Abstract
Objective:
Endoscopic retrograde cholangiopancreatography (ERCP) is a common procedure that combines the use of X-ray fluoroscopy and endoscopy for examination of the bile duct. Published data on ERCP doses are limited, including staff eye dose from ERCP. Occupational eye doses are of particular interest now as the International Commission on Radiological Protection (ICRP) has recommended a reduction in the dose limit to the lens of the eye. The aim of this study was to measure occupational eye doses obtained from ERCP procedures.
Methods:
A new eye lens dosemeter (EYE-D™, Radcard, Krakow, Poland) was used to measure the ERCP eye dose, Hp(3), at two endoscopy departments in Ireland. A review of radiation protection practice at the two facilities was also carried out.
Results:
The mean equivalent dose to the lens of the eye of a gastroenterologist is 0.01 mSv per ERCP procedure with an undercouch X-ray tube and 0.09 mSv per ERCP procedure with an overcouch X-ray tube. Staff eye dose normalised to patient kerma area product is also presented.
Conclusion:
Staff eye doses in ERCP have the potential to exceed the revised ICRP limit of 20 mSv per annum when an overcouch X-ray tube is used. The EYE-D dosemeter was found to be a convenient method for measuring lens dose. Eye doses in areas outside of radiology departments should be kept under review, particularly in light of the new ICRP eye dose limit.
Advances in knowledge:
Occupational eye lens doses from ERCP procedures have been established using a new commercially available dedicated Hp(3) dosemeter.
Endoscopic retrograde cholangiopancreatography (ERCP) is a common interventional radiology (IR) procedure that is used for examination of the pancreatic duct and bile ducts. ERCP was initially used as a purely diagnostic procedure; however, over the last two decades, therapeutic applications have been developed [1, 2]. Therapeutic procedures typically require longer fluoroscopy times (than diagnostic procedures) and result in a higher radiation dose [3]. During interventional ERCP procedures, fluoroscopic and radiographic images are taken, with staff positioned near the patient [4]. This arrangement, as for all IR procedures, will constitute a radiation risk to endoscopic staff in the vicinity of the patient.
Radiation dose from ERCP
In comparison with other IR/interventional cardiology (IC) procedures, data on staff doses from ERCP are limited and, where they exist, dose estimates vary greatly [1, 3, 5, 6]. Recent publications on extremity and eye doses from ERCP have shown that there is potential for high staff doses, particularly if the X-ray tube is positioned over the operating table (overcouch) [3, 5, 7, 8].
Use of radiation outside the radiology department
ERCP procedures are carried out by a gastroenterologist (GE) and may be performed outside the radiology department (e.g. operating theatre or endoscopic suite) [9]. It is a requirement of the Euratom 97/43 directive [10] that staff performing the practical aspects of a medical exposure should have received adequate training in radiation protection. However, the GE may not have had in-depth training in radiation management using diverse forms of fluoroscopic equipment nor in the potential harmful effects to patients and staff [1, 2, 11].
Revised ICRP dose limit
In April 2011, the International Commission on Radiological Protection (ICRP) published a statement on tissue reactions [12]. For the lens of the eye, the threshold in absorbed dose for tissue reaction effects (radiation-induced cataracts) is now considered to be 0.5 Gy. Based on this new threshold, the ICRP has recommended an equivalent dose limit for the lens of the eye of 20 mSv (with scope to average over defined periods of 5 years). This is a considerable reduction from the previous equivalent dose limit of 150 mSv [13]. While the need for improved eye lens dosimetry has been acknowledged, there has been much commentary regarding the practical implications of this new limit for medical radiation protection [14–18].
Measurement of staff eye lens doses
The ICRU operational quantity Hp(3) is used to monitor dose to the lens of the eye [19]. However, in practice, Hp(3) is not measured and estimates based on the ICRU operational quantity Hp(0.07) are often used [20]. The need for reliable determination of eye doses was investigated by the European Union (EU) Optimization of Radiation protection for Medical staff (ORAMED) project [21], which concluded that specific conversion coefficients for eye lens dosimetry were not available. The ORAMED project team went on to develop these conversion coefficients along with proposals for calibration conditions and the first dosemeter dedicated to measuring Hp(3) [18, 22, 23].
Based on (1) the lack of data on staff doses from ERCP procedures, (2) the need for more radiation protection focus on areas outside the radiology department, (3) the revised ICRP eye dose limit and (iv) the availability of a new Hp(3) dosemeter, this study was undertaken to investigate these issues. The primary aim was to obtain an accurate measure of occupational Hp(3) eye doses during ERCP procedures and to assess these data in the context of the revised ICRP lens limit. Along with the measurement of eye doses, a review of radiation protection arrangements at two ERCP facilities was carried out.
Methods and materials
Occupational eye doses from ERCP procedures at two endoscopy departments in Ireland were measured over a 6-week period during June and July 2011.
Clinical settings and room shielding
Two acute public hospitals were asked to participate in the study. Both hospitals have an ERCP workload of approximately 130 cases per GE per year, which is comparable to published values [1, 3]. The two hospitals, however, have quite different ERCP facilities.
Hospital A is an academic teaching hospital with a large dedicated X-ray room within the endoscopy department. The X-ray room is equipped with a fixed image-intensifier (II)-TV fluoroscopy X-ray system with an undercouch X-ray tube (Mecall Mecascope; Mecall SRL, Lissone, Italy). This hospital is a national tertiary referral centre for ECRP in Ireland. The size of the X-ray room (36 m2) is reasonably similar to recommended guidelines (for specialised radiology rooms, a range spanning from 38 m2 to 50 m2 has been recommended) [24, 25]. There is a ceiling-mounted lead glass screen that is usually (but not always) positioned in front of the GE. There are also lead drapes in front of the II-TV fluoroscopy X-ray system and tableside lead shields. The radiographer stands behind a fixed protective lead glass screen, which is sufficiently large to accommodate two or three people.
Hospital B is a large regional academic hospital. It is an ERCP referral centre for the region. The room used for ERCP cases in Hospital B is a small lead-lined procedure room, which is also used for other endoscopy procedures and is not solely dedicated to X-ray use. A mobile C-arm II-TV fluoroscopy system (OEC Flexiview 8800™; GE Healthcare, Chalfont St Giles, UK) is brought into the room for ERCP cases. The size of the room is very restrictive (16.75 m2). There is no fixed or mobile lead glass screen for staff protection; however, the radiographer is able to stand a reasonable distance back from the patient table and use the foot pedal to operate the fluoroscopy system. There is no ceiling-mounted screen present in this room. A lead skirt is fitted to the patient trolley for lower limb protection. It was intended that the C-arm would be used with undercouch geometry; however, the X-ray tube on this system is used overcouch.
The X-ray systems in both hospitals had recently undergone routine quality assurance testing and were operating satisfactorily based on recommended EU/UK standards.
Personal protective equipment and personal monitoring
All ERCP staff included in this study wore personal protective equipment (PPE) in the form of lead aprons (0.25–0.5 mm lead equivalence) and a thyroid collar (0.5 mm lead equivalence). None of the staff wore lead glasses. Personal monitoring with whole-body thermo-luminescent dosemeters (TLDs) has already been carried out in accordance with legal requirements in Ireland [26]. The staff in Hospital A are issued with two dosemeters, one to be worn on the trunk under the lead apron and one to be worn at the neck level unprotected (outside the thyroid collar). The staff in Hospital B are issued with one dosemeter to be worn on the trunk under the lead apron.
EYE-D dosemeter specifications
In addition to the personal monitoring already in place, a new dedicated eye lens dosemeter (EYE-D™) was issued to the staff participating in this study. The EYE-D was developed as part of the EU ORAMED project, specifically to measure eye lens dose or Hp(3) [22]. It was designed and manufactured by RADCARD (Krakow, Poland) and is commercially available. The EYE-D unit consists of a TLD pellet (MCP-N, LiF:Mg, Cu, P), a plastic capsule and an adjustable headband. The dosemeter is sensitive to low doses (specified range: 10 μSv–10 Sv). It has a satisfactory photon energy response and angular response (within 20%, 30 keV–1.3 MeV). The ORAMED partners have stated that it fulfils all requirements for its application in IR [22].
Calibration and readout
The supply, calibration and readout of the EYE-D dosemeters were provided by the laboratory at RADCARD and arranged via their distributor, RadPro International GmbH (Wermelskirchen, Germany). 20 dosemeters were purchased for staff measurements, and an additional 10 dosemeters were purchased for use as reference dosemeters (to remain unirradiated). All 30 dosemeters were calibrated at the RADCARD laboratory in Poland before use. They were calibrated using an ICRU slab phantom (30×30×15 cm) with Cs-137. Ideally the dosemeters would have been calibrated using the newly developed cylindrical phantom, which is more representative of the head [18, 23].
Following the 6-week measurement period, all dosemeters (including reference dosemeters) were returned to RADCARD in Poland to be read out.
Eye dose protocol
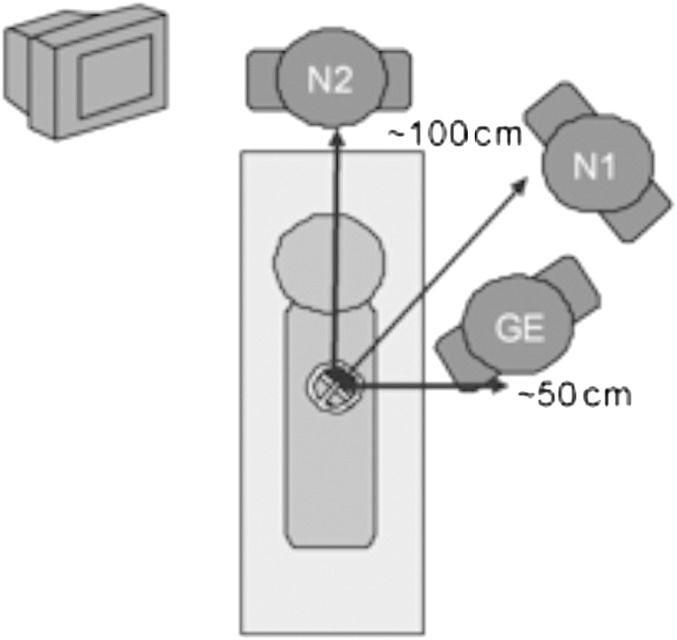
Several ERCP cases were observed by the authors at both Hospital A and B. The position of staff (within the X-ray room) in both hospitals was comparable, and the typical arrangement with approximate distances from the patient can be seen in Figure 1. For all ERCP cases, the patient remains in a recumbent left-lateral position. It was clear that the EYE-D should be worn at the outer edge of the left eye, as this side of the GE is closest to the patient and should receive the worst-case eye dose. The nurses were advised to wear the dosemeter close to their left eye also for consistency, but it should be noted that the nurses move around during the procedure and do not remain in one fixed position.
Figure 1.
Sketch of typical staff positions during endoscopic retrograde cholangiopancreatography procedures (GE, gastroenterologist; N1, nurse assisting the GE; N2, nurse assisting the patient).
Sample size of staff
In Hospital A, there are five consultant GEs and five registrar doctors in training who perform all the ERCPs. In Hospital B, there are two consultant GEs who perform all the ERCPs. All GEs (n=12) were issued with their own unique EYE-D dosemeter, and the ID number of their dosemeter was recorded for tracking purposes.
In both hospitals, there are a large number of nursing staff who rotate their duties in the ERCP room. A significant number of dosemeters would have been required to provide individual monitoring of nursing staff. It was also possible that, owing to the rotation of duties, the dose recorded would have been negligible. Therefore, nursing staff were assigned a dosemeter to wear based on whether they were assisting the GE (Nurse 1) or assisting the patient (Nurse 2). These two nursing roles were assigned a dosemeter in both Hospital A (n=2) and Hospital B (n=2). Again the unique ID number of these dosemeters was recorded.
Five reference dosemeters were left (unirradiated) at each hospital (n=10). Prior to the start of each ERCP case, the radiographer/nurse in charge issued the EYE-D dosemeters to the relevant staff. A cumulative dose reading over the 6-week measurement period was obtained.
Patient dosimetry
The focus of this study was occupational eye lens dosimetry. Nonetheless, as staff exposure is strongly correlated with patient exposure, a record of kerma area product (KAP) (Gy cm2) and fluoroscopy screening time (s) was obtained for each examination. A distinction was not made in this study between diagnostic and therapeutic examinations. Both sets of X-ray equipment used have KAP meters installed at the X-ray tube housing. The accuracy of the KAP meters was confirmed to be within the recommended tolerance [27].
Results
Radiation protection measures that are in place in the two ERCP facilities are shown in Table 1. This information was used in conjunction with the results of eye lens dosimetry to assess the radiation risk to staff carrying out ERCP procedures.
Table 1.
Radiation protection arrangements at two endoscopic retrograde cholangiopancreatography facilities
| Hospital A | Hospital B |
| Fixed X-ray system | Mobile C-arm X-ray system |
| Undercouch X-ray tube | Overcouch X-ray tube |
| Ceiling-mounted lead glass screen | No ceiling-mounted lead glass screen |
| Tableside lead shielding | Tableside lead shielding |
| Lead aprons worn | Lead aprons worn |
| Lead thyroid collars worn | Lead thyroid collars worn |
| No lead glasses | No lead glasses |
| Fixed protective lead screen for radiographer | No protective lead screen for radiographer |
| Large dedicated X-ray room (36 m2) | Very small room (16.75 m2) |
| Two TLDs (one under apron and one outside thyroid collar) | Single TLD (under apron) |
TLD, thermoluminescent dosemeter.
Occupational Hp(3) dose from ERCP
The results of monitoring with the EYE-D dosemeter are shown in Table 2. At Hospital A, 48 ERCP procedures in total were monitored. 10 GEs were issued with dosemeters; however, at the end of the 6-week monitoring period, it was confirmed that only 7 GEs wore the EYE-D dosemeter for ERCP cases. The dosemeters belonging to the remaining three GEs were returned from RADCARD with a zero dose reading, and these were excluded from further data analysis. In some ERCP cases at Hospital A, two GEs were present, and the role of primary operator would rotate between the two GEs as dictated by training needs. At Hospital B, 14 ERCP procedures in total were monitored. Two GEs were issued with EYE-D dosemeters, and both wore them for seven cases each.
Table 2.
Results of equivalent dose to the lens of the eye per endoscopic retrograde cholangiopancreatography (ERCP) procedure
| Staff | Mean equivalent dose to left eye Hp(3) per ERCP (mSv) | Range of equivalent dose to left eye Hp(3) per ERCP (mSv) | Number of monitored staff (n1) and the number of cases (n2) | Annuala equivalent dose to left eye, Hp(3) (mSv) |
| Hospital A | ||||
| GE | 0.01 | 0.01–0.03 | n1=7 | 1.3 |
| n2=48 | ||||
| Nurse 1 | <0.01b | — | n1=1 | <1.3b |
| n2=48 | ||||
| Nurse 2 | <0.01b | — | n1=1 | <1.3b |
| n2=48 | ||||
| Hospital B | ||||
| GE | 0.09 | 0.09–0.10 | n1=2 | 11.7 |
| n2=14 | ||||
| Nurse 1 | 0.02 | — | n1=1 | 2.6 |
| n2=14 | ||||
| Nurse 2 | 0.03 | — | n1=1 | 3.9 |
| n2=14 | ||||
GE, gastroenterologist.
Annual doses based on a typical workload of 130 cases per year.
Results limited by sensitivity of the dosemeter (10 μSv).
The results for Nurse 1 and Nurse 2 at both hospitals are also shown in Table 2. The 48 cases in Hospital A were performed with a rotation of 15 different nurses. The 14 cases in Hospital B were performed with a rotation of 7 different nurses. As only one dosemeter was assigned for the role of Nurse 1 and Nurse 2, a single result was obtained and no range is presented in Table 2. Overall, the dose to the nursing staff is less than the GE. This is to be expected owing to the increased distance from the patient and the fact that the nurses typically move around the room during the procedure. It is useful to note that the dose to Nurse 1 is comparable to that to Nurse 2. This shows that the staff member in the role of Nurse 2 (who is required at the patient’s head) does not receive a significantly different dose from the nurse who is assisting the doctor.
It can be seen from the results that the mean eye dose per ERCP is significantly higher in Hospital B (overcouch X-ray tube) than in Hospital A (undercouch X-ray tube and ceiling-mounted screen). The estimated equivalent dose to the eye of a GE working with an overcouch X-ray tube is 11.7 mSv per annum (based on a typical workload of 130 cases per GE per year). In comparison, for the same workload, using a ceiling-mounted shield and with the X-ray tube undercouch, the estimated equivalent dose to the eye of a GE is 1.3 mSv per annum.
Patient KAP from ERCP
The patient KAP per ERCP at Hospital A was measured to be 14.5 Gy cm2 (mean) and 19.6 Gy cm2 (third quartile) (patient size not recorded, both diagnostic and therapeutic cases, n=48). The third quartile result is just above the UK diagnostic reference level of 16.7 Gy cm2 [28]. In comparison, the patient KAP per ERCP at Hospital B was measured to be 5.4 Gy cm2 (mean) and 7.9 Gy cm2 (third quartile) (patient size not recorded, both diagnostic and therapeutic cases, n=14).
The mean fluoroscopy screening time was 225 s (Hospital A) and 87 s (Hospital B). The difference in patient KAP and screening time between the two hospitals may be partly attributable to the difference in patient referrals to each centre. However, further work on patient dose would be required to fully investigate other factors such as the skill of the operator, the percentage of teaching/training cases and the clinical protocols used.
Normalised dose data, Hp(3) per unit KAP
The patient dose data were used to normalise the staff dose results by KAP as shown in Table 3. An estimation of Hp(3)/KAP (mSv/Gy cm2) was calculated for four GEs. The eye dose for two GEs from Hospital A was used, as they recorded the largest Hp(3) result and carried out the greatest number of ERCP cases (n=20). Their individual cumulative Hp(3) result was divided by the total patient KAP that they were exposed to during the monitoring period. It is likely that this is an overestimation of the total patient KAP as, for some procedures, both these GEs were present in the X-ray room, and it has been assumed that they were both exposed equally to the measured patient KAP.
Table 3.
Results of occupational eye dose normalised by patient kerma area product (KAP)
| Staff | Total equivalent dose to left eye, Hp(3) (mSv) | Patient KAP (Gy cm2) | Hp(3)/KAP (mSv/Gy cm2) |
| Hospital A | |||
| GE A.1 | 0.53 | 370.85 | 1.43×10−3 |
| GE A.2 | 0.38 | 386.72 | 0.98×10−3 |
| Hospital B | |||
| GE B.1 | 0.67 | 46.1 | 14.5×10−3 |
| GE B.2 | 0.62 | 29.3 | 21.2×10−3 |
GE, gastroenterologist.
The results for the two GEs in Hospital A were compared with the normalised Hp(3)/KAP values for the two GEs in Hospital B. The results show that although the patient KAP per procedure is approximately three times higher in Hospital A, when this is taken into account, the eye dose Hp(3) per unit KAP is much lower than in Hospital B (by a factor of between 10 and 20).
Discussion
The results obtained from this study highlight the potential for staff to receive high eye lens doses in areas outside of the “typical” IR/IC setting, for example in gastroenterology departments. Staff in gastroenterology settings should be aware that key factors in reducing eye doses are (1) positioning the X-ray tube under the patient table and (2) installing and using ceiling-mounted screens. This approach is already well established in IR, and the results of this study further confirm the dose reductions that can be achieved.
Although it can be seen that overall the dose to assisting nurses is considerably lower than the dose to the GE, it is interesting to note that the nurses in Hospital B (overcouch) recorded higher doses than the GE in Hospital A. The actual dose to one individual nurse will, however, be much less owing to rotation of duties; nonetheless, the results show that there is scope to improve practice.
Patient dose (KAP) was recorded as part of this study. The results show that the staff eye dose per ERCP in Hospital B is higher than in Hospital A. However, the patient KAP per procedure is much lower in Hospital B. The eye doses in Hospital B (overcouch X-ray tube) could be considerably higher if the KAP per patient increased, owing to more complex ERCP cases or less experienced operators/trainee GEs.
The review of radiation protection practice at both hospitals identified opportunities for further optimisation of eye doses. It was clear from the radiation risk assessment and the dose recorded at Hospital B that follow-up was required. The main recommendations for this hospital were to (1) position the X-ray tube undercouch, (2) install a ceiling-mounted lead glass screen and (3) issue ERCP staff with a second TLD to be worn unprotected at the neck level to give an indication of eye dose. Also, although it does not pose an immediate risk, the size of the room is restrictive. Staff were reminded to increase their distance from the patient as much as possible without compromising clinical care.
Issues with personal monitoring compliance have also been highlighted. ERCP staff in Hospital A are already issued with a second TLD to be worn at the neck. This arrangement has been in place since the ERCP suite was first opened several years ago; however, the second TLD is not worn consistently by all staff. 30% of the GEs in Hospital A did not wear the EYE-D dosemeter that had been issued to them for this study. It is hoped that this work will raise awareness of the importance of wearing personal dosemeters and using PPE in the IR room, particularly because of new evidence regarding the lower threshold for eye damage.
Some limitations of the study are acknowledged. The period of monitoring was relatively short at 6 weeks and the number of cases monitored was low (particularly for Hospital B). The number of staff monitored was also relatively small but did encompass all the relevant GEs at both hospitals. The calibration phantom used by RADCARD for this study was a slab phantom, and a more accurate approach would be to use the newly designed cylindrical head-shaped phantom developed at Institut CEA-LIST, Gif sur Yvette, France [23].
Conclusions
Occupational eye doses from ERCP procedures may have the potential to exceed the new ICRP equivalent dose limit of 20 mSv per annum, particularly with the use of an overcouch X-ray tube. With the equipment in this configuration, a GE may also exceed the proposed threshold for Category A workers of 15 mSv to the lens of the eye. The EYE-D dosemeter was found to be a convenient method for measuring eye dose in terms of Hp(3), and it should have applications in other high-dose IR areas.
There is scope to improve radiation protection awareness in areas such as gastroenterology. Radiation risk assessments should establish the clinical configuration of the X-ray equipment (i.e. the X-ray tube used predominantly overcouch or undercouch), particularly for mobile systems used outside of radiology. In line with the established practice for IR/IC, the X-ray tube in ERCP should be undercouch and ceiling-mounted lead glass screens should be used. This has been partly achieved in Hospital B, where the X-ray tube is now positioned undercouch. Consideration should be given to the use of lead glasses by the primary operator. Lead glasses may also be advised for nurses who must remain close to the patient but who are not protected by the ceiling-mounted screen.
Compliance with dose monitoring was an issue, even during the short period of time required for this study. This is likely to persist, which will make it difficult to obtain good baseline data on eye doses in advance of the new ICRP limit being adopted into legislation and to assess compliance with the limit once it is introduced.
Future work will include carrying out repeat eye dose measurements with the new equipment configuration. Eye doses in areas outside of radiology should be kept under review, particularly in light of the new ICRP eye dose limit.
Acknowledgments
The authors are grateful to the gastroenterology teams and the ERCP radiographers in both hospitals for participating in this project. The authors also wish to thank Ms Anita Dowling, Medical Physics and Bioengineering Department, St. James’s Hospital, for her assistance.
References
- 1.Buls N, Pages J, Mana F, Osteaux M. Patient and staff exposure during endoscopic retrograde cholangiopancreatography. Br J Radiol 2002;75:435–43 [DOI] [PubMed] [Google Scholar]
- 2.Tsapaki V, Paraskeva KD, Mathou N, Andrikopoulos E, Tentas P, Triantopoulou C, et al. Patient and endoscopist radiation doses during ERCP procedures. Radiat Prot Dosimetry 2011;147:111–13 [DOI] [PubMed] [Google Scholar]
- 3.Naidu L, Singhal S, Preece D, Vohrah A, Loft D. Radiation exposure to personnel performing endoscopic retrograde cholangiopancreatography. Postgrad Med J 2005;81:660–2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cohen RV, Aldred MA, Paes WS, Fausto AMF, Nucci JR, Yoshimura EM, et al. How safe is ERCP to the endoscopist? Surg Endosc 1997;11:615–17 [DOI] [PubMed] [Google Scholar]
- 5.Sulieman A, Elzaki M, Khalil M. Occupational exposure to staff during endoscopic retrograde cholangiopancreatography in Sudan. Radiat Prot Dosimetry 2011;144:530–3 [DOI] [PubMed] [Google Scholar]
- 6.Campbell N, Sparrow K, Fortier M, Ponich T. Practical radiation safety and protection for endoscopist during ERCP. Gastrointest Endosc 2002;55:552–7 [DOI] [PubMed] [Google Scholar]
- 7.Vanhavere F, Carinou E, Domienik J, Donadille L, Ginjaume M, Gualdrini G, et al. Measurements of eye lens doses in interventional radiology and cardiology: final results of the ORAMED project. Radiat Meas 2011;46:1243–7 [DOI] [PubMed] [Google Scholar]
- 8.Nikodemova D, Brodecki M, Carinou E, Domienik J, Donadille L, Koukorava C, et al. Staff extremity doses in interventional radiology. Results of the ORAMED measurements campaign. Radiat Meas 2011;46:1210–15 [DOI] [PubMed] [Google Scholar]
- 9.National Council on Radiation Protection Radiation protection for procedures performed outside the radiology department. NCRP report no. 133. Bethesda, MD: NCRP; 2000 [Google Scholar]
- 10.Council Directive 97/43/EURATOM of 30 June 1997 on health protection of individuals against the dangers of ionizing radiation in relation to medical exposure, and repealing Directive 84/466/EURATOM. OJ L 180, 9.7.1997, pp. 22–7
- 11.European Medical ALARA Network. WG3-synthesis report. November 2010 – version 1.0 [cited 12 April 2012]. Available from: http://www.eman-network.eu/spip.php?article166 [Google Scholar]
- 12.International Commission on Radiological Protection. Statement on tissue reactions. ICRP 4825-3093-1464. Ottawa (ON): ICRP; 2011. [Google Scholar]
- 13.International Commission on Radiological Protection. The 2007 Recommendations of the International Commission on Radiological Protection. Annals of the ICRP publication 103. Oxford, UK: Pergamon Press; 2007. [DOI] [PubMed]
- 14.Martin CJ. What are the implications of the proposed revision of the eye dose limit for interventional operators? Br J Radiol 2011;84:961–2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Martin CJ. A 20 mSv dose limit for the eye: sense or no sense? J Radiol Prot 2011;31:385–7 [DOI] [PubMed] [Google Scholar]
- 16.Englefield C. Is the new ICRP eye dose limit justified? J Radiol Prot 2011;31:499–500 [DOI] [PubMed] [Google Scholar]
- 17.Society for Radiological Protection (London) Impact on the medical sector of revised dose limit for the eye. Conference Presentations; 8 February 2012 [cited 12 April 2012]. Available from: http://www.srp-uk.org/news/news-items/467-february-2012. [Google Scholar]
- 18.Gualdrini G, Mariotti F, Wach S, Bilski P, Denoziere M, Daures J, et al. Eye lens dosimetry: task 2 within the ORAMED project. Radiat Prot Dosimetry 2011;144:473–7 [DOI] [PubMed] [Google Scholar]
- 19.International Commission on Radiological Units. Quantities and units in radiation protection dosimetry. ICRU Report no. 51. Bethesda, MD; ICRU; 1993 [Google Scholar]
- 20.Martin CJ. Personal dosimetry for interventional operators: when and how should monitoring be done? Br J Radiol 2011;84:639–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.EU-ORAMED (Optimisation of Radiation protection for MEDical staff) 7th EU Framework Programme, EURATOM [cited 12 April 2012]. Available from: http://www.oramed-fp7.eu/en.
- 22.Bilski P, Bordy J-M, Daures J, Denoziere M, Fantuzzi E, Ferrari P, et al. The new EYE-D™ dosemeter for measurements of Hp(3) for medical staff. Radiat Meas 2011;46:1239–42 [Google Scholar]
- 23.Bordy JM, Gualdrini G, Daures J, Mariotti F. Principles for the design and calibration of radiation protection dosemeters for operational and protection quantities for eye lens dosimetry. Radiat Prot Dosimetry 2011;144:257–61 [DOI] [PubMed] [Google Scholar]
- 24.NHS Estates. Facilities for diagnostic imaging and interventional radiology. HBN 6. London, UK: Stationery Office; 2001. [Google Scholar]
- 25.Sutton DG, Williams JR, editors. Radiation shielding for diagnostic X-rays. Report of a joint BIR/IPEM working party. London, UK: British Institute of Radiology; 2000. [Google Scholar]
- 26.Radiological Protection Act. Order SI. 125 (2000) Dublin, Ireland: The Stationery Office; 1990. [Google Scholar]
- 27.Dosimetry Working Party of the Institute of Physical Sciences in Medicine, National Radiological Protection Board and College of Radiographers. National protocol for patient dose measurements in diagnostic radiology. Chilton, UK: National Radiological Protection Board; 1997. [Google Scholar]
- 28.Hart D, Hillier MC, Wall BF. Doses to patients from radiographic and fluoroscopic X-ray imaging procedures in the UK—2005 review. HPA-RPD-029. London, UK: Health Protection Agency; 2007. [Google Scholar]