Abstract
Purpose
The cardiac and renal protective effects of phosphodiesterase-5 (PDE-5) inhibitors against ischemia-reperfusion injury have recently been demonstrated in animal studies. We evaluated the effect of pretreatment with the PDE-5 inhibitor zaprinast on warm renal ischemia in a rat model.
Methods
Female Sprague-Dawley rats underwent concomitant right nephrectomy and left renal hilar occlusion for 30 minutes. Twelve animals were equally divided into three groups: Group 1 received no pharmacologic pretreatment, group 2 was pretreated with zaprinast 10 mg/kg, and group 3 was pretreated with zaprinast 20 mg/kg. Zaprinast was dissolved in 25% dimethyl sulfoxide and given as a single intraperitoneal injection 30 minutes before surgery. Serum blood urea nitrogen (BUN) and creatinine levels, histopathology, and TUNEL staining for apoptosis were assessed 24 hours postoperatively.
Results
The mean creatinine level for groups 1, 2, and 3 was 0.73 mg/dL, 0.55 mg/dL, and 0.38 mg/dL, respectively. These values were not statistically different (P=0.099). The mean BUN levels of 35.8 mg/dL for group 1, 27.3 mg/dL for group 2, and 23.3 mg/dL for group 3 were also statistically similar (P=0.278). There were no objective differences in histopathologic evaluation or TUNEL staining between the groups.
Conclusion
This study did not demonstrate a beneficial effect of zaprinast pretreatment on renal parameters after warm ischemic injury.
Introduction
Partial nephrectomy has become a standard of care for the treatment of patients with many small renal masses1 with similar oncologic outcomes to those of radical nephrectomy.2 To help improve visualization and minimize blood loss during this procedure, the renal vasculature is often occluded. Although kidney parenchyma is surgically spared, the induced ischemia and subsequent reperfusion can lead to functional nephron injury and loss.3
Several mechanisms are involved in the kidney dysfunction that follows a hypoxic insult.4 There is a vascular component in which endothelial damage induces intrarenal vasoconstriction, vascular smooth muscle dysfunction, and vascular congestion. This further reduces renal blood flow and potentiates tubular epithelial injury.5 A tubular component develops as injured and dead epithelial cells are sloughed into the tubules, obstructing urine flow, causing transtubular backleak of filtrate and interstitial inflammation.6 The net effect is a decrease in glomerular filtration rate (GFR) and cessation of urine production.5 On a molecular level, the local hypoxia leads to a variety of secondary processes causing cellular injury, including the intracellular accumulation of calcium, the generation of reactive oxygen species, depletion of adenosine triphosphate, and cellular apoptosis or necrosis.7
Phosphodiesterases (PDEs) are a large group of structurally related enzymes that catalyze the hydrolysis of 3,5-cyclic mononucleotides to the corresponding inactive nucleotide 5-monophosphate. There are 11 different families of PDEs that differ in structure, tissue distribution, and affinity for substrates.8 The PDE-5 enzyme catalyzes the breakdown of cyclic guanosine monophosphate (cGMP), one of the primary potentiators of smooth muscle relaxation. It is particularly abundant in vascular smooth muscle9 and has also been identified in the kidney.10,11 In the penis, nitric oxide released by parasympathetic neurons leads to the production of cGMP and the resultant smooth muscle relaxation that causes erection. Because of their vasoactive properties, PDE-5 inhibitors have received considerable attention in recent years for their potential protective effect against ischemia-reperfusion injury.9
The aim of this study was to evaluate the effect of zaprinast, a PDE-5 inhibitor, on renal ischemic injury in a rat model.
Methods
Animals
Pathogen-free, adult female Sprague-Dawley rats (150–200 g; Harlan Laboratories, Madison, WI) were housed in temperature (23°±3°C) and light (12-hours light; 12-hours dark cycle) controlled rooms with standard rodent chow and water available ad libitum. All experiments were approved by the Institutional Animal Care and Use Committee (IACUC) of Loyola University Chicago. All procedures were conducted in accordance with the Guide for Care and Use of Laboratory Animals published by the National Institutes of Health, and handling of animals was in accordance with institutional guidelines and approved by the Loyola University IACUC.
Drug treatment and surgical methods
Animals were anesthetized with inhalational isoflurane (Baxter, Deerfield, IL). They were placed on a warm heating pad to maintain body temperature. Through a midline laparotomy incision, right nephrectomy was performed. The left renal hilum was then dissected, and a nontraumatic vascular clamp was applied en bloc to the artery and vein. The clamp was left in place for 30 minutes, during which time a warm saline-soaked gauze pad was used to cover the abdominal incision. After clamp removal, the abdomen was assessed for hemostasis, and the incision was closed with 4-0 polyglactin suture.
Rats were divided into three groups. Group 1 (n=4) underwent surgery with no pharmacologic pretreatment. Group 2 (n=4) was treated with zaprinast 10 mg/kg, and group 3 (n=4) was treated with zaprinast 20 mg/kg. Zaprinast (Tocris Bioscience, Bristol, United Kingdom) was dissolved in 0.5 mL of 25% dimethyl sulfoxide and given as a single intraperitoneal injection 30 minutes before the surgical procedure. Two animals underwent a sham procedure during which a laparotomy incision was made and the abdomen was left open for 30 minutes with no nephrectomy or renal ischemia.
Histopathology and laboratory analysis
Twenty-four hours postoperatively, all animals were euthanized. Blood samples were drawn for determination of serum blood urea nitrogen (BUN) and creatinine (Cr) levels using the VetScan Classic automated machine (Abaxis, Union City, CA). The animals were transcardially perfused with saline followed by fixative. The left kidney was removed, bisected along the nonhilar axis, and further fixed in formalin. One half of the kidney was then embedded in paraffin, sectioned, and stained with hematoxylin and eosin. The other half was frozen in liquid nitrogen and stored at −80°C.
Histologic changes were examined by light microscopy. Two different sections of each kidney were examined at 200X power, and each section was divided into 10 different fields. The histologic features of each field (20 fields per kidney) were scored in a blinded fashion by a single renal pathologist (MP) using the system outlined in Table 1.
Table 1.
Scoring System for Histopathological Change
| Score | Microscopic pattern |
|---|---|
| 0 | Normal architecture |
| 1 | Microvacuolar change |
| 2 | Macrovacuolar change |
| 3 | Tubular attenuation <50% |
| 4 | Tubular attenuation >50% |
| 5 | Frank necrosis |
Assay
Terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) staining was performed using the ApopTag® Fluorescein In Situ Apoptosis Detection Kit (Chemicon International) on tissue cryosections. The methodology was performed in accordance with the manufacturer's guidelines with several minor alterations. Hoechst was used as a counterstain. Positive staining was evaluated with fluorescence microscopy, and samples were compared in a blinded fashion by the senior investigator (FW).
Statistical analysis
Statistical analysis was performed using SPSS 16.0 for Windows (SPSS, Chicago, IL). Mean serum BUN and Cr levels and histologic scores were compared using the analysis of variance (ANOVA) test. Statistical significance was considered at a P value of < 0.05.
Results
Laboratory data
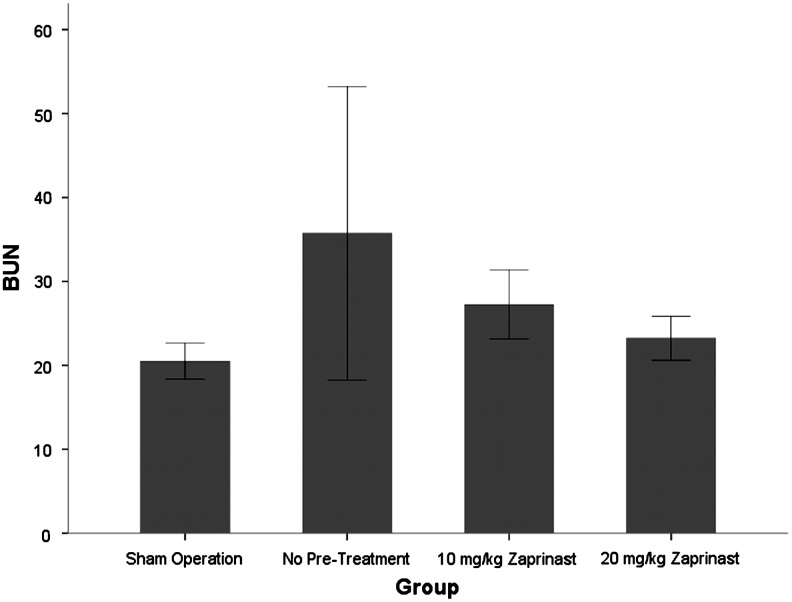
The mean BUN level of animals in group 1 (no pretreatment), group 2 (pretreatment with zaprinast 10 mg/kg), and group 3 (pretreatment with zaprinast 20 mg/kg) was 35.8, 27.3, and 23.3 mg/dL, respectively (Fig. 1). An ANOVA test comparing these values did not demonstrate a significant difference between the groups (F2=1.48, P=0.278). The mean Cr level for Groups 1, 2, and 3 was 0.73, 0.55, and 0.38 mg/dL, respectively (Fig. 2). Although the pretreated animals, especially at the higher dose, displayed a trend toward lower Cr levels, the difference between the groups was not statistically significant (F2=3.02, P=0.099).
FIG. 1.
The mean serum blood urea nitrogen (BUN) level of animals in group 1 (no pretreatment), group 2 (pretreatment with zaprinast 10 mg/kg), and group 3 (zaprinast 20 mg/kg). Animals in the sham group underwent anesthesia for 30 minutes with no right nephrectomy or left hilar clamping.
FIG. 2.
The mean serum creatinine (Cr) level of animals in group 1 (no pretreatment), group 2 (pretreatment with zaprinast 10 mg/kg), and group 3 (zaprinast 20 mg/kg).
Histopathology
The mean histologic scores were 2.7 (group 1), 1.5 (group 2), and 2.2 (group 3). These were not statistically different (P=0.174). Figure 3 demonstrates a box plot of the scores for each group and highlights the variability of histologic findings within groups, particularly group 1.
FIG. 3.
A box plot depicting the histologic scores in each group. There was wide variability in the histology observed among group 1 animals.
TUNEL assay
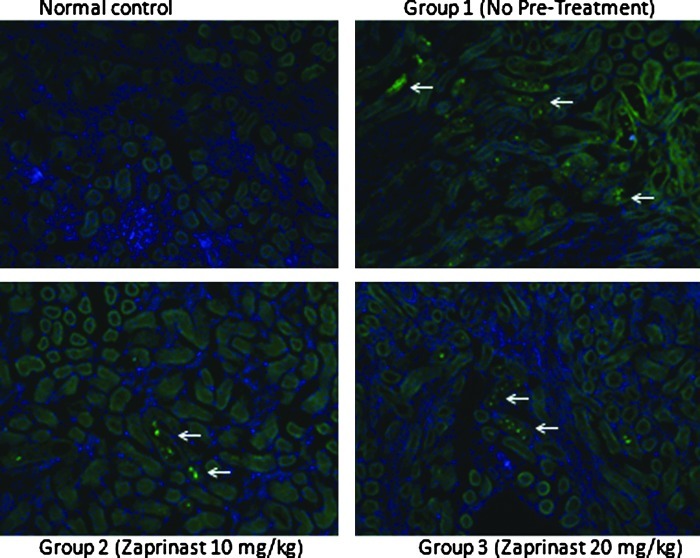
The number of TUNEL positive cells in the kidneys of nontreated ischemia-reperfusion animals was similar to those pretreated with zaprinast 10 mg/dL and zaprinast 20 mg/dL. A representative example from each group is demonstrated in Figure 4.
FIG. 4.
Representative samples of TUNEL staining from control and groups 1–3. White arrows point to TUNEL positive cells.
Discussion
Several recent studies have examined the protective effect of PDE-5 inhibitors on renal ischemia-reperfusion injury. In a swine model, Lledo-Garcia and associates12 found that renal vascular flow was higher and vascular resistance was lower in animals treated with sildenafil citrate before renal autotransplantation with 45 minutes of warm ischemia. Choi and colleagues13 used a rat model to evaluate the renoprotective effects of sildenafil after ischemic injury. They found that sildenafil treated animals had more favorable functional and histologic parameters than controls. Furthermore, they provided evidence that this protection may primarily be from the inhibition of apoptosis and necrosis.13
Zaprinast, which was a lead compound for sildenafil, is an inhibitor of cGMP-phosphodiesterases, particularly PDE-5 and PDE-6.14,15 Previously, zaprinast was shown to accelerate the recovery from established acute renal failure in the rat because of its ability to stimulate regional blood flow and raise intracellular cGMP levels.16 We selected zaprinast for the current study because of its wide commercial availability, ease of use, and reliability in delivering a predefined dose. We also hypothesized that its modest activity against other PDE isoforms might enhance its renoprotective effect.
The medication was dosed based on previous work that suggested that beneficial effects on regional blood flow and cyclic mononucleotide levels without a significant reduction in mean arterial pressure could be achieved at 10 to 20 mg/kg doses.17–19 It is possible that the concentration of zaprinast used in this study was too low to achieve a meaningful effect.
The 30-minute ischemic time for this experiment was based on several of our own pilot studies. Using male Sprague-Dawley rats weighing 250 to 350 g, Jablonski and coworkers20 concluded that 60 minutes of warm renal ischemia with concomitant contralateral nephrectomy was the most suitable model for testing the efficacy of pretreatment regimens. When we used considerably smaller females, however, this same insult was survivable by only half of the animals, and complete coagulative necrosis was observed histologically in the experimental kidney. We then tested ischemia times of 15, 30, and 45 minutes and found 30 minutes to be ideal based on functional and histologic data.
Although statistical significance was not achieved, the BUN and Cr levels of zaprinast-treated animals were lower than those of control animals, particularly at the higher dose of 20 mg/kg. The power to detect a difference was limited by the small number of animals in each treatment group. We suspect that these differences may have reached significance with a larger sample size.
Our study did not find a difference in histology or in TUNEL staining between kidneys of treated and untreated animals. Interestingly, in his swine model of warm-ischemic transplants, despite markedly improved vascular flow and nitric oxide levels in the sildenafil group, Lledo-Garcia and associates12 did not observe a histologic difference between sildenafil- and placebo-treated animals. In contrast, in addition to significantly improved histologic parameters, Choi and colleagues13 found that the number of TUNEL positive cells was lower in sildenafil-treated animals. This led them to conclude that the primary renoprotective effect was through inhibition of apoptosis and necrosis.
There are several limitations of our study. The small sample size in each group limited the statistical power. The comparatively simpler anatomy of the rat kidney limits direct translation to humans.5 We used serum BUN and Cr as biomarkers of GFR, which have known limitations. Furthermore, the bioavailability of zaprinast given by intraperitoneal injection and its duration of action could not be directly assessed.
Conclusion
This study with a limited sample size did not demonstrate a beneficial effect of zaprinast on renal ischemic injury. Although there was a trend toward improvement of functional and histologic parameters, significant differences were not observed.
Abbreviations Used
- ANOVA
analysis of variance
- BUN
blood urea nitrogen
- cGMP
cyclic guanosine monoposphate
- Cr
creatinine
- GFR
glomerular filtration rate
- IACUC
Institutional Animal Care and Use Committee
- PDE
phosphodiesterase
- TUNEL
terminal deoxynucleotidyl transferase dUTP nick end labeling
Disclosure Statement
No competing financial interests exist.
References
- 1.Campbell SC. Novick AC. Belldegrun A, et al. Guideline for management of the clinical T1 renal mass. J Urol. 2009;182:1271–1279. doi: 10.1016/j.juro.2009.07.004. [DOI] [PubMed] [Google Scholar]
- 2.Leibovich BC. Blute ML. Cheville JC, et al. Nephron sparing surgery for appropriately selected renal cell carcinoma between 4 and 7 cm results in outcome similar to radical nephrectomy. J Urol. 2004;171:1066–1070. doi: 10.1097/01.ju.0000113274.40885.db. [DOI] [PubMed] [Google Scholar]
- 3.Martin GL. Warner JN. Nateras RN, et al. Comparison of total, selective, and nonarterial clamping techniques during laparoscopic and robot-assisted partial nephrectomy. J Endourol. 2012;26:152–156. doi: 10.1089/end.2011.0304. [DOI] [PubMed] [Google Scholar]
- 4.Trof RJ. Di Maggio F. Leemreis J. Groeneveld AB. Biomarkers of acute renal injury and renal failure. Shock. 2006;26:245–253. doi: 10.1097/01.shk.0000225415.5969694.ce. [DOI] [PubMed] [Google Scholar]
- 5.Simmons MN. Schreiber MJ. Gill IS. Surgical renal ischemia: A contemporary overview. J Urol. 2008;180:19–30. doi: 10.1016/j.juro.2008.03.022. [DOI] [PubMed] [Google Scholar]
- 6.Lameire N. Van Biesen W. Vanholder R. Acute renal failure. Lancet. 2005;365:417–430. doi: 10.1016/S0140-6736(05)17831-3. [DOI] [PubMed] [Google Scholar]
- 7.Thadhani R. Pascual M. Bonventre JV. Acute renal failure. N Engl J Med. 1996;334:1448–1460. doi: 10.1056/NEJM199605303342207. [DOI] [PubMed] [Google Scholar]
- 8.Kulkarni SK. Patil CS. Phosphodiesterase 5 enzyme and its inhibitors: Update on pharmacological and therapeutical aspects. Methods Find Exp Clin Pharmacol. 2004;26:789–799. doi: 10.1358/mf.2004.26.10.872561. [DOI] [PubMed] [Google Scholar]
- 9.Kukreja RC. Salloum F. Das A, et al. Pharmacological preconditioning with sildenafil: Basic mechanisms and clinical implications. Vascul Pharmacol. 2005;42:219–232. doi: 10.1016/j.vph.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 10.Rotella DP. Phosphodiesterase 5 inhibitors: Current status and potential applications. Nat Rev Drug Discov. 2002;1:674–682. doi: 10.1038/nrd893. [DOI] [PubMed] [Google Scholar]
- 11.Kotera J. Fujishige K. Michibata H, et al. Characterization and effects of methyl-2- (4-aminophenyl)-1, 2-dihydro-1-oxo-7- (2-pyridinylmethoxy)-4-(3,4, 5-trimethoxyphenyl)-3-isoquinoline carboxylate sulfate (T-1032), a novel potent inhibitor of cGMP-binding cGMP-specific phosphodiesterase (PDE5) Biochem Pharmacol. 2000;60:1333–1341. doi: 10.1016/s0006-2952(00)00457-3. [DOI] [PubMed] [Google Scholar]
- 12.Lledó-Garcia E. Subirá-Rios D. Rodriguez-Martinez D, et al. Sildenafil as a protecting drug for warm ischemic kidney transplants: Experimental results. J Urol. 2009;182:1222–1225. doi: 10.1016/j.juro.2009.05.006. [DOI] [PubMed] [Google Scholar]
- 13.Choi DE. Jeong JY. Lim BJ, et al. Pretreatment of sildenafil attenuates ischemia-reperfusion renal injury in rats. Am J Physiol Renal Physiol. 2009;297:F362–F370. doi: 10.1152/ajprenal.90609.2008. [DOI] [PubMed] [Google Scholar]
- 14.Taniguchi Y. Tonai-Kachi H. Shinjo K. Zaprinast, a well-known cyclic guanosine monophosphate-specific phosphodiesterase inhibitor, is an agonist for GPR35. FEBS Lett. 2006;580:5003–5008. doi: 10.1016/j.febslet.2006.08.015. [DOI] [PubMed] [Google Scholar]
- 15.Dundore RL. Clas DM. Wheeler LT, et al. Zaprinast increases cyclic GMP levels in plasma and in aortic tissue of rats. Eur J Pharmacol. 1993;249:293–297. doi: 10.1016/0014-2999(93)90525-m. [DOI] [PubMed] [Google Scholar]
- 16.Guan Z. Miller SB. Greenwald JE. Zaprinast accelerates recovery from established acute renal failure in the rat. Kidney Int. 1995;47:1569–1575. doi: 10.1038/ki.1995.220. [DOI] [PubMed] [Google Scholar]
- 17.Ibrahim MA. Asai H. Satoh S, et al. Effect of zaprinast on nitric oxide levels in serum and aortic tissue. Methods Find Exp Clin Pharmacol. 2004;26:19–24. doi: 10.1358/mf.2004.26.1.793468. [DOI] [PubMed] [Google Scholar]
- 18.Ibrahim MA. Satoh N. Ueda S. Possible impact of nitric oxide on the antihypertensive effect of captopril and zaprinast. Adv Ther. 2003;20:143–148. doi: 10.1007/BF02850201. [DOI] [PubMed] [Google Scholar]
- 19.Beierwaltes WH. cGMP stimulates renin secretion in vivo by inhibiting phosphodiesterase-3. Am J Physiol Renal Physiol. 2006;290:F1376–1381. doi: 10.1152/ajprenal.00209.2005. [DOI] [PubMed] [Google Scholar]
- 20.Jablonski P. Howden BO. Rae DA, et al. An experimental model for assessment of renal recovery from warm ischemia. Transplantation. 1983;35:198–204. doi: 10.1097/00007890-198303000-00002. [DOI] [PubMed] [Google Scholar]