SUMMARY
Sleep is composed of an alternating sequence of REM and non-REM episodes, but their respective roles are not known. We found that the overall firing rates of hippocampal CA1 neurons decreased across sleep concurrent with an increase in the recruitment of neuronal spiking to brief “ripple” episodes, resulting in a net increase in neural synchrony. Unexpectedly, within non-REM episodes, overall firing rates gradually increased together with a decrease in the recruitment of spiking to ripples. The rate increase within non-REM episodes was counteracted by a larger and more rapid decrease of discharge frequency within the interleaved REM episodes. Both the decrease in firing rates and the increase in synchrony during the course of sleep were correlated with the power of theta activity during REM episodes. These findings assign a prominent role of REM sleep in sleep-related neuronal plasticity.
INTRODUCTION
Although sleep is a fundamental physiological process, no overarching hypothesis has emerged to explain its functions (Siegel, 2005). The putative roles of sleep vary from “adaptive inactivity” (Siegel, 2005), to memory consolidation (Born et al., 2006; Buzsáki, 1989; McClelland et al., 1995; Stickgold, 2005; Walker, 2010), to “homeostatic regulation” of neuronal activity. Homeostatic (or “two-process”) models of sleep suggest that sleep serves a largely recuperative function for the brain (Feinberg, 1974; Borbély, 1982; Tononi and Cirelli, 2006). According to these models, neocortical excitability, used broadly to refer to several statistical aspects of neural activity, including firing rate and synaptic strength, increases cumulatively during waking behavior, associated with increasing power of delta activity, which may exhaust energy resources within the brain (Borbély, 1982). Conversely, sleep is hypothesized to decrease delta power and reduce firing rates and neuronal excitability (Borbély, 1982; Tononi and Cirelli, 2006). These models inspired large numbers of experiments in both humans and other animals (Tononi and Cirelli, 2006; Vyazovskiy et al., 2009; Miyamoto and Hensch, 2003; Greene and Frank, 2010), although the mechanisms by which these changes are brought about during sleep have largely remained unexplored (Tononi and Cirelli, 2006). The implications of the sleep homeostatic model on neuronal excitability have recently been examined in the barrel cortex of the rat. In agreement with the model, the global firing rates of neocortical neurons increased during the wake-active cycle, accompanied by increased synchrony of the recorded neurons, whereas both firing rates and synchrony decreased during the sleep cycle (Vyazovskiy et al., 2009), largely in accordance with the in vitro synaptic “scaling” model (Turrigiano, 1999).
To establish the general validity of the homeostatic model, it is essential to test its predictions in multiple cortical areas. Moreover, since sleep consists of two competing physiological processes, non-REM and REM sleep, it is important to learn how these distinct sleep stages contribute to the hypothesized homeostatic function of sleep. Notably, homeostatic models do not attribute an explicit role to REM sleep, even though alterations of REM sleep are intricately related to cognitive and affective disorders manifested in the waking brain (Born et al., 2006; Campbell and Gillin, 1987; Gierz et al., 1987; Walker, 2010).
Neurons in the hippocampal cortex display distinct firing patterns during different behaviors (O’Keefe, 2007). Waking exploration and REM sleep are characterized by theta oscillations and neural firing “episodes” in which individual cells sustain elevated firing rates for several hundreds of milliseconds (Buzsáki, 2002; Louie and Wilson, 2001; Montgomery et al., 2008). In contrast, during immobility and non-REM sleep, hippocampal neural firing is concentrated in short (~120 ms) sharp-wave ripple events, which synchronize activity across much of the network and have been suggested to reflect reactivation of learned firing patterns (Buzsáki, 1989; Wilson and McNaughton, 1994). Between ripples, neural firing is sparse and asynchronous for hundreds of milliseconds (Buzsáki et al., 1992; Csicsvari et al., 1999; Sullivan et al., 2011; cf. Carr et al., 2011). During sleep, hippocampal ripples are weakly correlated with neocortical slow oscillations (Steriade et al., 1993), although hippocampal activity is often dissociated from that of the neocortex (Hahn et al., 2007; Wolansky et al., 2006; Isomura et al., 2006).
We examined the evolution of population firing patterns in the CA1 hippocampal region during sleep. Our findings show that discharge rates of both pyramidal cells and interneurons gradually ramp up during non-REM episodes, interrupted by larger rate decreases during the interleaving REM epochs. This “sawtooth” pattern of rate changes across non-REM and REM episodes results in an overall downscaling of discharge rates over the course of sleep. In contrast, synchrony during non-REM ripple events increases from the early to late stages of sleep. The concurrent decrease of firing rates and increased population synchrony from one non-REM episode to the next are correlated with the power of theta oscillations during the intervening REM sleep. Our findings, therefore, suggest a central role of REM sleep in regulating both discharge rates and synchrony in the hippocampus.
RESULTS
Local field potentials (LFPs) and spiking activity of isolated CA1 putative pyramidal cells and putative interneurons were recorded in the home cage while the rat was immobile and assumed a characteristic sleep posture. The ratio of theta (5–11 Hz) and delta (1–4 Hz) power was used to identify non-REM and REM episodes (Figure 1A; see Supplemental Experimental Procedures available online), as described previously (Montgomery et al., 2008). Twenty-two sleep sessions (38.2 ± 5.8 min, SEM) with at least one non-REM-REM-non-REM cycle were recorded in five rats. Mean firing rates of pyramidal cells (n = 618) were similar between non-REM and REM episodes, whereas firing rates of interneurons (n = 111) were significantly higher during REM (p < 0.00018; sign-rank test; Csicsvari et al., 1999). In the majority of our analyses, we focused on the following comparisons. First, changes “across sleep” were defined as differences between the first and the last non-REM episodes in a sleep session. Second, changes in “within non-REM” episodes refer to differences between the first and the last thirds of each non-REM. Third, changes in “within REM” episodes refer to differences between the first and the last thirds of each REM. Finally, we examined the relationship between these categories.
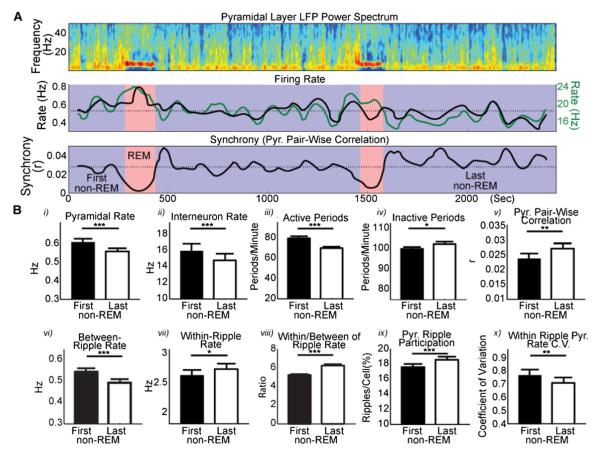
Figure 1. Excitability Changes across Sleep.
(A) Time-resolved spectrum (top) and smoothed (60 s) mean firing rate changes of pyramidal cells (black) and interneurons (green; middle) and mean pyramidal pairwise correlation (“synchrony”; bottom) across representative non-REM (blue) and REM (pink) episodes from one uninterrupted sleep session (dotted lines show session means). (B) Significant changes across sleep, calculated between the first (black bars) and last (white bars) non-REM episodes of sleep, firing rates (i and ii), incidence of periods of high-frequency LFP activity (iii) and inactivity (iv), synchrony (v), firing rate of pyramidal cells between ripples (vi) and within ripples (vii), ripple-induced firing rate modulation (viii), percentage of ripples in which individual pyramidal cells participated (i.e., fired at least one spike; ix), and coefficient of variation of within-ripple firing rate across cells (x). Note that the decrease in firing rates across sleep is concomitant with increasing synchrony. All comparisons were carried out as sign-rank (paired) tests. *p < 0.05; **p < 0.005; ***p < 0.0005.
Excitability Changes across Sleep
Since non-REM sleep is characterized by alternating periods of population activity and inactivity in both the neocortex (Steriade et al., 1993) and hippocampus (Ji and Wilson, 2007; Isomura et al., 2006), we defined active periods as those in which smoothed gamma and epsilon band (30–300 Hz) LFP activity was at least 0.5 SDs above the mean for at least 50 ms. Conversely, inactive periods were detected as those in which gamma and epsilon band activity was 0.5 SDs below the mean for at least 50 ms (see Supplemental Experimental Procedures, see also Figure S2 for an analogous spike-based analysis). The incidence of active periods decreased, whereas the incidence of inactive periods increased significantly from the first to the last non-REM episodes of each session (i.e., across sleep; Figure 1B; Table S1). The firing rates of both pyramidal cells and interneurons decreased significantly across sleep (Figure 1B). These findings are in accord with the two-process model of sleep and indicate similarities between sleep-related activity of neurons between the neocortex and hippocampus (Borbély, 1982; Tononi and Cirelli, 2006; Vyazovskiy et al., 2009).
During sleep, the hippocampal neural population fires synchronously during sharp-wave ripple events and relatively asynchronously between ripples (Buzsáki et al., 1992). The discharge rate of pyramidal neurons between ripples decreased significantly across sleep (Figure 1B), similar to the decrease in global firing rate. Conversely, the mean firing rate of pyramidal cells within the short-lived ripple events increased during the course of sleep (Figure 1B). This increase in ripple-related activity across sleep was the result of an increase in the percentage of ripples within which pyramidal cells participated (i.e., fired at least one spike) rather than an increase of the within-ripple firing rates of individual neurons in individual ripples (Figure 1B; Figure S3). Concurrent with the increase of within-ripple participation, the coefficient of variation of within-ripple firing rate across cells decreased (Figure 1B; Figure S3), suggesting that the within-ripple participation was more evenly distributed across the population of pyramidal cells at the end compared to the beginning of sleep. Synchrony, as measured by the correlation strength of pyramidal cell pairs in nonoverlapping 100 ms bins (Wilson and McNaughton, 1994), also increased across sleep (Figure 1B), probably due to the more consistent participation of pyramidal cells in ripples. In short, the decreased firing rate across sleep was associated with a “paradoxical” increase in pyramidal cell synchrony and more consistent recruitment of spikes to ripple events (Table S1).
Excitability Changes within Non-REM and within REM Episodes
Next, we investigated which sleep state might be responsible for the global across-sleep changes of firing patterns. Since the duration of individual non-REM and REM episodes vary, their lengths were normalized (see Experimental Procedures) and the pattern of changes within episodes was quantified. In non-REM episodes, we found that firing rates significantly increased between the first and last thirds of the episodes, both in pyramidal cells (p < 1.99 × 10−14, n = 618) and in interneurons (p < 4.6 × 10−5, n = 111) (Figure 2A; Figure S2). Other measures, such as incidence of active and inactive epochs, the percentage of ripples in which pyramidal cells participated, and population synchrony, as measured by pyramidal cell pairwise correlations, also showed significant and opposite changes within non-REM compared to those observed across sleep (Figure 2B). In contrast, firing rates significantly decreased within REM epochs, both in pyramidal cells (p < 0.012, n = 618) and in interneurons (p < 1.23 × 10−5, n = 111) (Figure 2A; Figure S2).
Figure 2. Excitability Changes within Non-REM Episodes Are Opposite to Those across Sleep.
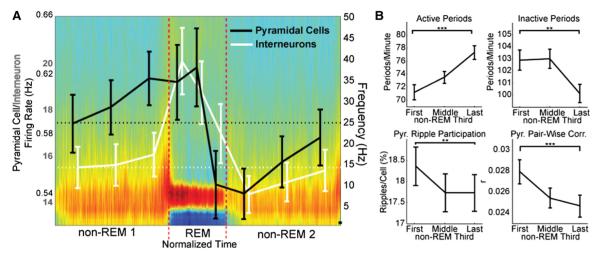
(A) Time-normalized power spectra of adjacent non-REMn-REM-non-REMn+1 episodes (mean of n = 45 non-REMn-REM-non-REMn+1 cycles) and corresponding firing rates (±SEM) of pyramidal cells and interneurons shown within thirds of non-REM and REM episodes. Note that firing rates increase within non-REM episodes and decrease within REM episodes (Figure S2). Horizontal lines represent mean rates at the beginning of the non-REMn-REM-non-REMn+1 cycle. (B) Incidence of LFP high-frequency activity and inactivity epochs, percentage of ripples in which pyramidal cells participate, and pairwise correlation of pyramidal cells across thirds of non-REM. Note opposite changes as those observed across sleep (Figure 1). *p < 0.05; **p < 0.005; ***p < 0.0005.
LFP Spectral Changes across Sleep and within Non-REM and within REM Episodes
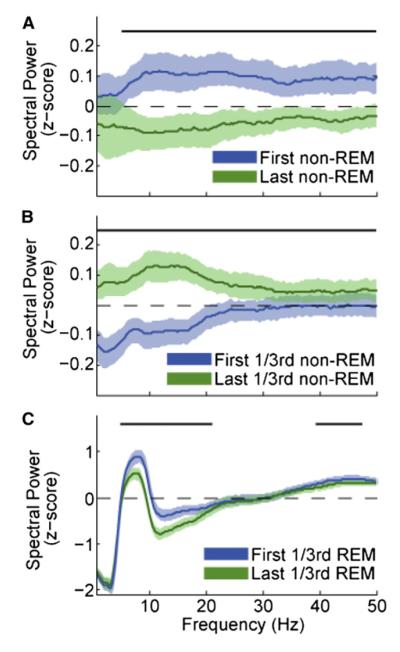
In addition to unit firing, the LFP spectral changes across sleep were also calculated. For each sleep session, the LFP spectra in individual non-REM and REM episodes, recorded from the CA1 pyramidal layer, were normalized independently for each frequency by the power of concatenated non-REM episodes and expressed as a Z score. Spectral power decreased significantly in a broad range of frequencies (4–50 Hz) across sleep (i.e., from the first to last non-REM episode; Figure 3A; n = 22 sleep sessions; change in 0–50 Hz integrated power; p < 0.0024; sign-rank test). In contrast, a significant increase in power (0–50 Hz) was present within non-REM episodes (Figure 3B; n = 82 non-REM episodes; p < 2.11 × 10−9; sign-rank test). Within REM episodes, a power decrease was observed in the theta-beta (5–20 Hz) and lower gamma (40–50 Hz) band (Figure 3C; n = 45 REM episodes; 0–50 Hz power; p < 2.85 × 10−4; sign-rank test). Changes in the delta band (1–4 Hz) may reflect changes in the hippocampus or volume-conducted LFP from the neocortex (Wolansky et al., 2006; Isomura et al., 2006).
Figure 3. LFP Spectral Power Decreases across Sleep and within REM Episodes and Increases within Non-REM Episodes.
For each session, pyramidal layer LFP spectra were normalized for each frequency bin as the Z score of non-REM power. (A) Spectral power decreases from the first to last non-REM episode of a sleep session across a wide range of frequencies (n = 22 sleep sessions). Shaded regions show 95% confidence intervals. Top black bars show frequencies for which sign-rank test was p < 0.05. (B) Spectral power changes within non-REM episodes (n = 82). (C) Spectral power changes within REM episodes (n = 45).
Relationship between Non-REM and REM Sleep
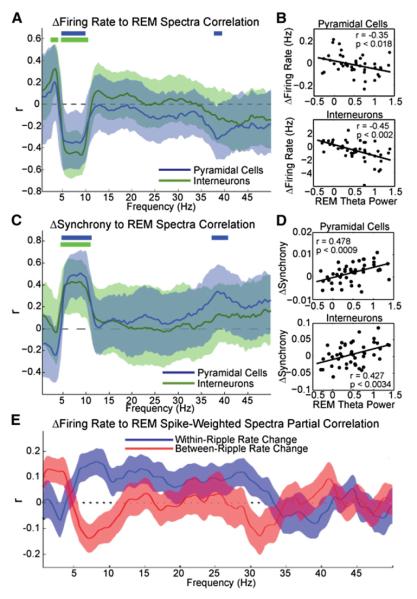
Since the evolution of firing patterns and LFP across sleep was similar to those observed within REM sleep but dissimilar to the changes observed within non-REM episodes, we examined how REM episodes might contribute to the overall reorganization of firing patterns during the course of sleep. The mean firing rate decrease of both the pyramidal cell and interneuron populations from the non-REM episode preceding a REM (non-REMn) to the non-REM episode after a REM episode (non-REMn+1) was significantly correlated with the theta power of the interleaving REM episode but not the power of other frequencies (Figures 4A and 4B), except for the lower gamma band for pyramidal cells. Similar calculations were performed to examine the relationship between population synchrony (pairwise correlation) during non-REM and spectral power of REM. The increase in synchrony of both pyramidal cells and interneurons from non-REMn to non-REMn+1 was significantly correlated with the theta and gamma (around 40 Hz) power of the interleaving REM episode but not the power of other frequencies (Figures 4C and 4D).
Figure 4. REM Sleep Affects Firing Patterns in Non-REM.
(A) Correlation values between firing rate changes between non-REMn and non-REMn+1 episodes versus LFP power of the intervening REM (mean and 95% confidence intervals) in the 0–50 Hz frequency range. Top solid bars indicate frequency bands in 0.25 Hz steps of significant correlation. Note significant effect of REM theta power on rate changes between successive non-REM episodes for both pyramidal cells and interneurons. (B) Mean firing rate changes (Hz) between non-REMn and non-REMn+1 episodes (y axis) as a function of the theta power during the interleaving REM episode. Power during REM was normalized by the power of concatenated non-REM episodes (Z score). (C and D) Same as (A) and (B), respectively, but for synchrony change (pairwise correlation) between successive non-REM episodes. (E) Correlation values between firing rate changes across sleep and spike-weighted spectra (SpWS; see Supplemental Experimental Procedures) during REM for pyramidal cells in the 0–50 Hz frequency range. Results are shown separately for spikes that occurred between ripples (red) and in within-ripple (blue) events.
To examine how the rate change of individual neurons across sleep was related to their network pattern-related activity during REM sleep, we introduced the method of spike-weighted spectra (SpWS) by relating the instantaneous firing rates of single cells to the power distribution of the simultaneously detected LFP. LFP spectra and firing rates of individual pyramidal cells were computed in 1 s bins with 0.5 s overlap during REM (Figure S4 and Supplemental Experimental Procedures). For normalization purposes, the LFP spectrograms were Z scored independently for each frequency band and the LFP power spectrum was multiplied bin-by-bin by the neuron’s within-bin firing rate and divided by its overall REM rate (see Figure S4). Since power in each frequency of SpWS is first Z scored, stochastic firing results in power nearing zero, while positive values for a given SpWS frequency band reflect a cell’s selective firing preference in that band. To quantify the relationship between the neuron’s frequency preference during REM sleep and its firing pattern change across sleep, we normalized the correlation between the neuron’s SpWS in REM and its rate change between the first and last non-REM episodes of sleep by the neuron’s REM mean firing rate (see Supplemental Experimental Procedures for the “partialization” procedure). These partial correlations were computed separately for changes occurring across sleep in either within-ripple or between-ripple firing rates (n = 22 sleep sessions). Pyramidal cells with firing rates less than 0.4 Hz during REM (n = 281 of 618 cells) were excluded from the SpWS analysis. The SpWS analyses (Figure 4E; see also Figure S4) demonstrated that within the same population of simultaneously recorded pyramidal cells, the across-sleep decrease of between-ripple firing rate was correlated with the pyramidal neurons’ preference to discharge selectively during high-power theta (~5–10 Hz) and gamma epochs during REM. Similarly, a neuron’s theta and gamma power preference reliably predicted its across-sleep firing rate increase within ripples (Figure 4E).
DISCUSSION
We found that firing rate changes during sleep display a sawtooth pattern, so that the modest increase in discharge activity within non-REM episodes are overcome by the larger rate deceleration within the intervening REM episodes, resulting in an overall rate decrease during the course of sleep. Theta power of REM sleep is coupled with an increase in synchrony and decrease in rate variability of pyramidal cells during the brief ripple events across sleep. REM mechanisms are thus implicated in both the rate and synchrony changes. These findings suggest that different stages of sleep make different contributions to firing pattern changes. Moreover, a simple global discharge rate measure in the hippocampus does not faithfully characterize the firing pattern reorganization that takes place during the course of sleep.
There are two dominant views on the role of sleep in firing pattern regulation. According to the “consolidation” model, neurons that are activated by recent waking experience remain selectively active during sleep, firing mainly within hippocampal ripples and neocortical sleep spindles (cf. Buzsáki, 1989; Carr et al., 2011; McClelland et al., 1995; Stickgold, 2005; Born et al., 2006; Sejnowski and Destexhe, 2000). The increased firing of the active neurons is balanced by a commensurate decrease in the remaining neuronal population so that the global firing rates and population excitability remain relatively constant (Dragoi et al., 2003). In contrast, “homeostatic” models suggest that waking experience-related neurons add to the overall excitability of the cortical networks and sleep (i.e., non-REM) serves to equalize and reduce rates (Borbély, 1982; Tononi and Cirelli, 2006; Lubenov and Siapas, 2008). Thus, both models attribute importance to sleep-related plasticity, as manifested in the rate changes of individual neurons and/or synaptic weight changes. While our findings do not provide direct information on these issues, they show that rate and synchrony effects should be treated separately (Wilson and McNaughton, 1994) and that it is REM sleep that may be instrumental in bringing about both rate effects and increased synchrony.
An important aspect of our findings was the opposing firing rate changes between non-REM and REM episodes of sleep, as found in both pyramidal cells and interneurons. One potentially linked factor to the observed firing rate changes during sleep is a parallel change in core and brain temperature. As observed in rabbits, the temperature of the brain decreases during sleep, interrupted by rapid increases of up to 0.4°C during REM episodes (Kawamura and Sawyer, 1965; Baker and Hayward, 1967). However, temperature change is unlikely to be the sole cause of the sawtooth discharge pattern of non-REM and REM, since in the waking, exploring rat, elevation of brain temperature during running is associated with increased neuronal discharge rate and higher excitability (Moser et al., 1993).
Of the three brain states (waking, non-REM, and REM), only REM episodes are associated with decreasing firing rates in the hippocampus (Montgomery et al., 2008). Although both active waking and REM sleep are associated with similar network states, characterized by theta oscillations and sustained neuronal firing, these states are fundamentally different when viewed from the perspective of the brain stem (Vertes, 1984; McCarley, 2007). Exploration is strongly linked to elevated activity of cholinergic, serotoninergic, histaminergic, and noradrenergic neurons, whereas during REM sleep only the cholinergic tone is high (Steriade, 2004). It is thus possible that serotonin and/or norepinephrine are responsible for producing different directions of rate and excitability changes during waking and REM, especially because these neuromodulators have been shown to strongly affect long-term synaptic plasticity (Bliss et al., 1983) and REM sleep deprivation results in impaired synaptic plasticity (McDermott et al., 2006).
Another unexpected observation in our experiments was the parallel changes of decreased global firing rates and increased synchrony during sharp-wave ripples across sleep (Diekelmann et al., 2011). Increased firing rates are typically accompanied by spurious increases in synchrony measures (Perkel et al., 1967). However, in the hippocampus, large, nonlinear increases in population synchrony are brought about by ripples (Buzsáki et al., 1992), and increased synchrony in our experiments occurred almost exclusively during hippocampal ripples. In fact, within non-REM episodes, firing rates between ripples decreased in parallel with the increased participation of neurons in ripples. We hypothesize that the two types of changes, i.e., decreasing firing rates and increased synchrony during the course of sleep, are due to the same mechanism(s) since both changes were significantly correlated with the power of theta oscillations during REM episodes.
It remains to be demonstrated whether the described sleep-related firing pattern changes are unique to the hippocampal CA1 region or can be generalized to other cortical regions. According to a current influential model, the most important role of non-REM sleep is to decrease firing rates (Tononi and Cirelli, 2006). Since this prediction is opposite to the present observations in the hippocampus, one potential outcome is that firing rate regulations in the neocortex and hippocampus follow different rules. Another alternative is that downscaling of neocortical firing rates is also brought about by the intervening REM episodes, as observed in the hippocampus. In either case, the present findings imply a fundamental physiological role for REM sleep.
EXPERIMENTAL PROCEDURES
Animals, Surgery, and Data Collection
LFP and unit firing were recorded by multiple-shank silicon probes (Mizuseki et al., 2009) from the septal third of hippocampal CA1 region in five male rats. Histological localization of the electrodes, criteria for clustering of single units, and separation of pyramidal cells and interneurons in these animals have been described in detail previously (Mizuseki et al., 2009, 2011). Recordings were carried out in the home cage of the animal during sleep, including several epochs of REM and non-REM episodes, while the behavior of the rat and LFPs from several channels were monitored by the experimenter (Montgomery et al., 2008). Head movements were detected by LEDs mounted on the head stage and recorded by a video camera.
Analysis Methods
REM and non-REM episodes were identified offline using the ratio of the power in theta band (5–11 Hz) to delta band (1–4 Hz) of LFP (Sirota et al., 2008;Mizuseki et al., 2009, 2011). Spike sorting was carried out offline from the digitally high-pass filtered (0.8–5 kHz) data using an automatic clustering algorithm (http://klustakwik.sourceforge.net) (Mizuseki et al., 2009). Principal cells and interneurons were separated on the basis of their autocorrelograms, combination of trough-to-peak latency, and the asymmetry index of the filtered spike waveform, bursting properties, and mean firing rates (Supplemental Experimental Procedures).
Two major comparisons of firing patterns and LFP were used. First, changes across sleep were defined as differences between the first and the last non-REM episodes in a sleep session. Second, the duration of REM and non-REM episodes was normalized (100% each epoch) and divided into equal normalized thirds. Changes within the episodes were then analyzed by comparing the first and the last thirds of each REM and non-REM episode. Third, changes within REM episodes refer to differences between the first and the last thirds of each REM.
Ripple events were detected during nontheta periods from the band-pass filtered (120–250 Hz) trace by defining periods during which ripple power is continuously greater than mean 2 SD, and peak of power in the periods was greater than mean 3 SD of ripple power. Three approaches were used to characterize firing patterns associated with ripples. (1) Within-ripple firing rate: all ripples within a given epoch E were concatenated. Within-ripple firing rate for each cell C is defined as the number of spikes detected in the concatenated ripple epochs divided by the total concatenated time (Figure 1Bvii). (2) Ripple participation: for each cell C, ripple participation is the percentage of ripples in E in which C fired at least one spike (Figures 1Bix and 2B, bottom, left). (3) Ripple participant firing rate: for each pyramidal cell C, only those ripples in a given epoch E in which C fired at least one spike were concatenated. Ripples in which C fired no spikes were excluded. Ripple participant firing rate was the total number of spikes divided by the total time in the concatenated subset of ripples (Figure 1B). These methods were designed to disambiguate ripple-related firing rate changes due to increased spiking of the neuron C in the same number of ripples in different sleep episodes from increased participation of the neuron in more ripple events without changing the firing rate within individual ripple participation events.
The relationship between LFP and firing patterns were examined using spectral methods. Further details about the experimental techniques are available in the Supplemental Experimental Procedures.
Supplementary Material
ACKNOWLEDGMENTS
We thank Mariano Belluscio, Adrien Peryache, and Richard W. Tsien for comments on the manuscript. This work was supported by the International Human Frontiers Science Program Organization, the U.S. National Institutes of Health (NS34994; MH54671), James S. McDonnell Foundation, the Japan Society of Promotion for Sciences (K.M.), and the Minority Biomedical Research Support Program (1R25GM096161).
Footnotes
SUPPLEMENTAL INFORMATION Supplemental Information includes four figures, one table, and Supplemental Experimental Procedures and can be found with this article online at http://dx.doi.org/10.1016/j.neuron.2012.08.015.
REFERENCES
- Baker MA, Hayward JN. Autonomic basis for the rise in brain temperature during paradoxical sleep. Science. 1967;157:1586–1588. doi: 10.1126/science.157.3796.1586. [DOI] [PubMed] [Google Scholar]
- Bliss TV, Goddard GV, Riives M. Reduction of long-term potentiation in the dentate gyrus of the rat following selective depletion of monoamines. J. Physiol. 1983;334:475–491. doi: 10.1113/jphysiol.1983.sp014507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borbély AA. A two process model of sleep regulation. Hum. Neurobiol. 1982;1:195–204. [PubMed] [Google Scholar]
- Born J, Rasch B, Gais S. Sleep to remember. Neuroscientist. 2006;12:410–424. doi: 10.1177/1073858406292647. [DOI] [PubMed] [Google Scholar]
- Buzsáki G. Two-stage model of memory trace formation: a role for “noisy” brain states. Neuroscience. 1989;31:551–570. doi: 10.1016/0306-4522(89)90423-5. [DOI] [PubMed] [Google Scholar]
- Buzsáki G. Theta oscillations in the hippocampus. Neuron. 2002;33:325–340. doi: 10.1016/s0896-6273(02)00586-x. [DOI] [PubMed] [Google Scholar]
- Buzsáki G, Horváth Z, Urioste R, Hetke J, Wise K. High-frequency network oscillation in the hippocampus. Science. 1992;256:1025–1027. doi: 10.1126/science.1589772. [DOI] [PubMed] [Google Scholar]
- Campbell SS, Gillin JC. Sleep measures in depression: How sensitive? How specific? Psychiatr. Ann. 1987;17:647–653. [Google Scholar]
- Carr MF, Jadhav SP, Frank LM. Hippocampal replay in the awake state: a potential substrate for memory consolidation and retrieval. Nat. Neurosci. 2011;14:147–153. doi: 10.1038/nn.2732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csicsvari J, Hirase H, Czurkó A, Mamiya A, Buzsáki G. Oscillatory coupling of hippocampal pyramidal cells and interneurons in the behaving Rat. J. Neurosci. 1999;19:274–287. doi: 10.1523/JNEUROSCI.19-01-00274.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diekelmann S, Büchel C, Born J, Rasch B. Labile or stable: opposing consequences for memory when reactivated during waking and sleep. Nat. Neurosci. 2011;14:381–386. doi: 10.1038/nn.2744. [DOI] [PubMed] [Google Scholar]
- Dragoi G, Harris KD, Buzsáki G. Place representation within hippocampal networks is modified by long-term potentiation. Neuron. 2003;39:843–853. doi: 10.1016/s0896-6273(03)00465-3. [DOI] [PubMed] [Google Scholar]
- Feinberg I. Changes in sleep cycle patterns with age. J. Psychiatr. Res. 1974;10:283–306. doi: 10.1016/0022-3956(74)90011-9. [DOI] [PubMed] [Google Scholar]
- Gierz M, Campbell SS, Gillin JC. Sleep disturbances in various nonaffective psychiatric disorders. Psychiatr. Clin. North Am. 1987;10:565–581. [PubMed] [Google Scholar]
- Greene RW, Frank MG. Slow wave activity during sleep: functional and therapeutic implications. Neuroscientist. 2010;16:618–633. doi: 10.1177/1073858410377064. [DOI] [PubMed] [Google Scholar]
- Hahn TTG, Sakmann B, Mehta MR. Differential responses of hippocampal subfields to cortical up-down states. Proc. Natl. Acad. Sci. USA. 2007;104:5169–5174. doi: 10.1073/pnas.0700222104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isomura Y, Sirota A, Ozen S, Montgomery S, Mizuseki K, Henze DA, Buzsáki G. Integration and segregation of activity in entorhinal-hippocampal subregions by neocortical slow oscillations. Neuron. 2006;52:871–882. doi: 10.1016/j.neuron.2006.10.023. [DOI] [PubMed] [Google Scholar]
- Ji D, Wilson MA. Coordinated memory replay in the visual cortex and hippocampus during sleep. Nat. Neurosci. 2007;10:100–107. doi: 10.1038/nn1825. [DOI] [PubMed] [Google Scholar]
- Kawamura H, Sawyer CH. Elevation in brain temperature during paradoxical sleep. Science. 1965;150:912–913. doi: 10.1126/science.150.3698.912. [DOI] [PubMed] [Google Scholar]
- Louie K, Wilson MA. Temporally structured replay of awake hippocampal ensemble activity during rapid eye movement sleep. Neuron. 2001;29:145–156. doi: 10.1016/s0896-6273(01)00186-6. [DOI] [PubMed] [Google Scholar]
- Lubenov EV, Siapas AG. Decoupling through synchrony in neuronal circuits with propagation delays. Neuron. 2008;58:118–131. doi: 10.1016/j.neuron.2008.01.036. [DOI] [PubMed] [Google Scholar]
- McCarley RW. Neurobiology of REM and NREM sleep. Sleep Med. 2007;8:302–330. doi: 10.1016/j.sleep.2007.03.005. [DOI] [PubMed] [Google Scholar]
- McClelland JL, McNaughton BL, O’Reilly RC. Why there are complementary learning systems in the hippocampus and neocortex: insights from the successes and failures of connectionist models of learning and memory. Psychol. Rev. 1995;102:419–457. doi: 10.1037/0033-295X.102.3.419. [DOI] [PubMed] [Google Scholar]
- McDermott CM, Hardy MN, Bazan NG, Magee JC. Sleep deprivation-induced alterations in excitatory synaptic transmission in the CA1 region of the rat hippocampus. J. Physiol. 2006;570:553–565. doi: 10.1113/jphysiol.2005.093781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto H, Hensch TK. Reciprocal interaction of sleep and synaptic plasticity. Mol. Interv. 2003;3:404–417. doi: 10.1124/mi.3.7.404. [DOI] [PubMed] [Google Scholar]
- Mizuseki K, Diba K, Pastalkova E, Buzsáki G. Hippocampal CA1 pyramidal cells form functionally distinct sublayers. Nat. Neurosci. 2011;14:1174–1181. doi: 10.1038/nn.2894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuseki K, Sirota A, Pastalkova E, Buzsáki G. Theta oscillations provide temporal windows for local circuit computation in the entorhinal-hippocampal loop. Neuron. 2009;64:267–280. doi: 10.1016/j.neuron.2009.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montgomery SM, Sirota A, Buzsáki G. Theta and gamma coordination of hippocampal networks during waking and rapid eye movement sleep. J. Neurosci. 2008;28:6731–6741. doi: 10.1523/JNEUROSCI.1227-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser E, Mathiesen I, Andersen P. Association between brain temperature and dentate field potentials in exploring and swimming rats. Science. 1993;259:1324–1326. doi: 10.1126/science.8446900. [DOI] [PubMed] [Google Scholar]
- O’Keefe J. Hippocampal neurophysiology in the behaving animal. In: Andersen P, Morris R, Amaral D, Bliss T, O’Keefe J, editors. The Hippocampus Book. Oxford University Press; New York: 2007. pp. 475–548. [Google Scholar]
- Perkel DH, Gerstein GL, Moore GP. Neuronal spike trains and stochastic point processes. II. Simultaneous spike trains. Biophys. J. 1967;7:419–440. doi: 10.1016/S0006-3495(67)86597-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sejnowski TJ, Destexhe A. Why do we sleep? Brain Res. 2000;886:208–223. doi: 10.1016/s0006-8993(00)03007-9. [DOI] [PubMed] [Google Scholar]
- Siegel JM. Clues to the functions of mammalian sleep. Nature. 2005;437:1264–1271. doi: 10.1038/nature04285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirota A, Montgomery S, Fujisawa S, Isomura Y, Zugaro M, Buzsáki G. Entrainment of neocortical neurons and gamma oscillations by the hippocampal theta rhythm. Neuron. 2008;60:683–697. doi: 10.1016/j.neuron.2008.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steriade M. Acetylcholine systems and rhythmic activities during the waking—sleep cycle. Prog. Brain Res. 2004;145:179–196. doi: 10.1016/S0079-6123(03)45013-9. [DOI] [PubMed] [Google Scholar]
- Steriade M, Nuñez A, Amzica F. A novel slow (< 1 Hz) oscillation of neocortical neurons in vivo: depolarizing and hyperpolarizing components. J. Neurosci. 1993;13:3252–3265. doi: 10.1523/JNEUROSCI.13-08-03252.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stickgold R. Sleep-dependent memory consolidation. Nature. 2005;437:1272–1278. doi: 10.1038/nature04286. [DOI] [PubMed] [Google Scholar]
- Sullivan D, Csicsvari J, Mizuseki K, Montgomery S, Diba K, Buzsáki G. Relationships between hippocampal sharp waves, ripples, and fast gamma oscillation: influence of dentate and entorhinal cortical activity. J. Neurosci. 2011;31:8605–8616. doi: 10.1523/JNEUROSCI.0294-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tononi G, Cirelli C. Sleep function and synaptic homeostasis. Sleep Med. Rev. 2006;10:49–62. doi: 10.1016/j.smrv.2005.05.002. [DOI] [PubMed] [Google Scholar]
- Turrigiano GG. Homeostatic plasticity in neuronal networks: the more things change, the more they stay the same. Trends Neurosci. 1999;22:221–227. doi: 10.1016/s0166-2236(98)01341-1. [DOI] [PubMed] [Google Scholar]
- Vertes RP. Brainstem control of the events of REM sleep. Prog. Neurobiol. 1984;22:241–288. doi: 10.1016/0301-0082(84)90020-0. [DOI] [PubMed] [Google Scholar]
- Vyazovskiy VV, Olcese U, Lazimy YM, Faraguna U, Esser SK, Williams JC, Cirelli C, Tononi G. Cortical firing and sleep homeostasis. Neuron. 2009;63:865–878. doi: 10.1016/j.neuron.2009.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker MP. Sleep, memory and emotion. Prog. Brain Res. 2010;185:49–68. doi: 10.1016/B978-0-444-53702-7.00004-X. [DOI] [PubMed] [Google Scholar]
- Wilson MA, McNaughton BL. Reactivation of hippocampal ensemble memories during sleep. Science. 1994;265:676–679. doi: 10.1126/science.8036517. [DOI] [PubMed] [Google Scholar]
- Wolansky T, Clement EA, Peters SR, Palczak MA, Dickson CT. Hippocampal slow oscillation: a novel EEG state and its coordination with ongoing neocortical activity. J. Neurosci. 2006;26:6213–6229. doi: 10.1523/JNEUROSCI.5594-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.