Abstract
Objective:
Scapular free flap harvesting for oral cavity cancer reconstruction is an increasingly used and versatile option. We aim to describe the appearance of the scapula harvest site on chest radiograph and CT.
Methods:
We retrospectively reviewed a surgical database of 82 patients who underwent scapular osteocutaneous flap harvesting for oral cavity cancer reconstruction and had imaging performed at our institution. We searched the picture archiving and communications system for all associated imaging.
Results:
Characteristic radiographic appearance in the immediate post-operative period as well as in the remote post-operative period is described, including an upside-down V-shaped paraglenoid notch, rectangular (or triangular) lateral border defects and a sharply pointed inferior scapular body. Additionally, common CT appearances are discussed, including an abrupt gleno-scapular interval, an absent axillary rim bulge and a Z-shaped scapula.
Conclusion:
The altered appearance of the scapular defect following surgical harvest is easily recognised. Although the description of this defect may not alter management and may reasonably be omitted, a radiologist’s comfort with these appearances may potentially enhance the understanding of patient management and recognition of superimposed complications, such as infection.
Advances in knowledge:
Scapular osteocutaneous free flap reconstruction is an increasingly used technique after oral cavity surgery.
Very few radiologists reported in our review the surgical scapular defects, and there is apparent ignorance of their appearance.
We described characteristic radiographic and CT signs of scapular free flap harvesting to increase radiologists’ familiarity with these defects, which may provide clinical information and possibly contribute to detection of complications.
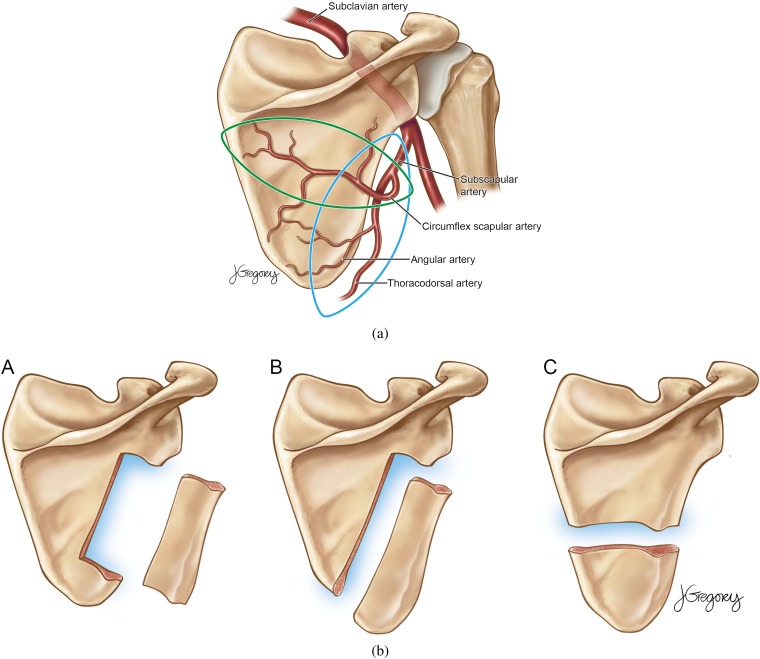
The scapular osteocutaneous flap, although first described in the 1980s [1, 2], is increasingly used for reconstruction after head and neck surgery [3, 4]. The flap offers several advantages, including ease of harvesting, an extensive and varied subscapular arterial and venous system (Figure 1a), up to 14 cm of bone, and a multitude of soft tissue as well as bone flaps (Figure 1b). The scapular tip, because of its shape, has been described as ideally suited both for mandibular angle [5] and palatomaxillary [6] reconstruction.
Figure 1.
(a) Drawing of a posterior view of the scapula demonstrating potential myocutaneous paddles that can be harvested: a more traditional circumflex artery flap (horizontal) and the newer thoracodorsal artery angular branch flap (vertical). (b) Illustrations of the three possible bone flaps, which can be combined with the myocutaneous flaps for various composite flaps. © Jill Gregory, Continuum Health Partners. Permission has been granted by the illustrator to publish this figure.
There is increasing recognition among surgeons of the versatility of osteocutaneous scapular free flaps after oral cavity surgery, even outside tertiary care centres. The option was initially used in patients with multiple surgeries whose traditional donor sites were already used. However, they are increasingly selected because they are ideally suited to repair defects of the lateral mandible that include skin, mucosal or “through-and-through” soft-tissue loss [3]. When harvesting the bone, the lateral border and inferior angle can be harvested together or separately with independent blood supplies, based on the subscapular system [1, 2]. The circumflex scapular artery provides a short associated vascular pedicle, and the angular branch of the thoracodorsal artery provides a longer pedicle [4].
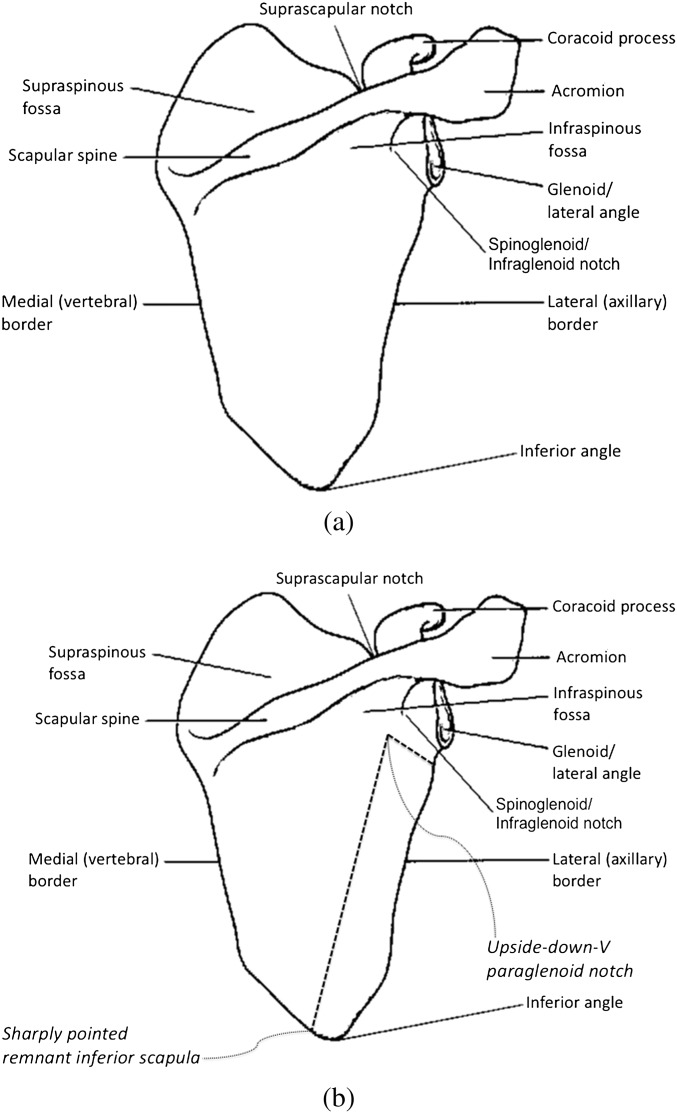
Knowledge of normal anatomy (Figure 2a) can improve the detection of surgical defects (Figure 2b). Embryogenesis of the scapula is poorly understood, with some evidence that its portions derive from different elements of the mesoderm, for instance the blade and spine originating from dermomyotomal mesenchyme and the glenoid and coracoid developing from the lateral plate mesoderm [7]. The scapula, or shoulder blade, is a thin, translucent, triangular flat bone of the posterolateral thorax, overlying the second through seventh ribs, and comprising a major portion of the shoulder girdle. The anterior or costal surface is referred to as the subscapular fossa. The posterior surface is divided by the scapular spine into a larger infraspinous fossa and a smaller supraspinous fossa. The thick horizontally oriented spine continues laterally as the acromion [Greek (G.) akros, for point]. The adjacent truncated superolateral portion of the scapula, or the lateral angle, is the glenoid tubercle, which contains the glenoid fossa (G. socket) for articulation with the humeral head. The thickest portion of the glenoid is also called the scapular head, and the thinnest portion the scapular neck. Between the glenoid and the acromion is the spinoglenoid (or infraglenoid) notch, running anteromedial and just inferior to the glenoid. The coracoid process (G. korakodes, like a crow’s beak) projects anterolaterally, above the glenoid. The lateral scapular border, also called the axillary border, is the thickest of the borders, bearing more stress than the thinner medial and superior borders. The suprascapular notch is on the superior scapular border at the base of the coracoid process, between the lateral and the middle thirds. The superior and inferior angles mark the extremes of the medial border, where many of the muscles insert [8].
Figure 2.
(a) Normal scapular anatomy. (b) Scapular anatomy with superimposed surgical defects.
Patients with these flaps commonly receive chest radiographs, both immediately following surgery and later, as well as multiple cross-sectional studies for surveillance and diagnosis, including for suspected pulmonary embolism. The altered appearance of the scapula may be easily overlooked on routine imaging or mistaken for scapular pathology in the absence of appropriate surgical history. Thus, our objective was to describe the appearance of the scapular harvest site on chest radiograph and CT, in the post-operative and later periods, and to assess how these findings have been previously reported at our institution.
METHODS
We retrospectively reviewed our surgical database of 82 patients who underwent scapular osteocutaneous flap harvesting for oral cavity cancer reconstruction at our institution between November 2004 and November 2011. We searched our picture archiving and communications system (PACS) for all associated imaging. We characterised and quantified the early and late appearances of the defects on chest radiograph and cross-sectional imaging, including multiplanar sagittal and coronal reconstructions.
2 of the 82 patients had bilateral scapular free flaps at different times, effectively increasing our population to 84. The patient population consisted of 40 males and 42 females, ranging in age from 15 to 93 years (mean of 65 and median of 66) at the time of surgery.
At least 12 (12/84=14.3%) of the patients underwent segmental mandibular resection and reconstruction owing to osteoradionecrosis of the jaw, and at least 25 (25/84=29.8%) underwent resection and reconstruction owing to recurrence of disease. Clear history was not available in 23 (23/84=27.4%) cases. The remaining 24 patients (24/84=28.6%) underwent a primary reconstruction of the oral cavity following initial cancer resection.
This retrospective Health Insurance Portability and Accountability Act-compliant study was performed after the institutional review board deemed the study to be exempt from review, not requiring patient informed consent.
RESULTS
At least two portable chest radiographs beginning on post-operative Day 1 were accessible on PACS for all but one patient who had their surgery prior to management at our institution (and only for the second operations in both patients with bilateral defects). 37 patients (37/84=44.0%) received delayed follow-up radiographs (defined as at least 1 month post surgery). 39 patients (39/84=46.4%) had additional cardiothoracic cross-sectional imaging [chest CT or positron emission tomography (PET) scans] after surgery, including 6 of the patients who did not have delayed radiographs. 7 (7/84=8.3%) patients received a chest CT within the first post-operative week for clinical suspicion of pulmonary embolism or pneumonia.
Imaging
Nine patients were subsequently imaged with conventional posterior-to-anterior and lateral projections. The indications for these radiographs were largely not provided, but they were typically performed in the outpatient as opposed to inpatient setting. Despite better evaluation of the lungs, the abduction of the scapulae away from the thorax was less suited for the recognition of the scapular resection defect when compared with the portable technique. Lateral radiographs did not prove useful in the identification of a scapular free flap resection.
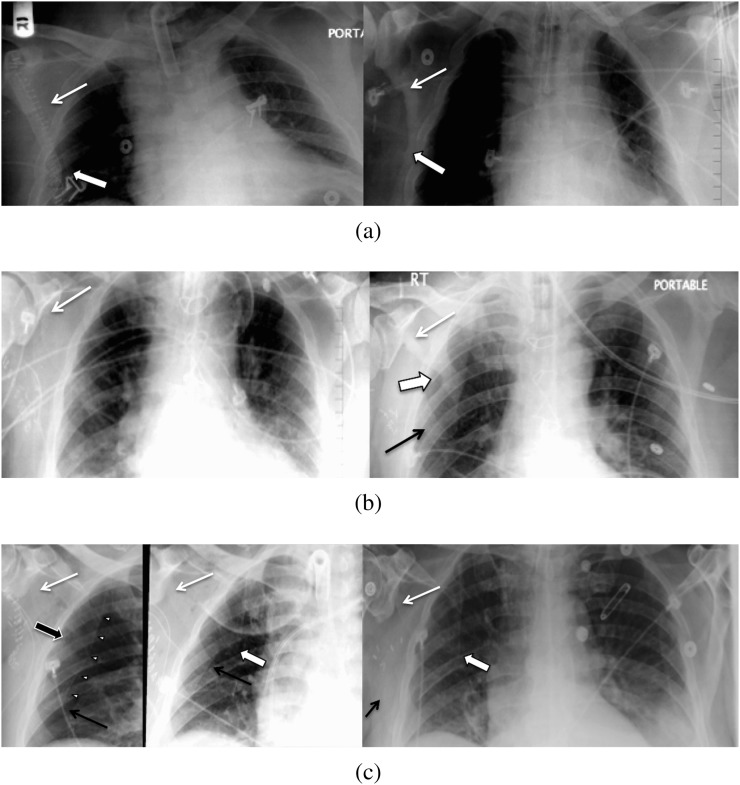
A characteristic upside-down V-shaped notch immediately medial and slightly caudal to the glenoid tubercle (which we called an upside-down-V paraglenoid notch) was the most recognisable defect on radiographs seen in 100% of patients in the immediate and more remote post-operative periods (Figure 3).
Figure 3.
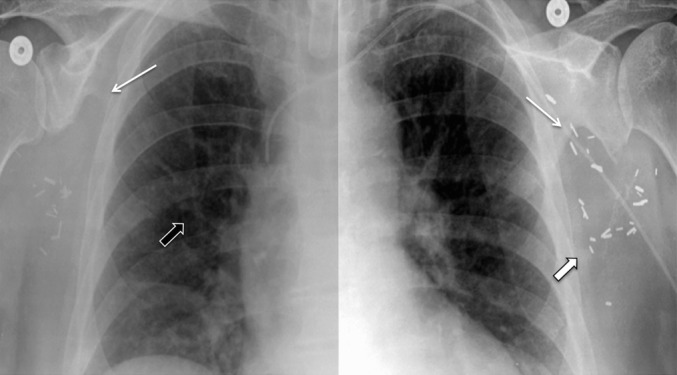
Two-week post-operative chest radiograph of a 61-year-old female with multifocal oral cancer who had a left scapular free flap oral reconstruction in 2003 and required an additional right scapular free flap reconstruction in 2010 for recurrence. The single radiograph (bisected with different brightness and contrast levels on each side) reveals bilateral paraglenoid notches (thin white arrows) with a discrete lateral scapular border (shallow triangular) defect on the left (first surgery; thick white arrow indicates inferior corner) and a sharply pointed inferior scapular body on the right (second surgery) with early callus formation (thick black arrow).
The lateral border of the scapula was ill-defined, with varying degrees of lucency in comparison with the well-corticated, rounded and thickened lateral border of the normal scapula, in the immediate post-operative period in 100% of patients (Figures 2 and 3). Increased conspicuity of the lateral and inferior scapula, in the later post-operative period, revealed different appearances of the residual bone (relating both to the choice of vascular pedicle and the surgical utility of the inferior tip in reconstruction; Figures 1b and 3).
The most common appearance of the inferior border, a sharply pointed remnant inferior scapula (with a somewhat shark-tooth appearance; Figure 4), was seen in 49 patients (49/84=58.3%) and was best recognised on follow-up imaging. An additional 6 cases (6/84=7.1%) were considered likely to have this feature, allowing for the lack of imaging beyond the first few post-operative days. This feature was best recognised on early radiographs by the lack of a well-defined and rounded inferior scapular angle, asymmetric in comparison with the contralateral side, seen in a total of 55 cases (55/84=65.5%; Figure 4). A relative generalised lucency of one hemithorax was also an indirect sign of the resection.
Figure 4.
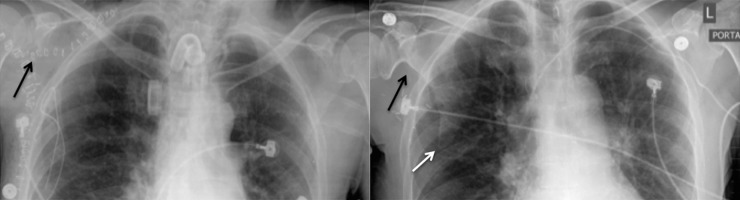
51-year-old female with oral cavity cancer requiring a right scapular free flap reconstruction with immediate post-operative and 1-year follow-up chest radiographs. An upside-down-V paraglenoid notch is present, both early and late (black arrows), but becomes more conspicuous on the later radiographs. Haziness of the lateral border and the remnant scapular body become better defined upon follow-up, with lateral border callus formation and a sharply pointed inferior scapular body remnant (white arrow).
Less often, the remnant inferior scapular tip was preserved, seen in 26 cases (26/84=31%). In 18 of these cases (18/84=21.4%), a second inferior 90° angle notch was perceived on the lateral scapular border, creating a well-defined rectangular defect (Figure 5a). Interestingly, the remnant tip seemed particularly prone to propagation of the defect, or fracture, particularly when there was a more pronounced or deeper triangular lateral wall defect, seen in the remaining 8 of those 26 patients (8/84=9.5%; Figure 5b). 3 cases (3/26=11.5%), which initially demonstrated this deeper triangular defect, eventually resulted in migrated inferior tips from propagation of the resection defect, creating an appearance similar to those with tip harvesting and a pointed shark-tooth-appearing inferior scapula body. There was progressive inferolateral migration of the inferior angle on later radiographs (presumably from muscular traction) and diminishing visualisation of the separated inferior portion (Figure 5c).
Figure 5.
(a) 29-year-old male with oral cavity cancer requiring a right scapular free flap reconstruction with immediate post-operative and 14-month follow-up chest radiographs. There are paraglenoid notches (thin white arrows) in both radiographs and haziness of the lateral border on the late film, not unlike the case from Figure 2. However, the later studies reveal preservation of the inferior scapular angle and an additional angular notch more inferiorly on the lateral border (thick white arrows), creating a discrete rectangular lateral defect. (b) 70-year-old male with oral cavity cancer requiring scapular free flap reconstruction with immediate and 11-month post-operative chest radiographs. Both radiographs also reveal paraglenoid notches (white arrows) and haziness of the lateral borders, not unlike the case from Figure 2. However, later studies reveal preservation of the inferior scapular angle (black arrow) and an additional angular notch more inferiorly on the lateral border (thick white arrow), creating a discrete triangular lateral border defect. (c) 71-year-old male with mandibular trigone cancer requiring scapular free flap reconstruction with post-operative Day 1 and Day 2 as well as 5-month follow-up chest radiographs. The two immediate post-operative radiographs demonstrate the typical paraglenoid notches (thin white arrows) and haziness of the lateral borders. There is preservation of the inferior scapular angle (thin black arrows) and the entire medial border (white arrowheads). A triangular lateral border defect (thick black arrow) is also seen on the first radiograph (on the left). However, in the second post-operative radiograph and on the later follow-up study (on the right), there is fracture or separation of the inferior angle at the inferior surgical notch with a sharply pointed remnant inferior scapular body and a step-off from the medial border (thick white arrows). Progressive lateral migration of the inferior angle is noted (thin black arrows) with poor visualisation on the late follow-up radiograph (on the right).
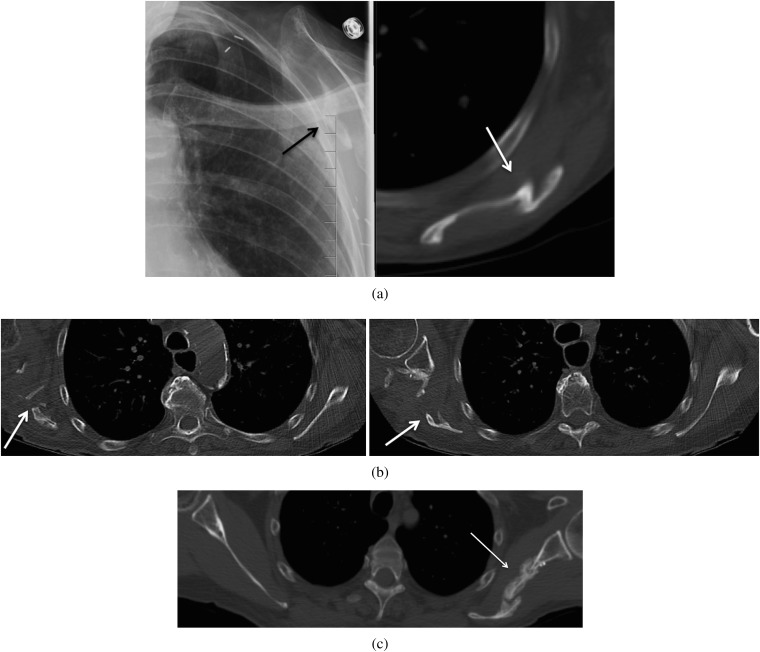
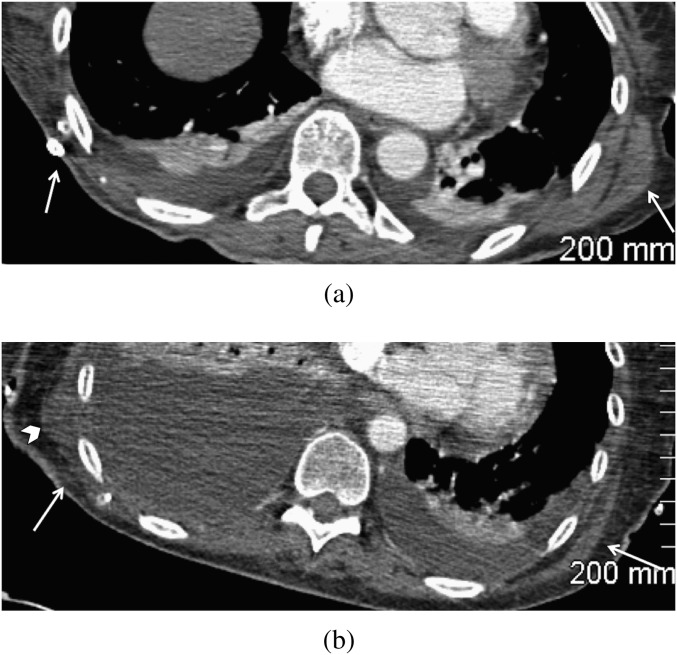
The most characteristic findings in the axial plane on CT were an abrupt defect interposed between the glenoid tubercle and the body of the scapula (Figure 6) and deficiency of the lateral and inferior borders with loss of the characteristic bulging axillary rim (normally the thickest border; Figure 7). A normal scapular appearance in the axial plane, from craniad to caudad, will never demonstrate a soft tissue interval between the glenoid tubercle and the body of the scapula (owing to the sloping of the lateral border from the lateral angle). Therefore, the presence of a sharply demarcated defect, medial to the glenoid tubercle (corresponding to the radiographic paraglenoid notch), is particularly suggestive of this surgery, and it was seen in 34 patients (34/39=87.2%) who had post-operative CT imaging of the chest. However, this may be present on only one or two slices and is easily missed. As such, inspection of the lateral border of the scapula for loss of the normally thickened axillary border (which we called an absent axillary bulge) and generalised tapering is important in recognising the defect on CT, as this was present in 100% of patients, to varying degrees. In 2 of the patients (2/5=40%) without a gleno-scapular interval defect, slight excrescent irregularity of the infero-lateral aspect of the glenoid was seen (Figure 8). This may also serve as an indicator to alert a reader to the possibility of this resection defect.
Figure 6.
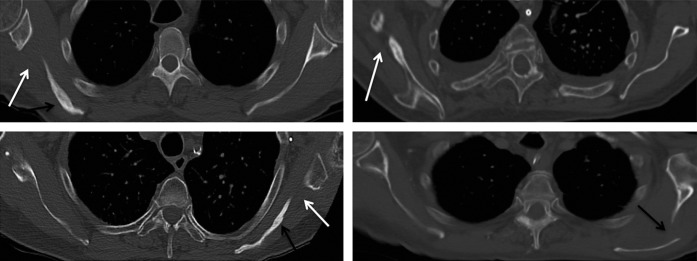
Axial non-contrast chest CTs in multiple patients with scapular free flap harvesting at remote follow-up: a 70-year-old male at 1-year follow-up (top left), a 70-year-old female at 6-month follow-up (top right), an 81-year-old female at 2-year follow-up (bottom left) and a 76-year-old male at 4-year follow-up (bottom right). There are abrupt well-demarcated defects interposed between the glenoid tubercle and the scapular body (white arrows). No portion of the glenoid tubercle should be seen separate from the scapular body on any cut of an axial scan in the normal anatomy. Note, the fissuring and irregular sclerosis sometimes seen, which can aid in detection (black arrows).
Figure 7.
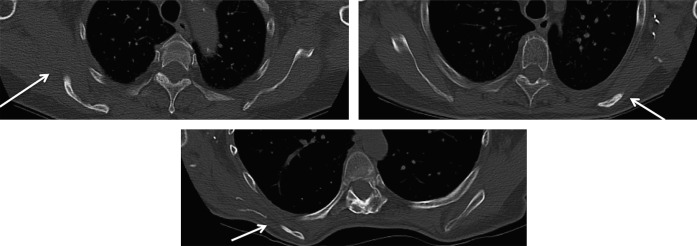
Axial non-contrast chest CTs in multiple patients with scapular free flap harvesting at remote follow-up: a 70-year-old female at 6-month follow-up (top left), an 81-year-old female at 2-year follow-up (top right) and a 58-year-old female at 16-month follow-up (bottom). There is deficiency of the lateral borders and inferior angles with loss of the normal rounded and bulging lateral border. It is replaced by post-surgical irregular heterogeneous sclerosis and callus formation (arrow). Note the surgical material laterally, which can aid detection.
Figure 8.
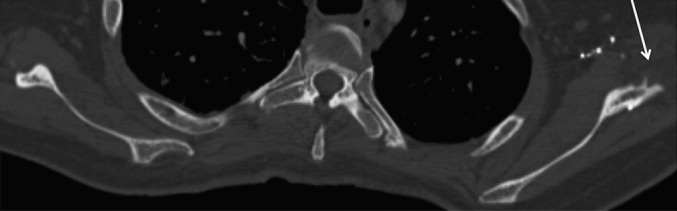
47-year-old male with squamous cell carcinoma of the oral cavity requiring scapular free flap reconstruction with a 2-year follow-up axial non-contrast CT of the chest demonstrating a rare appearance of the remaining scapula. There is no defect seen between the glenoid and the scapular body on the axial CT, with only mild excrescence of the infero-lateral aspect of the glenoid (arrow).
Occasionally, scapular fissuring was seen, to a moderate degree in 7 patients, and minimally in 3 patients (10/39=25.6%). Fissuring was extensive enough to involve overriding of medial and lateral portions of a vertical fracture defect in 8 cases (8/39=20.5%), which we called a Z-shaped scapula (Figure 9a), enhancing detection. This feature was pronounced enough to be recognisable on a portable chest radiograph in one patient (Figure 9a). Most often, scapular body irregularity was very slight, with mild healed fracture remodelling, seen in 14 patients (14/39=35.9%), requiring a more focused inspection to be recognised (Figure 9b). Extensive mature periostial remodelling was seen in one patient (Figure 9c), presumably from atypical post-surgical callus formation, as there was no historical record of infection.
Figure 9.
(a) 73-year-old female with maxillary cancer requiring scapular reconstruction. One-year follow-up axial non-contrast CT and 2-month follow-up radiograph reveal a Z-shaped scapula. Overriding healed deformities from vertical propagation of lateral border resection are seen on the radiograph (black arrow) and CT (white arrow). (b) 88-year-old female with squamous cell carcinoma of the retromolar trigone and subsequent osteonecrosis and osteomyelitis of a fibular graft, requiring scapular free flap reconstruction. Four-month follow-up non-contrast axial CT scan shows extensive irregular sclerosis and fissuring, which was occasionally seen and can aid detection (white arrows). (c) 47-year-old male with osteonecrosis of the jaw from oral cavity radiation requiring scapular free flap reconstruction. Follow-up non-contrast axial chest CT shows mature periostial remodelling along the scapular defect on the left (arrow).
Some degree of muscular deficiency was also seen in all cases most often related to latissimus dorsi harvesting (Figures 10a,b).
Figure 10.
(a) Contrast-enhanced axial CT scan of a 55-year-old female on post-operative Day 3 after scapular free flap harvesting for recurrent oral cancer. The latissimus dorsi is absent on the right and preserved on the left (white arrows). Subjacent to the skin staples, there is minimal fat stranding. (b) Contrast-enhanced axial CT scan of a 61-year-old female on post-operative Day 5 after scapular free flap harvesting for oral cancer. The latissimus dorsi is largely absent on the right and preserved on the left (white arrows). The deficient remnant right latissimus dorsi is retracted (arrow head) with minimal intramuscular oedema and fat stranding without a markedly shaggy muscle contour or infiltration of the fat to suggest infection.
The 7 (7/84=8.3%) patients receiving early post-operative CT exhibited clean, non-corticated surgical margins and a mild, occasionally moderate, amount of adjacent fat stranding (Figure 11). The surrounding muscle borders were faintly ill-defined, with minimal low attenuation of the muscular bellies, presumably from oedema (Figures 10 and 11). As expected, neither cortical erosion or faint periostitis nor extensive, nodular or enhanced soft tissue swelling was seen (which may potentially be distinguishing features in infection; Table 1).
Figure 11.
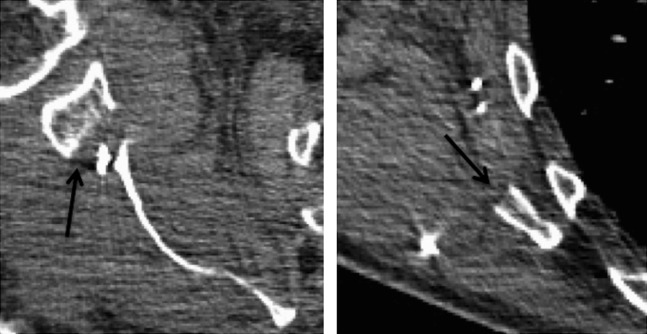
Post-operative Day 2 contrast-enhanced chest CT of a 73-year-old male after scapular free flap reconstruction of a loose mandibular resection fixation plate for oral cancer. Note the straight, clean surgical margins (black arrows) of the gleno-scapular interval superiorly (on the left image) and the absent axillary bulge inferiorly (on the right image) in this patient <1 week after surgery.
Table 1.
Characteristic appearances
| Characteristic | Frequency (%) | Description | Association |
| Plain radiograph | |||
| Upside-down V-shaped paraglenoid notch | 100 | Notch immediately medial and caudal to the glenoid, representing superior resection edge | Abrupt gleno-scapular interval (below) |
| Sharply pointed inferior scapular remnant | 66 | Shark-tooth appearance of inferior scapular tip from resection of the angle | Decreased density of the lateral scapular border |
| Generalised lucency of hemi-thorax | |||
| Rectangular lateral border defects | 21 | Second inferior 90° angle notch on the lateral scapular border inferior to the paraglenoid notch, creating a well-defined rectangular defect | Remnant inferior scapular angle |
| Triangular lateral border defects | 10 | Deep and inferior extension of the lateral border resection, creating a triangular defect, with preservation of the inferior scapular angle | Prone to fracture (12% of our population) |
| CT findings | |||
| Absent axillary bulge | 100 | Loss of the thickened axillary border. Tapering of the lateral border | Irregular border excrescence (36%), bone fissuring (26%) and periostial reaction/callus formation |
| Abrupt gleno-scapular interval | 87 | Sharply demarcated defect interposed between the glenoid tubercle and the body of the scapula | Upside-down V-shaped paraglenoid notch (above) |
| Z-shaped scapula | 21 | Overriding of medial and lateral portions of a vertical fracture defect of scapular body, creating a Z-shaped appearance | |
Although there were distinct changes seen on delayed follow-up CT imaging, such as smoothing and remodelling of the defect margins, it is difficult to make an inference with regard to the natural progression and timing of healing changes given the small group with early cross-sectional imaging and the variability of later imaging timing. An expected rate of new bone formation and chronic periostial thickening also cannot be reliably estimated.
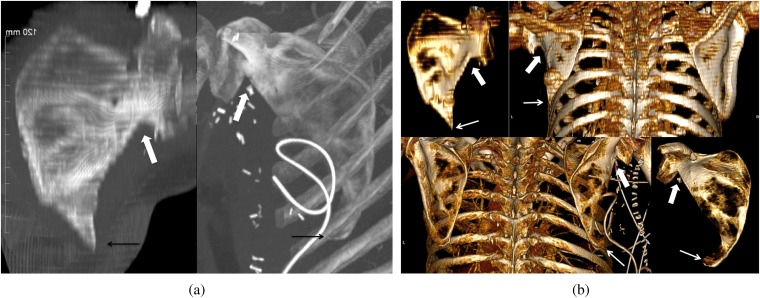
Oblique or paracoronal reconstructed CT images did not successfully approximate the radiographic appearance or allow easy identification of the defects. Although double-oblique maximal intensity projection (Figure 12a) as well as three-dimensional volume-rendered reconstructions (Figure 12b) were useful in recognising the defects, they are not practical on routine imaging.
Figure 12.
(a) Reconstructed double-oblique maximal intensity projection images of the scapula showing the paraglenoid notches (white arrows) and sharply pointed inferior scapular tips (black arrows). 47-year-old female (in left image) after scapular reconstruction for osteoradionecrosis of the jaw (patient from Figure 9c). 73-year-old male (in right image) on post-operative Day 2 after scapular reconstruction (patient from Figure 11). (b) Three-dimensional volume-rendered reconstructions of the posterior thorax and isolated oblique reconstructions of the scapula showing the paraglenoid notches (thick arrows) and sharply pointed inferior scapular remnants (thin arrows).
Reporting
Imaging for the reviewed patients included 884 chest radiographs, 3 shoulder radiographs, 36 chest CTs and 32 whole-body PET/CTs. These were read by 29, 3, 11 and 4 different radiologists, respectively. However, there was overlap between these groups and a total of 33 different radiologists read post-operative imaging that included the scapula. Only 1 (1/884=0.1%) of the original chest radiograph readings described the defect, identifying it as a resection. The report was made by a resident describing a defect on an early post-operative film with limited conspicuity; as such, specific knowledge of the patient’s history is suspected in this case. The defect was accurately reported on one of the three shoulder radiographs, which provided the clearest views of the scapula and happened to be interpreted by a head and neck radiologist. Four of the CT reports included descriptions of the surgical defects (describing them as “distorted”, “fragmented” or “post-surgical changes”). In many cases, readers would describe other bone defects or adjacent post-surgical changes but fail to recognise or describe any scapular deformity, even when markedly fragmented. Two faculties described the defects accurately on CT, as partial scapular resections, for a total of 6 descriptions out of 36 reports (6/36=16.7%). 1 case out of 32 reports (1/32=3.1%) was described on PET/CT. Interestingly, all these descriptions came from different radiologists, and these descriptions were not repeated by subsequently reporting radiologists (except for one of the instances on chest CT). Therefore, 8 (24.2%) of the 33 radiologists recognised and described the defect on one occasion. Only 4 (12.1%) accurately described a resection defect. However, none of the recognised defects was mistaken for possible acute pathology.
DISCUSSION
The rise in prevalence of scapular osteocutaneous flap repairs has been accompanied by an apparent lack of awareness of their appearance among radiologists. We hope this initial characterisation will alert radiologists to the presence of these defects. Although a description of the defects has no impact on management and may reasonably be omitted, their recognition may provide further history (to the radiologist or other referring clinicians) and will more importantly increase radiologist comfort with the normal post-operative appearances. This, in turn, may lead to better recognition of complications, such as infection. One would not expect metastases to occur in association with this surgical bed, and this has not been reported. However, imaging of complications was not performed for the patients we studied, and it may be interesting to evaluate their characteristic findings in future studies.
CONCLUSIONS
On radiograph, we found an upside-down-V paraglenoid notch was present in all cases. A sharply pointed inferior remnant scapular body was seen in up to 65.5% of cases, while a rectangular or triangular defect of the lateral border was seen in 21.4% of cases. This lateral border defect appeared most prone to defect propagation with fragment displacement, seen in 11.5% of all cases. On axial CT, a gleno-scapular interval was present in 87.2%. Scapular fissuring was seen in 25.6% and overriding healed fracture remodelling, or a Z-shaped scapula, was seen in 20.5%. Absence of the normal axillary rim bulge was seen in all cases and generalised minimal irregularity was seen in 35.9% of all cases.
REFERENCES
- 1.Batchelor AG, Sully L. A multiple territory free tissue transfer for reconstruction of a large scalp defect. Br J Plast Surg 1984;37:76–9 [DOI] [PubMed] [Google Scholar]
- 2.Baker SR, Sullivan MJ. Osteocutaneous free scapular flap for one-stage mandibular reconstruction. Arch Otolaryngol Head Neck Surg 1988;114:267–77 [DOI] [PubMed] [Google Scholar]
- 3.Takushima A, Harii K, Asato H, Momosawa A, Okazaki M, Nakatsuka T. Choice of osseous and osteocutaneous flaps for mandibular reconstruction. Int J Clin Oncol 2005;10:234–42 [DOI] [PubMed] [Google Scholar]
- 4.Deleyiannis FW, Rogers C, Lee E, Russavage J, Gastman B, Dunklebarger J, et al. Reconstruction of the lateral mandibulectomy defect: management based on prognosis and location and volume of soft tissue resection. Laryngoscope 2006;116:2071–80 [DOI] [PubMed] [Google Scholar]
- 5.Yoo J, Dowthwaite SA, Fung K, Franklin J, Nichols A. A new angle to mandibular reconstruction: the scapular tip free flap. Head Neck Jul. 2012 doi: 10.1002/hed.23065. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 6.Pagedar NA, Gilbert RW, Chan H, Daly MJ, Irish JC, Siewerdsen JH. Maxillary reconstruction using the scapular tip free flap: A radiologic comparison of 3D morphology. Head Neck 2012;34:1377–82 [DOI] [PubMed] [Google Scholar]
- 7.Capellini TD, Vaccari G, Ferretti E, Fantini S, He M, Pellegrini M, et al. Scapula development is governed by genetic interactions of Pbx1 with its family members and with Emx2 via their cooperative control of Alx1. Development 2010;137:2559–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moore KL, Dalley AF. Upper limb. In: Kelly PJ, ed. Clinically oriented anatomy. 4th edn. Philadelphia, PA: Lippincot Williams and Wilkins; 1999. pp. 668–9 [Google Scholar]