasodilator agent. This was confirmed by administering increasing doses of the H2S donor, NaHS, which decreased vascular resistance in a dose-dependent manner. We found the effects of NaHS to be significantly attenuated in the presence of the KATP channel blocker glibenclamide in terms of perfusion pressure and maximal relaxation; however, the effect of treatment with the NO synthesis blocker l-NAME was intermediate between the effects of treatment with glibenclamide and treatment with NaHS alone. These observations imply that the primary dilator actions of H2S in the human placenta are mediated via KATP channels and that there is an additional interaction between H2S and NO that functions to modulate vascular tone. There are potential interactions between NO and H2S for NO donor treatment–enhanced endogenous production of H2S from rat aortic tissues. NO can increase CSE activity in vascular tissues via cGMP-dependent protein kinases and by regulating CSE expression.20
Patel et al22 confirmed endogenous H2S production and the presence of CSE and CBS in rat and human intrauterine tissues. These authors additionally reported endogenous H2S production in the human placenta but did not identify the cellular source. Herein, we immunolocalized CSE to the SMCs of large placental vessels and to the syncytiotrophoblast; however, CBS expression was confined to the outer syncytiotrophoblast layer of placental villi. This pattern matched reports of different tissue localizations of the two enzymes in other systems. CSE is expressed in vascular SMCs but not in the endothelium,17, 20, 35 whereas CBS is predominantly found in the brain. CBS protein localizes in most areas of the brain but is predominantly expressed in the cell bodies and neuronal processes of Purkinje cells and Ammon’s horn neurons,36, 37 and it does not play an important role in cardiovascular tissues.38
We investigated whether the potential for synthesis of H2S was deficient in placentas from pathologic pregnancies. PE and IUGR are the most serious perinatal complications of human pregnancy that adversely affect maternal and fetal health. They are associated with deficient uteroplacental vascular conversion, leading to maternal placental malperfusion.26, 27 In the more severe cases, there is evidence of associated increased resistance in the umbilical circulation, resulting in reduced, or even reversed, end-diastolic flow.1
Therefore, it was of interest to determine the localization of the two H2S-generating enzymes in these placentas. We found that the protein and RNA expression levels of CSE were significantly decreased in IUGR placentas and in PE-AD placentas, which also had a trajectory for IUGR, compared with cesarean-delivered healthy placentas or PE-ND placentas. These are striking results and suggest that reduced H2S production could contribute to fetoplacental vasoconstriction in these growth-restricted placentas. There were no differences in CBS expression among the four different groups. In the human placenta, the terminal villi are the principal sites of gaseous exchange. The capillary network within them is supplied by the umbilical arteries that branch into a series of resistance arteries contained in the distributing stem villi. As there is no nerve supply in the placenta, vasomotor control of the resistance arteries is performed by locally produced vasoactive molecules. The syncytiotrophoblast expresses endothelial NO synthase, heme oxygenase, and CBS and, as such, produces NO, CO, and H2S locally. The half-life of these gaseous molecules is seconds (NO) to minutes (CO and H2S). In addition, NO and CO are produced by vascular endothelial cells, whereas H2S is produced by vascular SMCs. These vasodilators are likely to interact, mediate the vascular tone locally, and act as signaling molecules.39
We then investigated a potential mechanism that controls CSE suppression. Yang et al25 recently reported the involvement of miR-21 in down-regulation of the SMC expression of CSE. miRNAs are endogenous small single-strand noncoding RNAs of approximately 22 nucleotides that control gene expression by targeting the 3′ untranslational regions of mRNAs for degradation and/or translational repression.40, 41, 42 miR-21, an oncogenic antiapoptotic miRNA that can affect proliferation, migration, and invasion,43, 44 has been shown to be aberrantly overexpressed in some cardiovascular conditions, such as cardiac hypertrophy induced by aortic banding, which could be reversed by miR-21 down-regulation,45 or the heart tissue of patients with end-stage heart failure.46 Angiotensin II can increase miR-21 expression in neonatal rat cardiomyocytes in vitro.47 These data suggest that miR-21 could be a new pathologic factor and potentially a therapeutic target in cardiovascular disorders, including complicated pregnancies with increased vascular resistance.
Most of the pathologic placentas were associated with abnormal umbilical Doppler velocimetry. Altered vascular reactivity in IUGR could be a consequence of reduced generation of vasodilators, increased expression of constrictors, modified oxygen tension, and/or oxidative stress. These findings suggest that reduced CSE expression and consequent reduced H2S bioavailability contribute to the IUGR pathology by increasing resistance in the umbilical circulation, which manifests as reduced or even reversed end-diastolic blood flow. The study design did not allow us to test this hypothesis directly. Such experiments would require the administration of an enzyme substrate to placentas from healthy and complicated pregnancies, the worthy subject of a future investigation.
Note that in the perfusion protocol, the perfusion medium was gassed with 95% O2. Recently, some researchers have moved away from using 95% O2. However, it is yet to be determined what is the best percentage of O2 to use for a perfusion preparation to compensate for the lack of hemoglobin. Most perfusion setups are focused on placental transfer and metabolism, and few protocols have been published to assess vascular chorionic reactivity. We used this 95% O2 mixture based on our previous unpublished experiments and those of others.48, 49 Although there is evidence that some vasoconstrictors may have better responses at low oxygen levels in isolated arteries and veins,50, 51 there is no consensus as to different responses to oxygenation changes.
Holwerda et al23 also studied the expression of CBS and CSE in early-onset PE samples and reported a reduction in the mRNA expression of CBS and no changes in the protein or RNA expression of CSE or in the protein expression of CBS. The reasons for the differences in these findings are unclear, but they could be a reflection of several factors, including antibody specificity, timing of tissue collection, and/or variation in the severity of the pathology. To reinforce these findings in the pathologic placentas, we studied changes in placental CBS and CSE expression in placental explants subjected to H/R. This stimulus is recognized to mimic the ischemia-reperfusion consequences of deficient conversion of maternal spiral arteries, the underlying causes of the oxidative stress, which precipitates the symptoms of IUGR and PE.26, 27 We found that compared with normoxic explants, the protein and mRNA expression of CSE was significantly reduced and the expression of miR-21 increased by H/R, confirming that ischemia-reperfusion could be the mechanism that leads to up-regulation of miR-21 and subsequent inhibition of placental CSE expression. We found no significant differences in the expression levels of CBS in these explants, again matching the in vivo findings.
The mechanism of the vasodilator action of H2S has been established to be primarily mediated by KATP channels, which can be potentiated by NO. For example, an intravenous H2S injection transiently decreased the arterial blood pressure of rats,20 an effect that could be mimicked by a KATP channel opener, pinacidil, and blocked by glibenclamide. H2S might induce reduction of disulfide bonds of the KATP channel protein.52 H2S also induced in vitro relaxation of the aorta and the portal vein of rats,20, 21 acting mainly through the opening of KATP channels in vascular SMCs and partially through a K+ conductance in endothelial cells. Unlike NO and CO, which mediate vasorelaxation largely by cGMP pathway activation, the vasorelaxant effect of H2S is independent of the cGMP pathway.20 In addition, NO synthesis and effects depend largely on endothelial function integrity, whereas H2S is an endothelium-independent vasodilator and exerts its direct effect on SMCs. In fact, H2S is the only endogenous gaseous KATP channel opener known in these cells, and, notably, the CSE enzyme was immunolocalized largely to the SMCs surrounding the stem villus arteries. The interactions between NO and H2S are highlighted by experiments in which treatment with a NO donor enhanced endogenous production of H2S in rat aortic tissues.20 NO can also increase CSE activity in vascular tissues via cGMP-dependent protein kinases and by regulating CSE expression.20 In addition, the vascular effect of H2S can be potentiated by NO.20, 21 We, therefore, tested the effect of the KATP channel blocker glibenclamide and the NO synthesis blocker l-NAME when applied with NaHS in perfused human placentas. We found the effects of NaHS to be significantly attenuated in the presence of glibenclamide in terms of perfusion pressure and maximal relaxation, whereas the effect of treatment with l-NAME was intermediate between the effects of treatment with glibenclamide and treatment with NaHS alone. This, again, implies that the primary vasodilator mechanism of H2S in the human placenta is mediated via KATP channels and an additional interaction between H2S and NO.
Collectively, the three experiments show that H2S is a potent vasodilator of the human placenta and that its synthesis is impaired under conditions of hypoxia and reoxygenation and in pathologic PE and IUGR pregnancies with abnormal umbilical artery Doppler waveforms. These data suggest an epigenetic mechanism whereby impaired placental H2S generation occurs in complicated pregnancy. Furthermore, the bioavailability of H2S might be a novel mechanistic target for therapeutic interventions to improve placental perfusion and protect fetal growth and development in complicated pregnancies.
Acknowledgments
We thank Prof. Irene Cetin for supplying the IUGR placental samples and the Cambridge Comprehensive Biomedical Research Centre, the staff of the Rosie Hospital, and Melanie Monk for their help in collecting the normal term placentas.
Footnotes
Supported by the Wellcome Trust (084804/2/08/Z). E.A.H. was partially funded by the U-Apoya Program, Universidad de Chile.
T.C-D. and E.A.H. contributed equally to this work.
References
- 1.Toal M., Keating S., Machin G., Dodd J., Adamson S.L., Windrim R.C., Kingdom J.C. Determinants of adverse perinatal outcome in high-risk women with abnormal uterine artery Doppler images. Am J Obstet Gynecol. 2008;198:330.e1–330.e7. doi: 10.1016/j.ajog.2007.09.031. [DOI] [PubMed] [Google Scholar]
- 2.Kingdom J.C., Burrell S.J., Kaufmann P. Pathology and clinical implications of abnormal umbilical artery Doppler waveforms. Ultrasound Obstet Gynecol. 1997;9:271–286. doi: 10.1046/j.1469-0705.1997.09040271.x. [DOI] [PubMed] [Google Scholar]
- 3.Geerts L., Odendaal H.J. Severe early onset pre-eclampsia: prognostic value of ultrasound and Doppler assessment. J Perinatol. 2007;27:335–342. doi: 10.1038/sj.jp.7211747. [DOI] [PubMed] [Google Scholar]
- 4.Proctor L.K., Toal M., Keating S., Chitayat D., Okun N., Windrim R.C., Smith G.C., Kingdom J.C. Placental size and the prediction of severe early-onset intrauterine growth restriction in women with low pregnancy-associated plasma protein-A. Ultrasound Obstet Gynecol. 2009;34:274–282. doi: 10.1002/uog.7308. [DOI] [PubMed] [Google Scholar]
- 5.Baschat A.A. Neurodevelopment following fetal growth restriction and its relationship with antepartum parameters of placental dysfunction. Ultrasound Obstet Gynecol. 2011;37:501–514. doi: 10.1002/uog.9008. [DOI] [PubMed] [Google Scholar]
- 6.Karsdorp V.H., van Vugt J.M., Jakobs C., Dekker G.A., van Geijn H.P. Amino acids, glucose and lactate concentrations in umbilical cord blood in relation to umbilical artery flow patterns. Eur J Obstet Gynecol Reprod Biol. 1994;57:117–122. doi: 10.1016/0028-2243(94)90053-1. [DOI] [PubMed] [Google Scholar]
- 7.Pardi G., Cetin I., Marconi A.M., Lanfranchi A., Bozzetti P., Ferrazzi E., Buscaglia M., Battaglia F.C. Diagnostic value of blood sampling in fetuses with growth retardation. N Engl J Med. 1993;328:692–696. doi: 10.1056/NEJM199303113281004. [DOI] [PubMed] [Google Scholar]
- 8.Steiner H., Staudach A., Spitzer D., Schaffer K.H., Gregg A., Weiner C.P. Growth deficient fetuses with absent or reversed umbilical artery end-diastolic flow are metabolically compromised. Early Hum Dev. 1995;41:1–9. doi: 10.1016/0378-3782(94)01596-h. [DOI] [PubMed] [Google Scholar]
- 9.Myatt L. Control of vascular resistance in the human placenta. Placenta. 1992;13:329–341. doi: 10.1016/0143-4004(92)90057-z. [DOI] [PubMed] [Google Scholar]
- 10.Barber A., Robson S.C., Myatt L., Bulmer J.N., Lyall F. Heme oxygenase expression in human placenta and placental bed: reduced expression of placenta endothelial HO-2 in preeclampsia and fetal growth restriction. FASEB J. 2001;15:1158–1168. doi: 10.1096/fj.00-0376com. [DOI] [PubMed] [Google Scholar]
- 11.Szabo C. Hydrogen sulphide and its therapeutic potential. Nat Rev Drug Discov. 2007;6:917–935. doi: 10.1038/nrd2425. [DOI] [PubMed] [Google Scholar]
- 12.Bukovska G., Kery V., Kraus J.P. Expression of human cystathionine beta-synthase in Escherichia coli: purification and characterization. Protein Expr Purif. 1994;5:442–448. doi: 10.1006/prep.1994.1063. [DOI] [PubMed] [Google Scholar]
- 13.Erickson P.F., Maxwell I.H., Su L.J., Baumann M., Glode L.M. Sequence of cDNA for rat cystathionine gamma-lyase and comparison of deduced amino acid sequence with related Escherichia coli enzymes. Biochem J. 1990;269:335–340. doi: 10.1042/bj2690335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhong G., Chen F., Cheng Y., Tang C., Du J. The role of hydrogen sulfide generation in the pathogenesis of hypertension in rats induced by inhibition of nitric oxide synthase. J Hypertens. 2003;21:1879–1885. doi: 10.1097/00004872-200310000-00015. [DOI] [PubMed] [Google Scholar]
- 15.Clarke R., Daly L., Robinson K., Naughten E., Cahalane S., Fowler B., Graham I. Hyperhomocysteinemia: an independent risk factor for vascular disease. N Engl J Med. 1991;324:1149–1155. doi: 10.1056/NEJM199104253241701. [DOI] [PubMed] [Google Scholar]
- 16.Eberhardt R.T., Forgione M.A., Cap A., Leopold J.A., Rudd M.A., Trolliet M., Heydrick S., Stark R., Klings E.S., Moldovan N.I., Yaghoubi M., Goldschmidt-Clermont P.J., Farber H.W., Cohen R., Loscalzo J. Endothelial dysfunction in a murine model of mild hyperhomocyst(e)inemia. J Clin Invest. 2000;106:483–491. doi: 10.1172/JCI8342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang G., Wu L., Jiang B., Yang W., Qi J., Cao K., Meng Q., Mustafa A.K., Mu W., Zhang S., Snyder S.H., Wang R. H2S as a physiologic vasorelaxant: hypertension in mice with deletion of cystathionine gamma-lyase. Science. 2008;322:587–590. doi: 10.1126/science.1162667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reiffenstein R.J., Hulbert W.C., Roth S.H. Toxicology of hydrogen sulfide. Annu Rev Pharmacol Toxicol. 1992;32:109–134. doi: 10.1146/annurev.pa.32.040192.000545. [DOI] [PubMed] [Google Scholar]
- 19.Kimura H. Hydrogen sulfide induces cyclic AMP and modulates the NMDA receptor. Biochem Biophys Res Commun. 2000;267:129–133. doi: 10.1006/bbrc.1999.1915. [DOI] [PubMed] [Google Scholar]
- 20.Zhao W., Zhang J., Lu Y., Wang R. The vasorelaxant effect of H(2)S as a novel endogenous gaseous K(ATP) channel opener. EMBO J. 2001;20:6008–6016. doi: 10.1093/emboj/20.21.6008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hosoki R., Matsuki N., Kimura H. The possible role of hydrogen sulfide as an endogenous smooth muscle relaxant in synergy with nitric oxide. Biochem Biophys Res Commun. 1997;237:527–531. doi: 10.1006/bbrc.1997.6878. [DOI] [PubMed] [Google Scholar]
- 22.Patel P., Vatish M., Heptinstall J., Wang R., Carson R.J. The endogenous production of hydrogen sulphide in intrauterine tissues. Reprod Biol Endocrinol. 2009;7:10. doi: 10.1186/1477-7827-7-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Holwerda K.M., Bos E.M., Rajakumar A., Ris-Stalpers C., van Pampus M.G., Timmer A., Erwich J.J., Faas M.M., van Goor H., Lely A.T. Hydrogen sulfide producing enzymes in pregnancy and preeclampsia. Placenta. 2012;33:518–521. doi: 10.1016/j.placenta.2012.02.014. [DOI] [PubMed] [Google Scholar]
- 24.You X.J., Xu C., Lu J.Q., Zhu X.Y., Gao L., Cui X.R., Li Y., Gu H., Ni X. Expression of cystathionine beta-synthase and cystathionine gamma-lyase in human pregnant myometrium and their roles in the control of uterine contractility. PLoS One. 2011;6:e23788. doi: 10.1371/journal.pone.0023788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang G., Pei Y., Cao Q., Wang R. MicroRNA-21 represses human cystathionine gamma-lyase expression by targeting at specificity protein-1 in smooth muscle cells. J Cell Physiol. 2012;227:3192–3200. doi: 10.1002/jcp.24006. [DOI] [PubMed] [Google Scholar]
- 26.Redman C.W., Sargent I.L. Latest advances in understanding preeclampsia. Science. 2005;308:1592–1594. doi: 10.1126/science.1111726. [DOI] [PubMed] [Google Scholar]
- 27.Burton G.J., Jauniaux E. Placental oxidative stress: from miscarriage to preeclampsia. J Soc Gynecol Investig. 2004;11:342–352. doi: 10.1016/j.jsgi.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 28.Todros T., Ferrazzi E., Groli C., Nicolini U., Parodi L., Pavoni M., Zorzoli A., Zucca S. Fitting growth curves to head and abdomen measurements of the fetus: a multicentric study. J Clin Ultrasound. 1987;15:95–105. doi: 10.1002/jcu.1870150203. [DOI] [PubMed] [Google Scholar]
- 29.Parazzini F., Cortinovis I., Bortolus R., Fedele L. Standards of birth weight in Italy. Ann Ostet Ginecol Med Perinat. 1991;112:203–246. [in Italian] [PubMed] [Google Scholar]
- 30.Cindrova-Davies T., Spasic-Boskovic O., Jauniaux E., Charnock-Jones D.S., Burton G.J. Nuclear factor-kappa B, p38, and stress-activated protein kinase mitogen-activated protein kinase signaling pathways regulate proinflammatory cytokines and apoptosis in human placental explants in response to oxidative stress: effects of antioxidant vitamins. Am J Pathol. 2007;170:1511–1520. doi: 10.2353/ajpath.2007.061035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Romero-Calvo I., Ocon B., Martinez-Moya P., Suarez M.D., Zarzuelo A., Martinez-Augustin O., de Medina F.S. Reversible Ponceau staining as a loading control alternative to actin in Western blots. Anal Biochem. 2010;401:318–320. doi: 10.1016/j.ab.2010.02.036. [DOI] [PubMed] [Google Scholar]
- 32.Brownbill P., Sibley C.P. Regulation of transplacental water transfer: the role of fetoplacental venous tone. Placenta. 2006;27:560–567. doi: 10.1016/j.placenta.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 33.Solanky N., Requena Jimenez A., D’Souza S.W., Sibley C.P., Glazier J.D. Expression of folate transporters in human placenta and implications for homocysteine metabolism. Placenta. 2010;31:134–143. doi: 10.1016/j.placenta.2009.11.017. [DOI] [PubMed] [Google Scholar]
- 34.Burton G.J., Woods A.W., Jauniaux E., Kingdom J.C. Rheological and physiological consequences of conversion of the maternal spiral arteries for uteroplacental blood flow during human pregnancy. Placenta. 2009;30:473–482. doi: 10.1016/j.placenta.2009.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang R. Two’s company, three’s a crowd: can H2S be the third endogenous gaseous transmitter? FASEB J. 2002;16:1792–1798. doi: 10.1096/fj.02-0211hyp. [DOI] [PubMed] [Google Scholar]
- 36.Abe K., Kimura H. The possible role of hydrogen sulfide as an endogenous neuromodulator. J Neurosci. 1996;16:1066–1071. doi: 10.1523/JNEUROSCI.16-03-01066.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Robert K., Vialard F., Thiery E., Toyama K., Sinet P.M., Janel N., London J. Expression of the cystathionine beta synthase (CBS) gene during mouse development and immunolocalization in adult brain. J Histochem Cytochem. 2003;51:363–371. doi: 10.1177/002215540305100311. [DOI] [PubMed] [Google Scholar]
- 38.Chen P., Poddar R., Tipa E.V., Dibello P.M., Moravec C.D., Robinson K., Green R., Kruger W.D., Garrow T.A., Jacobsen D.W. Homocysteine metabolism in cardiovascular cells and tissues: implications for hyperhomocysteinemia and cardiovascular disease. Adv Enzyme Regul. 1999;39:93–109. doi: 10.1016/s0065-2571(98)00029-6. [DOI] [PubMed] [Google Scholar]
- 39.Kajimura M, Fukuda R, Bateman RM, Yamamoto T, Suematsu M: Interactions of multiple gas-transducing systems: hallmarks and uncertainties of CO, NO, and H2S gas biology. Antioxid Redox Signal 13:157–192 [DOI] [PMC free article] [PubMed]
- 40.Kim V.N., Han J., Siomi M.C. Biogenesis of small RNAs in animals. Nat Rev Mol Cell Biol. 2009;10:126–139. doi: 10.1038/nrm2632. [DOI] [PubMed] [Google Scholar]
- 41.Ambros V. The functions of animal microRNAs. Nature. 2004;431:350–355. doi: 10.1038/nature02871. [DOI] [PubMed] [Google Scholar]
- 42.Bartel D.P. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 43.Zhu S., Wu H., Wu F., Nie D., Sheng S., Mo Y.Y. MicroRNA-21 targets tumor suppressor genes in invasion and metastasis. Cell Res. 2008;18:350–359. doi: 10.1038/cr.2008.24. [DOI] [PubMed] [Google Scholar]
- 44.Yao Q., Xu H., Zhang Q.Q., Zhou H., Qu L.H. MicroRNA-21 promotes cell proliferation and down-regulates the expression of programmed cell death 4 (PDCD4) in HeLa cervical carcinoma cells. Biochem Biophys Res Commun. 2009;388:539–542. doi: 10.1016/j.bbrc.2009.08.044. [DOI] [PubMed] [Google Scholar]
- 45.Cheng Y., Ji R., Yue J., Yang J., Liu X., Chen H., Dean D.B., Zhang C. MicroRNAs are aberrantly expressed in hypertrophic heart: do they play a role in cardiac hypertrophy? Am J Pathol. 2007;170:1831–1840. doi: 10.2353/ajpath.2007.061170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Naraba H., Iwai N. Assessment of the microRNA system in salt-sensitive hypertension. Hypertens Res. 2005;28:819–826. doi: 10.1291/hypres.28.819. [DOI] [PubMed] [Google Scholar]
- 47.Tatsuguchi M., Seok H.Y., Callis T.E., Thomson J.M., Chen J.F., Newman M., Rojas M., Hammond S.M., Wang D.Z. Expression of microRNAs is dynamically regulated during cardiomyocyte hypertrophy. J Mol Cell Cardiol. 2007;42:1137–1141. doi: 10.1016/j.yjmcc.2007.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ong S.S., Moore R.J., Warren A.Y., Crocker I.P., Fulford J., Tyler D.J., Gowland P.A., Baker P.N. Myometrial and placental artery reactivity alone cannot explain reduced placental perfusion in pre-eclampsia and intrauterine growth restriction. BJOG. 2003;110:909–915. [PubMed] [Google Scholar]
- 49.Donoso M.V., Lopez R., Miranda R., Briones R., Huidobro-Toro J.P. A2B adenosine receptor mediates human chorionic vasoconstriction and signals through arachidonic acid cascade. Am J Physiol Heart Circ Physiol. 2005;288:H2439–H2449. doi: 10.1152/ajpheart.00548.2004. [DOI] [PubMed] [Google Scholar]
- 50.Cooper E.J., Wareing M., Greenwood S.L., Baker P.N. Oxygen tension and normalisation pressure modulate nifedipine-sensitive relaxation of human placental chorionic plate arteries. Placenta. 2006;27:402–410. doi: 10.1016/j.placenta.2005.04.012. [DOI] [PubMed] [Google Scholar]
- 51.Wareing M., Greenwood S.L., Baker P.N. Reactivity of human placental chorionic plate vessels is modified by level of oxygenation: differences between arteries and veins. Placenta. 2006;27:42–48. doi: 10.1016/j.placenta.2004.11.008. [DOI] [PubMed] [Google Scholar]
- 52.Warenycia M.W., Steele J.A., Karpinski E., Reiffenstein R.J. Hydrogen sulfide in combination with taurine or cysteic acid reversibly abolishes sodium currents in neuroblastoma cells. Neurotoxicology. 1989;10:191–199. [PubMed] [Google Scholar]