Summary
We aimed to investigate how early and late work shifts influenced the diurnal cortisol rhythm using a within-subjects study design. Participants were 30 healthy male non-smoking pilots, mean age 39.4, employed by a short-haul airline. The standard rotating shift pattern consisted of 5 early shifts (starting before 0600 h), followed by 3 rest days, 5 late shifts (starting after 1200 h) and 4 rest days. Pilots sampled saliva and completed subjective mood ratings in a logbook 6 times over the day on two consecutive early shift days, two late days and two rest days. Sampling was scheduled at waking, waking + 30 m, waking + 2.5 h, waking + 8 h, waking + 12 h and bedtime. Waking time, sleep duration, sleep quality and working hours were also recorded. Cortisol responses were analysed with repeated measures analysis of variance with shift condition (early, late, rest) and sample time (1–6) as within-subject factors. Early shifts were associated with a higher cortisol increase in response to awakening (CARi), a greater total cortisol output over the day (AUCG) and a slower rate of decline over the day than late shifts or rest days. Early shifts were also associated with shorter sleep duration but co-varying for sleep duration did not alter the effects of shift on the cortisol rhythm. Both types of work shift were associated with more stress, tiredness and lower happiness than rest days, but statistical adjustment for mood ratings did not alter the findings. Early shift days were associated with significantly higher levels of circulating cortisol during waking hours than late shifts or rest days.
Keywords: Shift work, Cortisol, Hpa axis, Time of waking, Sleep
1. Introduction
In industrialised countries, almost one in five workers participates in shift work, in which different groups of workers replace each other in the same role (ILO, 2004). The most common shift pattern in the UK is a two-shift double-day system, consisting of early and late day shifts (such as 6 am–2 pm and 2 pm–10 pm), with workers alternating on a routine basis (Steel, 2011). Large-scale prospective cohort studies have found that rotating shift work is associated with increased risks of weight gain (Suwazono et al., 2008), diabetes (Pan et al., 2011), heart disease (Fujino et al., 2006), stroke (Brown et al., 2009) and some cancers (Kubo et al., 2006). Adverse health consequences are thought to arise as a result of chronic misalignment between endogenous circadian timing systems and behavioural sleep/wake and feeding cycles (Ruger and Scheer, 2009). Controlled laboratory studies, designed to mimic shift work sleep/wake disruption, have demonstrated disruptions to the normal 24-h cycle of a number of metabolic, autonomic and endocrine system indicators (Griefahn and Robens, 2010; Ribeiro et al., 1998; Scheer et al., 2009).
The influence of shift work on the hypothalamic–pituitary–adrenal (HPA) axis has been identified as a potential mechanism through which circadian desynchrony may lead to ill-health (Nader et al., 2010). Circulating cortisol levels naturally follow a circadian rhythm. Cortisol peaks in response to morning waking, declines gradually over the day, falls further after the onset of sleep and rises gradually in the early hours of the morning before waking (Spath-Schwalbe et al., 1992; Wilhelm et al., 2007). The HPA axis also reacts to physical or perceived stressors, leading to temporary increases in circulating cortisol. The awakening response or CAR is thought to be a discrete phenomenon superimposed on the circadian cycle of cortisol release, and is a phasic response to the sleep-wake transition (Kudielka et al., 2012). Total cortisol output over the day or area under the curve (AUC) and the slope or rate of decline over the day are also frequently studied as indicators of HPA axis function (Adam and Kumari, 2009). For example, the magnitude of the awakening response was positively correlated with job stress in a recent meta-analysis (Chida and Steptoe, 2009); increased cortisol secretion over the day was predictive of depression (Stetler and Miller, 2011) and a flatter diurnal slope predicted cardiovascular mortality in over 4000 community-dwelling adults (Kumari et al., 2011).
Studies which have examined the association between waking time and the CAR have found contrasting results. In the series of studies which first characterised the awakening response, Pruessner et al. (1997) reported that the cortisol increase after waking showed moderate intra-individual stability (r = 0.39–0.70, p < 0.001) and, importantly, was independent of time of waking. This latter finding has been replicated by a number of other studies (e.g. Born et al., 1999; Kunz-Ebrecht et al., 2004; Wust et al., 2000a,b). In direct contrast, two small studies in shift workers (Federenko et al., 2004; Williams et al., 2005) and two studies in community-dwelling adults reported that earlier waking was associated with a significantly heightened cortisol increase on awakening (Edwards et al., 2001; Kudielka and Kirschbaum, 2003). A recent case study measured the CAR on 50 occasions in one student and reported a wide range of values for the waking increase (3.6–39.0 nmol/l). In this individual, waking cortisol, but not the mean increase, was significantly associated with waking time (Stalder et al., 2009).
Salivary cortisol is increasingly used as an indicator of HPA function in epidemiological studies (Adam and Kumari, 2009). Clarification of time of waking effects is important not only for interpreting shift work effects but also for the design and interpretation of future studies. It is possible that between-subjects differences in the CAR attributed to early waking above (Edwards et al., 2001; Kudielka and Kirschbaum, 2003) were confounded by unmeasured individual trait characteristics such as coping style (O’Donnell et al., 2008) or preferred ‘morningness’ chronotype (Kudielka et al., 2006). To remove potential for systematic differences between groups of early and late ‘awakeners’, within-subject comparisons may be most appropriate design for understanding time of waking effects. Kudielka and Kirschbaum (2003) used a within-subjects design but did not account for time-varying factors such as sleep duration (Kumari et al., 2009), exercise (Scheen et al., 1998), positive affect or negative affective states on the day of testing (Jacobs et al., 2007; Steptoe et al., 2005). A previous study from our group showed that the same workers waking for an early shift (mean waking time 0400 h ± 41 m) had a higher CAR than on later day shifts (waking time 0739 h ± 94 m) or rest days (waking time 0804 h ± 78 m) (Williams et al., 2005). Shift differences in the CAR were no longer significant after controlling statistically for perceived stress and lower sleep quality on early shift mornings.
The CAR is thought to be controlled independently of cortisol output over the remainder of the day so waking for an early shift may have different implications for other aspects of the cortisol rhythm (Schmidt-Reinwald et al., 1999; Wust et al., 2000a). Edwards et al. (2001) reported that earlier waking was followed by prolonged elevation of cortisol for several hours. A recent study comparing waking and evening cortisol samples in two groups of bus drivers suggested that there was less decline over the day in early shift workers than afternoon shift workers, but the samples were not timed in relation to waking (Diez et al., 2011).
We aimed to investigate how early and late shift patterns influenced the CAR, the rate of diurnal decline and total cortisol output over the day using a within-subjects study design. We recruited UK-based airline pilots working a rotating two-shift pattern consisting of 5 early shifts (starting before 0600 h), 3 rest days, 5 late shifts (starting after 1200 h) and 4 rest days. To reduce variability in the cortisol responses, we included only healthy non-smoking men (Kudielka and Kirschbaum, 2003; Steptoe and Ussher, 2006). Pilots’ work hours are regulated and therefore accurate records of work-related hours were available. We considered the influence of a range of potential confounding factors including work demands, stress and happiness over the day, sleep duration and sleep quality, alcohol and exercise.
2. Methods
2.1. Participants
Participants were healthy male non-smoking pilots employed by one short-haul passenger airline based in the United Kingdom. An invitation to participate in a research study to explore work fatigue was emailed to trade union members. To be eligible, participants had to work full-time on the standardised shift pattern, consisting of 5 early shifts, followed by 3 rest (non-working) days, 5 late shifts and 4 rest days (5-3-5-4 pattern). Early shifts required the pilot to start work before 0600 h and late shifts after 1200 h, followed by 7–12 h on duty. Pilots typically flew 2 or 4 flights per day, of between 45 m and 5 h in length, returning home overnight. Approximately 85% of pilots fulfilled these criteria, according to a prior workforce survey. Pilots routinely taking steroid medication were excluded. During the 4 weeks allocated for recruitment, 36 pilots volunteered to participate, of whom 30 were eligible. All 30 were sent detailed study information and gave written consent to participate. The study was approved by the UCL Research Ethics Committee.
2.2. Procedure
Participants received a sampling pack by post containing instructions, a logbook and 36 labelled Salivettes (Sarstedt, Germany). Participants collected saliva 6 times over the day on 2 consecutive days for each of 3 shift conditions: early shifts, late shifts and rest days. Although participants predominantly worked a 5-3-5-4 pattern, a computerised allocation system assigned occasional variations. To allow for this, participants were permitted to separate each 2-day sampling block by up to 10 days. An established sampling schedule was used capture the diurnal cortisol rhythm: waking (S1), waking + 30 m (S2), waking + 2.5 h (S3), waking + 8 h (S4), waking + 12 h (S5) and bedtime (S6) (Kumari et al., 2009). Participants were asked to avoid eating, drinking caffeinated drinks or brushing their teeth for 15 m before each sample and to record alcohol or exercise on the sampling day. Waking was defined as ‘as soon as you open your eyes and while you are still in bed’. Bedtime was defined as ‘the time at which you try to go to sleep’. At the time of the first sample, participants recorded time of going to bed the previous night, time of waking and time of sample 1. As a measure of sleep quality, pilots were asked ‘Did you sleep well last night?’ with possible responses: ‘1’ (yes, I slept well), ‘2’ (quite well) or ‘3’ (no, I did not sleep well). At each subsequent sampling point they used the logbook to record the time and ratings of stress, happiness and tiredness over the previous 30 m on a scale from ‘0’ (not at all) to ‘4’ (extremely high). On work days, pilots also recorded duty hours, flying hours and the number of sectors (landings). Participants provided demographic information via a questionnaire. Salivettes were refrigerated and returned to the investigators by post. Salivary cortisol was measured using an enzyme-linked immunosorbent assay (ELISA) (SLV-2930, DRG International, Inc., USA) at the Technical University Dresden, Germany. The intra- and inter-assay coefficients of variation were less than 8%.
2.3. Analysis
Samples taken more than 15 m from the timed protocol were excluded, including wake-up samples delayed >15 m post awakening (Dockray et al., 2008). Extreme cortisol values (≥±3 SD from the mean) were excluded. Paired t-tests were used to test for differences between pairs of samples at the same time point within each shift condition. There were no significant differences and values were all positively correlated so mean values were calculated for each sample point. Cortisol results were skewed so logarithmic transformations were computed to generate distributions suitable for parametric analyses. Analyses were carried out on transformed values, but raw data are presented in the figures. Where data were missing from one of the 2 days within a shift, data from the alternate day was used to maximise data availability. Cortisol values were incomplete for 3 participants; analyses are presented for 27 complete cases. The cortisol awakening response (CARi) was calculated as the mean increase between sample 1 (waking) and sample 2 (waking + 30 m). Cortisol output over the day (AUCG) was calculated using the trapezoid formula for area under the curve with respect to ground (Pruessner et al., 2003), based on all 6 samples. Diurnal slopes for each participant were calculated by regressing cortisol values on sample time using a piecewise approach to generate a mean rate of reduction in cortisol per hour. The slope was anchored on the waking sample and excluded the second (waking + 30 m) value (Cohen et al., 2006).
Cortisol responses were analysed with repeated measures analysis of variance (ANOVA) with shift condition (early, late, rest) and sample time (1–6) as within-subject factors. The Greenhouse–Geisser correction was applied where assumptions of sphericity were violated. Aspects of the diurnal cortisol rhythm which may have clinical significance (the CARi, AUCG, diurnal slope and bedtime sample) were also analysed in separate repeated measures ANOVAs, with shift condition as the within-subject factor.
Paired t-tests were used to test differences between duty hours, flying hours and sectors across early and late work shifts. Maximum sleep duration was estimated as the time between waking and bedtime the previous evening. Waking hours were the difference between waking and bedtime the same evening. Shift differences in wake-up time, bedtime, waking hours, sleep duration and sleep quality were investigated using repeated measures ANOVAs. Mean values for subjective mood ratings at each time point within each shift condition were calculated. Subjective ratings were analysed with repeated measures ANOVAs with shift condition (early, late, rest) and sample time (1–6) as within-subject factors. Paired t-tests were used to confirm significant differences between pairs of values. Correlations between cortisol and subjective ratings both at individual sampling points and mean stress/happiness/tiredness over the day for each shift condition were calculated. If work factors, time of day factors or subjective ratings differed significantly by shift or were significantly correlated with cortisol responses, we investigated whether these factors could explain shift differences in the CARi, AUCG or diurnal slope by entering mean values for each shift as time-varying covariates in repeated measures ANOVAs, using the mixed command. SPSS version 18.0 was used for all statistical analyses.
3. Results
Characteristics of participants with complete cortisol data are outlined in Table 1. All pilots had worked at least 3 years for their employer and had worked as a pilot for 3–37 years.
Table 1.
Characteristics of participants with complete cortisol data, n = 27.
| n (%) | |
|---|---|
| Rank | |
| Captain | 14 (51.9) |
| First officer | 13 (48.1) |
| Age | |
| 20–29 | 4 (14.8) |
| 30–39 | 11 (40.7) |
| 40–49 | 8 (29.6) |
| 50–59 | 4 (14.8) |
| BMI, mean (SD) | 25.6 (2.52) |
| Years as pilot, mean (SD) | 12.7 (10.0) |
| Years working for current employer, mean (SD) | 5.9 (2.3) |
3.1. Shift characteristics
Waking times ranged from 0340 to 0530 h on an early shift, 0621 to 1225 h on a late shift and 0641 to 0925 h on a rest day. Early shifts were associated with significantly earlier waking, a longer waking day and shorter sleep duration than late shifts or rest days (Table 2). Bed-time was significantly later on a late shift. Sleep quality did not differ significantly between conditions. Objective work demands did not differ between work shifts. Drinking alcohol was more common on rest days.
Table 2.
Characteristics of early and late work shifts and rest days, mean ± standard deviation or percentage (%) of participants. a,b,cSignificantly higher value than earlya, lateb or restc days (p < 0.05).
| Early | Late | Rest | |
|---|---|---|---|
| Waking hours and sleep quality | |||
| Time of waking (h) | 0426 ± 0023 | 0840 ± 0123a | 0752 ± 0100a |
| Bed-time (h) | 2131 ± 0043 | 0052 ± 0117a,c | 2316 ± 0042 |
| Waking hours (h) | 17.0 ± 0.8b,c | 16.1 ± 1.1 | 15.4 ± 1.0 |
| Sleep duration (h) | 7.0 ± 0.7 | 7.8 ± 1.4a | 8.2 ± 1.0a |
| Poor sleep quality | 2.1 ± 0.6 | 1.9 ± 0.6 | 1.7 ± 0.6 |
| Work demands | |||
| Duty hours (h) | 8.0 ± 1.5 | 8.9 ± 1.6 | – |
| Flying hours (h) | 5.4 ± 1.2 | 5.7 ± 1.4 | – |
| Sectors (landings) | 2.7 ± 0.7 | 2.9 ± 0.9 | – |
| Behaviours on day of shift | |||
| Consumed alcohol, % | 29.6 | 20.3 | 51.9a,b |
| Exercised for >10 min, % | 20.4 | 16.7 | 27.8 |
3.2. Subjective mood ratings
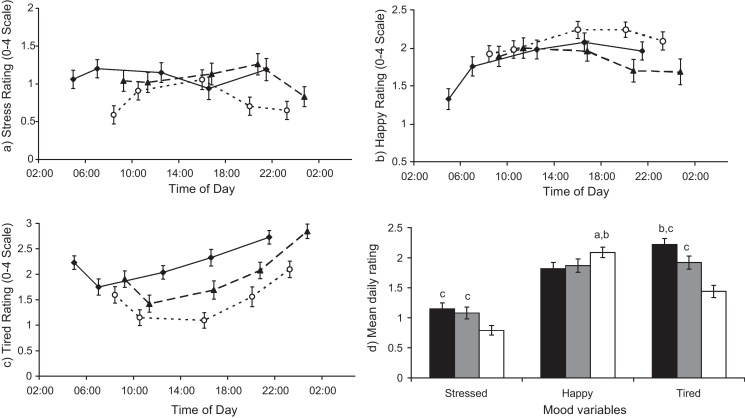
In separate repeated measures ANOVAs, there were significant main effects of shift condition and time for both happiness and tiredness (F(2,52) > 4.94–6.86, p < 0.005); and a significant main effect of shift (F(2,52) = 5.80, p = 0.005) but not time on stress, illustrated in Fig. 1a–c. There were significant shift-time interactions for stress (F(2,52) = 2.73, p = 0.007) and happiness (F(2,52) = 5.98, p < 0.001). Happiness was at a low-point at the start of an early shift, but peaked on all days mid-afternoon. Stress decreased at the bedtime sample on rest and late days, but peaked before bed on early shifts. Tiredness tended to reduce from waking + 30 m to waking + 2.5 h, and then increase throughout every day. Mean daily ratings correlated positively within individuals between work shifts and to a lesser extent rest days; for example, mean happiness on an early shift correlated with late (r = 0.785, p < 0.001) and rest day happiness (r = 0.575, p = 0.002). There were no consistent correlations between different mood states within a shift condition that neared significance; for example, daily stress was not correlated with daily happiness (r = −0.017 to 0.235, p > 0.364). Overall, both work shifts were associated with significantly greater mean stress and tiredness than a rest day, and lower happiness (paired t-tests, p < 0.01), Fig. 1d.
Figure 1.
Subjective mood ratings over the day for (a) stress, (b) happiness, (c) tiredness at waking + 30 min, waking + 2.5 h, waking + 8 h, waking + 12 h and bedtime for early shifts (♦, full black line), late shifts (▴, dashed line) and rest days (○, dotted line). (d) Mean daily ratings for early (black bars), late (grey bars) and rest days (white bars). a,b,cSignificantly higher than earlya, lateb or restc days (p < 0.05). Error bars denote standard error of the mean.
3.3. Cortisol responses
3.3.1. Main shift effects
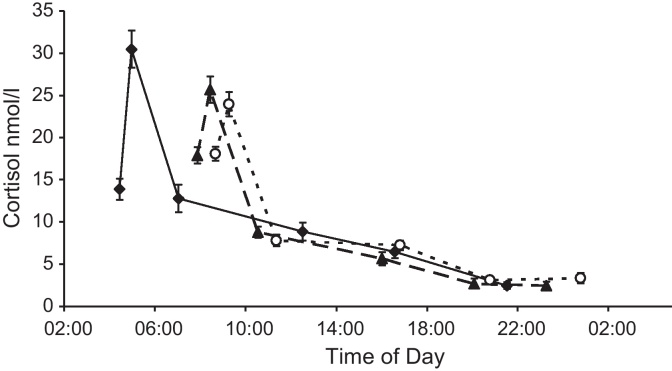
Cortisol responses on each of the three conditions are illustrated in Fig. 2. In unadjusted analyses, there were significant main effects of shift condition (F(2,52) = 10.15, p < 0.001), time of sample and a shift by time interaction (p < 0.001). There were main effects of shift on the CARi (F(2,52) = 17.82, p < 0.001), output over the day (AUCG) (F(2,52) = 22.58, p < 0.001) and diurnal slope (F(2,52) = 5.09, p = 0.024). The awakening response was greatest on an early shift, resulting from both a lower waking value and a higher peak 30 m later (Table 3). Early shifts were also associated with a greater area under the curve and a slower rate of decline over the day than late shifts or rest days. Late shifts were associated with higher cortisol at bedtime than the other conditions.
Figure 2.
Mean cortisol at waking, waking + 30 min, waking + 2.5 h, waking + 8 h, waking + 12 h and bedtime for early shifts (♦, full black line), late shifts (▴, dashed line) and rest days (○, dotted line). Error bars represent standard error of the mean.
Table 3.
Salivary cortisol on early and late work shifts and rest days, mean ± standard deviation. a,b,cSignificantly higher value than earlya, lateb or restc days (p < 0.05).
| Early | Late | Rest | |
|---|---|---|---|
| Cortisol (nmol/l), natural log | |||
| Waking cortisol, S1 | 2.50 ± 0.54 | 2.85 ± 0.31a | 2.85 ± 0.26a |
| Waking + 30 m, S2 | 3.34 ± 0.43b | 3.11 ± 0.40 | 3.20 ± 0.32 |
| Bed-time cortisol, S6 | 0.68 ± 0.64 | 0.98 ± 0.78c | 0.45 ± 0.92 |
| Increase on waking, CARi | 0.83 ± 0.38b,c | 0.25 ± 0.41 | 0.35 ± 0.35 |
| Area under curve, AUCG | 32.8 ± 6.80b,c | 26.4 ± 6.50 | 23.7 ± 7.29 |
| Diurnal slope | 0.11 ± 0.06 | 0.15 ± 0.07a | 0.20 ± 0.18a |
3.3.2. Impact of sampling delays
An increase in cortisol after waking was observed in the majority of participants but a minority had a negative response to waking: n = 1 on an early shift, n = 7 on a late shift and n = 4 on a rest day. All participants with a negative CARi had a positive CARi on at least one other occasion. In case delays of up to 15 m between waking and the first sample influenced the negative CARs, we excluded participants with 10 m or more delay between waking and sample 1 on any shift condition (Griefahn and Robens, 2011). Based on this subsample, all 17 pilots recorded a positive CARi, on an early shift, but a minority still recorded a negative CAR on a late shift (n = 3) or a rest day (n = 2). The effects of shift on cortisol responses did not differ from those described above with a higher CARi, a higher AUCG and a slower rate of decline on an early shift (p < 0.01). Only the effect of the late shift on higher bedtime samples was no longer statistically significant (F(2,32) = 3.08, p = 0.060). Owing to the similarities in data with the larger sample size, we report data for all 27 respondents (with delays of up to 15 m before the sample) in remaining analyses.
3.3.3. Correlations between cortisol and other variables within each shift condition
There were no correlations between the CARi, AUCG, or diurnal slope with waking time, time of bedtime sample, sleep duration or sleep quality with that were consistent across all three shift conditions. Cortisol values on waking (S1) and bedtime (S2) were not consistently associated with waking times or sleep variables. There were no consistent patterns of association between mood states and cortisol across shifts. There was no association between cortisol indicators and age, rank or daily reports of alcohol or exercise.
3.3.4. Shift differences in cortisol in multivariable models
Shift differences in the CARi, AUCG and diurnal slope persisted when mean stress and happiness ratings over the day for each shift condition were included as time-varying covariates (CARi F(2,53.6) = 16.3; AUCG F(2,52.6) = 24.4; slope F(2,54.4) = 3.24; all models p < 0.050). There was no difference to the main effects when subjective ratings were limited to morning values, or if tiredness ratings were included. The addition of subjective ratings did not significantly improve model fit, based on the −2 restricted log likelihood test. The effect of shift condition on the CARi, AUCG and slope remained robust after adjusting for sleep quality or sleep duration. Shift differences in the AUCG and diurnal slope were also independent of the CARi when entered into the same model (p < 0.001). Adjustment for age, rank or years experience as a pilot did not alter any of the significant shift effects described above.
4. Discussion
Early work shifts, which involved waking before 0530 h, were associated with a higher cortisol increase in response to awakening (CARi), a greater total cortisol output over the day (AUCG) and a slower rate of decline over the day than late shifts or rest days, which involved waking after 0620 h. Early shifts were also associated with shorter sleep duration but co-varying for sleep duration did not alter the effects of shift on the cortisol rhythm. Both types of work shift were associated with more stress, tiredness and lower happiness than rest days, but statistical adjustment for mood ratings did not explain the increased cortisol output on early shift days.
We discuss the results relating to the cortisol awakening response followed by the two other cortisol measures. Two previous studies using a within-subject design similarly found that waking before 0530 h for an early shift was associated with a higher CARi: compared to afternoon shifts and rest days in London underground rail workers (Williams et al., 2005) and compared to afternoon and night shifts in nurses (Federenko et al., 2004). Kudielka and Kirschbaum (2003) also reported between-subject effects of early waking on the CARi in 102 community dwelling subjects, when ‘early’ was defined through cluster analysis as earlier than 0803 h (early mean waking time 0649 h ± 0.06; late mean 0943 h ± 0.09). Between-subjects effects were reported in a study of non-shift workers (n = 36) where early waking was defined as before the median waking time, 0721 h, but the range of waking times was not specified (Edwards et al., 2001).
In spite of the ‘early’ versus ‘late’ group effects described above, a number of large studies have reported that the CAR is independent of time of waking (Kunz-Ebrecht et al., 2004; Pruessner et al., 1997; Stalder et al., 2009). For example, Wust et al. (2000b), found no association between waking times and the CARi or awakening response calculated as area under the curve (CARAUC) in over 500 adults, with a wide range of waking times (0420–1245 h). In the current study, we also found no correlation between time of waking and CARi, AUCG or diurnal slope within any single shift condition. The apparent group (early vs. late) effects in the absence of a linear association between waking time and the CARi might be explained by a threshold effect. There could be a threshold time, or a stage of sleep, before which the HPA axis is hypersensitive to awakening. Two studies argue against a purely physiological threshold. Hucklebridge et al. (2000) found a smaller CARAUC when 11 volunteers were woken at 0400 h than 0800 h. Born et al. (1999) reported no difference in blood cortisol levels in 15 volunteers sleeping in a laboratory woken at 0600 h or 0900 h. However, neither of these studies was conducted under typical work conditions. Born et al. specified that subjects stay in bed for 3 h post waking and Hucklebridge et al. did not specify work day sampling. Born et al., importantly showed that anticipation of the earlier waking time was associated with an ACTH surge before waking, proving that anticipation pervades sleep. On an early shift, pilots were typically due to start work within 90 min of waking. Waking for imminent work might be associated with greater anticipation of stress on the preceding evening, which may be indicated by the peak in stress levels observed before bedtime on early shifts. If anticipation of stress pervades sleep, it might also be that HPA axis reactivity to this psychological stress is greater during the circadian low. It has been shown that lower basal levels of cortisol are associated with heightened cortisol reactivity to standardised laboratory stressors during the day (Kudielka et al., 2004). Cortisol reaches its nadir in the early hours of the morning during slow wave sleep (Spath-Schwalbe et al., 1991), so theoretically, waking from these low levels could be associated with a hyper-reactivity to psychological stress. Williams et al. (2005) reported that shift differences in the CARi were no longer significant after adjustment for ratings of stress at 30 m post waking. Shift differences in the CARi did persist after co-varying for stress and happiness in the current study, but we only asked participants to rate their stress over the previous 30 m, not anticipated stress (Fries et al., 2009). In a case study of one subject over 50 mornings, Stalder et al. (2010), reported that the area under the curve 45 m post waking was predicted by anticipated study day obligations or anticipated lack of leisure, but not by stress measured on the same day. Future research could incorporate alternative methods such as the day reconstruction method for assessing psychological states before and during different work shifts, in association with cortisol monitoring over more than two consecutive days, to further investigate psychobiological interactions (Daly et al., 2010; Kahneman et al., 2004).
Another factor which may have contributed to the elevated CARi on early shifts is lack of sleep. Early shifts in this study were associated with shorter sleep duration. Animal studies have shown that sleep restriction is a mild activator of the HPA axis and may also alter reactivity to stress (Meerlo et al., 2002). In a cross sectional study of over 2750 older adults, self-reported short sleep duration was associated with an increased CARi and a shallow diurnal slope, after adjusting for a number of covariates, including stress on the day of testing. Poor sleep quality over the last month was also associated with a less steep diurnal slope (Kumari et al., 2009). We did not find reliable correlations between sleep duration or quality and cortisol responses within shifts, but this may be owing to the small sample or the narrow distribution of sleep durations within each shift. Shift differences in the CARi, AUCG and diurnal slope and persisted after adjustment for sleep duration and quality, indicating that sleep was not the primary driver of between shift differences in cortisol metrics.
To our knowledge, this is the first study to systematically compare the cortisol profile from waking to bedtime on early versus late day shifts and rest days in a naturalistic setting. Early shifts were associated with greater overall cortisol output over the day, in line with the study by Edwards et al. (2001), in which the early waking group showed greater mean cortisol when measurements were taken up to 12 h post waking. A recent study in bus drivers reported a less marked decline over the day for early shifts (n = 16) compared to afternoon shifts (n = 31) (Diez et al., 2011). The present study confirms this relationship using a more robust research design; the study by Diez et al. (2011), was limited by the single day of sampling, a between-groups design with limited adjustment for potential confounding variables and failure to specify sampling times in relation to waking.
Shift differences in the AUCG and diurnal slope were independent of the CARi in this study which reinforces the assumption that these metrics are influenced by separate mechanisms (Wilhelm et al., 2007). Greater 24-h cortisol output and a flatter cortisol slope have been linked to an increased risk of cardiovascular mortality in two recent large-scale epidemiological studies (Kumari et al., 2011; Vogelzangs et al., 2010), increasing the potential prognostic significance of these indicators. Heightened cortisol responses to acute psychosocial stress have been linked to increased risks of hypertension (Hamer and Steptoe, 2012) and progression of coronary artery calcification (Hamer et al., 2012). Chronic oversecretion of cortisol in Cushing's syndrome is associated with hyperglycaemia, insulin resistance and dyslipidaemia (Whitworth et al., 2005). Elevated exposure to cortisol over the day on early shifts, if repeated routinely on a chronic basis, might promote pathogenic processes including insulin resistance (Anagnostis et al., 2009). Longitudinal studies which include repeated measurements of cortisol profiles by shift condition and changes in cardiovascular risk factors over time will be necessary to investigate whether cortisol exposure is one mechanism contributing to increased risks of heart disease in rotating shift workers (Fujino et al., 2006).
A striking feature of this study is the large within-individual variation in the diurnal cortisol rhythm depending on early shift, late shift or rest days, illustrated in Fig. 2. There were no significant differences in mean cortisol values for the key metrics on consecutive days within a shift condition, but overall the CAR on an early shift (for example), was not significantly correlated with the CAR on a rest or late day (p > 0.400). Wide individual variability in the CAR was also reported over 50 days in the case series by Stalder and colleagues (3.6–39.0 nmol/l) (2009), raising questions about the reliability of ‘normal’ values for the CAR without reference to waking time and anticipated work requirements (Wust et al., 2000b). In order to assess ‘trait’ differences in the CAR, a minimum of 2 days of consecutive measures have been suggested, with 6 days recommended (Hellhammer et al., 2007). Studies measuring cortisol in population studies should identify shift workers and specify work or rest day sampling for all participants. In order to improve current understanding of waking time effects on the diurnal cortisol rhythm, it would be helpful for future studies to report sleep and waking times, sleep duration and where possible, psychosocial factors, in relationship to cortisol indicators.
The study was conducted on a relatively small convenience sample of male airline pilots and findings may not generalise to women or other professions. However similar findings relating to the CAR have been reported in male and female nurses and train workers, suggesting that the findings may be applicable to other professions working a two-shift system (Federenko et al., 2004; Williams et al., 2005). A strength of this study was the continuation of sampling from waking to bedtime, over 6 days, enabling us to investigate the diurnal slope and total daily output. Despite the small sample, adherence to the protocol by pilots was high compared to salivary cortisol studies in shift workers. For example, Federenko et al. (2004) reported that 9 out of 24 nurses provided complete data and Williams et al. (2005) obtained complete data from 22 out of 32 London underground workers. We relied on subjective ratings of sleep quality and duration which may not accurately reflect objective measures (Jackowska et al., 2011). Similarly we used self-reported waking time and records of sampling times. Previous studies have found good agreement between objective and self-reported waking (Dockray et al., 2008). Subjective mood ratings in this study were limited to recent stress, tiredness and happiness, while future studies could consider prior day anticipation of work demands.
In conclusion, we report that male pilots show a heightened cortisol awakening response, greater cortisol output over the day and a slower rate of decline on early shift days compared to late shift work days and rest days. The mechanisms leading to elevated cortisol on early shift days are unclear but early waking, anticipated stress and short sleep may play a role.
Role of funding sources
The funding bodies had no role in the collection, analysis and interpretation of data or the writing of this report.
Conflict of interest
The authors declare that they have no conflicts of interest.
Acknowledgements
SB and AS are funded by the British Heart Foundation. This work was partly funded by BALPA, the British Airline Pilots’ Association.
References
- Adam E.K., Kumari M. Assessing salivary cortisol in large-scale epidemiological research. Psychoneuroendocrinology. 2009:34. doi: 10.1016/j.psyneuen.2009.06.011. [DOI] [PubMed] [Google Scholar]
- Anagnostis P., Athyros V.G., Tziomalos K., Karagiannis A., Mikhailidis D.P. Clinical review: the pathogenetic role of cortisol in the metabolic syndrome: a hypothesis. J. Clin. Endocrinol. Metab. 2009;94:2692–2701. doi: 10.1210/jc.2009-0370. [DOI] [PubMed] [Google Scholar]
- Born J., Hansen K., Marshall L., Molle M., Fehm H.L. Timing the end of nocturnal sleep. Nature. 1999;397:29–30. doi: 10.1038/16166. [DOI] [PubMed] [Google Scholar]
- Brown D.L., Feskanich D., Sánchez B.N., Rexrode K.M., Schernhammer E.S., Lisabeth L.D. Rotating night shift work and the risk of ischemic stroke. Am. J. Epidemiol. 2009;169:1370–1377. doi: 10.1093/aje/kwp056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chida Y., Steptoe A. Cortisol awakening response and psychosocial factors: a systematic review and meta-analysis. Biol. Psychol. 2009;80:265–278. doi: 10.1016/j.biopsycho.2008.10.004. [DOI] [PubMed] [Google Scholar]
- Cohen S., Schwartz J.E., Epel E., Kirschbaum C., Sidney S., Seeman T. Socioeconomic status, race and diurnal cortisol decline in the Coronary Artery Risk Development in Young Adults (CARDIA) study. Psychosom. Med. 2006;68:41–50. doi: 10.1097/01.psy.0000195967.51768.ea. [DOI] [PubMed] [Google Scholar]
- Daly M., Delaney L., Doran P.P., Harmon C., MacLachlan M. Naturalistic monitoring of the affect-heart rate relationship: a day reconstruction study. Health Psychol. 2010;29:186–195. doi: 10.1037/a0017626. [DOI] [PubMed] [Google Scholar]
- Diez J.J., Vigo D.E., Lloret S.P., Rigters S., Role N., Cardinali D.P., Chada D.P. Sleep habits, alertness, cortisol levels, and cardiac autonomic activity in short-distance bus drivers: differences between morning and afternoon shifts. J. Occup. Environ. Med. 2011;53:806–811. doi: 10.1097/JOM.0b013e318221c6de. [DOI] [PubMed] [Google Scholar]
- Dockray S., Bhattacharya M.R., Molloy G.J., Steptoe A. The cortisol awakening response in relation to objective and subjective measures of waking in the morning. Psychoneuroendocrinology. 2008;33:77–82. doi: 10.1016/j.psyneuen.2007.10.001. [DOI] [PubMed] [Google Scholar]
- Edwards S., Evans A., Hucklebridge F., Clow A. Association between time of awakening and diurnal cortisol secretory activity. Psychoneuroendocrinology. 2001;26:613–622. doi: 10.1016/s0306-4530(01)00015-4. [DOI] [PubMed] [Google Scholar]
- Federenko I., Wust S., Hellhammer D.H., Dechoux R., Kumsta R., Kirschbaum C. Free cortisol awakening responses are influenced by awakening time. Psychoneuroendocrinology. 2004;29:174–184. doi: 10.1016/s0306-4530(03)00021-0. [DOI] [PubMed] [Google Scholar]
- Fries E., Dettenborn L., Kirschbaum C. The cortisol awakening response (CAR): facts and future directions. Int. J. Psychophysiol. 2009;72:67–73. doi: 10.1016/j.ijpsycho.2008.03.014. [DOI] [PubMed] [Google Scholar]
- Fujino Y., Iso H., Tamakoshi A., Inaba Y., Koizumi A., Kubo T., Yoshimura T., Group f.t.J.C.C.S. A prospective cohort study of shift work and risk of ischemic heart disease in Japanese male workers. Am. J. Epidemiol. 2006;164:128–135. doi: 10.1093/aje/kwj185. [DOI] [PubMed] [Google Scholar]
- Griefahn B., Robens S. The normalization of the cortisol awakening response and of the cortisol shift profile across consecutive night shifts – an experimental study. Psychoneuroendocrinology. 2010;35:1501–1509. doi: 10.1016/j.psyneuen.2010.05.002. [DOI] [PubMed] [Google Scholar]
- Griefahn B., Robens S. Cortisol awakening response: are sampling delays of 15 minutes acceptable? Int. J. Psychophysiol. 2011;82:202–205. doi: 10.1016/j.ijpsycho.2011.08.005. [DOI] [PubMed] [Google Scholar]
- Hamer M., Endrighi R., Venuraju S.M., Lahiri A., Steptoe A. Cortisol responses to mental stress and the progression of coronary artery calcification in healthy men and women. PLoS One. 2012;7:e31356. doi: 10.1371/journal.pone.0031356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamer M., Steptoe A. Cortisol responses to mental stress and incident hypertension in healthy men and women. J. Clin. Endocrinol. Metab. 2012;97:E29–E34. doi: 10.1210/jc.2011-2132. [DOI] [PubMed] [Google Scholar]
- Hellhammer J., Fries E., Schweisthal O.W., Schlotz W., Stone A.A., Hagemann D. Several daily measurements are necessary to reliably assess the cortisol rise after awakening: state- and trait components. Psychoneuroendocrinology. 2007;32:80–86. doi: 10.1016/j.psyneuen.2006.10.005. [DOI] [PubMed] [Google Scholar]
- Hucklebridge F.H., Clow A., Rahman H., Evans P. Cortisol response to nocturnal awakening. J. Psychophysiol. 2000;14:24–28. [Google Scholar]
- ILO . International Labour Organisation; 2004. Shift Work Factsheet. http://ilo.org/travail/info/fs/WCMS_170713/lang-en/index.htm (accessed June 2012) [Google Scholar]
- Jackowska M., Dockray S., Hendrickx H., Steptoe A. Psychosocial factors and sleep efficiency: discrepancies between subjective and objective evaluations of sleep. Psychosom. Med. 2011;73:810–816. doi: 10.1097/PSY.0b013e3182359e77. [DOI] [PubMed] [Google Scholar]
- Jacobs N., Myin-Germeys I., Derom C., Delespaul P., van Os J., Nicholson N.A. A momentary assessment study of the relationship between affective and adrenocortical stress responses in daily life. Biol. Psychol. 2007;74:60–66. doi: 10.1016/j.biopsycho.2006.07.002. [DOI] [PubMed] [Google Scholar]
- Kahneman D., Krueger A., Schkade D., Schwarz N., Stone A. A survey method for characterizing daily life experience: the day reconstruction method. Science. 2004;306:1776–1780. doi: 10.1126/science.1103572. [DOI] [PubMed] [Google Scholar]
- Kubo T., Ozasa K., Mikami K., Wakai K., Fujino Y., Watanabe Y., Miki T., Nakao M., Hayashi K., Suzuki K., Mori M., Washio M., Sakauchi F., Ito Y., Yoshimura T., Tamakoshi A. Prospective cohort study of the risk of prostate cancer among rotating-shift workers: findings from the Japan Collaborative Cohort Study. Am. J. Epidemiol. 2006;164:549–555. doi: 10.1093/aje/kwj232. [DOI] [PubMed] [Google Scholar]
- Kudielka B.M., Federenko I., Hellhammer D.H., Wust S. Morningness and eveningness: the free cortisol rise after awakening in “early birds” and “night owls”. Biol. Psychol. 2006;72:141–146. doi: 10.1016/j.biopsycho.2005.08.003. [DOI] [PubMed] [Google Scholar]
- Kudielka B.M., Gierens A., Hellhammer D.H., Wust S., Schlotz W. Salivary cortisol in ambulatory assessment – some dos, some don’ts, and some open questions. Psychosom. Med. 2012;74:418–431. doi: 10.1097/PSY.0b013e31825434c7. [DOI] [PubMed] [Google Scholar]
- Kudielka B.M., Kirschbaum C. Awakening cortisol responses are influenced by health status and awakening time but not by menstrual cycle phase. Psychoneuroendocrinology. 2003;28:35–47. doi: 10.1016/s0306-4530(02)00008-2. [DOI] [PubMed] [Google Scholar]
- Kudielka B.M., Schommer N.C., Hellhammer D.H., Kirschbaum C. Acute HPA axis responses, heart rate, and mood changes to psychosocial stress (TSST) in humans at different times of day. Psychoneuroendocrinology. 2004;29:983–992. doi: 10.1016/j.psyneuen.2003.08.009. [DOI] [PubMed] [Google Scholar]
- Kumari M., Badrick E., Ferrie J.E., Perski A., Marmot M., Chandola T. Self-reported sleep duration and sleep disturbance are independently associated with cortisol secretion in the Whitehall II study. J. Clin. Endocrinol. Metab. 2009;94:4801–4809. doi: 10.1210/jc.2009-0555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumari M., Shipley M.J., Stafford M., Kivimaki M. Association of diurnal patterns in salivary cortisol with all-cause and cardiovascular mortality: findings from the Whitehall II study. J. Clin. Endocrinol. Metab. 2011;96:1478–1485. doi: 10.1210/jc.2010-2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunz-Ebrecht S.R., Kirschbaum C., Marmot M., Steptoe A. Differences in cortisol awakening response on work days and weekends in women and men from the Whitehall II cohort. Psychoneuroendocrinology. 2004;29:516–528. doi: 10.1016/s0306-4530(03)00072-6. [DOI] [PubMed] [Google Scholar]
- Meerlo P., Koehl M., van der Borght K., Turek F.W. Sleep restriction alters the hypothalamic–pituitary–adrenal response to stress. J. Neuroendocrinol. 2002;14:397–402. doi: 10.1046/j.0007-1331.2002.00790.x. [DOI] [PubMed] [Google Scholar]
- Nader N., Chrousos G.P., Kino T. Interactions of the circadian CLOCK system and the HPA axis. Trends Endocrinol. Metab. 2010;21:277–286. doi: 10.1016/j.tem.2009.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Donnell K., Badrick E., Kumari M., Steptoe A. Psychological coping styles and cortisol over the day in healthy older adults. Psychoneuroendocrinology. 2008;33:601–611. doi: 10.1016/j.psyneuen.2008.01.015. [DOI] [PubMed] [Google Scholar]
- Pan A., Schernhammer E.S., Sun Q., Hu F.B. Rotating night shift work and risk of type 2 diabetes: two prospective cohort studies in women. PLoS Med. 2011;8:e1001141. doi: 10.1371/journal.pmed.1001141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruessner J.C., Kirschbaum C., Meinlschmid G., Hellhammer D.H. Two formulas for computation of the area under the curve represent measures of total hormone concentration versus time-dependent change. Psychoneuroendocrinology. 2003;28:916–931. doi: 10.1016/s0306-4530(02)00108-7. [DOI] [PubMed] [Google Scholar]
- Pruessner J.C., Wolf O.T., Hellhammer D.H., Buske-Kirschbaum A., von Auer K., Jobst S., Kaspers F., Kirschbaum C. Free cortisol levels after awakening: a reliable biological marker for the assessment of adrenocortical activity. Life Sci. 1997;61:2539–2549. doi: 10.1016/s0024-3205(97)01008-4. [DOI] [PubMed] [Google Scholar]
- Ribeiro D., Hampton S., Morgan L., Deacon S., Arendt J. Altered postprandial hormone and metabolic responses in a simulated shift work environment. J. Endocrinol. 1998;158:305–310. doi: 10.1677/joe.0.1580305. [DOI] [PubMed] [Google Scholar]
- Ruger M., Scheer F.A. Effects of circadian disruption on cardiometabolic system. Rev. Endocr. Metab. Disord. 2009;10:245–260. doi: 10.1007/s11154-009-9122-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheen A.J., Buxton O.M., Jison M., Van Reeth O., Leproult R., L’Hermite-Beleriaux M., Van Cauter E. Effects of exercise on neuroendocrine secretions and glucose regulation at different times of day Am. J. Physiol. Endocrinol. Metab. 1998;274:E1040–E1049. doi: 10.1152/ajpendo.1998.274.6.E1040. [DOI] [PubMed] [Google Scholar]
- Scheer F.A., Hilton M.F., Mantzoros C.S., Shea S.A. Adverse metabolic and cardiovascular consequences of circadian misalignment. Proc. Natl. Acad. Sci. U. S. A. 2009;106:4453–4458. doi: 10.1073/pnas.0808180106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt-Reinwald A., Pruessner J.C., Hellhammer D.H., Federenko I., Rohleder N., Schürmeyer T.H., Kirschbaum C. The cortisol response to awakening in relation to different challenge tests and a 12-hour cortisol rhythm. Life Sci. 1999;63:1653–1660. doi: 10.1016/s0024-3205(99)00103-4. [DOI] [PubMed] [Google Scholar]
- Spath-Schwalbe E., Gofferje M., Kern W., Born J., Fehm H.L. Sleep disruption alters nocturnal ACTH and cortisol secretory patterns. Biol. Psychol. 1991;29:575–584. doi: 10.1016/0006-3223(91)90093-2. [DOI] [PubMed] [Google Scholar]
- Spath-Schwalbe E., Scholler T., Kern W., Fehm H.L., Born J. Nocturnal adrenocorticotropin and cortisol secretion depends on sleep duration and decreases in association with spontaneous awakening in the morning. J. Clin. Endocrinol. Metab. 1992;75:1431–1435. doi: 10.1210/jcem.75.6.1334495. [DOI] [PubMed] [Google Scholar]
- Stalder T., Evans P., Hucklebridge F., Clow A. Associations between psychosocial state variables and the cortisol awakening response in a single case study. Psychoneuroendocrinology. 2010;35:209–214. doi: 10.1016/j.psyneuen.2009.06.006. [DOI] [PubMed] [Google Scholar]
- Stalder T., Hucklebridge F., Evans P., Clow A. Use of a single case study design to examine state variation in the cortisol awakening response: relationship with time of awakening. Psychoneuroendocrinology. 2009;34:607–614. doi: 10.1016/j.psyneuen.2008.10.023. [DOI] [PubMed] [Google Scholar]
- Steel, M., 2011. Changes in shift work patterns over the last ten years (1999 to 2009). Prepared for the Health and Safety Executive by the Office of National Statistics Newport.
- Steptoe A., Ussher M. Smoking, cortisol and nicotine. Int. J. Psychophysiol. 2006;59:228–235. doi: 10.1016/j.ijpsycho.2005.10.011. (Epub 6 December, 2005) [DOI] [PubMed] [Google Scholar]
- Steptoe A., Wardle J., Marmot M. Positive affect and health-related neuroendocrine, cardiovascular, and inflammatory processes. Proc. Natl. Acad. Sci. U. S. A. 2005;102:6508–6512. doi: 10.1073/pnas.0409174102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stetler C., Miller G.E. Depression and hypothalamic–pituitary–adrenal activation: a quantitative summary of four decades of research. Psychosom. Med. 2011;73:114–126. doi: 10.1097/PSY.0b013e31820ad12b. [DOI] [PubMed] [Google Scholar]
- Suwazono Y., Dochi M., Sakata K., Okubo Y., Oishi M., Tanaka K., Kobayashi E., Kido T., Nogawa K. A longitudinal study on the effect of shift work on weight gain in male japanese workers. Obesity. 2008;16:1887–1893. doi: 10.1038/oby.2008.298. [DOI] [PubMed] [Google Scholar]
- Vogelzangs N., Beekman A.T., Milaneschi Y., Bandinelli S., Ferrucci L., Penninx B.W. Urinary cortisol and six-year risk of all-cause and cardiovascular mortality. J. Clin. Endocrinol. Metab. 2010;95:4959–4964. doi: 10.1210/jc.2010-0192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitworth J.A., Williamson P.M., Mangos G., Kelly J.J. Cardiovascular consequences of cortisol excess. Vasc. Health Risk Manage. 2005;1:291–299. doi: 10.2147/vhrm.2005.1.4.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilhelm I., Born J., Kudielka B.M., Schlotz W., Wust S. Is the cortisol awakening rise a response to awakening? Psychoneuroendocrinology. 2007;32:358–366. doi: 10.1016/j.psyneuen.2007.01.008. [DOI] [PubMed] [Google Scholar]
- Williams E., Magid K., Steptoe A. The impact of time of waking and concurrent subjective stress on the cortisol response to awakening. Psychoneuroendocrinology. 2005:139–148. doi: 10.1016/j.psyneuen.2004.06.006. [DOI] [PubMed] [Google Scholar]
- Wust S., Federenko I., Hellhammer D.H., Kirschbaum C. Genetic factors, perceived chronic stress, and the free cortisol response to awakening. Psychoneuroendocrinology. 2000;25:707–720. doi: 10.1016/s0306-4530(00)00021-4. [DOI] [PubMed] [Google Scholar]
- Wust S., Wolf J., Hellhammer D.H., Federenko I., Schommer N., Kirschbaum C. The cortisol awakening response – normal values and confounds. Noise Health. 2000;2:79–88. [PubMed] [Google Scholar]