Abstract
Vascular endothelial growth factor A (VEGF-A) is a validated therapeutic target in several angiogenic- and vascular permeability–related pathological conditions, including certain cancers and potentially blinding diseases, such as age-related macular degeneration and diabetic retinopathy. We and others have shown that VEGF-A also plays an important role in neuronal development and neuroprotection, including in the neural retina. Antagonism of VEGF-A function might therefore present a risk to neuronal survival as a significant adverse effect. Herein, we demonstrate that VEGF-A acts directly on retinal ganglion cells (RGCs) to promote survival. VEGF receptor-2 signaling via the phosphoinositide-3-kinase/Akt pathway was required for the survival response in isolated RGCs. These results were confirmed in animal models of staurosporine-induced RGC death and experimental hypertensive glaucoma. Importantly, we observed that VEGF-A blockade significantly exacerbated neuronal cell death in the hypertensive glaucoma model. Our findings highlight the need to better define the risks associated with use of VEGF-A antagonists in the ocular setting.
Vascular endothelial growth factor A (VEGF-A) was initially identified as a vascular permeability factor and endothelial cell mitogen. Since then, it has been shown to have numerous roles outside the vasculature, perhaps most significantly in the nervous system. Neurons express VEGF receptor (VEGFR)-1 and VEGFR-2, and are able to respond to VEGF-A.1 Furthermore, neuropilins, which are important receptors for neuronal development and function, are also coreceptors for the heparin-binding VEGF164 and VEGF188 isoforms.2 Studies have revealed neurodevelopmental, neurotrophic, and neuroprotective roles for VEGF-A in a variety of nervous tissues. In vitro, VEGF-A can protect neurons against hypoxia, glutamate excitotoxicity, and deprivation of serum, oxygen, or glucose,3, 4, 5 and mediate neuronal migration, axonal outgrowth, and Schwann cell proliferation.6, 7 In vivo, VEGF-A can rescue retinal neurons after optic nerve axotomy,8 protect neural tissues through hypoxic preconditioning in ischemia-reperfusion injury,9 improve function in rodent models of amyotrophic lateral sclerosis10 and cerebral ischemia,11 and mediate neuroprotection during development via the coreceptor neuropilins.12 VEGF-A appears to exert these effects directly on neuronal cells, independently of its vascular actions, and may even be important for maintenance of neuronal circuitry.13
Given that antagonism of VEGF-A function is used as a therapeutic strategy for numerous pathological conditions, including various types of cancer, choroidal neovascularization associated with age-related macular degeneration, and macular edema associated with diabetes mellitus and retinal vein occlusion, a better understanding of the roles of VEGF-A in the nervous system is critical.14, 15 This therapeutic strategy is also being explored for additional conditions in which vascular growth and permeability are important, such as neovascular glaucoma and fibrotic complications of glaucoma filtration surgery.16 Given the functional and protective roles of VEGF-A in the nervous system, these treatments might have unexpected adverse effects on neural function, particularly in the eye.
With this in mind, we sought to explore the mechanism by which VEGF-A exerts its neuroprotective effects. We first determined if VEGF-A can act directly on isolated retinal ganglion cells (RGCs). Having established that VEGF-A directly prevents RGC apoptosis via VEGFR-2 and phosphoinositide-3-kinase (PI3K)/Akt signaling, we used two different animal models to study RGC death in vivo. Our findings suggest a neuroprotective role for VEGF-A in models of acute toxicity and hypertensive glaucoma, and highlight the need for rigorous assessment of the long-term impact of VEGF-A inhibition on retinal neurons.
Materials and Methods
Animals
All animals were obtained from Harlan Laboratories (Shardlow, United Kingdom) and used according to Home Office (http://www.homeoffice.gov.uk/science-research/animal-research, last accessed February 17, 2013) and the Association for Research in Vision and Ophthalmology Statement for the Use of Animals in Ophthalmic and Vision Research guidelines (http://www.arvo.org/About_ARVO/Policies/Statement_for_the_Use_of_Animals_in_Ophthalmic_and_Visual_Research, last accessed February 17, 2013).
RGC Isolation and Culture
We used an immunomagnetic cell separation protocol based on Sappington et al,17 with modifications. Retinas from postnatal day 1 Sprague-Dawley rats were dissociated as previously described. To ensure purity of RGCs, we removed macrophages first. The pellet was resuspended in Dulbecco’s modified essential media (Invitrogen, Paisley, UK) with rabbit anti–rat-macrophage antiserum (1:100; Accurate Chemical, Westbury, NJ). The solution was then incubated with goat anti-rabbit secondary antibody conjugated to magnetic microbeads and separated using an automagnetic activated cell sorter (Miltenyi Biotec, Cologne, Germany). The negative fraction was incubated with mouse anti-rat Thy1.1 antibody (1:125; BD Pharmingen, San Diego, CA), followed by secondary rat anti-mouse IgG1 antibody conjugated to magnetic beads (Miltenyi Biotec). Automagnetic activated cell sorter separation was performed, leaving Thy1.1-positive RGCs, which are reported to comprise 93% of Thy1.1-positive cells in the retina.18
Before seeding, culture vessels were coated with 0.01 mg/mL poly-d-lysine (Sigma-Aldrich, Dorset, UK) and 0.01 mg/mL laminin (Roche Applied Science, West Sussex, UK). Cells were seeded in 4-well plates (Nunc, Roskilde, Denmark) on 13-mm glass coverslips at 2.5 × 104 cells per well, and 5 × 105 cells per well on 12-well plates for real-time PCR. Cells were grown in serum-free Neurobasal-A medium, as previously described,17 and maintained at 37°C in 5% CO2.
Because RGCs require growth factors to survive, it was necessary to dissect protective properties of VEGF-A from those offered by growth factors already present. Cells received full medium at day in vitro (DIV) 0 and DIV 1; then, no further medium was used until treatment on DIV 5. This ensured sufficient cells survived for assays without masking the beneficial effects of VEGF-A by other neuroprotectants. Mouse VEGF164, VEGF120 (R&D Systems, Abingdon, UK), VEGF-E (Isolate D1701 with His tag, CRV007; Cell Sciences, Canton, MA), placental growth factor (PlGF)-1, and PlGF-2 (Peprotech, London, UK), at 2.5 nmol/L final concentration, were added in Neurobasal-A (Invitrogen) on DIV 5, 24 hours before toxicity treatment. These cells were added in media minus supplements or growth factors to media covering the monolayer, because removal of all survival factors was too damaging. For H2O2 treatment, cell culture medium was removed, and 500 μL of 10 μmol/L H2O2, with or without VEGFR ligands in Neurobasal-A, was added for 5 hours. Because of staurosporine (SSP) potency, it was necessary to add this onto media already present. SSP, with or without VEGFR ligands (1 μmol/L), was added for 24 hours in Neurobasal-A. The PI3K inhibitors, LY-294,002 (0.1 to 10 μmol/L) and wortmannin (0.3 to 30 nmol/L), were added 10 minutes before VEGFR agonist pretreatment in Neurobasal-A. Pan-caspase inhibitors Z-VAD-Fmk and Q-VD-Oph, used individually or in combination, were added simultaneously with H2O2 or SSP at 100 μmol/L. Equivalent concentrations of dimethyl sulfoxide (DMSO) were included as controls for SSP, PI3K, and caspase inhibitor experiments.
Cell Survival Assay
Cell survival was determined using calcein AM dye (Invitrogen) to quantify viable cells remaining after treatments, based on previously published methods.19 Calcein AM is a cell-permeable, fluorogenic esterase substrate, which is hydrolyzed by intracellular esterases in living cells and converted into the fluorescent product, calcein. We imaged three random nonoverlapping fields of each well, on duplicate coverslips at ×10 magnification using a BX51 epifluorescence microscope with a Retiga SRV camera (QImaging, Surrey, BC, Canada). At least 200 cells were counted per N, using an automated cell counting program (Image Pro Plus 6.2; Media Cybernetics, San Diego, CA). The survival rate was expressed as a percentage of the total number of cells in control wells at each time point.
Real-Time PCR
For in vitro real-time PCR, cells received full media, plus or minus 2.5 nmol/L VEGF164 or PlGF-1, at DIV 1, 2, and 5. At DIV 7, total RNA was isolated using the RNEasy kit (Qiagen, Sussex, UK). For in vivo studies, eyes were stored in RNAlater (Invitrogen) until RNA was extracted. Real-time PCR was conducted using the Taq-Man Gene Expression Assay (Applied Biosystems, Warrington, UK). To detect expression of the target gene, the following assays were used: VEGF (Rn00582935_m1), VEGFR-2 (Rn00564986_m1), VEGFR-1 (RN00570815_m1), and β-actin (RN00667869_m1). Expression levels of target genes were determined by the relative quantification method using β-actin as an endogenous control.
TUNEL Staining
The TUNEL assay quantified apoptotic cells in vitro and in whole mount retinas, according to manufacturer’s instructions (Promega, Southampton, UK). For RGCs in vitro, cells were fixed in 4% paraformaldehyde (PFA) for 15 minutes, permeabilized with 0.2% Triton X-100 in PBS (T-PBS), before the TUNEL reaction. Coverslips were mounted onto glass slides, and images were obtained of six nonoverlapping fields taken from duplicate coverslips at ×10 magnification using an Olympus BX51 microscope (Olympus, Essex, UK) with a Retiga SRV camera (QImaging). DAPI- and TUNEL-positive cells were counted (minimum, 500 cells per coverslip). The number of TUNEL-positive cells was subtracted from DAPI-positive cells to give TUNEL-negative (nonapoptosing) cells and averaged per field. Each N represents independent cell separations.
For whole mounts, animals were CO2 asphyxiated, then eyes were fixed in 4% PFA. Retinas were permeabilized in 3% T-PBS for 2 hours. The TUNEL protocol was performed, and retinas were washed in 0.3% T-PBS with 5 μmol/L DAPI and then flat mounted in Vectashield (Vector Laboratories, Peterborough, UK). To quantify TUNEL-positive neurons, we used a Zeiss 700 confocal microscope (Zeiss, Oberkochen, Germany), taking 10-μm Z-stacks through the ganglion cell layer (GCL) at ×20 magnification. Morphological criteria discriminated nonneuronal (endothelial and glial) cells from neuronal cells. We took three images on each of the four petals: close to the optic nerve, the middle, and periphery of the retina, giving 12 images per whole mount and sampling approximately 7000 cells. Areas were selected using only DAPI, and investigators (R.F. and A.F.) were blinded to treatment groups.
Acute Toxicity Model
Male 10-week-old C57/Bl6 mice were anesthetized with 100 mg/kg ketamine and 0.5 mg/kg xylazine, and pupils were dilated with 2.5% phenylephrine hydrochloride and 1.0% tropicamide (Bausch and Lomb, Surrey, UK). For pretreatment, bilateral intravitreal injections of 4 pmol VEGF120 or PBS vehicle were administered in a 1-μL volume before injecting SSP or vehicle. Mice recovered for 24 hours after initial injection; then, 1 nmol SSP or 10% DMSO vehicle, with or without wortmannin in 1 μL, was administered intravitreally. Animals were sacrificed 24 hours later by CO2 asphyxiation. Eyes were enucleated and fixed in 4% PFA for 4 hours for staining. Investigators (R.F. and A.F.) were masked to treatment groups until analysis was complete.
Ocular Hypertension Model
Experimental glaucoma was induced by elevating the intraocular pressure (IOP) via injection of paramagnetic microspheres into the anterior chamber, based on Samsel et al.20 Briefly, 250 to 300 g female exbreeder Brown Norway rats were housed for 1 week in a constant low-light environment (40 to 60 lux) to minimize diurnal fluctuations in IOP. Rats were anesthetized with 37.5 mg/kg ketamine and 0.25 mg/kg medetomidine hydrochloride, and a toroidal magnet (Supermagnete, Gottmadingen, Germany) was placed around the eye, before 25 μL of a solution containing 30 mg/mL 8-μm magnetic microspheres (Bangs Laboratories, Fisher, IN) in HBSS was injected into the anterior chamber. The magnet drew the beads into the iridocorneal angle, to impede aqueous drainage from the trabecular meshwork. Right eyes acted as unoperated on controls. IOP measurements were taken in awake animals before bead injection, then every 2 to 3 days using a TonoLab rebound tonometer (Tiolat, Oy, Finland). The IOP was taken as the mean of five readings. To investigate VEGF-A neuroprotection, 20 pmol of VEGF120, VEGFR-2 Fc chimera (R&D Systems), IgG, or vehicle controls was injected intravitreally on days 3 and 10 after induction. Animals were sacrificed on day 17 after induction, and eyes were enucleated and fixed in 4% PFA overnight for TUNEL. All experiments were performed masked.
Immunostaining
For RGC cultures, cells were fixed in 4% PFA and blocked with 5% normal goat serum in 0.1% T-PBS for 2 hours. Primary antibodies were rabbit anti-Thy1 (1:200; Santa Cruz Biotechnology, Santa Cruz, CA), rabbit anti–βIII-tubulin (1:500; Abcam, Cambridge, UK), rabbit anti–VEGFR-2 (1:200; Abcam), goat anti-VEGFR1 (1:100; Santa Cruz Biotechnology), rabbit anti–phospho-Akt (1:200; Cell Signaling, Beverly, MA), and rabbit anti–active caspase-3 (1:250; R&D Systems). Specificity for VEGFR-2 was confirmed using blocking peptide (Abcam). Secondary antibodies were goat anti-rabbit or donkey anti-goat conjugated to Alexa Fluor 488 or 594 (Invitrogen) used at a 1:500 dilution for VEGFR staining and a 1:200 dilution for all other experiments. Coverslips were mounted onto glass slides in ProLong Gold with DAPI (Invitrogen).
For retinal whole mounts, animals were sacrificed and retinas were prepared as for TUNEL staining. The tissue was blocked for 2 hours in 5% donkey serum and 0.3% T-PBS before primary antibodies were applied overnight. Secondary antibodies were added for 2 hours in 0.3% T-PBS. After secondary incubation, the tissue was rinsed in 0.3% T-PBS plus 5 μmol/L DAPI and then flat mounted in Vectashield (Vector Laboratories, Peterborough, UK). Primary antibodies used were rabbit anti–phospho-Akt (1:500; Cell Signaling) and goat anti-Brn3a (1:200; Santa Cruz Biotechnology); secondary antibodies were donkey anti-rabbit conjugated to Alexa Fluor 633 and donkey anti-goat conjugated to Alexa Fluor 594 (1:1000; Invitrogen). Fluorescein-conjugated Griffonia simplicifolia isolectin B4 (1:400; Vector Laboratories, Peterborough, UK) stained blood vessels. Controls were no primary antibody and relevant IgG isotypes. Images of cells and retinas were taken on a Zeiss 700 confocal microscope.
Optic Nerve Sections
Optic nerves were fixed overnight in Karnovsky’s solution at 4°C. Specimens were osmicated for 2 hours in 1% (w/v) osmium tetroxide and then dehydrated in 100% ethanol. Optic nerves were then incubated in propylene oxide for 30 minutes and placed in a 50:50 mixture of propylene oxide:araldite overnight. This solution was changed to 100% araldite and incubated overnight at 60°C. Semithin sections (0.75 μm thick) were cut and stained with 1% toluidine blue/borax (TB) in 50% ethanol before examination by light microscopy. For quantification, three nonoverlapping images were taken at ×63 magnification, in the center, midway, and periphery of the optic nerve, using an Olympus BX51 microscope with a Retiga 2000R camera (QImaging). Nonviable/dying axons with TB accumulated were counted in two sections per optic nerve, averaged per section, and then expressed as dying axons per mm2. At least three optic nerves were quantified per treatment group.
Statistical Analysis
Statistical analyses were performed using GraphPad Prism software version 4 (GraphPad Software, La Jolla, CA). In all instances, one-way analysis of variance with a Newman-Keul’s post hoc test was used, except for RGC cultures, when analysis of variance with repeated measures was used. To analyze real-time PCR results, CT values were normalized to β-actin and statistics were performed on ΔCT values. Results are means ± SEM unless stated, with each N representing an individual cell culture separation or retina; N was at least three for each statistical analysis. P < 0.05 was considered significant.
Results
Characterization of Primary RGC Cultures
We have previously demonstrated that VEGF-A can protect retinal neurons from death induced by ischemia-reperfusion injury.9 Because this in vivo model involves potential indirect effects of blood flow and paracrine-mediated protection from endothelial or other retinal cell types, we used primary RGC cultures to probe the mechanisms of VEGF-A–mediated neuroprotection. RGCs were used because relatively homogeneous cultures can be obtained compared with other retinal neurons, and they are relevant to many retinal pathological conditions.21 We confirmed the purity of our primary RGC cultures by immunostaining for the RGC marker, Thy-1, and neuron-specific βIII-tubulin. At DIV 5, RGC cultures were >95% positive for both markers (Supplemental Figure S1). The cells formed dense networks of neurites and were capable of surviving for weeks in culture.
Expression and Function of VEGFRs in Cultured RGCs
In vivo, RGCs express both VEGF-A and its receptors.1 By using real-time quantitative PCR (qPCR), we demonstrated that VEGFR expression was maintained in our cultured RGCs. VEGFR-2 was the most abundant receptor, with relative levels 17-fold higher than those of VEGFR-1 (P < 0.001) (Supplemental Figure S2A). When compared with primary rat brain microvascular endothelial cells in culture (donated by Dr. Patric Turowski, UCL Institute of Ophthalmology, London, UK)), a cell type known to express functional VEGFRs, the relative levels of VEGFR-2 were fivefold higher and of VEGFR-1 were fourfold lower in RGCs and ratios of VEGFR-2/VEGFR-1 were approximately 17:1 in RGCs and 1:1 in endothelial cells (Supplemental Figure S2, A and B).
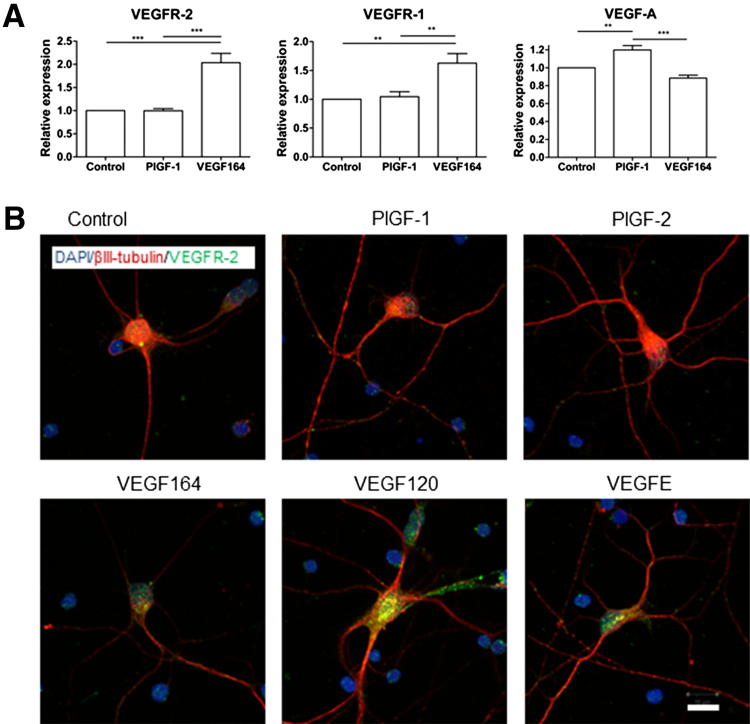
To determine whether the receptors for VEGF-A are functional in cultured RGCs, we treated cells with VEGF-A isoforms VEGF120 and VEGF164; VEGFR-1–specific PlGF-1 and PlGF-2; and VEGF-E, which is specific for VEGFR-2. VEGF164 induced a twofold increase in VEGFR-2 expression (P < 0.001) (Figure 1A). This was accompanied by increased VEGFR-2 immunostaining, particularly along the neurites and perinuclear region: VEGFR-2 staining in RGCs was predominantly perinuclear (Figure 1B). Increased immunostaining was also observed with VEGF120 and VEGF-E treatments (Figure 1B). VEGFR-1 expression increased approximately 1.6-fold (P < 0.01) after VEGF164 treatment (Figure 1A). PlGF-1 did not significantly modify mRNA expression of either VEGFR (Figure 1A), nor was the qualitative immunostaining pattern altered for PlGF-1 or PlGF-2 (Figure 1B). Together, these data demonstrate that cultured RGCs express VEGFR-2 and that receptors are functional.
Figure 1.
Primary RGC cultures express VEGF-A and its receptors. A: Real-time qPCR analysis of VEGFR-2, VEGFR-1, and VEGF-A. Relative expression levels of VEGFR-1 and VEGFR-2 were significantly elevated after VEGF164 treatment compared with control, whereas PlGF had no effect. In contrast, VEGF-A RNA levels were slightly attenuated with VEGF164 supplementation, and enhanced in response to PlGF-1. **P < 0.01, ***P < 0.001 (N = 6). Data are given as means ± SD. B: Immunolabeling of VEGFR-2 (green) and βIII-tubulin (red) in RGCs cultured in control medium or with 2.5 nmol/L PlGF-1, PlGF-2, VEGF120, VEGF164, or VEGF- E for 5 days. Original magnification, ×63. VEGFR-2 immunoreactivity increased after VEGF120, VEGF164, or VEGF-E supplementation, with punctate staining observed both perinuclearly and on neurites. In contrast, VEGFR-2 expression did not increase and remained perinuclear in control and PlGF-1– and PlGF-2–treated RGCs. Scale bar = 10 μm.
RGC Responses to Cell Death Agents
To assess the neuroprotective properties of VEGF-A, it was necessary to identify agents that effectively induce RGC death in culture. We evaluated nine conditions described in the literature3, 4, 22, 23, 24, 25, 26, 27, 28 for inducing RGC death (Supplemental Table S1). RGCs in culture were surprisingly resilient; agents that induce receptor-mediated apoptosis, including tumor necrosis factor α, Fas ligand, and IL-1β, failed to cause RGC death after 1 to 5 days of treatment. Excitotoxic ligands, N-methyl-d-aspartate, 2-amino-3-(5-methyl-3-oxo-1,2-oxazol-4-yl) propanoic acid, and glutamate, also failed to cause significant cell death, even with 5 days of 500 μmol/L ligand. However, RGCs in culture have previously been invulnerable to N-methyl-d-aspartate–mediated cell death.29 Even hypoxia for up to 24 hours did not induce significant cell death (1% O2) (Supplemental Table S1).
Conditions that caused significant RGC death were growth factor withdrawal and exposure to paraquat, SSP, or H2O2 (Supplemental Table S1). From these, H2O2, used to model oxidative stress in vitro, and SSP, a non-specific protein kinase inhibitor that broadly activates cellular death pathways,30 were chosen because they were optimal for our assays. Both induced consistent, dose-dependent RGC death (P < 0.05).
VEGF-A Protects RGCs via VEGFR-2, Independent of Neuropilins
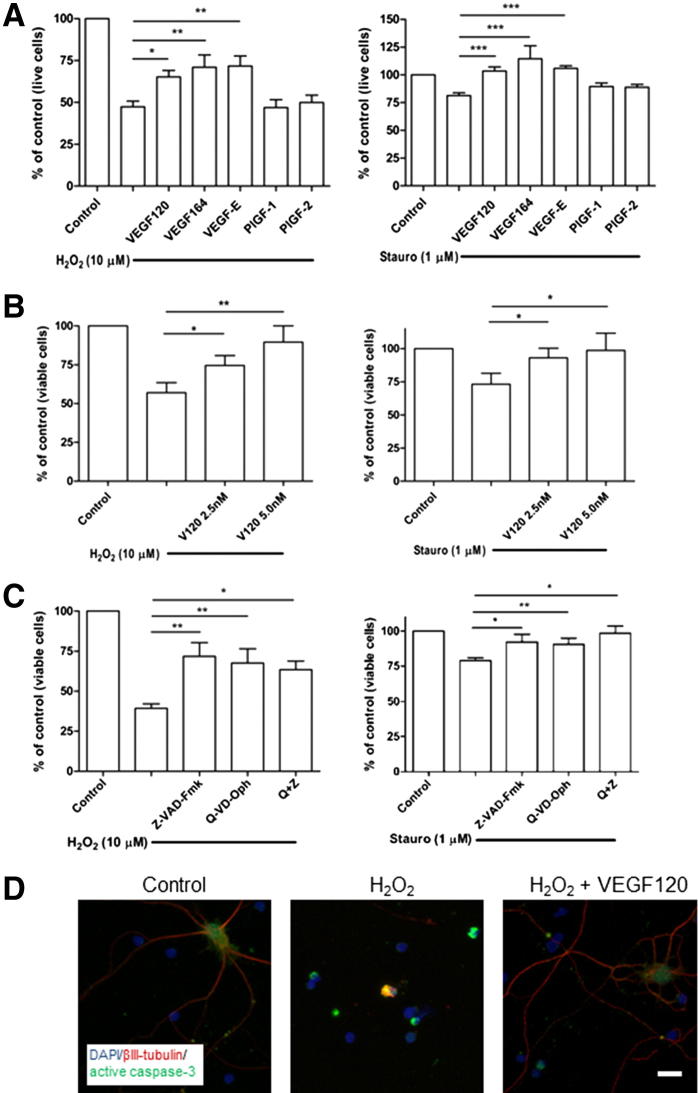
To examine VEGF-mediated effects on RGC survival, cultures were pre-treated with VEGF family ligands with different binding properties for VEGFR-1, VEGFR-2, and neuropilins. RGCs were pre-treated at DIV 5 for 24 hours with media supplemented with a final concentration to 2.5 nmol/L of the agonists, followed by addition of 10 μmol/L H2O2 for 5 hours or SSP for 24 hours. Of the different VEGFR ligands tested, VEGF164 (P < 0.01), VEGF120 (P < 0.05), and VEGF-E (P < 0.01) all increased RGC survival by approximately 50% (Figure 2A). In contrast, PlGF-1 and PlGF-2 did not protect against H2O2-mediated cell death. A similar pattern emerged for SSP treatment. SSP (1 μmol/L) induced approximately 25% death of RGCs, which was completely reversed by 24-hour pretreatment with VEGF164, VEGF120, or VEGF-E (all P < 0.001). Again, both PlGF-1 and PlGF-2 failed to offer detectable protection.
Figure 2.
VEGF-A protects against apoptotic, caspase-dependent death via VEGFR-2, independent of neuropilins. A: The RGCs at DIV 5 were pre-treated for 24 hours in media, with or without VEGF120, VEGF164, VEGF-E, PlGF-1, or PlGF-2 (2.5 nmol/L). H2O2 (10 μmol/L; left panel) was added to the cells for 5 hours, and SSP (1 μmol/L; right panel) was added for 24 hours. VEGF164, VEGF120, and VEGF-E all increased survival of the cultures, whereas neither PlGF-1 nor PlGF-2 prevented RGC death. *P < 0.05, **P < 0.01, and ***P < 0.001 (N = 5 to 6). B: Cells were treated with H2O2 (left panel) or SSP (right panel) and TUNEL stained. The percentage of TUNEL-negative, viable RGCs increased after pretreatment with 2.5 and 5.0 nmol/L VEGF120. *P < 0.05, **P < 0.01 (N = 8). Data are given as means ± SEM. C: RGCs were incubated with pan-caspase inhibitors, Z-VAD-Fmk and Q-VD-Oph, before H2O2 or SSP exposure. Caspase inhibitors largely abolished the toxic response, both independently and in combination. *P < 0.05, **P < 0.01 (N = 4 to 8). D: Immunocytochemistry for active caspase-3 (green) showed increased staining in cells treated with H2O2 (note condensed caspase-3 staining around apoptotic nuclei; middle panel) compared with control cells (left panel). Staining was reduced after VEGF120 pretreatment (right panel). Cells were counterstained with βIII-tubulin (red) and DAPI (blue). Original magnification, ×20. Scale bar = 10 μm.
These data indicate that VEGFR-2 is required for protection of RGCs, consistent with our findings in vivo.9 It appeared that the heparin-binding domain of VEGF-A was not essential, because the VEGF120 isoform and VEGF-E that lack this domain rescued cells with similar potency to heparin-binding VEGF164. These data further suggest that neuropilin receptors are not required for protection, because VEGF120 and VEGF-E, which exhibit little or no binding to neuropilin-1 and neuropilin-2,2, 31, 32 were protective, whereas PlGF-2, which binds to both neuropilins, did not enhance survival. We decided to use VEGF120 in subsequent experiments because this isoform produced consistent protection (Figure 2A) and induced fewer adverse effects on intravitreal injection in vivo.9
VEGF-A Is Able to Protect against Apoptotic, Caspase-Dependent Cell Death
We sought to characterize the mechanism of VEGF-A–mediated neuroprotection in further detail. TUNEL staining was used to define whether cell death was associated with DNA fragmentation, commonly associated with apoptosis. Inhibitors and immunostaining were also used to determine whether modulation of caspase signaling was associated with VEGF-A–mediated neuroprotection. TUNEL staining revealed that H2O2 treatment significantly reduced viable (TUNEL-negative) cell number by 42% relative to control (P < 0.001) and that VEGF120 dose dependently augmented survival compared with control, by 31% at 2.5 nmol/L (P < 0.05) and 50% at 5.0 nmol/L (P < 0.05) (Figure 2B). These effects were also observed with SSP treatment, for which VEGF120 exposure significantly reduced cell death (P < 0.05 compared with SSP alone).
Caspase activation is an early step in the initiation of apoptosis. To determine the role of caspases in H2O2- and SSP-mediated RGC death, two different caspase inhibitors, Z-VAD-Fmk and Q-VD-Oph, were used. These inhibitors have differential affinities for individual caspases; therefore, they must be used in combination to fully differentiate between caspase-dependent and caspase-independent death.33 Both Z-VAD-Fmk and Q-VD-Oph significantly increased the percentage of viable RGCs from 39% to 72% and 68%, respectively (both P < 0.01), and to 63% (P < 0.05) when combined (Figure 2C). Findings were similar for SSP-induced cell death (Figure 2C).
Because caspase activity and DNA fragmentation are involved in H2O2- and SSP-induced RGC death, we sought to determine whether the neuroprotective effects of VEGF-A involve modulation of caspase activation. Immunostaining confirmed an increase in activated caspase-3 levels in the presence of H2O2, and pretreatment of cells with VEGF120 markedly reduced the amount of activated caspase-3 (Figure 2D). Taken together, these data suggest that H2O2 and SSP initiate apoptotic, caspase-dependent death in RGCs, and that VEGF-A signaling via VEGFR-2 inhibits caspase-3 activation to promote RGC survival.
PI3K/Akt Signaling Pathways Mediate VEGF-A Neuroprotection in Vitro
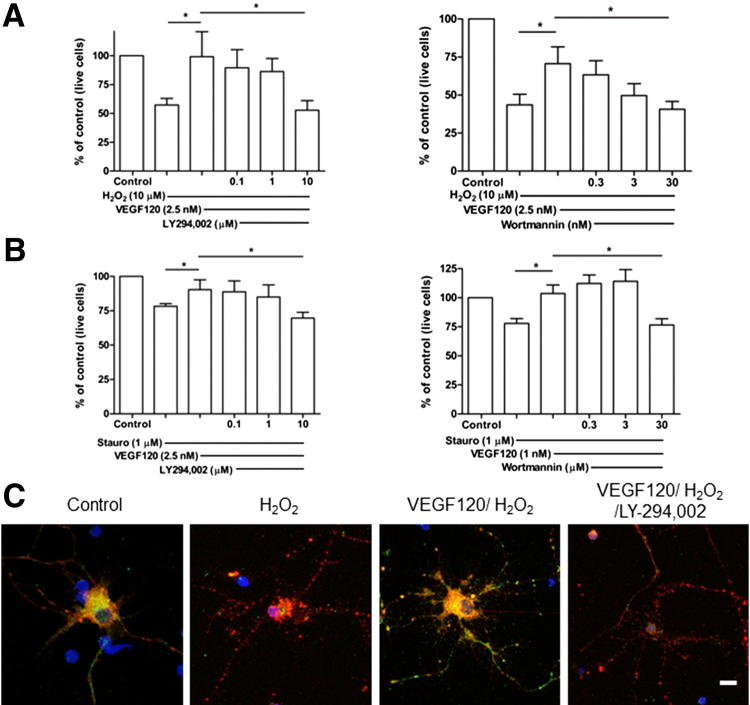
The PI3K/Akt signaling pathway is involved in numerous cellular functions, and has been identified as central for survival of many cell types, including neurons.5 To determine whether VEGF-A mediates neuroprotection via PI3K/Akt in RGCs, we first explored the activation status of Akt in cultured cells. In the presence of H2O2, RGC phospho-Akt levels were reduced, an effect prevented by VEGF120 pretreatment (Figure 3C). Furthermore, pretreatment of RGCs with the PI3K inhibitor, LY294,002, blocked VEGF120-induced Akt phosphorylation. These data indicate that Akt signaling is activated during VEGF120-mediated protection of RGCs.
Figure 3.
Neuroprotection by VEGF120 is PI3K/Akt mediated. A and B: Increasing concentrations of PI3K inhibitors, LY294,002 (left panel) and wortmannin (right panel), were added to RGCs immediately before VEGF120 pretreatment, before H2O2 (A) or SSP (B) was added to kill the cells. Both LY294,002 and wortmannin dose dependently abolished the protective effect of VEGF120. *P < 0.05 (N = 5 to 6). Data are given as means ± SEM. C: Immunocytochemistry revealed cytoplasmic expression of phospho-Akt (green) in control cultures, which was reduced after H2O2 exposure. In cells rescued with VEGF120, phospho-Akt (pAkt) reactivity was similar to control and was reduced with LY294,002 treatment. Cells were counterstained with βIII-tubulin (red) and DAPI (blue). Original magnification, ×63. Scale bar = 10 μm.
To confirm that VEGF-A acts via the PI3K/Akt signaling axis, cells were exposed to PI3K inhibitors during VEGF120 pretreatment and the effects on neuroprotection were monitored. LY294,002 or wortmannin alone did not induce RGC death (Supplemental Figure S3, A and B). When added to RGCs immediately preceding VEGF120, LY294,002 dose dependently abolished the survival-enhancing properties of VEGF120 against H2O2 (P < 0.05) (Figure 3A). Similar results were obtained with wortmannin (P < 0.05). When the corresponding experiments were conducted using SSP, attenuation of VEGF120 protection was observed at the highest inhibitor doses tested (P < 0.05) (Figure 3B).
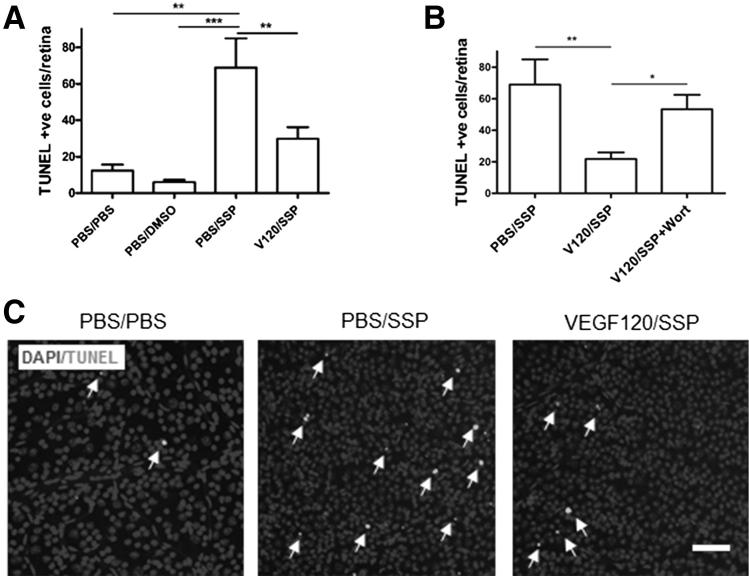
VEGF-A Protects RGCs in an in Vivo Acute Toxicity Model via PI3K-Dependent Pathways
Using primary RGC cell cultures, we have illustrated the direct neuroprotective function of VEGF-A, and implicated signaling pathways involved. To examine the applicability of these findings in vivo, acute toxin-induced retinal cell death was initiated by intravitreal injection of SSP. Mice were pre-treated with an intravitreal injection of 4 pmol VEGF-A or vehicle for 24 hours before receiving 1 nmol SSP or vehicle for a further 24 hours. Injection of SSP significantly increased the number of TUNEL-positive cells in the GCL (68.9 ± 16.8 cells per retina), compared with saline-injected (12.3 ± 3.3 cells per retina; P < 0.01) or DMSO vehicle controls (6.0 ± 1.2 cells per retina; P < 0.001) (Figure 4, A and C). VEGF120 pretreatment significantly protected against SSP-induced toxicity, reducing apoptotic nuclei by 57% compared with vehicle control (29.8 ± 6.4 cells per retina; P < 0.01). To explore if VEGF-A–mediated neuroprotection is mediated by PI3K signaling in vivo, the PI3K inhibitor wortmannin, was injected simultaneously with SSP, after VEGF120 pretreatment. Wortmannin alone did not increase RGC apoptosis compared with controls (Supplemental Figure S3C), but it fully reversed the protective effects of VEGF120 against SSP toxicity (21.79 ± 4.2 versus 53.29 ± 9.1 cells per retina; P < 0.05) (Figure 4B), suggesting a fundamental role for PI3K in VEGF-A–mediated neuroprotection.
Figure 4.
VEGF120 protects against SSP-induced retinal cell death in vivo via the PI3K/Akt pathway. A: VEGF120 protects against SSP-induced cell death in the mouse retina. DMSO vehicle did not increase the number of TUNEL-positive cells higher than PBS vehicle control (N = 6 to 8), whereas SSP elevated apoptotic nuclei counts by approximately 5.5-fold (P < 0.01, N = 12). Pretreatment with VEGF120 protected against SSP toxicity, reducing TUNEL-positive cells by 57%. **P < 0.01, ***P < 0.001 (N = 10). B: Treatment with 1 nmol of the PI3K inhibitor, wortmannin, reversed VEGF120-mediated neuroprotection (*P < 0.05, N = 14). The PBS/SSP data in this figure were taken from the experiment shown in Figure 4A. Data are given as means ± SEM. C: Representative images of PBS/PBS- (left panel), PBS/SSP- (middle panel), and VEGF120/SSP- (right panel) injected retinas, stained for DAPI and TUNEL (white; arrows). Original magnification, ×20. Scale bar = 50 μm.
VEGF-A Protects against RGC Death in an Ocular Hypertension Model
The protective effect of VEGF-A was also explored in an in vivo model of experimental glaucoma, in which RGC death was induced by mechanically increasing IOP. In patients with ocular hypertensive glaucoma, elevated IOP caused by obstruction of aqueous outflow is a key risk factor in disease pathophysiological characteristics.34 Animal models have been developed to mimic blockage of the trabecular meshwork, including use of magnetic microspheres drawn into the iridocorneal angle to reduce outflow.20 This model was used herein to validate the neuroprotective properties of VEGF-A in rats.
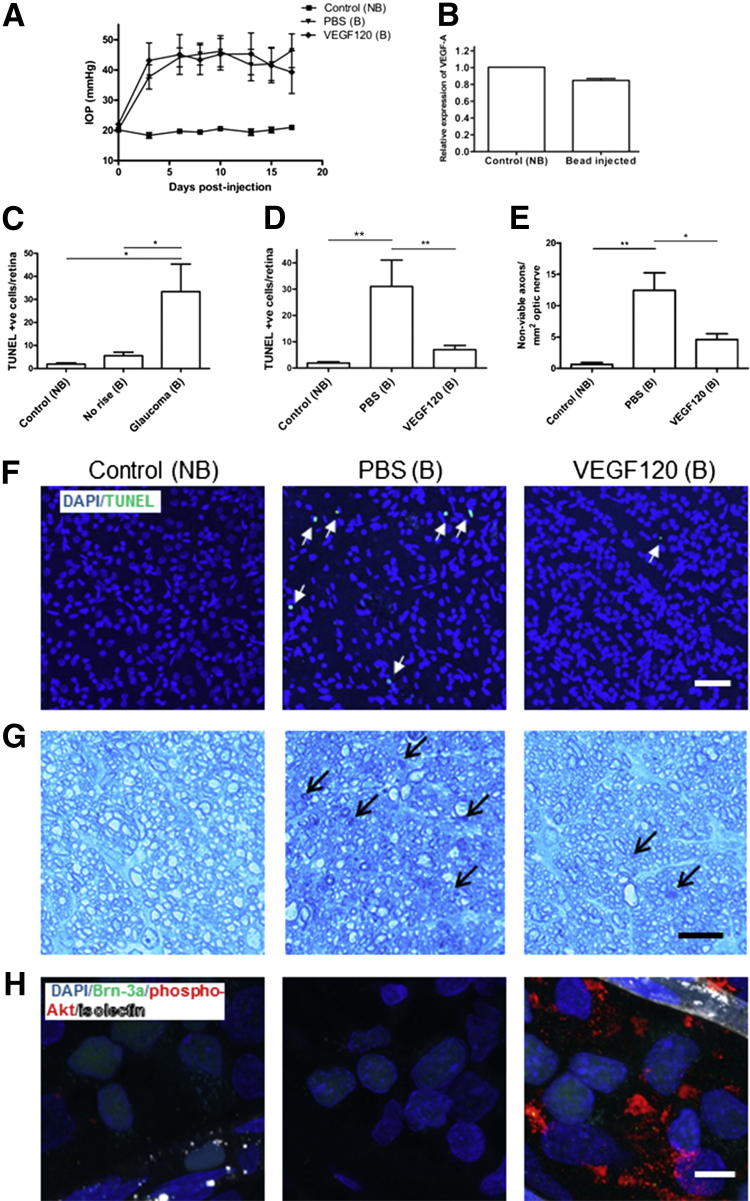
Injection of magnetic beads into the anterior chamber triggered a significant and prolonged increase in IOP. The mean IOP averaged over the full length of the experiment for control, non–bead-injected eyes was 19.8 ± 0.6 mmHg, compared with 43.3 ± 3.3 mmHg for bead-injected eyes (P < 0.001) (Figure 5A). The peak IOP was 22.8 ± 0.6 mmHg for control eyes versus 55.2 ± 3.5 mmHg for bead-injected eyes (P < 0.001). Intravitreal injection of VEGF120 did not affect IOP in bead-injected eyes (Figure 5A); the mean and peak IOP levels were all similar for VEGF120- and vehicle-injected eyes.
Figure 5.
VEGF120 protects RGCs against apoptosis in experimental hypertensive glaucoma, with a corresponding increase in phospho-Akt. A: Bead injection triggers a significant increase in IOP, in both PBS- and VEGF120-bead–injected (B) compared with control, non–bead-injected (NB) rat eyes (P < 0.001, N = 10). B: VEGF mRNA remained at control levels in bead-injected eyes (N = 4). C: A significant increase in TUNEL-positive nuclei in the GCL was observed for bead-injected eyes with an increase in IOP, but not for bead-injected eyes in which pressure did not increase (N = 5 to 7). D: In PBS bead-injected eyes (B), apoptotic cell number increased 16-fold (N = 8) higher than control (NB), but was markedly reduced by 77% in eyes treated with 20 pmol VEGF120 (N = 8). Data are given as means ± SEM. E: VEGF120 also protected against optic nerve damage. Extensive damage was found to the optic nerve in histologically stained transverse sections from hypertensive eyes, as determined by TB staining of semithin nerve segment sections. Approximately 20-fold more degenerating axons were found in animals with a high IOP, from 0.6 ± 0.3 to 12.4 ± 2.8 axons per mm2 optic nerve. This was reduced by 63% to 4.6 ± 0.94 axons per mm2 optic nerve in eyes treated with intravitreal VEGF120 (E). Data are given as means ± SEM. *P < 0.05, **P < 0.01 (N = 3 to 5) (C–E). F: Representative images of control (left panel), PBS bead-injected (middle panel), and VEGF120 bead-injected (right panel) retinas stained for DAPI (blue) and TUNEL (green), showing that VEGF120 treatment reduces TUNEL staining (arrows) to near-control levels. G: Representative images of optic nerve staining, showing, compared to controls, an increase in TB accumulation from control (left panel), in PBS bead-injected animals with a high IOP (middle panel), which was reduced with VEGF120 administration (right panel). Arrows denote degenerating axons. H: Representative images of whole-mount staining for phospho-Akt (pAkt; red). In control (left panel) eyes, pAkt was barely detectable within the GCL, indicating Akt is not constitutively phosphorylated in RGCs. This staining pattern was also observed in PBS bead-injected (middle panel) groups. However, in VEGF120 treated eyes (right panel), strong pAkt immunoreactivity was observed around Brn-3a (green) positive RGCs, and vessels stained with isolectin B4 (white). These images confirm VEGF120 stimulates pAkt signaling in correlation with its neuroprotective activity. Original magnification: ×20 (F); ×63 (G and H). Scale bars: 50 μm (F and G); 10 μm (H).
To investigate if expression of endogenous VEGF-A and its receptors was altered after IOP elevation, retinas were analyzed by real-time qPCR. No change in mRNA levels for VEGF-A (Figure 5B), VEGFR-2, or VEGFR-1 (Supplemental Figure S4) was detected between control and retinas from hypertensive eyes.
Cell death in GCL was assessed using TUNEL staining, which has been shown to increase in patients with glaucoma35, 36 and correlates with RGC loss and optic nerve degeneration in animal glaucoma models.37, 38, 39 In eyes in which magnetic beads were injected but pressure did not increase (because of incomplete blockage of the iridocorneal angle), the numbers of TUNEL-positive cells were not significantly different from those of non–bead-injected control (Figure 5C). These eyes were excluded from our studies. In eyes in which the IOP increased after microsphere injection, there was a significant elevation in TUNEL-positive apoptotic nuclei in the GCL, which is mostly composed of RGCs, as previously reported.20, 40 The number of apoptotic nuclei increased by approximately 16-fold, from 1.9 ± 0.5 to 31.0 ± 10.0 cells per retina (P < 0.01) (Figure 5C), confirming that a high IOP leads to apoptosis of RGCs. Treatment with an intravitreal injection of 20 pmol VEGF120 on days 3 and 10 after glaucoma induction reduced apoptotic cell counts by 77% (P < 0.01), from 31.0 ± 10.0 to 7.0 ± 1.6 cells per retina (Figure 5, D and F), indicating that VEGF120 protects retinal neurons against apoptotic cell death in experimental glaucoma. Furthermore, we observed extensive damage to the optic nerve in histologically stained transverse sections from hypertensive eyes. There were approximately 20-fold more degenerating axons in animals with a high IOP, from 0.6 ± 0.3 to 12.4 ± 2.8 axons per mm2 optic nerve (P < 0.01). This damage was reduced by 63% (P < 0.05) to 4.6 ± 0.94 axons per mm2 optic nerve in eyes treated with intravitreal VEGF120 (Figure 5, E and G).
To determine whether VEGF-A affects PI3K/Akt signaling during neuroprotection in vivo, we stained whole mount retinas from the ocular hypertension model for phospho-Akt. Immunostaining revealed barely detectable levels of phospho-Akt in control and PBS-treated glaucomatous retinas (Figure 5H). In response to VEGF120 injection, phospho-Akt immunoreactivity in glaucomatous retinas increased considerably, particularly in the cell cytoplasm, suggesting that the PI3K/Akt pathway is involved in mediating VEGF120 protection in this model.
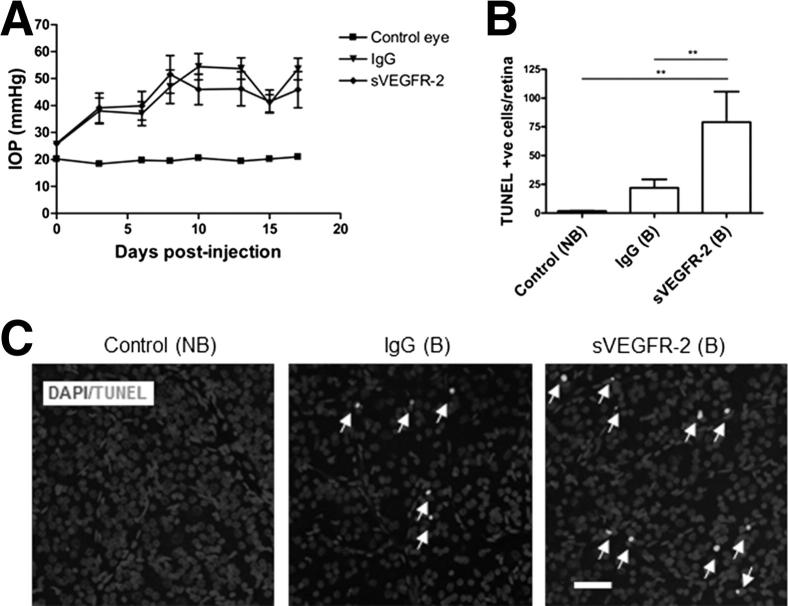
VEGF Neutralization Exacerbates Cell Death in the Ocular Hypertension Model
Finally, to probe the role of endogenous VEGF-A on RGC survival in our experimental hypertensive glaucoma model, soluble human VEGFR-2/Fc chimera (sVEGFR-2) was injected intravitreally to neutralize endogenous VEGF-A. Injection of sVEGFR-2 and human IgG control did not influence IOP, when compared with control PBS bead-injected hypertensive eyes (Figure 6A). However, a comparison of cell death in IgG- and sVEGFR-2–treated hypertensive eyes revealed that TUNEL-positive apoptotic cells markedly increased as a result of VEGF neutralization. Apoptosis in the GCL of the retina was significantly elevated by approximately 3.5-fold higher than IgG treatment, from 22.0 ± 7.4 to 79.2 ± 26.5 cells per retina (P < 0.01) (Figure 6, B and C), indicating that neutralization of endogenous VEGF-A further exacerbates neuronal death in this model.
Figure 6.
Anti-VEGF treatment exacerbates neuronal injury in experimental hypertensive glaucoma. A: Bead injection triggers a significant increase in IOP over time, in both IgG- and sVEGFR-2 bead-injected (B) eyes compared with control, non–bead-injected (NB) rat eyes (P < 0.001, N = 8). B: sVEGFR-2 initiated a large increase in TUNEL-positive cells in the GCL, higher than contralateral NB (N = 6 to 9) and IgG vehicle (N = 6 to 7) groups. Endogenous VEGF-A is, therefore, neuroprotective under conditions in which ocular hypertension provokes neuronal damage. **P < 0.01. Data are given as means ± SEM. C: Representative images of control (left panel), IgG bead-injected (middle panel), and sVEGFR-2 bead-injected (right panel) retinas stained for DAPI and TUNEL (white; arrows). Original magnification, ×20. Scale bar = 50 μm.
Discussion
VEGF-A has long been identified as a critical survival factor for endothelium,41 but this role has been significantly expanded over recent years to involve other cell types, including those in both the peripheral and central nervous systems. In the retina, VEGF-A has been shown to reduce retinal neuron loss9, 42; these findings have led to discussion about the long-term neuronal risk of VEGF-A antagonists,43 which are being widely used to treat various ocular conditions.14 In this study, we have used several approaches to determine the mechanistic basis of neuroprotective effects of VEGF-A, both in vitro and in vivo. Our findings highlight the need to further define risks that may be associated with inhibition of VEGF-A in ocular conditions.
In our isolated RGC model, the neuroprotective effects of VEGF-A were mediated by VEGFR-2. This finding is consistent with other published data, including in vitro models using hypoxia or serum withdrawal,4, 44 oxidative stress,45 and glutamate toxicity,22 plus in vivo models, such as optic nerve transection.8 Several studies have suggested that neuropilin-1 may be involved in VEGF-A–mediated neuroprotection,46 particularly during embryonic development,12 but it has not yet been established in the adult. Given that VEGF164, VEGF120, and VEGF-E had comparable neuroprotective potency in our study, despite the fact that VEGF-E does not bind and VEGF120 interacts weakly, if at all, with neuropilin-1, and that the neuropilin ligand PlGF-2 was not neuroprotective, neuropilin-1 may not be necessary for VEGF-A–mediated neuroprotection.
In terms of mechanisms downstream of VEGFR-2, several pathways have been shown to initiate survival in neuronal tissues. Outside the ocular setting, mitogen-activated kinase/mitogen-activated protein kinase/extracellular signal-regulated kinase,47 protein kinase A,4 and PI3K/Akt,5 alone or acting together, have been shown to mediate neuroprotection. In the retina, PI3K/Akt alone9 and dual activation of PI3K/Akt and ERK-1/28 were shown to enhance survival. We showed that H2O2- and SSP-triggered RGC death in vitro was caspase dependent and accompanied by DNA fragmentation and was, therefore, likely the result of apoptosis. VEGF-A–mediated cell rescue was prevented by the PI3K inhibitors, LY-294,002 and wortmannin, which was validated using wortmannin in the SSP-induced neuronal cell death model in vivo. These PI3K inhibitors have been reported to have off-target effects48; however, future experiments could confirm specificity using cell-specific VEGFR-2 phosphorylation site mutant mice, to prevent PI3K signal transduction after VEGFR-2 activation, and also an RGC-selective inducible VEGFR-2 inactivation model to confirm the role of VEGFR-2 in this pathway. Nevertheless, phospho-Akt staining in the experimental glaucoma model corroborated the results from RGC cultures and further strengthened the case for involvement of PI3K/Akt signaling.
Importantly, our studies using the ocular hypertension model of glaucoma demonstrate that VEGF-A signaling is a critical part of the endogenous response to neural damage. Administration of a VEGFR-2–soluble receptor significantly increased the number of TUNEL-positive cells in the GCL during ocular hypertension. We have previously shown that VEGF-A acts as an endogenous neuroprotective factor as part of the adaptive response to acute (1-hour) ischemia.9 In our experimental glaucoma model, there was no change in VEGF-A or VEGFR levels, yet the data demonstrate that VEGF-A is required for neuronal survival during a relatively prolonged insult (>2 weeks) to retinal neurons. Taken together, these data suggest that VEGF-A may play a constitutive role in RGC neuroprotection. These data are also consistent with those of previous studies, in which VEGF-A depletion via intravitreal or systemic injection of a neutralizing antibody, or adenoviral transfection of soluble VEGFR-1, did not affect normal adult vasculature but did lead to enhanced apoptosis of neurons of the inner and outer retina.9, 42
Do the data from animal models of acute and chronic retinal disease suggest there is a risk to the human retina exposed to VEGF-A antagonists? At a minimum, our findings suggest that risks to patients with glaucoma may need to be more systematically and rigorously assessed. Based on full-field electroretinographic results, it was recently reported that VEGF neutralization with bevacizumab regressed neovascularization and also reduced photoreceptor function in patients with neovascular glaucoma.49 Furthermore, a study observing 49 patients with age-related macular degeneration found that, in eyes treated with ranibizumab, nerve fiber layer thickness was significantly reduced after 1 year of treatment, whereas untreated control eyes displayed no change.50 However, determining the risk profile in glaucoma and in the broader retinal disease population is challenging. First, clinical evidence suggests that 25% to 35% of RGCs must be lost before there is a significant impact on visual acuity51; therefore, subclinical retinal neuron death could occur in patients being treated with VEGF-A antagonists. Second, even if loss of visual acuity is noted in patients, this could be attributed to the natural course of diseases, such as neovascular age-related macular degeneration, diabetes mellitus, and glaucoma.21, 52, 53 Given the enormous scale of these diseases and potential increasing use of VEGF antagonists in all of them, even a small effect would be significant.
Lastly, although focused on inherited disease, experimental analyses suggest that the rate of neurodegeneration in rodents could be as much as two orders of magnitude greater than in humans, and is related to maximum lifespan potential.54 Therefore, short-term rodent experiments may exaggerate the acute risks and long-term monitoring of patients may be required. Of note is the SEVEN-UP study, a small-scale (63 patients) follow-up study of patients with exudative age-related macular degeneration. Despite initial success in ranibizumab-treated patients in the first 24 months, after 7 to 8 years of follow-up and intermittent treatment, 37% of eyes had acuities of 20/200 or worse, with many patients exhibiting geographic atrophy.55 These data are the longest available follow-up of patients treated with VEGF-A antagonists and need to be expanded before strong conclusions can be made.
Given the remarkable impact of anti-VEGF strategies on near-term patient outcomes, one strategy for managing a potential trade-off between the positive vascular outcomes and longer-term neuronal risk may be to develop combination treatments for neovascular conditions that include neuroprotectants. Further elucidation of the details downstream of VEGF-A receptor activation could be critical in the development of a more holistic strategy for preserving the proper function of retinal neurons.
Acknowledgments
We thank Peter Lundh von Leithner, Meihua Ju, Shannon Bunker, and Claire Gregory (University College London) for providing technical assistance with animal experiments, Dr. Peter Munro for obtaining sections of optic nerve, and Anne Goodwin for providing editorial assistance.
Footnotes
Supported by the Medical Research Council (G0901303), in part by the Medical Research Council Dorothy Hodgkin Postgraduate Award, the Helen Hamlyn Trust, Fight for Sight, the National Institute for Health Research Biomedical Research Centre Moorfields, and the University College London Institute of Ophthalmology.
Supplemental material for this article can be found at http://dx.doi.org/10.1016/j.ajpath.2012.12.032.
Supplemental Data
RGC primary cultures are 95% homogeneous. RGC cultures counterstained with DAPI (blue) are 95% positive for Thy-1 (red) and βIII-tubulin (green) at DIV 5 (N = 3). Original magnification, ×63. Scale bar = 10 μm.
VEGF-A and VEGF receptor mRNA levels in RGCs and endothelial cells (ECs). A: Levels of VEGFR-1 and VEGFR-2 were compared using real-time qPCR in RGC cultures. VEGFR-2 was found to be the most abundantly expressed receptor, with relative levels 17-fold higher than VEGFR-1. ***P < 0.001, N = 6. B: Comparison of VEGF-A, VEGFR-1, and VEGFR-2 revealed that levels of VEGFR-2 were fivefold higher, levels of VEGFR-1 were fourfold lower, and levels of VEGF-A were 60-fold higher in RGCs compared with ECs. Ratios of VEGFR-2/VEGFR-1 were approximately 17:1 in RGCs and 1:1 in ECs. N = 3 of a triplicate set. Data are expressed as means ± SD.
PI3K inhibitors, wortmannin and LY294,002, had no effect on cell death in vitro or in vivo when administered alone. A: LY294,002 added at 10 μmol/L final concentration had no effect on cell viability in RGC cultures. Numbers of surviving cells were identical to PBS- or DMSO-treated controls. N = 3. B: This was also observed for cells treated with 30 nmol/L wortmannin. N = 3. C: When 1 nmol wortmannin was injected intravitreally, there was no increase higher than PBS- or DMSO vehicle–treated levels in the apoptotic TUNEL-positive cells in the ganglion cell layer (N = 6). Data are expressed as means ± SEM.
VEGFR-1 and VEGFR-2 mRNA remained at control levels in bead-injected eyes. A: VEGFR-1 expression was not significantly altered after ocular hypertension (N = 4). B: This effect was also observed for VEGFR-2 (N = 4). Data are expressed as means ± SD.
References
- 1.Kim I., Ryan A.M., Rohan R., Amano S., Agular S., Miller J.W., Adamis A.P. Constitutive expression of VEGF, VEGFR-1, and VEGFR-2 in normal eyes. Invest Ophthalmol Vis Sci. 1999;40:2115–2121. [PubMed] [Google Scholar]
- 2.Soker S., Takashima S., Miao H.Q., Neufeld G., Klagsbrun M. Neuropilin-1 is expressed by endothelial and tumor cells as an isoform-specific receptor for vascular endothelial growth factor. Cell. 1998;92:735–745. doi: 10.1016/s0092-8674(00)81402-6. [DOI] [PubMed] [Google Scholar]
- 3.Jin K.L., Mao X.O., Greenberg D.A. Vascular endothelial growth factor: direct neuroprotective effect in in vitro ischemia. Proc Natl Acad Sci U S A. 2000;97:10242–10247. doi: 10.1073/pnas.97.18.10242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gomes E., Papa L., Hao T., Rockwell P. The VEGFR2 and PKA pathways converge at MEK/ERK1/2 to promote survival in serum deprived neuronal cells. Mol Cell Biochem. 2007;305:179–190. doi: 10.1007/s11010-007-9542-2. [DOI] [PubMed] [Google Scholar]
- 5.Tolosa L., Mir M., Asensio V.J., Olmos G., Llado J. Vascular endothelial growth factor protects spinal cord motoneurons against glutamate-induced excitotoxicity via phosphatidylinositol 3-kinase. J Neurochem. 2008;105:1080–1090. doi: 10.1111/j.1471-4159.2007.05206.x. [DOI] [PubMed] [Google Scholar]
- 6.Sondell M., Lundborg G., Kanje M. Vascular endothelial growth factor has neurotrophic activity and stimulates axonal outgrowth, enhancing cell survival and Schwann cell proliferation in the peripheral nervous system. J Neurosci. 1999;19:5731–5740. doi: 10.1523/JNEUROSCI.19-14-05731.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Erskine L., Reijntjes S., Pratt T., Denti L., Schwarz Q., Vieira J.M., Alakakone B., Shewan D., Ruhrberg C. VEGF signaling through neuropilin 1 guides commissural axon crossing at the optic chiasm. Neuron. 2011;70:951–965. doi: 10.1016/j.neuron.2011.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kilic U., Kilic E., Jarve A., Guo Z., Spudich A., Bieber K., Barzena U., Bassetti C.L., Marti H.H., Hermann D.M. Human vascular endothelial growth factor protects axotomized retinal ganglion cells in vivo by activating ERK-1/2 and Akt pathways. J Neurosci. 2006;26:12439–12446. doi: 10.1523/JNEUROSCI.0434-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nishijima K., Ng Y.S., Zhong L., Bradley J., Schubert W., Jo N., Akita J., Samuelsson S.J., Robinson G.S., Adamis A.P., Shima D.T. Vascular endothelial growth factor-A is a survival factor for retinal neurons and a critical neuroprotectant during the adaptive response to ischemic injury. Am J Pathol. 2007;171:53–67. doi: 10.2353/ajpath.2007.061237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Azzouz M., Ralph G.S., Storkebaum E., Walmsley L.E., Mitrophanous K.A., Kingsman S.M., Carmeliet P., Mazarakis N.D. VEGF delivery with retrogradely transported lentivector prolongs survival in a mouse ALS model. Nature. 2004;429:413–417. doi: 10.1038/nature02544. [DOI] [PubMed] [Google Scholar]
- 11.Sun Y., Jin K., Xie L., Childs J., Mao X.O., Logvinova A., Greenberg D.A. VEGF-induced neuroprotection, neurogenesis, and angiogenesis after focal cerebral ischemia. J Clin Invest. 2003;111:1843–1851. doi: 10.1172/JCI17977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cariboni A., Davidson K., Dozio E., Memi F., Schwarz Q., Stossi F., Parnavelas J.G., Ruhrberg C. VEGF signalling controls GnRH neuron survival via NRP1 independently of KDR and blood vessels. Development. 2011;138:3723–3733. doi: 10.1242/dev.063362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brockington A., Heath P.R., Holden H., Kasher P., Bender F.L., Claes F., Lambrechts D., Sendtner M., Carmeliet P., Shaw P.J. Downregulation of genes with a function in axon outgrowth and synapse formation in motor neurones of the VEGFdelta/delta mouse model of amyotrophic lateral sclerosis. BMC Genomics. 2010;11:203. doi: 10.1186/1471-2164-11-203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chong V. Biological, preclinical and clinical characteristics of inhibitors of vascular endothelial growth factors. Ophthalmologica. 2012;227(Suppl 1):2–10. doi: 10.1159/000337152. [DOI] [PubMed] [Google Scholar]
- 15.Ferrara N., Hillan K.J., Novotny W. Bevacizumab (Avastin), a humanized anti-VEGF monoclonal antibody for cancer therapy. Biochem Biophys Res Commun. 2005;333:328–335. doi: 10.1016/j.bbrc.2005.05.132. [DOI] [PubMed] [Google Scholar]
- 16.Horsley M.B., Kahook M.Y. Anti-VEGF therapy for glaucoma. Curr Opin Ophthalmol. 2010;21:112–117. doi: 10.1097/ICU.0b013e3283360aad. [DOI] [PubMed] [Google Scholar]
- 17.Sappington R.M., Chan M., Calkins D.J. Interleukin-6 protects retinal ganglion cells from pressure-induced death. Invest Ophthalmol Vis Sci. 2006;47:2932–2942. doi: 10.1167/iovs.05-1407. [DOI] [PubMed] [Google Scholar]
- 18.Wang X., Archibald M.L., Stevens K., Baldridge W.H., Chauhan B.C. Cyan fluorescent protein (CFP) expressing cells in the retina of Thy1-CFP transgenic mice before and after optic nerve injury. Neurosci Lett. 2010;468:110–114. doi: 10.1016/j.neulet.2009.10.077. [DOI] [PubMed] [Google Scholar]
- 19.Tezel G., Yang X. Caspase-independent component of retinal ganglion cell death, in vitro. Invest Ophthalmol Vis Sci. 2004;45:4049–4059. doi: 10.1167/iovs.04-0490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Samsel P.A., Kisiswa L., Erichsen J.T., Cross S.D., Morgan J.E. A novel method for the induction of experimental glaucoma using magnetic microspheres. Invest Ophthalmol Vis Sci. 2011;52:1671–1675. doi: 10.1167/iovs.09-3921. [DOI] [PubMed] [Google Scholar]
- 21.Almasieh M., Wilson A.M., Morquette B., Cueva Vargas J.L., Di Polo A. The molecular basis of retinal ganglion cell death in glaucoma. Prog Retin Eye Res. 2012;31:152–181. doi: 10.1016/j.preteyeres.2011.11.002. [DOI] [PubMed] [Google Scholar]
- 22.Matsuzaki H., Tamatani M., Yamaguchi A., Namikawa K., Kiyama H., Vitek M.P., Mitsuda N., Tohyama M. Vascular endothelial growth factor rescues hippocampal neurons from glutamate-induced toxicity: signal transduction cascades. FASEB J. 2001;15:1218–1220. [PubMed] [Google Scholar]
- 23.Guo L., Salt T.E., Maass A., Luong V., Moss S.E., Fitzke F.W., Cordeiro M.F. Assessment of neuroprotective effects of glutamate modulation on glaucoma-related retinal ganglion cell apoptosis in vivo. Invest Ophthalmol Vis Sci. 2006;47:626–633. doi: 10.1167/iovs.05-0754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kortuem K., Geiger L.K., Levin L.A. Differential susceptibility of retinal ganglion cells to reactive oxygen species. Invest Ophthalmol Vis Sci. 2000;41:3176–3182. [PubMed] [Google Scholar]
- 25.Wang X., Zaidi A., Pal R., Garrett A.S., Braceras R., Chen X.W., Michaelis M.L., Michaelis E.K. Genomic and biochemical approaches in the discovery of mechanisms for selective neuronal vulnerability to oxidative stress. BMC Neurosci. 2009;10:12. doi: 10.1186/1471-2202-10-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fuchs C., Forster V., Balse E., Sahel J.A., Picaud S., Tessier L.H. Retinal-cell-conditioned medium prevents TNF-alpha-induced apoptosis of purified ganglion cells. Invest Ophthalmol Vis Sci. 2005;46:2983–2991. doi: 10.1167/iovs.04-1177. [DOI] [PubMed] [Google Scholar]
- 27.Kitaoka Y., Munemasa Y., Nakazawa T., Ueno S. NMDA-induced interleukin-1beta expression is mediated by nuclear factor-kappa B p65 in the retina. Brain Res. 2007;1142:247–255. doi: 10.1016/j.brainres.2007.01.097. [DOI] [PubMed] [Google Scholar]
- 28.Kim H.S., Park C.K. Retinal ganglion cell death is delayed by activation of retinal intrinsic cell survival program. Brain Res. 2005;1057:17–28. doi: 10.1016/j.brainres.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 29.Ullian E.M., Barkis W.B., Chen S., Diamond J.S., Barres B.A. Invulnerability of retinal ganglion cells to NMDA excitotoxicity. Mol Cell Neurosci. 2004;26:544–557. doi: 10.1016/j.mcn.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 30.Caballero-Benitez A., Moran J. Caspase activation pathways induced by staurosporine and low potassium: role of caspase-2. J Neurosci Res. 2003;71:383–396. doi: 10.1002/jnr.10493. [DOI] [PubMed] [Google Scholar]
- 31.Mackenzie F., Ruhrberg C. Diverse roles for VEGF-A in the nervous system. Development. 2012;139:1371–1380. doi: 10.1242/dev.072348. [DOI] [PubMed] [Google Scholar]
- 32.Wise L.M., Ueda N., Dryden N.H., Fleming S.B., Caesar C., Roufail S., Achen M.G., Stacker S.A., Mercer A.A. Viral vascular endothelial growth factors vary extensively in amino acid sequence, receptor-binding specificities, and the ability to induce vascular permeability yet are uniformly active mitogens. J Biol Chem. 2003;278:38004–38014. doi: 10.1074/jbc.M301194200. [DOI] [PubMed] [Google Scholar]
- 33.Chauvier D., Ankri S., Charriaut-Marlangue C., Casimir R., Jacotot E. Broad-spectrum caspase inhibitors: from myth to reality? Cell Death Differ. 2007;14:387–391. doi: 10.1038/sj.cdd.4402044. [DOI] [PubMed] [Google Scholar]
- 34.Sommer A., Tielsch J.M., Katz J., Quigley H.A., Gottsch J.D., Javitt J., Singh K. Relationship between intraocular pressure and primary open angle glaucoma among white and black Americans: the Baltimore Eye Survey. Arch Ophthalmol. 1991;109:1090–1095. doi: 10.1001/archopht.1991.01080080050026. [DOI] [PubMed] [Google Scholar]
- 35.Wax M.B., Tezel G., Edward P.D. Clinical and ocular histopathological findings in a patient with normal-pressure glaucoma. Arch Ophthalmol. 1998;116:993–1001. doi: 10.1001/archopht.116.8.993. [DOI] [PubMed] [Google Scholar]
- 36.Kerrigan L.A., Zack D.J., Quigley H.A., Smith S.D., Pease M.E. TUNEL-positive ganglion cells in human primary open-angle glaucoma. Arch Ophthalmol. 1997;115:1031–1035. doi: 10.1001/archopht.1997.01100160201010. [DOI] [PubMed] [Google Scholar]
- 37.Garcia-Valenzuela E., Shareef S., Walsh J., Sharma S.C. Programmed cell death of retinal ganglion cells during experimental glaucoma. Exp Eye Res. 1995;61:33–44. doi: 10.1016/s0014-4835(95)80056-5. [DOI] [PubMed] [Google Scholar]
- 38.Libby R.T., Li Y., Savinova O.V., Barter J., Smith R.S., Nickells R.W., John S.W. Susceptibility to neurodegeneration in a glaucoma is modified by Bax gene dosage. PLoS Genet. 2005;1:17–26. doi: 10.1371/journal.pgen.0010004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hanninen V.A., Pantcheva M.B., Freeman E.E., Poulin N.R., Grosskreutz C.L. Activation of caspase 9 in a rat model of experimental glaucoma. Curr Eye Res. 2002;25:389–395. doi: 10.1076/ceyr.25.6.389.14233. [DOI] [PubMed] [Google Scholar]
- 40.Jakobs T.C., Libby R.T., Ben Y., John S.W., Masland R.H. Retinal ganglion cell degeneration is topological but not cell type specific in DBA/2J mice. J Cell Biol. 2005;171:313–325. doi: 10.1083/jcb.200506099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gerber H.P., McMurtrey A., Kowalski J., Yan M., Keyt B.A., Dixit V., Ferrara N. Vascular endothelial growth factor regulates endothelial cell survival through the phosphatidylinositol 3′-kinase/Akt signal transduction pathway: requirement for Flk-1/KDR activation. J Biol Chem. 1998;273:30336–30343. doi: 10.1074/jbc.273.46.30336. [DOI] [PubMed] [Google Scholar]
- 42.Saint-Geniez M., Maharaj A.S., Walshe T.E., Tucker B.A., Sekiyama E., Kurihara T., Darland D.C., Young M.J., D’Amore P.A. Endogenous VEGF is required for visual function: evidence for a survival role on Müller cells and photoreceptors. PLoS One. 2008;3:e3554. doi: 10.1371/journal.pone.0003554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.D’Amore P.A. Vascular endothelial cell growth factor-A: not just for endothelial cells anymore. Am J Pathol. 2007;171:14–18. doi: 10.2353/ajpath.2007.070385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jin K., Mao X.O., Batteur S.P., McEachron E., Leahy A., Greenberg D.A. Caspase-3 and the regulation of hypoxic neuronal death by vascular endothelial growth factor. Neuroscience. 2001;108:351–358. doi: 10.1016/s0306-4522(01)00154-3. [DOI] [PubMed] [Google Scholar]
- 45.Cui W., Li W., Zhao Y., Mak S., Gao Y., Luo J., Zhang H., Liu Y., Carlier P.R., Rong J., Han Y. Preventing HO-induced apoptosis in cerebellar granule neurons by regulating the VEGFR-2/Akt signaling pathway using a novel dimeric antiacetylcholinesterase bis(12)-hupyridone. Brain Res. 2011;1394:14–23. doi: 10.1016/j.brainres.2011.02.006. [DOI] [PubMed] [Google Scholar]
- 46.Carmeliet P., Tessier-Lavigne M. Common mechanisms of nerve and blood vessel wiring. Nature. 2005;436:193–200. doi: 10.1038/nature03875. [DOI] [PubMed] [Google Scholar]
- 47.Ma Y., Liu W., Wang Y., Chao X., Qu Y., Wang K., Fei Z. VEGF protects rat cortical neurons from mechanical trauma injury induced apoptosis via the MEK/ERK pathway. Brain Res Bull. 2011;86:441–446. doi: 10.1016/j.brainresbull.2011.07.007. [DOI] [PubMed] [Google Scholar]
- 48.Ito K., Caramori G., Adcock I.M. Therapeutic potential of phosphatidylinositol 3-kinase inhibitors in inflammatory respiratory disease. J Pharmacol Exp Ther. 2007;321:1–8. doi: 10.1124/jpet.106.111674. [DOI] [PubMed] [Google Scholar]
- 49.Wittström E., Holmberg H., Hvarfner C., Andréasson S. Clinical and electrophysiologic outcome in patients with neovascular glaucoma treated with and without bevacizumab. Eur J Ophthalmol. 2012;22:563–574. doi: 10.5301/ejo.5000089. [DOI] [PubMed] [Google Scholar]
- 50.Martinez-de-la-Casa J.M., Ruiz-Calvo A., Saenz-Frances F., Reche-Frutos J., Calvo-Gonzalez C., Donate-Lopez J., Garcia-Feijoo J. Retinal nerve fiber layer thickness changes in patients with age-related macular degeneration treated with intravitreal ranibizumab. Invest Ophthalmol Vis Sci. 2012;53:6214–6218. doi: 10.1167/iovs.12-9875. [DOI] [PubMed] [Google Scholar]
- 51.Kerrigan-Baumrind L.A., Quigley H.A., Pease M.E., Kerrigan D.F., Mitchell R.S. Number of ganglion cells in glaucoma eyes compared with threshold visual field tests in the same persons. Invest Ophthalmol Vis Sci. 2000;41:741–748. [PubMed] [Google Scholar]
- 52.Adler R., Curcio C., Hicks D., Price D., Wong F. Cell death in age-related macular degeneration. Mol Vis. 1999;5:31. [PubMed] [Google Scholar]
- 53.Jackson G.R., Barber A.J. Visual dysfunction associated with diabetic retinopathy. Curr Diab Rep. 2010;10:380–384. doi: 10.1007/s11892-010-0132-4. [DOI] [PubMed] [Google Scholar]
- 54.Wright A.F., Jacobson S.G., Cideciyan A.V., Roman A.J., Shu X., Vlachantoni D., McInnes R.R., Riemersma R.A. Lifespan and mitochondrial control of neurodegeneration. Nat Genet. 2004;36:1153–1158. doi: 10.1038/ng1448. [DOI] [PubMed] [Google Scholar]
- 55.Bhisitkul R.B., Rofagha S., Boyer D.S., Sadda S., Zhang K. Year 7 outcomes for ranibizumab-treated subjects in anchor/marina: a multicenter, prospective cohort study. ARVO Meeting Abstracts. 2012;53:3679. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
RGC primary cultures are 95% homogeneous. RGC cultures counterstained with DAPI (blue) are 95% positive for Thy-1 (red) and βIII-tubulin (green) at DIV 5 (N = 3). Original magnification, ×63. Scale bar = 10 μm.
VEGF-A and VEGF receptor mRNA levels in RGCs and endothelial cells (ECs). A: Levels of VEGFR-1 and VEGFR-2 were compared using real-time qPCR in RGC cultures. VEGFR-2 was found to be the most abundantly expressed receptor, with relative levels 17-fold higher than VEGFR-1. ***P < 0.001, N = 6. B: Comparison of VEGF-A, VEGFR-1, and VEGFR-2 revealed that levels of VEGFR-2 were fivefold higher, levels of VEGFR-1 were fourfold lower, and levels of VEGF-A were 60-fold higher in RGCs compared with ECs. Ratios of VEGFR-2/VEGFR-1 were approximately 17:1 in RGCs and 1:1 in ECs. N = 3 of a triplicate set. Data are expressed as means ± SD.
PI3K inhibitors, wortmannin and LY294,002, had no effect on cell death in vitro or in vivo when administered alone. A: LY294,002 added at 10 μmol/L final concentration had no effect on cell viability in RGC cultures. Numbers of surviving cells were identical to PBS- or DMSO-treated controls. N = 3. B: This was also observed for cells treated with 30 nmol/L wortmannin. N = 3. C: When 1 nmol wortmannin was injected intravitreally, there was no increase higher than PBS- or DMSO vehicle–treated levels in the apoptotic TUNEL-positive cells in the ganglion cell layer (N = 6). Data are expressed as means ± SEM.
VEGFR-1 and VEGFR-2 mRNA remained at control levels in bead-injected eyes. A: VEGFR-1 expression was not significantly altered after ocular hypertension (N = 4). B: This effect was also observed for VEGFR-2 (N = 4). Data are expressed as means ± SD.