Summary
Background
New tuberculosis (TB) vaccines are being developed to combat the global epidemic. A phase IIb trial of a candidate vaccine, MVA85A, was conducted in a high burden setting in South Africa to evaluate proof-of-concept efficacy for prevention of TB in infants.
Objective
To describe the study design and implementation lessons from an infant TB vaccine efficacy trial.
Methods
This was a randomised, controlled, double-blind clinical trial comparing the safety and efficacy of MVA85A to Candin control administered to 4–6-month-old, BCG-vaccinated, HIV-negative infants at a rural site in South Africa. Infants were followed up for 15–39 months for incident TB disease based on pre-specified endpoints.
Results
2797 infants were enrolled over 22 months. Factors adversely affecting recruitment and the solutions that were implemented are discussed. Slow case accrual led to six months extension of trial follow up.
Conclusion
The clinical, regulatory and research environment for modern efficacy trials of new TB vaccines are substantially different to that when BCG vaccine was first evaluated in infants. Future infant TB vaccine trials will need to allocate sufficient resources and optimise operational efficiency. A stringent TB case definition is necessary to maximize specificity, and TB case accrual must be monitored closely.
Keywords: BCG, Vaccine, Tuberculosis, Lessons learnt, Implementation
1. Background
Nine million new cases of tuberculosis (TB) occur worldwide each year and it is estimated that 1 million (11%) are in children under 15 years.1 Universal infant vaccination with Mycobacterium bovis bacille Calmette-Guérin (BCG), the only licensed TB vaccine, is routine in TB endemic settings.2 BCG vaccination coverage is greater than 95% in South Africa3 where TB incidence is 981/100,000.4 BCG vaccination confers consistent, but partial protection against disseminated forms of TB2,5 but protection against pulmonary disease in children and adults is highly variable.6 A more effective TB vaccine is needed urgently.
Over ten candidate TB vaccines designed either to boost BCG or replace it are at different stages of clinical testing.7 One of these novel vaccines, MVA85A, is a recombinant strain of Modified Vaccinia virus Ankara expressing the Mycobacterium tuberculosis (Mtb) antigen 85A (Ag85A)8 and is designed to boost the immune response to BCG. The safety and immunogenicity of MVA85A have been extensively tested in clinical trials in adults, adolescents, children, and infants.9–19
The TB vaccine trial landscape has changed markedly in the half century since the infant BCG efficacy trials of Rosenthal and Aronson.20,21 Modern clinical trials are conducted in a highly regulated research environment, in which the safety of participants is paramount, guided by the principles of International Conference on Harmonisation Good Clinical Practice (ICH-GCP). The introduction of effective anti-tuberculous chemotherapy for TB disease and isoniazid preventive therapy (IPT) for TB-exposed children, coupled with the need for active surveillance for early detection and treatment, has dramatically shifted the TB phenotype observed in modern vaccine trials towards an early, mild presentation.22–24 These considerations impact negatively on the diagnosis of childhood TB as the efficacy endpoint of such trials, even in high TB burden countries, which have traditionally reported cases of advanced childhood TB disease.23,25 Further, vaccine-induced protection by any new TB vaccine must be demonstrated in addition to that conferred by BCG. It follows that case-finding strategies and definition of TB disease endpoints will be crucial to the successful demonstration of efficacy of any new infant TB vaccine.
A phase IIb proof-of-concept safety and efficacy trial of an infant BCG – prime and MVA85A – boost regimen began enrolment in July 2009, at the field site of the South African Tuberculosis Vaccine Initiative (SATVI) near Cape Town. This is the first infant efficacy trial of a new TB vaccine to be conducted since BCG. We report on the operational and scientific challenges experienced by the study team during the conduct of this trial.
2. Methods
2.1. Study design
This was a parallel randomized, controlled, double-blind clinical trial with a 1:1 ratio of intervention to control.
2.2. Study population
The SATVI field site is set in a region where the TB incidence across all ages is 1400/100,000 and the incidence in children younger than two years of age is estimated at 1500/100,000.26–28 The study area has a total population of 350,000 with an annual birth cohort of 7000. Healthy BCG-vaccinated infants aged 4–6 months, without evidence of individual or maternal HIV infection, without evidence of TB exposure or infection, and whose routine immunizations were up to date, were enrolled.
2.3. Recruitment, consenting and screening
Mothers of infants of 6 weeks or older were approached for possible participation in the trial. Once informed consent had been signed by the legal guardian, the infants were randomised sequentially into one of five cohorts starting with an initial safety cohort of at least 330 participants, followed by three immunogenicity cohorts of up to 60 participants each and the final, largest correlates of protection cohort of 2400.
2.4. Inclusion/Exclusion criteria at commencement of trial
Infants were required to be between 126 and 154 days old at randomisation, to have received BCG vaccination within 7 days of birth, to have received routine doses of EPI vaccines, including pneumococcal vaccine more than 28 days prior to randomisation, and have a weight >10th percentile at randomisation. Infants were excluded if they had an acute illness or fever ≥37.5 °C on Day 0, evidence of infant or maternal HIV infection, evidence of chronic hepatitis, a positive QuantiFERON TB Gold (in-tube) test (QFT) (Cellestis, Victoria, Australia) or a history of known TB disease, treatment of TB, or household TB contact.
2.5. Randomization and blinding
Infants confirmed eligible were randomized to receive 0.06 mL of either MVA85A vaccine candidate (1 × 108 plaque forming units [pfu]) or Candida skin test antigen control, Candin®, by intradermal injection into the left deltoid region using a masked labeled syringe. Candin was selected for its intradermal administration with similar local reactogenicity to MVA85A, thus ensuring unbiased safety and efficacy assessments. The study pharmacist was the only member of the field team unblinded to allocation of the investigational product. The infants' caregivers and study staff involved in all other aspects of the trial remained blinded to treatment allocation throughout the trial.
2.6. Outcomes
The primary objective of this study was to evaluate the safety profile of MVA85A in BCG-vaccinated, HIV-negative infants. The secondary objectives were: 1) to evaluate the efficacy of the MVA85A vaccine compared to placebo control in the prevention of TB disease using three clinical endpoints, 2) the evaluation of the immunogenicity of MVA85A compared to controls and 3) the evaluation of the rate of Mtb infection, as defined by QFT conversion at final study assessment in MVA85A recipients compared to controls in infants without a diagnosis of TB disease during the trial.
2.7. Follow up
All enrolled infants were followed for efficacy. Infants were to be followed up for between 9 months and 33 months (mean 18 months) by visits to the trial clinic and home visits. Legal guardians were interviewed about the health status of the participant with a focus on possible TB symptoms, and phlebotomy was done per protocol for safety and immunogenicity assessments. Blood was drawn for QFT at Day 336 and at the end of study visit.
2.8. Safety endpoints
Adverse events, both local and systemic, were recorded for the first twenty-eight days post-vaccination, and classified for severity and relationship to vaccine. Serious adverse events (SAEs) were collected for the duration of study participation through active surveillance and self-reporting at routine visits. SAEs were reported within specified time frames to the sponsor, regulatory authorities and the ethics committee. An unblinded safety review by the Safety and Monitoring Committee occurred after completion of Day 28 for the initial safety cohort and again after Day 84 for the 1000th participant.
2.9. Case accrual and efficacy endpoints
Follow-up visits and active surveillance of hospital records, clinic TB registers, radiology records and death certificates were used to identify suspected cases of TB. A household TB contact would also trigger a case-finding process.28 Any infant suspected of TB, or with a history of TB contact, or known tuberculin skin test (TST) or QFT conversion, was admitted to a case verification ward (CV ward) for standardized investigations.26,28 Children who were diagnosed as having TB and started on treatment by the health services were where possible admitted to the CV ward as soon as possible after diagnosis for these investigations. A trial-independent, hospital-based TB medical officer reviewed each case at discharge and prescribed appropriate care and treatment. Digital chest X-ray images were reviewed by an independent, blinded panel of three expert paediatric radiologists. A majority agreement on the presence of pre-specified radiological pathology was required for a chest X-ray to be classified as positive for inclusion in the primary efficacy endpoint. The endpoint definitions used to define a TB case are set out in Table 1.
Table 1.
Endpoint definitions.
| TB case definition Endpoint #1 |
|---|
Any of the following numerical categories
|
| TB case definition Endpoint #2 |
|---|
Any of the following numerical categories
|
| TB case definition Endpoint #3 |
|---|
| All individuals who are placed on anti-tuberculosis therapy with the intent of treating tuberculosis regardless of whether they have met the other efficacy endpoints |
Centers for Disease Control (CDC) Growth Charts (USA), developed by the National Center for Health Statistics in collaboration with the National Center for Chronic Disease Prevention and Health Promotion (2000).
2.10. Sample size determination
Given estimated tuberculosis cumulative incidence of 3% over 18 months in the control group, 1392 subjects per study group would provide a 90% chance of detecting a 60% reduction between the treated and control groups based on a two-sided log rank test at a significance level of 0.05. The enrolment target for the study was thus 2784 infants. A sample size of 1392 MVA85A vaccinated subjects would give a greater than 75% chance of observing an adverse event that has an approximately 1 in a 1000 actual rate of occurrence.
2.11. Study approvals
The trial was approved by the University of Cape Town (UCT) Faculty of Health Sciences Human Research Ethics Committee (REC), Oxford University Tropical Research Ethics Committee and the South African Medicines Control Council (MCC). Permission was obtained from the Western Cape Department of Health to access their health facilities.
2.12. Clinical trials registration
The trial was registered on South African National Clinical Trials Register in November 2008 (DOH-27-0109-2654) and on clinicaltrials.gov in July 2009 (NCT00953927).
3. Results
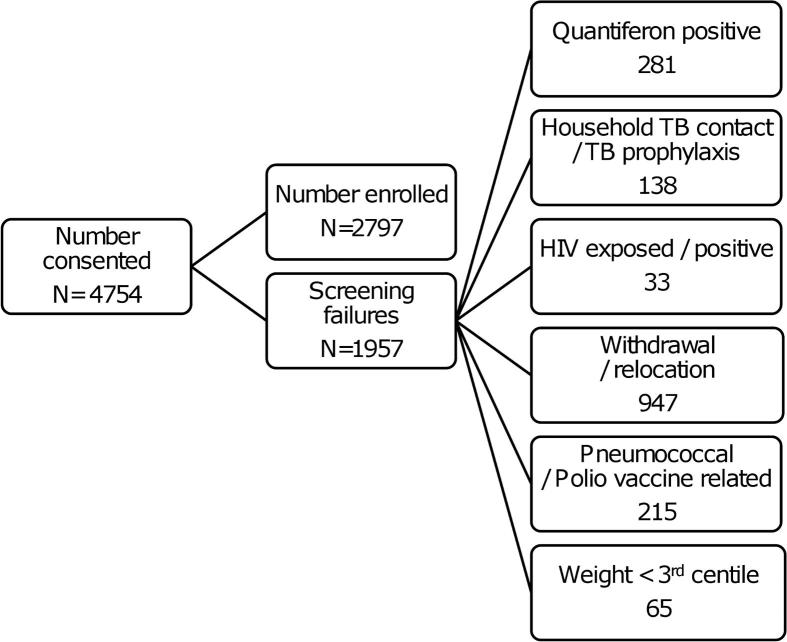
Enrolment commenced in July 2009 and was completed in May 2011. 4754 parents/legal guardians signed consent and 2797 infants were successfully screened and enrolled. The main reasons for exclusion are set out in Figure 1.Slightly more infants were enrolled than targeted because all infants that had already entered screening were allowed to be enrolled although the enrolment target of 2784 had been reached. Closeout visits were completed in September 2012, but evaluation of TB suspects detected through closeout visits continued until October 2012.
Figure 1.
Main reasons for screening failure.
Challenges arising from study design elements, operational lessons from implementation of the protocol, and alternative approaches and solutions are summarised in Table 2.
Table 2.
Study design and operational factors, implementation lessons learned, and alternative approaches and solutions.
| Category | Design/Operational factor | Alternative/Solution |
|---|---|---|
| Recruitment | Site of recruitment | Ensure conducive environment for informed consent |
| Sub-optimal field staff allocation | Ensure adequate staff allocation | |
| Informed consent | Train lay field workers in informed consent | |
| Screening procedures and application of inclusion/exclusion criteria | Community attitudes to phlebotomy | Ensure correct paediatric phlebotomy technique and counsel parents about procedure. |
| Specimen haemolysis | Centrifugation of specimens prior to transport | |
| Definition of TB exposure | Clear detailed definition – investigator assessment required | |
| Thrombocytosis – inappropriate laboratory reference ranges | Inclusion based on investigator clinical assessment | |
| Impact of routine immunisations | Widen vaccination window, be prepared for stockouts and mass immunisations campaigns | |
| Case accrual | TB case accrual | Monitor case accrual and extend follow up if needed. |
3.1. Recruitment
3.1.1. Site of recruitment
Previous experience at our site had taught us to expect an initial slow pace for screening and enrolment before gaining momentum, exhibiting an S-shaped enrolment rate curve.29 However, the initial pace was even slower than expected forcing a review of our recruitment methods. The initial recruitment plan was to approach mothers of infants attending public health clinics for the routine 6-week vaccination visit. This strategy failed due to limited space for study staff in the clinics and a reluctance of mothers to lengthen their clinic stay by engaging with our staff. A protocol amendment allowed us access to home addresses in the birth registers and vaccination records at local public health facilities which enabled our staff to visit parents at home resulting in substantially more effective recruitment.
3.1.2. Staffing
Initial planning underestimated the number of staff needed to cope with the large numbers to be recruited and vast distances to be travelled. The study area is approximately 10,000 km2 and included the inhabitants of fifteen towns and hundreds of surrounding farms. We increased staff as follows: four additional field workers for recruitment (initially n = 28) and ten more research nurses (initially n = 6) for screening, vaccination, follow-up activities and safety assessments. In addition, we assigned staff to purely administrative duties and re-organised the study staff into teams with team leaders. This had a positive impact on recruitment.
3.1.3. Informed consent
As professional nurses are a scarce resource needed for clinical tasks, we invited GCP-trained lay field workers to undergo training in the informed consent process. Competence was evaluated through role-play and successful candidates were accredited to conduct informed consent. This increased the pool of staff available to conduct informed consent which led to an increase in enrolment rates.
3.2. Screening procedures and application of inclusion/exclusion criteria
3.2.1. Phlebotomy technique
Approximately 3% of parents/caregivers who consented to participate in the trial withdrew during the screening phase, for a variety of reasons. About 20% of those who gave a reason said they were distressed by the phlebotomy process. Study nurses received additional training in infant phlebotomy from a paediatrician, who emphasised the use of appropriate anatomical sites (cubital fossa, hand or foot before external jugular vein), swaddling and the use of sucrose solution as an analgesic.30,31 A limit of maximum three phlebotomy attempts was set and the need to explain the procedure to the mother or caregiver was emphasised.
3.2.2. Haemolysis of blood specimens
Haemolysed safety blood samples could not be processed by the laboratory necessitating repeated phlebotomy. This was not always well received by participant's parents, placed an increased burden on the screening clinic numbers and had budgetary implications. We introduced centrifugation of all blood samples for biochemistry testing at 3750 rpm for 10 min at the trial site laboratory before transport and delivery of samples to the diagnostic laboratory 110 km away and this substantially reduced the rate of haemolysed samples.
3.2.3. TB exposure
We found that staff in first contact with potential participants was over-interpreting the exclusion criterion related to household TB contact. A clear definition of the term “household TB contact” was developed by the study medical team which defined a household contact as follows: one who has been diagnosed with TB after the infant's birth, and spent more than 6 h daily in the same living space. If the household member has been diagnosed with pulmonary TB before the infant's birth, they would qualify as a household contact if he/she had not yet completed 2 months of TB treatment or was a treatment defaulter or had drug resistant TB. The decision to exclude an infant based on “household TB contact” was made only by an investigator.
3.2.4. Thrombocytosis
Screening haematology tests revealed a high prevalence of thrombocytosis in otherwise healthy infants. More than 90% of infants screened had platelet counts above the local laboratory defined upper limit of normal (350 × 109/L) with 51% of screening values greater than 500 × 109/L. These reference ranges are derived from Western sources and not local data. Based on a haematologist's advice to interpret the results in a clinical context, we enrolled infants with thrombocytosis providing other haematological parameters were within the normal range and the infant was clinically well.
3.2.5. Impact of routine immunisations
We found that the 14-week routine immunisation doses were often delayed, resulting in study exclusions when the 28 day window between the receipt of routine immunisations and administration of the study vaccine fell after the 154 day age cut-off for study vaccination. A protocol amendment was approved to reduce the window between routine vaccines and the investigational vaccine to 14 days and to increase the age of eligibility for vaccination to 26 weeks. This reduced exclusion of infants due to being out of the vaccination window.
A temporary global shortage of BCG threatened enrolment, as eligibility for participation required receipt of BCG within seven days of birth. The local Department of Health arranged for areas with excess stock to share this with the birthing units in our study drainage area and arranged for the first new batch of BCG stock to be distributed in our study area to minimise the impact of this shortage.
A national polio eradication campaign, targeting all children from birth to 5 years regardless of immunisation status also threatened to disrupt enrolment. The campaign involved the administration of two doses of trivalent oral polio, four weeks apart. An arrangement was reached with the local health authorities whereby administration of polio vaccine was delayed until after study vaccine administration.
3.3. Case accrual
3.3.1. Efficacy follow up
Blinded case accrual was monitored throughout study follow up. About nine months prior to study closeout, it was determined that insufficient TB cases would be accrued by study end to ensure statistical significance for the efficacy objective. Twenty-one cases meeting the endpoint 1 definition had been accrued at that point, when 33–35 had been expected. An amendment to lengthen follow up by six months was submitted and approved to address this shortfall.
4. Discussion
Designing and operationalising this first efficacy trial of a TB vaccine candidate in infants was complicated by the lack of a single test to diagnose childhood TB. This necessitated a clear clinical endpoint definition, a lengthy follow-up period and a large sample size. In modern times, regulatory authorities and ethics committees, and investigators and sponsors are much more sensitised to participant rights particularly in trials conducted in vulnerable populations. We used the experience gained from the conduct of a large BCG trial26 and a neonatal cohort study of TB in28 infants to design and plan for this clinical trial. In practice, there was an evolutionary process with respect to study design and operational activities as the trial progressed.
4.1. Study design
A standard vaccine efficacy trial design has been described. An area warranting further discussion is the clinical endpoint selection. The diagnosis of TB in children is challenging as the disease is pauci-bacillary. Numerous scoring systems have been developed but no validated scoring system exists.32 Because there is no correlate of protection, TB vaccine efficacy trials depend on clinical disease endpoints.33 In a trial setting, it is imperative that endpoints are as specific as possible to ensure that efficacy measures are a true reflection of vaccine benefit. An attempt to determine an internationally agreed endpoint for TB vaccine efficacy trials in infants reached only a partial consensus.24 Using our experience from previous trials23,26 we developed a hierarchical, three tiered endpoint which we felt would be most suited to an infant TB vaccine efficacy trial (Table 1). While Graham et al.34 have published consensus endpoints for TB diagnostics studies, it was agreed that such endpoints may not be suitable for infant TB vaccine trials due to active case finding and a younger study population. Active case finding truncates further disease progression because of early isoniazid preventive therapy (IPT) and TB treatment; children younger than 2 years are at risk for rapid progression to military/meningitic TB in the absence of a classical respiratory symptomatic phase seen in older children. Our endpoints contain elements which are pathognomonic of TB – bacteriological confirmation by culture and/or nucleic acid amplification test (GeneXpert®, Cepheid) and clinical syndromes for TB meningitis, opthalmic TB and typical histological findings. However, these forms of TB are less common. A triad of features more commonly used to diagnose TB in children – evidence of latent TB infection, a chest X-ray compatible with TB and symptoms of TB – were added to the endpoint definition. All three elements were required to confirm a diagnosis of TB to ensure that the endpoint would be as specific as possible. Endpoint 2 allows a slightly less rigorous definition of TB disease than Endpoint 1, based upon the triad of TB exposure/infection, radiographic features and symptoms. Although the symptom and radiological criteria are identical, the Endpoint 2 criteria for TB exposure/infection are widened to include children with a lower TST threshold (10 mm) and children living in a household with a known smear positive sputum TB patient.
4.2. Operational activities
The study was more staff intensive than initially anticipated. Staff salaries comprise a large proportion of the costs of efficacy trials, but it would be false economy to reduce the staffing complement but then have to lengthen the trial to meet targets. On the other hand, we have shown that flexibility with regards to staff roles and responsibilities, changing the recruitment window, centrifugation of blood specimens prior to transport and good counselling with phlebotomy procedures are improvements that add minimally to trial costs but benefit recruitment progress.
The principal investigator is held accountable for informed consent. However, according to International Conference on Harmonisation (ICH) and South African GCP guidelines,35,36 he/she may delegate this to a suitably qualified person. Certain sponsors nevertheless require a medically qualified investigator to conduct consent. In developing countries, this is not always feasible, particularly when enrolling large numbers of participants. We have shown that non-medical staff when well trained with competency checks and frequent monitoring, were able to conduct informed consent which met international and local standards.
“Normal” ranges used by diagnostic laboratories around the world are often based on Western populations, as was the case with normal platelet ranges in our study. Other African research sites have experienced this problem as well.37–41 In clinical trials, this results in 1) the possible exclusion of healthy participants and 2) abnormal laboratory results being classified as adverse events in enrolled participants with subsequent repeat blood draws until ‘resolution’. Clinical trial data or specifically designed surveys of local healthy populations should be used to define normal ranges that are appropriate and relevant to the study setting and population for use in both patient care and clinical trials.
Stockouts of EPI vaccines do occur commonly in developing country settings and contingency plans need to be in place should trials require their prior administration as an inclusion criterion. National vaccination campaigns will affect trials where administration of other vaccines is an exclusion criterion. Unless solutions are found to these factors, a costly pause in enrolment may occur. Excellent communication channels between SATVI and the local health services at all levels served to minimise the impact of these potentially detrimental episodes during our enrolment phase.
5. Conclusion
We describe the first proof-of-concept trial of a new TB vaccine in infants since BCG. The rationale for specific efficacy endpoints is provided. We have described the challenges encountered by a site experienced in clinical trials with investigational medicinal products and the need for constant monitoring, evaluation and adaptation. The solutions we implemented will be of value in the planning of efficacy trials of other TB vaccine candidates.
Acknowledgments
We gratefully acknowledge all study staff and participants for the success of the trial. We thank the Department of Health for their support.
Contributor Information
Michele Tameris, Email: michele.tameris@uct.ac.za.
Helen McShane, Email: helen.mcshane@ndm.ox.ac.uk.
J. Bruce McClain, Email: t10b9@aol.com.
Stephen Lockhart, Email: lockharts@ebsi.com.
Angelique K.K. Luabeya, Email: angelique.luabeya@uct.ac.za.
Hennie Geldenhuys, Email: hennie.geldenhuys@uct.ac.za.
Jacqui Shea, Email: sheaj@ebsi.com.
Gregory Hussey, Email: gregory.hussey@uct.ac.za.
Linda van der Merwe, Email: linda.vandermerwe@uct.ac.za.
Marwou de Kock, Email: marwou.dekock@uct.ac.za.
Thomas Scriba, Email: thomas.scriba@uct.ac.za.
Robert Walker, Email: rwalker@aeras.org.
Willem Hanekom, Email: willem.hanekom@uct.ac.za.
Mark Hatherill, Email: mark.hatherill@uct.ac.za.
Hassan Mahomed, Email: hasssanmahomed1@gmail.com.
Funding
The trial was funded by Aeras, Wellcome Trust and Oxford-Emergent Tuberculosis Consortium.
Competing interests
SL and JS are employees of Emergent BioSolutions Inc. and own shares and stock options in the company. HMcS is a shareholder in the Oxford-Emergent Tuberculosis Consortium Limited (OETC). OETC is a Joint Venture between Emergent BioSolutions Inc. and the University of Oxford.
MT, JBM, BL, AKKL, HG, GH, LvdM, MdK, TS, RW, WAH, MH and HM declare that they have no conflicts of interest.
Ethical approval
The trial was approved by the University of Cape Town Faculty of Health Sciences Human Research Ethics Committee, Oxford University Tropical Research Ethics Committee and the Medicines Control Council of South Africa.
References
- 1.World Health Organisation . 2006. Guidance for national tuberculosis programmes on the management of tuberculosis in children. [PubMed] [Google Scholar]
- 2.Trunz B.B., Fine P., Dye C. Effect of BCG vaccination on childhood tuberculous meningitis and miliary tuberculosis worldwide: a meta-analysis and assessment of cost-effectiveness. Lancet. 2006;367:1173–1180. doi: 10.1016/S0140-6736(06)68507-3. [DOI] [PubMed] [Google Scholar]
- 3.Department of Health . Department of Health and Medical Research Council; Pretoria, South Africa: 1999. South Africa demographic and health survey 1998. [Google Scholar]
- 4.World Health Organisation . 2011. Global tuberculosis control. [Google Scholar]
- 5.Rodrigues L.C., Diwan V.K., Wheeler J.G. Protective effect of BCG against tuberculous meningitis and miliary tuberculosis: a meta-analysis. Int J Epidemiol. 1993;22:1154–1158. doi: 10.1093/ije/22.6.1154. [DOI] [PubMed] [Google Scholar]
- 6.Fine P.E.M., Carneiro I.A.M., Milstein J.B., Clements C.J. WHO; Geneva: 1999. Issues relating to the use of BCG in immunization programs. [Google Scholar]
- 7.Stop TB Working Group on TB vaccines . 2011. TB vaccine pipeline.http://wwwstoptborg/wg/new_vaccines/assets/documents/TB%20Vaccine%20Pipeline%202011_FINALpdf [accessed 14.08.12] [Google Scholar]
- 8.McShane H., Behboudi S., Goonetilleke N., Brookes R., Hill A.V. Protective immunity against Mycobacterium tuberculosis induced by dendritic cells pulsed with both CD8(+)- and CD4(+)-T-cell epitopes from antigen 85A. Infect Immun. 2002;70:1623–1626. doi: 10.1128/IAI.70.3.1623-1626.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Scriba T.J., Tameris M., Smit E., van der Merwe L., Hughes E.J. A phase IIa trial of the new tuberculosis vaccine, MVA85A, in HIV- and/or Mycobacterium tuberculosis-infected adults. Am J Respir Crit Care Med. 2012;185:769–778. doi: 10.1164/rccm.201108-1548OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Scriba T.J., Tameris M., Mansoor N., Smit E., van der Merwe L. Dose-finding study of the novel tuberculosis vaccine, MVA85A, in healthy BCG-vaccinated infants. J Infect Dis. 2011;203:1832–1843. doi: 10.1093/infdis/jir195. [DOI] [PubMed] [Google Scholar]
- 11.Scriba T.J., Tameris M., Mansoor N., Smit E., van der Merwe L. Modified vaccinia Ankara-expressing Ag85A, a novel tuberculosis vaccine, is safe in adolescents and children, and induces polyfunctional CD4+ T cells. Eur J Immunol. 2010;40:279–290. doi: 10.1002/eji.200939754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hawkridge T., Scriba T.J., Gelderbloem S., Smit E., Tameris M. Safety and immunogenicity of a new tuberculosis vaccine, MVA85A, in healthy adults in South Africa. J Infect Dis. 2008;198:544–552. doi: 10.1086/590185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sander C.R., Pathan A.A., Beveridge N.E., Poulton I., Minassian A. Safety and immunogenicity of a new tuberculosis vaccine, MVA85A, in Mycobacterium tuberculosis-infected individuals. Am J Respir Crit Care Med. 2009;179:724–733. doi: 10.1164/rccm.200809-1486OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brookes R.H., Hill P.C., Owiafe P.K., Ibanga H.B., Jeffries D.J. Safety and immunogenicity of the candidate tuberculosis vaccine MVA85A in West Africa. PLoS One. 2008;3:e2921. doi: 10.1371/journal.pone.0002921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Beveridge N.E., Price D.A., Casazza J.P., Pathan A.A., Sander C.R. Immunisation with BCG and recombinant MVA85A induces long-lasting, polyfunctional Mycobacterium tuberculosis-specific CD4+ memory T lymphocyte populations. Eur J Immunol. 2007;37:3089–3100. doi: 10.1002/eji.200737504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Minassian A.M., Rowland R., Beveridge N.E., Poulton I.D., Satti I. A Phase I study evaluating the safety and immunogenicity of MVA85A, a candidate TB vaccine, in HIV-infected adults. BMJ Open. 2011;1:e000223. doi: 10.1136/bmjopen-2011-000223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pathan A.A., Minassian A.M., Sander C.R., Rowland R., Porter D.W. Effect of vaccine dose on the safety and immunogenicity of a candidate TB vaccine, MVA85A, in BCG vaccinated UK adults. Vaccine. 2012;30:5616–5624. doi: 10.1016/j.vaccine.2012.06.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Odutola A.A., Owolabi O.A., Owiafe P.K., McShane H., Ota M.O. A new TB vaccine, MVA85A, induces durable antigen-specific responses 14 months after vaccination in African infants. Vaccine. 2012;30:5591–5594. doi: 10.1016/j.vaccine.2012.06.054. [DOI] [PubMed] [Google Scholar]
- 19.Ota M.O., Odutola A.A., Owiafe P.K., Donkor S., Owolabi O.A. Immunogenicity of the tuberculosis vaccine MVA85A is reduced by coadministration with EPI vaccines in a randomized controlled trial in Gambian infants. Sci Transl Med. 2011;3:88ra56. doi: 10.1126/scitranslmed.3002461. [DOI] [PubMed] [Google Scholar]
- 20.Aronson J.D., Palmer C.E. Experience with BCB vaccine in the control of tuberculosis among North American Indians. Public Health Rep. 1946;61:802–820. [PubMed] [Google Scholar]
- 21.Rosenthal S.R., Loewinsohne, Graham M.L., Liveright D., Thorne G. BCG vaccination against tuberculosis in Chicago. A twenty-year study statistically analyzed. Pediatrics. 1961;28:622–641. [PubMed] [Google Scholar]
- 22.Hatherill M. Prospects for elimination of childhood tuberculosis: the role of new vaccines. Arch Dis Child. 2011;96:851–856. doi: 10.1136/adc.2011.214494. [DOI] [PubMed] [Google Scholar]
- 23.Mulenga H., Moyo S., Workman L., Hawkridge T., Verver S. Phenotypic variability in childhood TB: implications for diagnostic endpoints in tuberculosis vaccine trials. Vaccine. 2011;29:4316–4321. doi: 10.1016/j.vaccine.2011.04.020. [DOI] [PubMed] [Google Scholar]
- 24.Hatherill M., Verver S., Mahomed H., Taskforce on Clinical Research Issues STBPWGoTBV Consensus statement on diagnostic end points for infant tuberculosis vaccine trials. Clin Infect Dis. 2012;54:493–501. doi: 10.1093/cid/cir823. [DOI] [PubMed] [Google Scholar]
- 25.Marais B.J., Gie R.P., Schaaf H.S., Starke J.R., Hesseling A.C. A proposed radiological classification of childhood intra-thoracic tuberculosis. Pediatr Radiol. 2004;34:886–894. doi: 10.1007/s00247-004-1238-0. [DOI] [PubMed] [Google Scholar]
- 26.Hawkridge A., Hatherill M., Little F., Goetz M.A., Barker L. Efficacy of percutaneous versus intradermal BCG in the prevention of tuberculosis in South African infants: randomised trial. BMJ. 2008;337:a2052. doi: 10.1136/bmj.a2052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.English R. Department of Health; 2010. Boland/Overberg region health status report 2007/2008. [Google Scholar]
- 28.Moyo S., Verver S., Hawkridge A., Geiter L., Hatherill M. Tuberculosis case finding for vaccine trials in young children in high-incidence settings: a randomised trial. Int J Tuberc Lung Dis. 2012;16:185–191. doi: 10.5588/ijtld.11.0348. [DOI] [PubMed] [Google Scholar]
- 29.Geldenhuys H., Waggie Z., Jacks M., Geldenhuys M., Traut L. Vaccine trials in the developing world: operational lessons learnt from a phase IV poliomyelitis vaccine trial in South Africa. Vaccine. 2012 doi: 10.1016/j.vaccine.2012.07.026. [DOI] [PubMed] [Google Scholar]
- 30.Harrison D., Stevens B., Bueno M., Yamada J., Adams-Webber T. Efficacy of sweet solutions for analgesia in infants between 1 and 12 months of age: a systematic review. Arch Dis Child. 2010;95:406–413. doi: 10.1136/adc.2009.174227. [DOI] [PubMed] [Google Scholar]
- 31.Willock J., Richardson J., Brazier A., Powell C., Mitchell E. Peripheral venepuncture in infants and children. Nurs Stand. 2004;18:43–50. doi: 10.7748/ns2004.03.18.27.43.c3571. quiz 52, 55–46. [DOI] [PubMed] [Google Scholar]
- 32.Hatherill M., Hanslo M., Hawkridge T., Little F., Workman L. Structured approaches for the screening and diagnosis of childhood tuberculosis in a high prevalence region of South Africa. Bull World Health Organ. 2010;88:312–320. doi: 10.2471/BLT.09.062893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mahomed H., Fourie P.B. Clinical trials of TB vaccines: harmonization and cooperation. Tuberculosis (Edinb) 2012;92(Suppl. 1):S21–S24. doi: 10.1016/S1472-9792(12)70008-2. [DOI] [PubMed] [Google Scholar]
- 34.Graham S.M., Ahmed T., Amanullah F., Browning R., Cardenas V. Evaluation of tuberculosis diagnostics in children: 1. Proposed clinical case definitions for classification of intrathoracic tuberculosis disease. Consensus from an expert panel. J Infect Dis. 2012;205(Suppl. 2):S199–S208. doi: 10.1093/infdis/jis008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Department of Health . Department of Health; Pretoria, South Africa: 2006. Guidelines for good practice in the conduct of clinical trials with Human participants in South Africa. [Google Scholar]
- 36.International conference on harmonisation of technical requirements for registration of pharmaceuticals for human use. 1996. ICH Harmonised Tripartite Guideline: Guideline for Good Clinical Practice E6(R1) Step 4 Version.
- 37.Lubega I.R., Fowler M.G., Musoke P.M., Elbireer A., Bagenda D. Considerations in using US-based laboratory toxicity tables to evaluate laboratory toxicities among healthy Malawian and Ugandan infants. J Acquir Immune Defic Syndr. 2011;55:58–64. doi: 10.1097/QAI.0b013e3181db059d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Buchanan A.M., Muro F.J., Gratz J., Crump J.A., Musyoka A.M. Establishment of haematological and immunological reference values for healthy Tanzanian children in Kilimanjaro Region. Trop Med Int Health. 2010 doi: 10.1111/j.1365-3156.2010.02585.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lugada E.S., Mermin J., Kaharuza F., Ulvestad E., Were W. Population-based hematologic and immunologic reference values for a healthy Ugandan population. Clin Diagn Lab Immunol. 2004;11:29–34. doi: 10.1128/CDLI.11.1.29-34.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Quinto L., Aponte J.J., Sacarlal J., Espasa M., Aide P. Haematological and biochemical indices in young African children: in search of reference intervals. Trop Med Int Health. 2006;11:1741–1748. doi: 10.1111/j.1365-3156.2006.01764.x. [DOI] [PubMed] [Google Scholar]
- 41.Troy S.B., Rowhani-Rahbar A., Dyner L., Musingwini G., Shetty A.K. Hematologic and immunologic parameters in Zimbabwean infants: a case for using local reference intervals to monitor toxicities in clinical trials. J Trop Pediatr. 2012;58:59–62. doi: 10.1093/tropej/fmr031. [DOI] [PMC free article] [PubMed] [Google Scholar]