Abstract
Objective:
Cardiac irradiation during left-sided breast radiotherapy may lead to deleterious cardiac side effects. Using image guided radiotherapy, it is possible to exclude the heart from treatment fields and monitor reproducibility of virtual simulation (VS) fields at treatment delivery using electronic portal imaging (EPI). Retrospectively, we evaluate the incidence of cardiac irradiation at VS and subsequent unintended cardiac irradiation during treatment.
Methods:
Patients receiving left-sided radiotherapy to the breast or chest wall, treated with a glancing photon field technique during a four-month period, were included. VS images and EPIs during radiotherapy delivery were visually assessed. The presence of any portion of the heart within the treatment field at VS or during treatment was recorded. Central lung distance and maximum heart distance were recorded.
Results:
Of 128 patients, 45 (35.1%) had any portion of the heart within the planned treatment field. Of these, inclusion of the heart was clinically unavoidable in 25 (55.6%). Of those with no heart included in the treatment fields at VS, 41 (49.4%) had presence of the heart as assessed on EPI during treatment.
Conclusion:
Unintended cardiac irradiation during left-sided breast radiotherapy treatment occurs in a sizeable proportion of patients.
Advances in knowledge:
Despite the use of three-dimensional computed tomography simulation and cardiac shielding, sizeable proportions of patients receiving left-sided breast cancer radiotherapy have unintended cardiac irradiation.
Post-operative radiotherapy is indicated following breast-conserving surgery and following mastectomy in those with high-risk features [1]. Following breast-conserving surgery, radiotherapy to residual breast tissue has been shown to lead to a significant reduction in local recurrence rates, with an associated reduction in breast cancer-specific mortality demonstrated in more recent analyses [2]. For women at higher risk of recurrence following mastectomy, such as those with heavily node-positive disease, post-operative radiotherapy also reduces local recurrence and improves survival [2]. The acute toxicities of breast or chest wall radiotherapy are well documented and usually resolve within the first few months following completion of treatment [3,4]. Over recent years, there has been an increasing awareness of the late effects of radiotherapy, particularly when treatment is given in an adjuvant or radical setting and long-term patient survival is anticipated [5,6].
Data from randomised trials have demonstrated excess mortality from cardiovascular disease among women treated with radiotherapy for early breast cancer, particularly where local nodal areas were irradiated [2,7]. A population-based study has also reported a significant association between fatal myocardial infarction and left-sided adjuvant radiotherapy given post lumpectomy [8]. During post-operative radiotherapy for breast cancer, cardiac musculature and its associated vasculature may be irradiated directly or indirectly via scatter radiation. The risk of cardiovascular disease was shown to be higher in those patients irradiated for a left-sided breast cancer, although there is a suggestion that this increased risk may not be present with newer radiotherapy techniques [2,9–11]. Other studies report that late cardiotoxicity still occurs with modern therapy when patient follow-up is over 10 years [12].
The total dose to cardiac structures appears to affect the degree of risk, although there is uncertainty as to the identity of the critical structures. Animal studies have shown the development of atherosclerotic changes in irradiated cardiac vessels, with human post-mortem examinations revealing similar changes, along with increased fibrotic change within cardiac tissue [8,13]. The left anterior descending artery may be important as it provides the blood supply to a significant area of myocardium, and ongoing studies are considering how the dose to this, and to the other main coronary arteries, affects risk of later cardiac events [14]. The α/β ratio for late effects on the coronary vessels has not as yet been fully determined, and the underlying radiobiology of heart damage remains poorly understood [15]. In terms of cardiac function, the most significant pathological process is believed to be myocardial damage resulting from diffuse interstitial fibrosis secondary to a cytokine-driven acute inflammatory response to radiation-induced endothelial damage [16]. Fibrotic change reduces cardiac compliance, and it may impair conduction pathways and lead to valvular dysfunction. Radiation induced atherosclerosis appears to be similar to atherosclerotic changes seen in the unirradiated population, except that it may occur at a younger age and in individuals without risk factors typical for the development of coronary artery disease [17].
Although the current gold standard for calculating cardiac dose is three-dimensional computed tomography (CT) radiotherapy planning with manual contouring of cardiac structures, this facility is not yet universally available in UK radiotherapy centres. Taylor et al [14] recently demonstrated that maximum heart distance (MHD) measured on simulation images provides a reliable surrogate for estimating dose to cardiac tissue. Using visual assessment and measurement of MHD, we retrospectively evaluate the incidence and extent of any cardiac irradiation at virtual simulation (VS). As the volume of heart included in radiation fields may change during treatment owing to factors such as patient relaxation and interfractional and intrafractional variation in respiration, we also evaluate any subsequent unintended cardiac irradiation during treatment using visual assessment and MHD measurements from electronic portal imaging (EPI).
Methods and materials
In an institutionally approved study, all patients completing left-sided breast or chest wall radiotherapy during the four-month period from October 2009 to February 2010 were selected. Baseline demographics including details of surgical procedure, pathological tumour information and systemic therapy received were obtained by retrospective review of patient records. Details of prescribed radiotherapy dosage and fractionation were recorded, including details of any electron boost given to the tumour bed.
Simulation was performed in the supine position with patients immobilised using a Med-TEC thorax immobilisation board and a knee rest (Med-TEC, Orange City, IA) with both arms positioned above the head. VS was performed using GE Advantage SIM software (GE Medical Systems, Milwaukee, WI). All patients were treated using tangential pair irradiation with 6-MV photon beams. Patients receiving whole breast radiotherapy following breast-conserving surgery were treated with divergence-matched tangential fields. Half beam blocking was used in cases of chest wall radiotherapy post-mastectomy. Irradiation of the ipsilateral supraclavicular fossa with a direct anterior field was included in patients with four or more positive axillary nodes at surgery as per the departmental protocol. Our protocol also states that “the heart should be excluded from the treatment field, except in cases with close or involved deep margins, where modification of the planning target volume to avoid the heart may lead to suboptimal coverage of the margins at risk”. It was at the treating clinician's discretion whether to modify the treatment field boundaries or create custom cardiac shielding with multileaf collimators (MLCs) in cases where the heart was included in the treatment field unnecessarily.
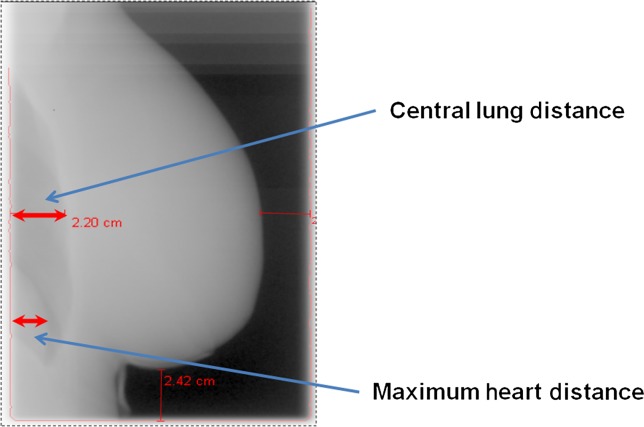
VS CT and digitally reconstructed radiograph (DRR) images for each patient were visually assessed by a therapeutic radiographer and clinical fellow in clinical oncology to determine if there was inclusion of the heart at treatment simulation. This assessment was undertaken jointly for each patient and agreement reached between the two observers for each of the parameters recorded. Central lung distance (CLD) and MHD were measured. The MHD has been defined as “the maximum distance between the anterior cardiac contour and the posterior tangential field border” [14] and is demonstrated in Figure 1.
Figure 1.
Maximum heart distance and central lung distance as viewed on electronic portal imaging during radiotherapy treatment.
Electronic portal images (EPIs) were obtained on Days 1, 2 and 3 of treatment and once weekly thereafter as per departmental policy. An EPI with a cine loop during treatment delivery was obtained on Day 3. All EPIs, including each frame from the Day 3 cine images, were assessed by the same therapeutic radiographer and clinical fellow who had interpreted the DRRs. The presence of any portion of the heart within the treatment field was recorded and CLD and MHD were measured for each image, with the two observers reaching a consensus for each measurement. Pathological information regarding surgical margins was analysed for any patient where there was inclusion of the heart within the treatment field on one or more recorded images to assess any potential reasons for inclusion of the heart within the treatment field. A significance level of 0.05 was used. Pearson’s correlation coefficient (r) was used to assess the significance of any correlation, the t test was used to estimate differences between mean values and the χ2 test was used to compare categorical data between two independent groups of data. Calculations were performed using SPSS® v. 15.0.1.1 (SPSS, Chicago, IL). Descriptive statistics were used where appropriate.
Results
132 patients were identified as having received left-sided radiotherapy for breast cancer during the study period. In one of these patients, the treatment was palliative in intent, the remainder received adjuvant radiotherapy following primary breast surgery. Full data were available for analysis in 128 patients; there was one male patient, and the median age at time of radiotherapy was 58.5 years, with a range of 29–87 years. 94 (73%) patients had breast-conserving surgery. As adjuvant therapy, 45 patients received an anthracycline-based adjuvant chemotherapy regimen, and eight were to receive adjuvant trastuzumab for 12 months following radiotherapy. The majority of patients (61%) were treated with a hypofractionated regimen of 40 Gy in 15 daily fractions, with the remainder receiving 50 Gy in 25 daily fractions. An electron boost to the tumour bed was delivered to 39 patients.
Of the 128 patients analysed, 45 (35.1%) had any portion of the heart included within the planned treatment field at simulation. For 25 of the 45 patients who had inclusion of the heart at simulation, inclusion of the heart was clinically unavoidable owing to close or involved surgical margins. Of the 83 patients for whom there was no inclusion of the heart within the treatment fields at simulation, 41 had evidence of the heart within treatment fields during radiotherapy delivery as assessed on EPIs obtained during treatment. This accounted for 32% of all patients assessed; therefore, almost one-third of the study group received unintended cardiac irradiation. Of note, of the 45 patients with the heart present at simulation, only 1 patient had no heart present as observed on EPI during treatment.
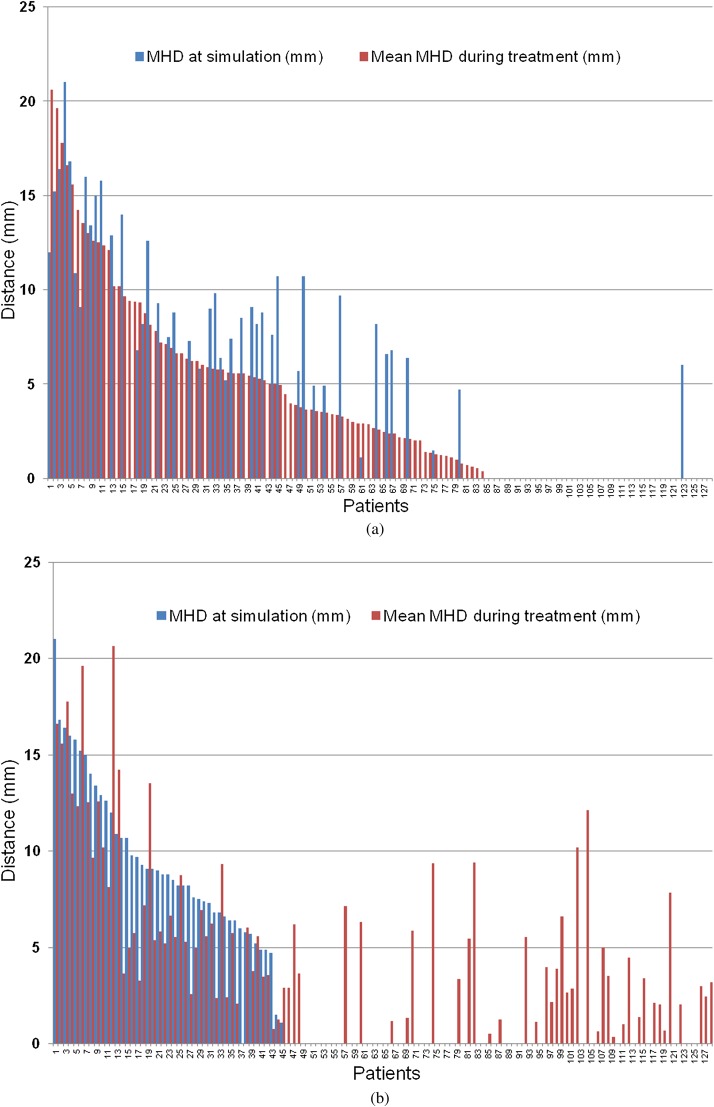
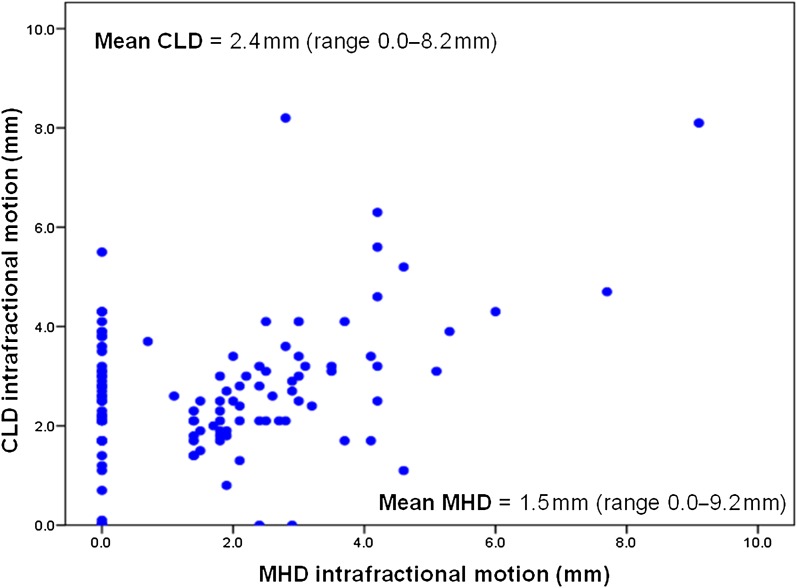
83 of the total 128 patients in this study had presence of the heart within the treatment fields as visualised on EPI at some point during their treatment. For all patients the mean MHD as measured on EPI was 3.9 mm (range 0–20.3 mm). The results for MHD at simulation and the average MHD during treatment for all patients are illustrated in Figure 2. Table 1 summarises the MHD results and associated treatment characteristics. In those patients with the heart included in the treatment fields at simulation, the heart was present during treatment in 91% of EPIs, with an average MHD of 8.36 mm [standard error (SE) ±0.51]. For those patients with no heart included at simulation but in whom the heart was present during treatment, the heart was present in 67.8% of EPIs, with an average MHD of 3.87 mm (SE ±0.51), which was significantly different from those with the heart present at simulation (p=0.0001). For all patients, and as expected, there was a significant positive correlation between MHD and CLD (Pearson’s correlation r=0.525, p<0.001), and interfractional motion was observed. Sizeable intrafractional motion was also detected on review of Day 3 cine loop EPIs (Figure 3).
Figure 2.
(a) Histogram illustrating the average (mean) measurement of the maximum heart distance (MHD) recorded for each patient during treatment as assessed on electronic portal imaging (EPI) and the associated MHD as measured on the digitally reconstructed radiograph (DDR) at simulation for each patient. The patients have been sorted in order of descending average MHD during treatment. (b) Histogram illustrating the average (mean) measurement of the MHD recorded during treatment as assessed on EPI and the associated MHD as measured on the DDR at simulation for each patient. The patients have been sorted in order of descending MHD at simulation.
Table 1.
Average maximum heart distance (MHD) during treatment groupings and associated relevant treatment characteristics
| Average MHD | Number of patients (n) | Number of patients with breast-conserving surgery n (%) | Number of patients with heart present at simulation n (%) | Number of patients treated with 3-week fractionation n (%) | Number of patients treated with a 5-week fractionation n (%) |
| 0.0–4.9 mm | 85 | 67 (78.8) | 15 (17.6) | 61 (71.8) | 24 (28.2) |
| 5.0–9.9 mm | 29 | 18 (62.1) | 18 (62.1) | 10 (34.4) | 19 (65.5) |
| 10.0–25.0 mm | 14 | 9 (64.3) | 12 (85.7) | 7 (50) | 7 (50) |
Figure 3.
Intrafraction motion for individual patients based on Day 3 cine image analysis. The range between the maximum and the minimum value for central lung distance (CLD) and the mean maximum heart distance (MHD) as viewed on the Day 3 cine images was used to provide a measure of intrafractional motion for each patient. The scatter plot illustrates the intrafractional motion values for each case, with the mean values denoted.
Comparing those patients who received radiotherapy following breast-conserving surgery with those who received radiotherapy after mastectomy, there was no significant difference in cardiac involvement between these subsets of patients (Table 2). To assess the variation in cardiac irradiation over the duration of a patient’s treatment, the MHD as measured on the Day 1 EPI was compared with the MHD on the last EPI image acquired for all patients (n=128). On Day 1, 66 patients had any degree of cardiac irradiation as compared with 64 patients at the last cine image. The average MHD on Day 1 was 4.36 (SE ±0.49) and this fell significantly to 3.72 (SE ±0.42) (p=0.033, two-tailed). There was strong positive correlation between the Day 1 and the last day EPI MHD measurements (Pearson’s correlation r=0.791, p<0.001).
Table 2.
Impact of surgery type on the presence of the heart at simulation and during treatment
| Presence of heart at simulation/during treatment | Mastectomy | Breast-conserving surgery | Significance of difference between groups | |
| n (%) | n (%) | |||
| Heart present during simulation | Yes | 11 (37.9) | 31 (33.0) | p=0.623 |
| No | 18 (62.1) | 63 (67.0) | ||
| Heart present during treatment | Yes | 21 (72.4) | 60 (63.8) | p=0.394 |
| No | 8 (27.6) | 34 (36.2) | ||
All 41 of the subgroup of patients who had evidence of unintentional cardiac irradiation as determined from EPI assessment were female. All received radiotherapy as an adjuvant treatment, three patients for a diagnosis of ductal carcinoma in situ and the remainder for invasive breast carcinoma. Seven patients, out of the total 128 patients, had custom cardiac shielding with MLCs designed at simulation. Six of these patients with custom cardiac shielding had complete avoidance of the heart on simulation images; however, four of these six patients had presence of the heart within the treatment field when EPIs were assessed.
Discussion
In our study population, 35% of patients had planned inclusion of the heart within the treatment field. In more than half of these cases, irradiation of a portion of the heart was unavoidable owing to close or involved surgical margins. Determination of the reason for inclusion of the heart within the treatment fields at simulation in the remainder of this patient group is outside the scope of our study. Of those patients whose radiotherapy plan avoided inclusion of the heart at simulation, 49% had unintended irradiation of the heart during treatment on review of EPIs recorded during treatment. Given the concerns regarding long-term cardiac toxicity from treatment, and that these risks may still be present with newer radiotherapy techniques, it is imperative that any dose to cardiac structures is minimised during adjuvant radiotherapy. This is particularly important as the increasing use of other cardiotoxic agents, such as anthracycline-based chemotherapy and trastuzumab as part of adjuvant therapy, may increase the risk of cardiotoxicity [6]. Our study highlights that a sizeable proportion of patients receive unintended cardiac irradiation. We suggest that in patients with left-sided breast cancers requiring adjuvant radiotherapy consideration should be given to advanced practice methods that allow direct cardiac irradiation to be reduced or avoided.
In our centre, the use of breast boards for simulation and treatment is standard practice. These simple devices are estimated to reduce mean cardiac dose by around 60%, and they also reduce by four times the cardiac volume that falls within the 50% isodose when compared with the use of a flat couch [18,19]. Delivering radiotherapy only when the patient is in deep inspiration with aid of a respiratory gating device has also been shown to significantly reduce the volume of cardiac tissue irradiated [20]. Use of the lateral decubitus position for electron boost fields may reduce dose to critical organs, including the heart and lungs [21]. Intensity modulated radiotherapy (IMRT) is gaining increasing acceptance as a treatment technique in many disease sites and is becoming more widely available within the UK [22,23]. Its application in adjuvant breast radiotherapy has been studied with regard to impact on cardiac irradiation, and it has been reported that the use of IMRT leads to a significant reduction in maximal dose to the left ventricle, which Lohr et al. believe could lead to a significant reduction in cardiac mortality [24]. IMRT may, however, lead to a larger volume of cardiac tissue receiving lower doses of radiation than conventional planning [24]. Given the continued uncertainty regarding the identity of the critical cardiac structures and appropriate dose constraints, further outcome data will be needed to assess the impact of IMRT on cardiac mortality.
Prone positioning is another potential treatment technique that may minimise dose to cardiac tissues. However, in a dosimetric study of prone radiotherapy where the impact of prone planning on heart and left anterior descending coronary artery doses was assessed, the impact of prone planning was variable. The effect of prone planning appeared to be dependent on the size of the breast tissue irradiated. A significant reduction in heart doses was seen in women with a larger breast volume and an increase in the dose to the heart observed in women with a smaller breast volume when the prone plans were compared with a supine planning technique [25]. An interesting finding from our study is the change in MHD during treatment with a significant reduction between the first and the last EPI MHD measurements. Perhaps, this may be a result of patient relaxation as treatment progresses, which may in turn be having the effect of positioning of the heart away from the treatment field. This finding may also reflect a reduction in respiratory motion amplitude as the patient relaxes, with the potential effect of reducing the amount of heart receiving treatment. The impact of strategies to help patients relax during treatment and thus improve patient alignment during treatment could be the focus of separate investigation.
In our study, good correlation between MHD and CLD was observed. This suggests that the MHD measurement of the proportion of the heart within the treatment field is feasible, and the correlation with CLD, which is an established method of assessing patient set-up during treatment, gives some reassurance that visual assessments of cardiac inclusion performed well. Both interfractional and intrafractional motion was observed, as evidenced by the mean range of both CLD and MHD illustrated in Figure 3. This has implications for the delivery of treatments that employ cardiac shielding. This suggests that, to attain the benefit of cardiac shielding, treatment must be subsequently delivered with assessment of daily patient set-up, the ability to correct for set-up errors and some management of respiratory motion.
The inclusion of modest patient numbers treated only in one centre is a limitation of our study. Although only two members of staff assessed the EPIs from each patient with the potential bias this may introduce, it was hoped that joint recording would minimise interobserver variation and improve consistency of recording. A further limitation is that a significant proportion of patients had only a small MHD included within the radiotherapy treatment field, and the clinical significance of inclusion of small parts of the heart is not fully understood [26]. Finally, although the MHD has been demonstrated in a previous study to provide a reliable surrogate for estimation of the dose to cardiac structures, our study did not include a calculation of the actual dose delivered to the cardiac structures during treatment [14]. It is also important to note that the use of MHD to predict for mean heart dose has been demonstrated only in women with no cardiac shielding. Seven patients in our study group had custom cardiac shielding created at simulation, and the use of MHD cannot therefore be assumed to be applicable to these patients. In the further investigation of unintended cardiac irradiation during treatment, images collected from cone beam imaging may permit a more accurate dose assessment than those gleaned from EPIs, and this may be a focus of further investigation.
Conclusion
Despite the exclusion of the heart from direct irradiation at radiotherapy treatment planning, a sizeable proportion of patients had unintended cardiac irradiation in this study. Advanced treatment techniques that permit reproducible cardiac shielding during breast radiotherapy treatments should be considered for patients with left-sided breast cancers.
References
- 1.National Institute for Health and Clinical Excellence NICE Clinical Guideline 80. Early and locally advanced breast cancer: diagnosis and treatment. NICE, 2009 [accessed 16 June 2012]. Available from: http://www.nice.org.uk/nicemedia/live/12132/43312/43312.pdf [Google Scholar]
- 2.Clarke M, Collins R, Darby S, Davies C, Elphinstone P, Evans E, et al. Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: an overview of the randomized trials. Lancet 2005;366:2087–106 [DOI] [PubMed] [Google Scholar]
- 3.Whelan TJ, Levine M, Julian J, Kirkbride P, Skingley P. The effects of radiation therapy on quality of life of women with breast carcinoma: results of a randomized trial. Ontario Clinical Oncology Group. Cancer 2000;88:2260–6 [PubMed] [Google Scholar]
- 4.Fiets WE, van Helvoirt RP, Nortier JWR, van der Tweel I, Struikmans H. Acute toxicity of concurrent adjuvant radiotherapy and chemotherapy (CMF or AC) in breast cancer patients. A prospective, comparative, non-randomised study. Eur J Cancer 2003;39:1081–8 [DOI] [PubMed] [Google Scholar]
- 5.Lind P. Clinical relevance of pulmonary toxicity in adjuvant breast cancer irradiation. Acta Oncol 2006;45:13–15 [DOI] [PubMed] [Google Scholar]
- 6.Healey Bird BRJ, Swain SM. Cardiac toxicity in breast cancer survivors: review of potential cardiac problems. Clin Cancer Res 2008;14:14–24 [DOI] [PubMed] [Google Scholar]
- 7.Early Breast Cancer Trialists’ Collaborative Group Favourable and unfavourable effects on long-term survival of radiotherapy for early breast cancer: an overview of the randomized trials. Lancet 2000;355:1757–70 [PubMed] [Google Scholar]
- 8.Paszat LF, Mackillop WJ, Groome PA, Schulze K, Holowaty E. Mortality from myocardial infarction following postlumpectomy radiotherapy for breast cancer: a population-based study in Ontario, Canada. Int J Radiat Oncol Biol Phys 1999;43:755–62 [DOI] [PubMed] [Google Scholar]
- 9.Paszat LF, Mackillop WJ, Groome PA, Boyd C, Schulze K, Holowaty E. Mortality from myocardial infarction after adjuvant radiotherapy for breast cancer in the surveillance, epidemiology, and end-results cancer registries. J Clin Oncol 1998;16:2625–31 [DOI] [PubMed] [Google Scholar]
- 10.Darby S, McGale P, Peto R, Granath F, Hall P, Ekbom A. Mortality from cardiovascular disease more than 10 years after radiotherapy for breast cancer: nationwide cohort study of 90 000 Swedish women. BMJ 2003;326:256–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Woodward WA, Strom EA, McNeese MD, Perkins GH, Outlaw EL, Hortobagyl GN, et al. Cardiovascular death and second non-breast cancer malignancy after postmastectomy radiation and doxorubicin-based chemotherapy. Int J Radiat Oncol Biol Phys 2003;57:327–35 [DOI] [PubMed] [Google Scholar]
- 12.Demirci S, Nam J, Hubbs JL, Nguyen T, Marks LB. Radiation-induced cardiac toxicity after therapy for breast cancer: interaction between treatment era and follow-up duration. Int J Radiat Oncol Biol Phys 2009;73:980–7 [DOI] [PubMed] [Google Scholar]
- 13.Gyenes G. Radiation-induced ischemic heart disease in breast cancer—a review. Acta Oncol 1998;37:241–6 [DOI] [PubMed] [Google Scholar]
- 14.Taylor CW, McGale P, Povall JM, Thomas E, Kumar S, Dodwell D, et al. Estimating cardiac exposure from breast cancer radiotherapy in clinical practice. Int J Radiat Oncol Biol Phys 2009;73:1061–8 [DOI] [PubMed] [Google Scholar]
- 15.Stewart JR, Fajardo LF, Gillette SM, Constine LS. Radiation injury to the heart. Int J Radiat Oncol Biol Phys 1995;31:1205–11 [DOI] [PubMed] [Google Scholar]
- 16.Rodemann HP, Bamberg M. Cellular basis of radiation-induced fibrosis. Radiother Oncol 1995;35:83–90 [DOI] [PubMed] [Google Scholar]
- 17.Basavaraju SR, Easterly CE. Pathophysiological effects of radiation on atherosclerosis development and progression, and the incidence of cardiovascular complications. Med Phys 2002;29:2391–403 [DOI] [PubMed] [Google Scholar]
- 18.Canney PA, Deehan C, Glegg M, Dickson J. Reducing cardiac dose in post-operative irradiation of breast cancer patients: the relative importance of patient positioning and CT scan planning. Br J Radiol 1999;72:986–93 [DOI] [PubMed] [Google Scholar]
- 19.Canney PA, Sanderson R, Deehan C, Wheldon T. Variation in the probability of cardiac complications with radiation technique in early breast cancer. Br J Radiol 2001;74:262–5 [DOI] [PubMed] [Google Scholar]
- 20.Korreman SS, Pedersen AN, Nottrup TJ, Specht L, Nystrom H. Breathing adapted radiotherapy for breast cancer: comparison of free breathing gating with the breath-hold technique. Radiother Oncol 2005;76:311–18 [DOI] [PubMed] [Google Scholar]
- 21.Ludwig MS, McNeese MD, Buchholz TA, Perkins GH, Strom EA. The lateral decubitus breast boost: description, rationale, and efficacy. Int J Radiat Oncol Biol Phys 2010;76:100–3 [DOI] [PubMed] [Google Scholar]
- 22.Staffurth J. A review of the clinical evidence for intensity-modulated radiotherapy. Clin Oncol (R Coll Radiol) 2010;22:643–57 [DOI] [PubMed] [Google Scholar]
- 23.Mayles WP. Survey of the availability and use of advanced radiotherapy technology in the UK. Clin Oncol (R Coll Radiol) 2010;22:636–42 [DOI] [PubMed] [Google Scholar]
- 24.Lohr F, El-Haddad M, Dobler B, Grau R, Wertz HJ, Kraus-Tiefenbacher U, et al. Potential effect of robust and simple IMRT approach for left-sided breast cancer on cardiac mortality. Int J Radiat Oncol Biol Phys 2009;74:73–80 [DOI] [PubMed] [Google Scholar]
- 25.Kirby AM, Evans PM, Donovan EM, Convery HM, Haviland JS, Yarnold JR. Prone versus supine positioning for whole and partial-breast radiotherapy: a comparison of non-target tissue dosimetry. Radiother Oncol 2010;96:178–84 [DOI] [PubMed] [Google Scholar]
- 26.Sardaro A, Petruzzelli MF, D’Errico MP, Grimaldi L, Pili G, Portaluri M. Radiation-induced cardiac damage in early left breast cancer patients: risk factors, biological mechanisms, radiobiology, and dosimetric constraints. Radiother Oncol 2012;103:133–42 [DOI] [PubMed] [Google Scholar]