Abstract
Background
The development of a ventricular septal defect(VSD) after myocardial infarction(MI) is an uncommon but highly lethal complication. We examined the Society of Thoracic Surgeons(STS) database to characterize patients undergoing surgical repair of post-MI VSD and to identify risk factors for poor outcomes.
Methods
This was a retrospective review of the STS database to identify adult(≥18 years) patients who underwent post-MI VSD repair between 1999–2010. Patients with congenital heart disease were excluded. The primary outcome was operative mortality. The covariates in the current STS model for predicted coronary artery bypass(CABG) operative mortality were incorporated in a logistic regression model in this cohort.
Results
There were 2,876 patients included. Mean age was 68±11 years, and 1,624(56.5%) were men. 215(7.5%) patients had prior CABG surgery, 950(33%) had prior percutaneous intervention, and 1,869(65.0%) were supported preoperatively with an intra-aortic balloon pump. Surgical status was urgent in 1,007(35.0%) and emergent in 1,430(49.7%). Concomitant CABG was performed in 1,837(63.9%). Operative mortality was 54.1%(1,077/1,990) if repair was ≤7 days from MI, and 18.4%(158/856) if >7 days from MI. Multivariable analysis identified several factors associated with increased odds of operative mortality.
Conclusions
In the largest study to date to examine post-MI VSD repair, ventricular septal rupture remains a devastating complication. As alternative therapies emerge to treat this condition, these results will serve as a benchmark for future comparisons.
Keywords: Ventricular septal defect, Outcomes, Society of Thoracic Surgeons database
Introduction
The development of a post-myocardial infarction ventricular septal defect(VSD) is an uncommon but frequently fatal complication, occurring in less than 1% of patients sustaining myocardial infarction(MI) in the modern era of early reperfusion therapy.1 In medically treated patients with this complication, mortality rates exceed 90%, while mortality in patients undergoing surgical repair ranges between 19 and 60%.1–7 More recently, percutaneous closure devices have permitted less invasive management of patients with post-MI VSD.8–11
As alternative technologies evolve to treat this highly lethal condition, the establishment of a benchmark for comparison is essential. Moreover, the identification of risk factors for poor outcomes after traditional surgical repair may help identify which patients are most likely to benefit from percutaneous intervention. The majority of previous studies on surgical outcomes have been confined to single series retrospective reviews with relatively small sample size.3–7 There is a single national registry report from Europe with 189 patients treated over a seven-year span.2 Therefore, we used national registry data provided by the Society of Thoracic Surgeons(STS) Adult Cardiac Surgery Database(ACSD) to examine risk factors for poor outcomes after surgical repair of post-MI VSD.
Material and Methods
Data Source
The STS ACSD encompasses clinical and demographic data on more than 4.5 million patients undergoing cardiac surgery at participating centers in North America since 1989. The Duke Clinical Research Institute(DCRI) remains the center for biostatistical analysis for all of the STS National Databases. The data used in analyses of the STS ACSD represent a limited dataset originally collected for non-research purposes, without direct patient identifiers, and therefore this study was considered exempt by the Duke University Health System institutional review board. The Johns Hopkins University institutional review board separately granted approval.
Patient Population
This study included all adult patients(≥18 years) who underwent surgical repair of a post-MI VSD between 1999–2010. Eligible patients were identified by an affirmative response in the STS data field for myocardial infarction. Patients who underwent concomitant coronary revascularization procedures or combined valvular procedures were included, however patients with congenital heart disease were excluded(n=65).
Outcomes
The primary outcome was operative mortality, defined as death from any cause either in-hospital or within 30 days of the index operation. Additional complications included length of stay, postoperative infection, respiratory failure, renal failure requiring renal replacement therapy, and cerebrovascular accident(CVA).
Study Design
Clinically relevant variables as well as previously reported predictors of 30-day post-CABG operative mortality according to the STS database were included as candidate variables in the full model of this analysis.12 These included demographic characteristics, medical co-morbidities(hypertension, hypercholesterolemia, chronic lung disease, diabetes, CVA, peripheral vascular disease, immunosuppressive treatment, renal function, left ventricular ejection fraction, present smoker, valvular disease, or arrhythmia), medical acuity(angina, timing of recent myocardial infarction(<6hr, 6–24hr, 1–7d, 8–21d, ≥21d), percutaneous coronary intervention ≤6hr, pre-operative intra-aortic balloon pump(IABP), inotropes, surgical status(emergent/salvage, urgent, elective), New York Heart Association(NYHA) classification, left main disease, previous cardiovascular intervention and cardiogenic shock), concomitant procedures, and year of surgery. Cardiogenic shock is defined as hypoperfusion with either (1) systolic blood pressure <80 mmHg or cardiac index <1.8L/min/m2 despite maximal treatment, or (2) intravenous inotropes and/or intra-aortic balloon pump necessary to maintain systolic blood pressure >80 mmHg or cardiac index >1.8 L/min/m2. Anatomic site of VSD rupture(posterior versus anterior) is not contained in the STS ACSD, and this operative factor was not analyzed.
Statistical analysis
Summary statistics for outcomes and baseline patient characteristics are presented as percentages for categorical variables and means with standard deviation(SD) for continuous variables. The Pearson chi-square test was used to compare categorical variables, whereas the Kruskal-Wallis test was used for continuous variables. SAS statistical software(version 9.1; SAS Institute, Cary, NC) was used for all calculations.
Missing data in the baseline characteristics were handled by multiple imputation under the assumption of missing at random and using Gibbs sampling.13 Variables included in the imputation models were operative mortality and the covariates in the full model (as described previously). Ten complete imputed datasets were created. A proposed model was then fitted to each of the completed datasets, and the ten sets of results were then combined.
Logistic regression modeling was used to estimate the risk of operative mortality as a function of patient baseline variables. Covariates were selected from the full list of candidate variables using a backward algorithm with a significance criterion of p=0.05 within each of the ten imputed data sets. Variables that were selected in any of the ten data sets were included in the reduced model. The year of surgery was forced into the reduced model to adjust time trends. Discrimination of full and reduced models was assessed by C-index. The enhanced bootstrap was used to estimate the bias(i.e. overestimated C-index) due to model overfitting in the original sample.15 Risk-adjusted odds ratios(ORs) of covariates were estimated. Robust sandwich variance estimates were used to obtain 95% confidence intervals(CI) to account for statistical dependence of patients within sites.16
Results
Demographics
In the cohort overall, there were 2,876 patients, with an average age of 68±11 years. There were 1,624(56.5%) men. Surgical status was listed as emergent in 1,430(49.7%), and 1,869(65.0%) were supported preoperatively with an IABP.
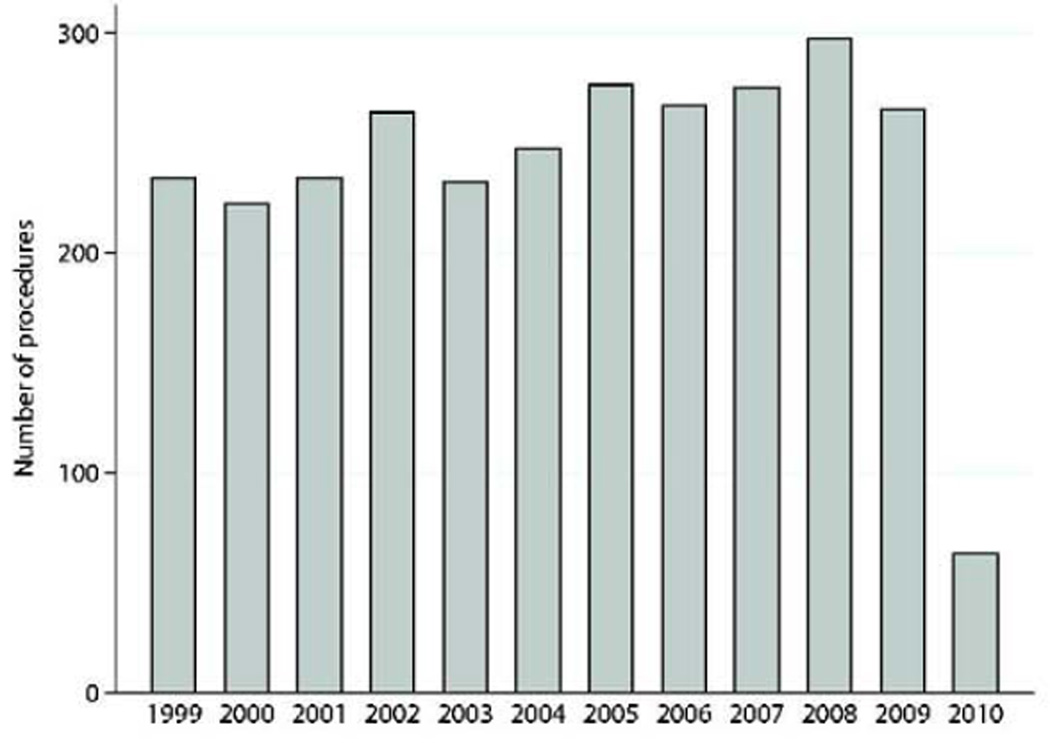
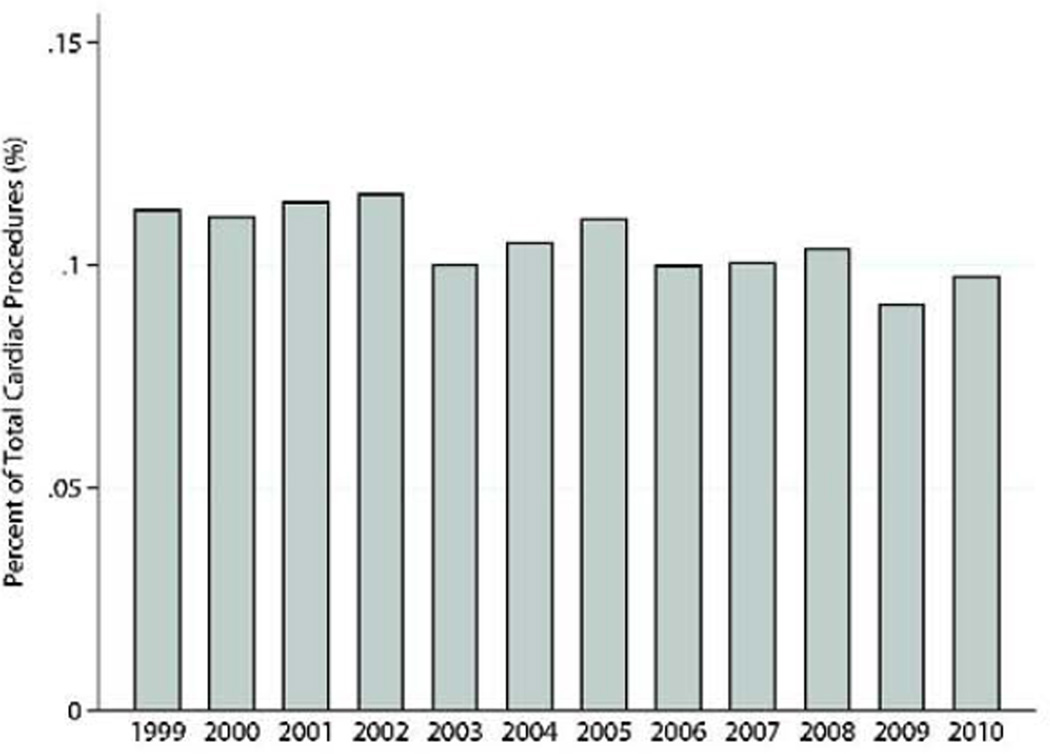
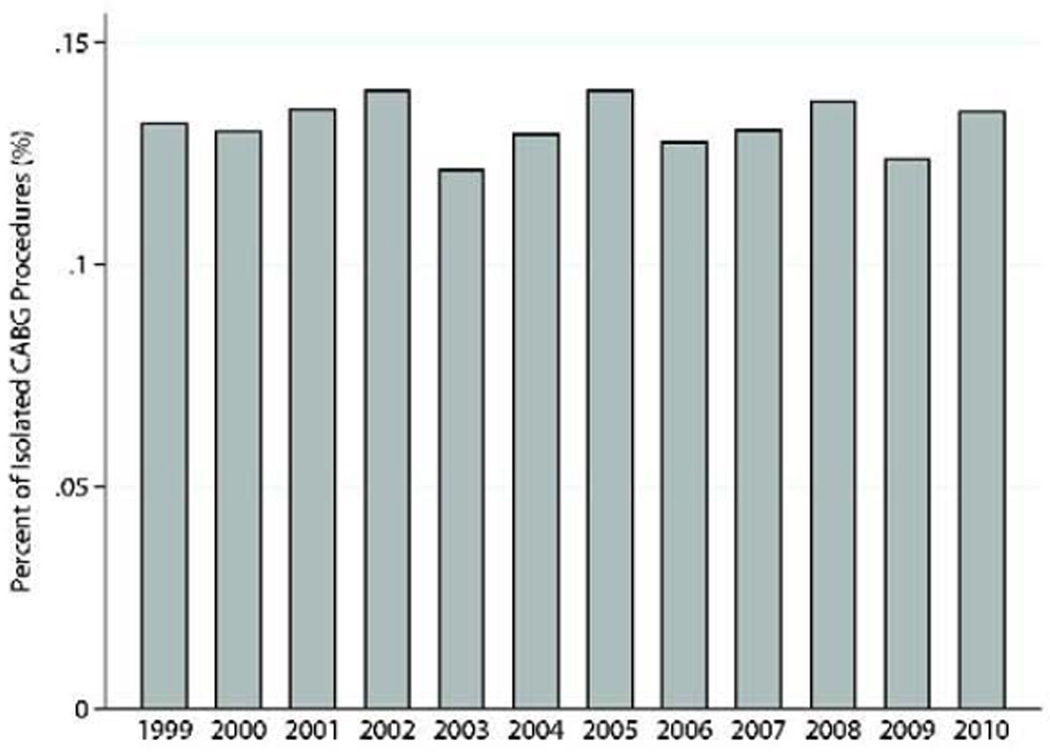
Concomitant coronary revascularization was performed in 1,837(63.9%). Mitral valve procedures were performed simultaneously in 211(7.3%) patients, and intraoperative ventricular assist device was required in 84(2.9%). One-third of the patients had undergone previous percutaneous coronary intervention(n=950,33.0%), and only 215(7.5%) had previous CABG. Additional clinical characteristics are presented in Table 1. The number of post-MI VSD operations performed annually remained relatively constant throughout the study(Range:232–297 procedures/year)(Figures 1a–c).
Table 1.
Clinical Characteristics of the Cohort Overall
| Variable | N=2,876 |
|---|---|
| Demographics | |
| Age, years(SD) | 68(±11) |
| Male gender, n(%) | 1,624(56.5%) |
| Caucasian race, n(%) | 2,504(87.1%) |
| Body mass index,(SD) | 27.8(±5.3) |
| Co-morbidities | |
| Hypertension, n(%) | 1,887(65.6%) |
| Insulin dependent diabetes, n(%) | 252(8.8%) |
| Smoking history, n(%) | 722(25.1%) |
| Chronic lung disease, n(%) | 499(17.4%) |
| Serum creatinine, mg/ml(SD) | 1.5(±0.9) |
| Dialysis, n(%) | 77(2.7%) |
| Cerebrovascular accident, n(%) | 269(9.4%) |
| Previous cardiac surgery, n(%) | 347(12.1%) |
| Prior percutaneous intervention, n(%) | 950(33.0%) |
| Acuity | |
| Preoperative IABP, n(%) | 1,869(65.0%) |
| Cardiogenic shock, n(%) | 1,487(51.7%) |
| Unstable angina, n(%) | 1,391(48.4%) |
| Triple vessel CAD, n(%) | 966(33.6%) |
| Ejection fraction, %(SD) | 43.1(14.1) |
| Operative Characteristics | |
| Emergent/salvage, n(%) | 1,430(49.7%) |
| Median CPB time, minutes(SD) | 163(125–213) |
| Median aortic cross clamp time, minutes(SD) | 109(80–147) |
| Intraoperative IABP, n(%) | 230(8.0%) |
| Concomitant CABG, n(%) | 1,837(63.9%) |
| Concomitant mitral valve procedure, n(%) | 211(7.3%) |
| Intraoperative VAD placement, n(%) | 84(2.9%) |
| Postoperative Outcomes | |
| Median length of stay, days(IQR) | 8(4–17) |
| CVA, n(%) | 104(3.6%) |
| Renal replacement therapy, n(%) | 343(11.9%) |
| Pneumonia, n(%) | 371(12.9%) |
| Heart block, n(%) | 114(4.0%) |
| Re-exploration for bleeding, n(%) | 224(7.8%) |
Figure 1.
a: Number of post-MI VSD surgical procedures by year. Data for 2010 only included first quarter data harvest.
b: Post-MI VSD surgical procedures performed as percentage of total cardiac operations reported to the STS database by year.
c: Post-MI VSD surgical procedures performed as percentage of total isolated CABG procedures reported to the STS database by year.
Several clinical variables differed according to surgical status(Table 2). Patients undergoing elective surgery were more likely to have a history of hypertension or CVA. Those patients operated upon in an emergency or salvage setting had worse renal function, more likely to smoke, and had greater acuity.
Table 2.
Clinical Characteristics According to Surgical Status
| Variable | Elective (N=425) |
Urgent (N=1,007) |
Emergent (N=1,215) |
Salvage (N=215) |
P-value* |
|---|---|---|---|---|---|
| Demographics | |||||
| Age, years(SD) | 68(±11) | 67(±11) | 69(±11) | 68(±10) | <0.01 |
| Male gender, n(%) | 264(62.1%) | 557(55.3%) | 683(56.2%) | 113(52.6%) | 0.06 |
| Caucasian race, n(%) | 372(87.5%) | 868(86.2%) | 1,061(87.3%) | 191(88.8%) | 0.9 |
| Body mass index, (SD) | 27.8(±5.1) | 27.6(±5.2) | 28.0(±5.4) | 28.0(5.2) | 0.4 |
| Co-morbidities | |||||
| Hypertension, n(%) | 304(71.6%) | 670(66.5%) | 772(63.5%) | 133(61.9%) | 0.03 |
| Insulin dependent diabetes, n(%) | 35(8.2%) | 102(10.1%) | 98(8.1%) | 17(7.9%) | 0.6 |
| Smoking history, n(%) | 80(18.8%) | 254(25.2%) | 336(27.7%) | 50(23.3%) | <0.01 |
| Serum creatinine, mg/ml(SD) | 1.3(±0.8) | 1.4(±0.9) | 1.6(±1.0) | 1.6(±0.9) | <0.01 |
| Dialysis, n(%) | 6(1.4%) | 36(3.6%) | 30(2.5%) | 5(2.3%) | 0.1 |
| Cerebrovascular accident, n(%) | 54(12.7%) | 99(9.8%) | 105(8.6%) | 10(4.7%) | 0.01 |
| Prior percutaneous intervention, n(%) | 146(34.4%) | 331(32.9%) | 417(34.3%) | 60(27.9%) | 0.09 |
| Acuity | |||||
| NYHA class IV, n(%) | 95(22.4%) | 543(53.9%) | 896(73.7%) | 168(78.1%) | <0.01 |
| Preoperative IABP, n(%) | 79(18.6%) | 561(55.7%) | 1,029(84.7%) | 189(87.9%) | <0.01 |
| Cardiogenic shock, n(%) | 34(8.0%) | 281(27.9%) | 970(79.8%) | 190(88.4%) | <0.01 |
| Unstable angina, n(%) | 97(22.8%) | 434(43.1%) | 716(58.9%) | 135(62.8%) | <0.01 |
| Triple vessel CAD, n(%) | 220(51.8%) | 651(64.7%) | 763(62.8%) | 129(60.0%) | <0.01 |
| Ejection fraction, %(SD) | 45.5(±14.2) | 44.1(±14.1) | 42.0(±13.6) | 36.6(±14.0) | <0.01 |
| Operative Characteristics | |||||
| Median CPB time, minutes(IQR) | 142(110–188) | 160(123–206) | 170(132–222) | 186(138–240) | <0.01 |
| Median aortic cross clamp time, minutes(IQR) | 99(70–137) | 108(78–145) | 113(83–150) | 116(85–154) | <0.01 |
| Intraoperative IABP, n(%) | 48(11.3%) | 99(9.8%) | 71(5.8%) | 11(5.1%) | <0.01 |
Pearson chi-square tests for categorical variables and Kruskal-Wallis nonparametric test for continuous variables.
Outcomes
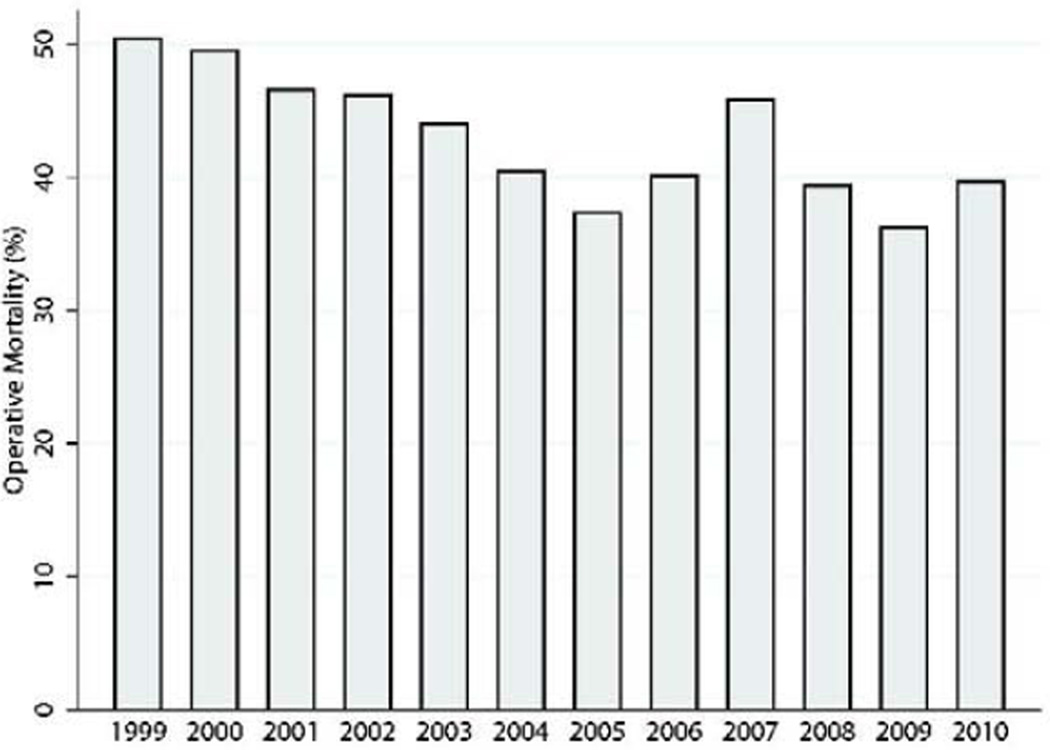
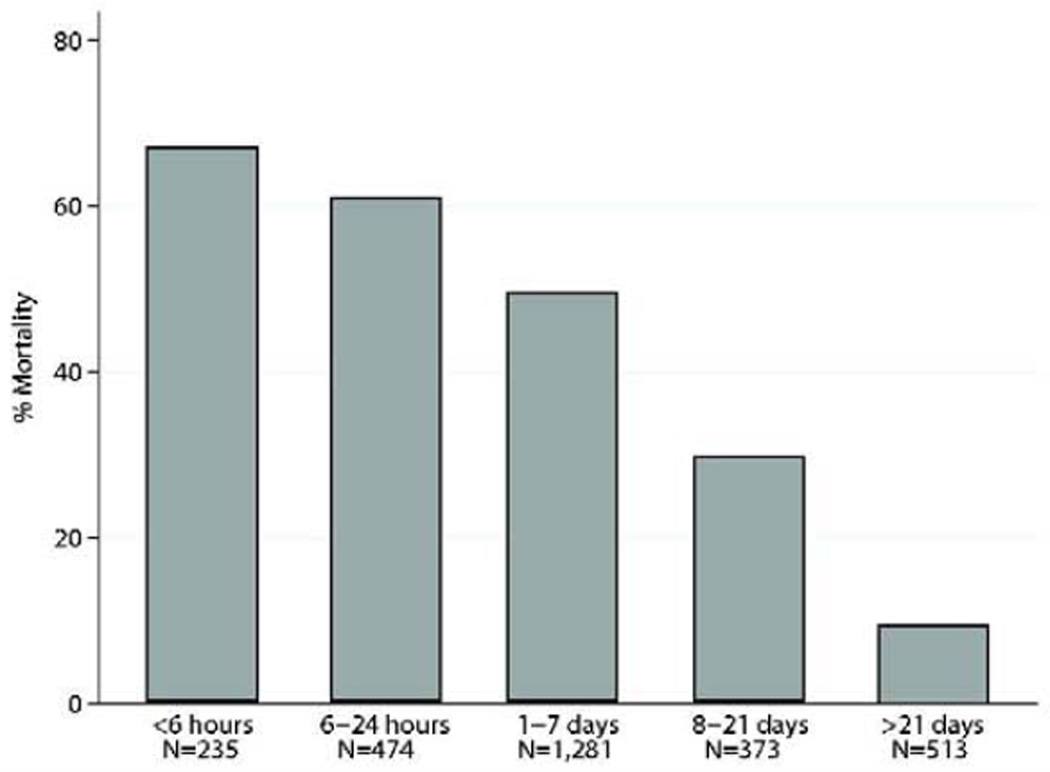
Overall operative mortality was 42.9%(n=1,235). There was a non-linear time trend with respect to operative mortality over the course of the study(Figure 2). If repair was ≤7 days from MI, operative mortality was 54.1%(1,077/1,990) compared with 18.4%(158/856) if >7 days from MI(Figure 3). The highest operative mortality rate was among patients whose VSD repair was within 6 hours from MI. When examining operative mortality by surgical status, 56(13.2%) patients undergoing elective surgery died, compared with 680(56.0%) emergent status patients and 173(80.5%) salvage patients who died. The most common cause of death was cardiac(n=947,76.7%), followed by pulmonary(n=43,3.5%), neurologic(n=40,3.2%), infectious(n=36,2.9%), renal(n=23,1.9%), and other(n=80,6.5%) complications.
Figure 2.
Operative mortality for each year of the study.
Figure 3.
Operative mortality according to timing of MI in relation to VSD repair. P<0.01 by univariate analysis.
A comparison of clinical characteristics between survivors and non-survivors is presented in Table 3. Patients who died within 30 days tended to be older, female, had higher serum creatinine, and markers of more severe clinical acuity. Patients who died were also less likely to be on a pre-operative B-blocker or lipid -lowering agent, and were also less likely to have hypertension, lung disease, or previous CABG. Longer cardiopulmonary bypass time was associated with higher mortality(159±64 vs 196±82 minutes, p<0.001).
Table 3.
Comparison of Clinical Characteristics Among Between Survivors and Non-Survivors
| Variable | Survivors (N=1,641) |
Non-Survivors (N=1,235) |
P-value* |
|---|---|---|---|
| Demographics | |||
| Age, years(SD) | 66(±11) | 71(±10) | <0.01 |
| Male gender, n(%) | 1,006(61.3%) | 618(50.0%) | <0.01 |
| Caucasian race, n(%) | 1,430(87.1%) | 1,074(87.0%) | 0.5 |
| Body mass index >28, n(%) | 722(44.0%) | 502(40.7%) | 0.07 |
| Co-morbidities | |||
| Hypertension, n(%) | 1,109(67.6%) | 778(63.0%) | 0.02 |
| Insulin dependent diabetes, n(%) | 151(9.2%) | 101(8.2%) | 0.8 |
| Smoking history, n(%) | 448(27.3%) | 274(22.2%) | <0.01 |
| Chronic lung disease, n(%) | 319(19.4%) | 180(14.6%) | <0.01 |
| Serum creatinine, mg/ml(SD) | 1.4(±0.9) | 1.6(±0.9) | <0.01 |
| Dialysis, n(%) | 40(2.4%) | 37(3.0%) | 0.3 |
| Cerebrovascular accident, n(%) | 154(9.4%) | 115(9.3%) | 0.9 |
| Previous CABG, n(%) | 137(8.4%) | 78(6.4%) | 0.04 |
| Prior percutaneous intervention, n(%) | 542(33.0%) | 408(33.0%) | 0.9 |
| Pre-operative B-blocker, n(%) | 913(55.6%) | 540(43.7%) | <0.01 |
| Pre-operative lipid-lowering agent, n(%) | 461(28.1%) | 228(18.5%) | <0.01 |
| Acuity | |||
| Preoperative IABP, n(%) | 876(53.4%) | 993(80.4%) | <0.01 |
| Cardiogenic shock, n(%) | 609(37.1%) | 878(71.1%) | <0.01 |
| NYHA class IV, n(%) | 617(61.5%) | 554(79.1%) | <0.01 |
| Triple vessel CAD, n(%) | 968(60.0%) | 804(65.1%) | <0.01 |
| Ejection fraction, %(SD) | 43.7(±13.8) | 41.9(±14.5) | <0.01 |
| Operative Characteristics | |||
| Elective status, n(%) | 369(22.5%) | 56(4.5%) | <0.01 |
| Urgent status, n(%) | 693(42.2%) | 314(25.4%) | <0.01 |
| Emergent status, n(%) | 535(32.6%) | 680(55.1%) | <0.01 |
| Salvage status, n(%) | 42(2.6%) | 173(14.0%) | <0.01 |
| Median CPB time, minutes(IQR) | 150(116–194) | 182(139–237) | <0.01 |
| Median aortic cross clamp time, minutes(IQR) | 103(75–140) | 117(86–158) | <0.01 |
| Intraoperative IABP, n(%) | 125(7.6%) | 105(8.5%) | <0.01 |
Pearson chi-square tests for categorical variables and Kruskal-Wallis nonparametric test for continuous variables.
Median overall hospital length of stay was 8 days(IQR:4–17). A majority of patients(n=2,204,76.6%) sustained major morbidity or mortality after surgical VSD repair(Table 1), with the most common complication being postoperative dialysis(n=343,11.9%).
Multivariable Analysis
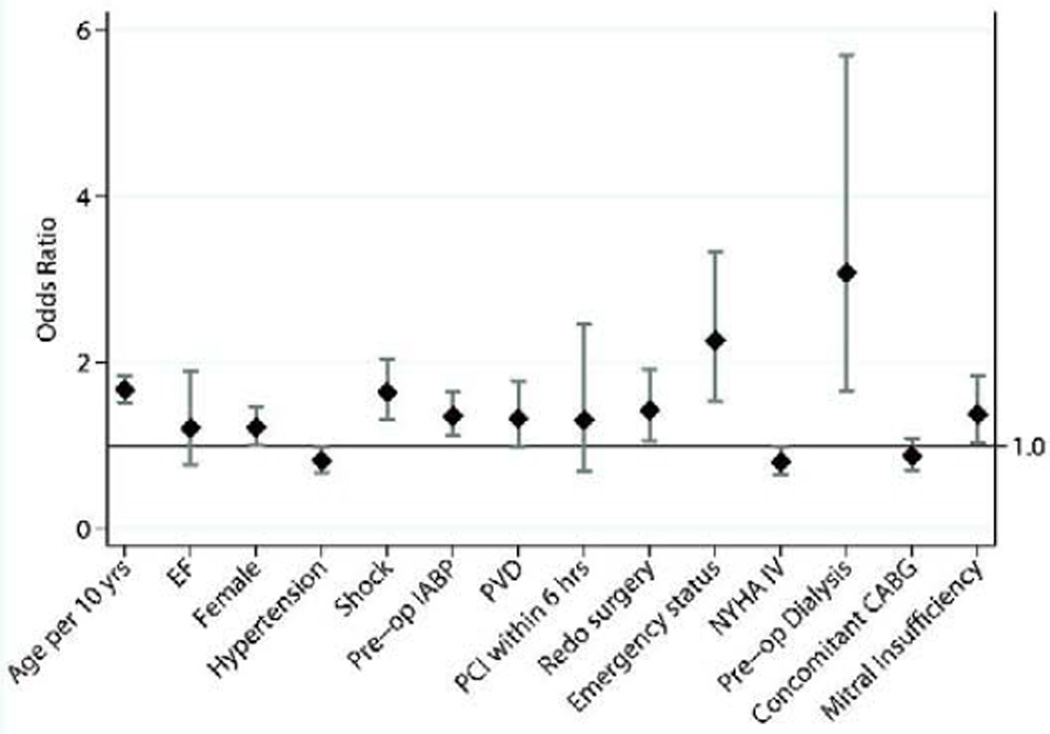
The full multivariable logistic regression model was initially performed using the 29 factors included in the model predicting operative mortality following CABG, as detailed in an earlier STS report.12 A more parsimonious model was constructed, and this reduced model included 14 exposure variables(figure 4) as well as surgical year. This simplified model retained similar predictive power as the full model(corrected C-index=0.79 for both full and reduced models).
Figure 4.
Odds ratio plot of variables included in the reduced risk-adjusted logistic regression model for operative mortality. Year of surgery controlled for in the regression model. Diamonds represent actual odds ratio values and gray bars denote 95%CI. C-index for the reduced model was 0.79.
The relationship between surgical year and operative mortality was non-linear. Only exposure variables are included in the odds ratio plot shown in Figure 4, and thus surgical year was not included. The factor with the highest magnitude of effect was preoperative dialysis(OR 3.07,95% CI[1.66–5.69],p=<0.01). Age(per 10 years), female gender, shock, pre-operative IABP, mitral insufficiency(moderate to severe), redo cardiac surgery, and emergency status were also independently associated with greater odds of operative mortality. When examining MI timing in relation to VSD repair, a longer time interval between MI and surgical repair(>21 days) was also associated with lower odds of operative mortality(p<0.01); there was a progressive increase in the odds of mortality with shorter time intervals(<6hrs:OR6.18; 6–24hrs:OR5.53; 1–7d:OR4.59; 8–21d:OR 2.37, all p<0.01). Histories of hypertension or CHF were associated with lower odds of operative mortality, whereas concomitant CABG was not associated with operative mortality(OR:0.87, 95%CI 0.70–1.08,p=0.2).
Comment
This study examined data provided by the STS ACSD to examine outcomes in over 2,800 patients with post-MI VSD who underwent surgical repair from 1999–2010. We observed a high operative mortality rate of 42.9% in the cohort overall, representing the highest risk of all cardiac procedures recorded in the STS ACSD. This high mortality rate is consistent with the 47% operative mortality rate seen in the GUSTO trial of over 41,000 patients treated for MI in the current thrombolytic era.1 Although earlier studies have reported a beneficial effect of concomitant CABG when indicated, in this study simultaneous CABG did not confer a protective effect.4, 6 An Italian study reported a decreasing incidence of ventricular septal rupture due to the advent of early reperfusion therapy and improved medical management of acute myocardial infarction.14 However, the absolute number of procedures for surgical repair of post-MI VSD has remained relatively constant over the study period. Furthermore, the advent of percutaneous treatment of VSD does not seem to have had an impact on the use of surgery.
In an earlier study, David et al. reported excellent operative results from the Toronto experience, with a surgical mortality rate of 19%.3 The authors describe technical aspects of anterior and posterior VSD repair through use of an infarct exclusion technique, and advocate early surgical repair prior to the development of cardiogenic shock. However, information regarding the time interval from MI to surgery was not reported, as most patients were referred for surgery from other hospitals.
Jeppsson et al. published a national registry series involving post-MI surgical VSD repair.2 This study included 189 patients operated on in ten different centers throughout Sweden during a seven-year period, with a 41% 30-day mortality rate. This study is strengthened by the inclusion of anatomic information regarding the location of septal rupture. The authors observed that posterior septal rupture was independently associated with an increased risk of operative mortality. While anatomic information regarding septal rupture and operative details are lacking from the STS database, our study complements these earlier reports by including a significantly larger sample size, and providing results from the North American experience with post-MI VSD repair.
The results in our study have important implications as newer percutaneous approaches for repair of VSD and valvular disease gain increasing traction within the cardiovascular community.8, 9, 11, 15 An alternative approach to post-MI VSD described use of biventricular mechanical support to temporize a patient until improved hemodynamics permitted definitive repair.16 When patients in our study underwent repair on an emergency basis operative mortality approached 60%, and perhaps direct circulatory support as a temporizing maneuver should be employed in sicker patients. Additional evidence is needed to better understand the cost-effectiveness of this approach, however. With ongoing advancements in percutaneous technology and development of alternative approaches, it will be incumbent on cardiovascular specialists to identify appropriate patients for surgical repair. The risk-adjusted results provided by this study will enable clinicians to have an appreciation of risk factors that increase the odds of operative mortality.
Patients who are hemodynamically stable without multiple organ failure perhaps could be carefully monitored, and then undergo a lower risk elective procedure at a later time. The advantages of delayed elective repair allow myocardial scar tissue formation and may facilitate the technical aspects of VSD repair. Or, in the case of a small VSD in a stable patient, early percutaneous treatment is an alternative option.
When simultaneous surgical procedures are not indicated, patients with a constellation of factors predisposing them to operative mortality such as cardiogenic shock, need for an IABP, and renal dysfunction may be best suited for percutaneous repair. Currently, data on percutaneous interventions for post-MI VSD repair are mainly limited to single center series and case reports, and report 30-day mortality rates as high as 65%.8–11 Maltais et al. compared 12 patients undergoing percutaneous repair with 39 patients who had surgical repair of a post-MI VSD repair.10 While not reaching significance, percutaneous closure patients tended to be older, and had smaller diameter ventricular defects. On multivariate analysis examining risk for 30-day mortality, the repair approach was not associated with increased risk for hospital mortality. Further studies are needed to validate the durability of the percutaneous approach and to compare results with surgical repair.
The association between center volume and outcomes following a wide range of surgical procedures is well-established.17, 18 However, it is difficult to account for center volume in this analysis because among the 666 centers that performed VSD repair during the study period, the average annual number of procedures ranged from 0.09 to 3.7. Because of small case volume range, we elected not to include center volume in this analysis.
Limitations
Due to the retrospective study design, we are unable to certify that all potential confounders have been examined. For example, there is no information in the database regarding the time interval between MI and VSD diagnosis, or the interval between VSD recognition and operative intervention. Furthermore, large registry databases such as the STS ACSD depend heavily on accurate coding. Being the prototype of national surgical subspecialty databases, the STS ACSD performs regular audits and internal data checking. We have assumed that any residual coding errors are random, and are thus unlikely to render any bias.
To minimize the effects of missing data, clinically relevant variables with >10% missing data were handled with multiple imputation. An alternative approach would be to omit factors with a substantial percentage of missing data from the analysis entirely. On bivariate analysis, ejection fraction and serum creatinine had nearly 10% missing data and these variables were deemed clinically relevant enough to warrant imputation.
Lack of mortality data among patients who died while awaiting surgery is an added limitation of this study. On univariate analysis, a shorter time interval between MI and surgical repair was associated with higher operative mortality, and this finding persisted on multivariable analysis. Other single center studies have observed the opposite finding.19 This time variable may introduce a double bias as more critically ill patients are likely dying before surgery and not included; conversely, more stable patients can be delayed and operated upon in a less urgent setting. Because of this inherent bias, these findings should be interpreted with caution. However, patients with multiple risk factors for operative mortality who are stable enough to delay immediate surgery may be better served waiting a period of several weeks prior to surgical repair.
This study is further limited by the relatively short follow-up period, and thus these data do not provide information on the durability of surgical closure of post-MI VSD. Furthermore, 30-day follow up from index hospitalization likely underestimates mortality and complications. The recent linking of the STS ACSD with the Medicare database may provide the opportunity for longer-term survival analysis in a future study.20
Conclusion
In this analysis of post-MI VSD repair using the STS national database, ventricular septal rupture remains a devastating complication after MI. As alternative therapies such as percutaneous closure devices emerge to treat this condition, these results will serve as a benchmark for future comparisons.
Acknowledgements
Dr. Arnaoutakis is the Irene Piccinini Investigator in Cardiac Surgery. Dr. George is the Hugh R. Sharp Cardiac Surgery Research Fellow. This research was supported by NIH Grant 1T32CA126607-01A2(GJA). The STS through use of the ACSD and the DCRI supported this work.
Abbreviations and Acronyms
- ACSD
Adult Cardiac Surgery Database
- CPB
Cardiopulmonary bypass
- CI
Confidence interval
- CABG
Coronary artery bypass graft
- CAD
Coronary artery disease
- DCRI
Duke Clinical Research Institute
- IQR
Interquartile range
- IABP
Intra-aortic balloon pump
- MI
Myocardial infarction
- NYHA
New York Heart Association
- OR
Odds ratio
- STS
Society of Thoracic Surgeons
- SD
Standard deviation
- VSD
Ventricular septal defect
Discussion
4. Richard E. Clark Paper: Surgical Repair of Ventricular Septal Defect After Myocardial Infarction — Outcomes From The Society of Thoracic Surgeons National Database. Paper presented by George Arnaoutakis, MD, Baltimore, MD. gja10@jhmi.edu
Discussion by Tirone E. David, MD, Canada tirone.david@uhn.on.ca
Dr. T. David (Toronto, ON, Canada):
I have no conflicts of interest to disclose.
Dr. Arnaoutakis and colleagues extracted from the Society of Thoracic Surgeons database all patients with postinfarction ruptured septum during a 12-year interval, 1999 to 2010. They identified 2,876 patients operated at 666 centers in the United States with an average of 0.09 to 3.7 patients per year per unit. Thus, very few of us operate on more than one patient per year. Most of us see one patient like this every 4 years.
I believe that practice makes perfect. It is extremely difficult to become an expert in treating this disease because of its relative rarity. It was not always like that. I treated at least one patient a month with a ruptured septum when I started practicing 30 years ago. It was so common that I was able to change the classical technique of infarctectomy and patches with a stiff Dacron graft developed by Bill Daggett in Boston to a more conservative approach whereby we simply opened the infarct and excluded the infarcted muscle and ruptured septum by using a soft patch such as pericardium secured to the healthy endocardium around the infarct. We named the technique "infarct exclusion" and we were able to dramatically reduce at least my personal mortality, particularly in patients with posterior septal rupture, who had the highest mortality 20–30 years ago.
Practice makes perfect. I reviewed our experience with postinfarction ruptured septum at Toronto General Hospital from 1990 to 2010. Our database contained 42,801 patients, and only 91 were operated on for postinfarction septal rupture. Coincidentally, our overall operative mortality was also 40%. Nine surgeons performed these 91 operations. Two surgeons performed 49 of them. The operative mortality of those two surgeons was just over 20%. The operative mortality among the other surgeons was in excess of 50%, and yet the clinical profiles of our patients were similar.
You have identified that timing of surgery following the acute myocardial infarction is likely the most important determinant of operative mortality. Operations performed under six hours from the infarction had a mortality in excess of 60%. With such a high operation mortality, isn't it time to stop and rethink? If we fail to save two-thirds of the patients who need surgery so soon after the infarction (I assume they were very sick and that’s the reason for emergency surgery) we should look for an alternative approach. And that’s my first question to you. In this era of newer left ventricular assist devices, percutaneous devices such as Impella, wouldn't be better to implant these devices and wait for a few weeks before definitive surgery is done? My second question is a bit more complicated and comes from a surgeon who has practiced in a more socialist health care system. Isn't it time to rethink again who should be taking care of critically ill patients such as these? Is it more appropriate to refer these patients to a regional center where more specialized service is available?
I would like to congratulate Dr. Arnaoutakis and his colleagues for an excellent presentation and thank the Society for the privilege to discuss this paper.
4. Richard E. Clark Paper: Surgical Repair of Ventricular Septal Defect After Myocardial Infarction — Outcomes From The Society of Thoracic Surgeons National Database. Response by George Arnaoutakis, MD, Baltimore, MD.
DR. ARNAOUTAKIS: Thank you, Dr. David, for those very kind comments, and I must add that it is a great honor to have you as the discussant for our paper. In preparing this presentation and the manuscript, I reviewed your manuscripts from the '90s, which, as you alluded to, have excellent operative mortality rates in high acuity patients, and I would also add that the illustrations and the technical descriptions were very useful.
Regarding your points, center volume and surgeon volume has been a matter of great interest to us in our research efforts, particularly related to thoracic transplantation, and we certainly would agree with you that center volume and individual surgeon volume are very important. That has been shown in a wide variety of surgical procedures. Because of that, we specifically in working with the statisticians when putting together this analysis went to look at center volume, but because, as you alluded, the volume ranges between .1 to 3.9 procedures performed at a given center, we determined that the number of procedures was too low to be meaningful, although we certainly appreciate the importance of center volume.
Regarding the timing, certainly looking at the univariate and the multivariate relationships there is a significant association, and that is adjusting for the other confounders. But what is left out by a retrospective study, unfortunately, is the number of patients who died while waiting. And so even though the graph shows the progressive improvement in mortality when you have a more spaced out time interval from the MI in relation to the VSD repair, there is a caveat in that there is the bias of not knowing how many patients died while waiting. Currently, many authors in past studies, including your paper, advocate early repair before the development of hemodynamic compromise and shock, and, currently, the American College of Cardiology recommendations are earlier or immediate repair in patients who developed post-MI VSD.
I think in the absence of a prospective study, even given these data presented today, I wouldn't advocate withholding early therapy. But what I would caution is that instead of advocating early surgical repair that we maybe shift to a paradigm of early intervention, as you alluded, either with percutaneous closure devices or there are some reports in the literature of placing biventricular support devices to temporize a patient so that their hemodynamics may recover. Percutaneous closure devices may, while not necessarily affecting pump failure, improve the pulmonary overcirculation, allow the pulmonary hemodynamics to improve, also allowing time for the myocardium to fibrose, and enhancing the chances of a good outcome with surgery. So I still think that in the absence of prospective data, early intervention in these patients is warranted, but, as you suggest, we may want to shift our focus in appropriate patients. In a patient who comes in without any evidence of shock and need for dialysis who would otherwise be a low-risk candidate, I think that probably surgical intervention may still be warranted. But someone who comes in with dialysis and a balloon pump and is elderly may be better served with a percutaneous or an alternative approach initially followed by definitive surgical repair if they don't achieve a satisfactory result with whatever initial approach.
Regarding referral, certainly regionalization is important with procedures that are complex and that have high associated morbidity and mortality. I do appreciate your point about referral to tertiary centers in patients who present with post-MI VSDs, with the caution that some patients may be too unstable to be transferred. I know in your series many of the patients were transferred from other centers to yours, but you do comment that some patients died before being able to be transferred. It is a difficult question in a center where there are appropriate services available. It may be better off that that patient undergo early surgery, especially if they have not developed signs of shock, instead of waiting for transfer when a few days may elapse and then they develop shock and their risk for surgery goes up. So it is a very difficult question. I don't think our data can definitively answer it, but I certainly think it is worth discussion.
4. Richard E. Clark Paper: Surgical Repair of Ventricular Septal Defect After Myocardial Infarction — Outcomes From The Society of Thoracic Surgeons National Database. Paper presented by George Arnaoutakis, MD, Baltimore, MD. gja10@jhmi.edu
Discussion by Ernesto Jimenez, MD, Florida ernesto.jimenez@va.gov
Dr. E. Jimenez (Tampa, FL):
My question is, did you look at the residual VSD leak rate and is the mortality associated with a good repair or if the repair fails and you still have a leak, does that increase mortality? And is there a difference in terms of leak rate if you operate early versus late? Presumably there is, but I was wondering what the data shows.
4. Richard E. Clark Paper: Surgical Repair of Ventricular Septal Defect After Myocardial Infarction — Outcomes From The Society of Thoracic Surgeons National Database. Response by George Arnaoutakis, MD, Baltimore, MD.
DR. ARNAOUTAKIS: Thank you for your important question. Unfortunately, as we heard a little earlier with respect to mitral valve operations, echocardiographic follow-up information in the database is lacking, and so we weren't able to assess whether the leak affected mortality in this study. Although I would advocate that echocardiographic information in other studies has been important and should be a possible consideration for future iterations of the STS database.
DR. JIMENEZ: I guess 40% of the patients were emergent operations, but only 8% had balloon pumps?
DR. ARNAOUTAKIS: Sixty-five percent of patients had balloon pumps placed preoperatively. In addition to that 65%, another 8% had balloon pumps placed intraoperatively. So presumably the overwhelming majority listed as emergent or salvage had balloon pumps in place prior to going to the operating room, and then the additional 8% required balloon pump placement in the operating room as well as an additional 3% who went on to require a VAD.
4. Richard E. Clark Paper: Surgical Repair of Ventricular Septal Defect After Myocardial Infarction — Outcomes From The Society of Thoracic Surgeons National Database. Paper presented by George Arnaoutakis, MD, Baltimore, MD. gja10@jhmi.edu
Discussion by Daniel H. Drake, MD, Michigan tcbulldog@charter.net Dr. D. Drake (Traverse City, MI):
I congratulate you on your presentation. It was excellent. For all of the attendees who haven't tried Dr. David's infarct exclusion procedure, it is elegant and saves clamp time. I strongly encourage you to become familiar with the technique.
Dr. Arnaoutakis, at your institution, what is the preferred procedure?
4. Richard E. Clark Paper: Surgical Repair of Ventricular Septal Defect After Myocardial Infarction — Outcomes From The Society of Thoracic Surgeons National Database. Response by George Arnaoutakis, MD, Baltimore, MD.
DR. ARNAOUTAKIS: Thank you for that question. I would like to add one point with respect to the infarct exclusion technique. When you compare the national cardiopulmonary bypass and aortic cross-clamp times in our study compared with Dr. David's, the cardiopulmonary bypass times are much less suggesting that the infarct exclusion technique does save time.
At our institution, we prefer the infarct exclusion technique as well. In the case of a severely extensive necrotic LV wall, we sometimes will put a patch to reconstruct the LV wall simultaneously as a double-patch technique. But where the LV wall has sufficient myocardium to allow for closure, then we prefer the infarct exclusion technique exactly as Dr. David described.
DR. DRAKE: One final point. Dr. David, your procedures are known to evolve. Have you down anything new or exciting with the infarct exclusion or do you still anchor it to the mitral valve and extend from there?
DR. DAVID: We do.
DR. DRAKE: And do you use CorMatrix now as the procedure?
DR. DAVID: Yes.
4. Richard E. Clark Paper: Surgical Repair of Ventricular Septal Defect After Myocardial Infarction — Outcomes From The Society of Thoracic Surgeons National Database. Paper presented by George Arnaoutakis, MD, Baltimore, MD. gja10@jhmi.edu
Discussion by William L. Holman, MD, Alabama wholman@uab.edu
Dr. W. Holman (Birmingham, AL):
The question I had stems from the surprise of only 3% of the patients being on something other than a balloon pump with regard to mechanical support. Do you have any idea of the outcome of those patients versus the other, and do you know if the use of the total artificial heart would be captured in the database, because that is one of the relative indications for the use of that device?
4. Richard E. Clark Paper: Surgical Repair of Ventricular Septal Defect After Myocardial Infarction — Outcomes From The Society of Thoracic Surgeons National Database. Response by George Arnaoutakis, MD, Baltimore, MD.
DR. ARNAOUTAKIS: In addition to VAD, there was 65% preoperatively and then another 8% who required a balloon pump.
DR. HOLMAN: No. Those were balloon pumps. What about VADs?
DR. ARNAOUTAKIS: In the database it's 3% that's listed as VAD, which we were a little surprised as well. I would caution that there were a fair number of patients with the field coded as "missing" for ventricular assist device and we are left unsure whether that signified a yes or no, but presumably it was a no.
With respect to univariate testing, VAD did not predict mortality, but, as I said, there is the caution that there were a fair number of patients "missing." Also, presumably, total artificial heart would be included in the ventricular assist device category, because, to my knowledge, there is not a separate code for total artificial heart in the STS database.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts: Dr. Conte receives research support from Thoratec and Medtronic.
References
- 1.Crenshaw BS, Granger CB, Birnbaum Y, et al. Risk factors, angiographic patterns, and outcomes in patients with ventricular septal defect complicating acute myocardial infarction. GUSTO-I (Global Utilization of Streptokinase and TPA for Occluded Coronary Arteries) Trial Investigators. Circulation. 2000;101(1):27–32. doi: 10.1161/01.cir.101.1.27. [DOI] [PubMed] [Google Scholar]
- 2.Jeppsson A, Liden H, Johnsson P, Hartford M, Radegran K. Surgical repair of post infarction ventricular septal defects: a national experience. Eur J Cardiothorac Surg. 2005;27(2):216–221. doi: 10.1016/j.ejcts.2004.10.037. [DOI] [PubMed] [Google Scholar]
- 3.David TE, Armstrong S. Surgical repair of postinfarction ventricular septal defect by infarct exclusion. Semin Thorac Cardiovasc Surg. 1998;10(2):105–110. doi: 10.1016/s1043-0679(98)70003-6. [DOI] [PubMed] [Google Scholar]
- 4.Muehrcke DD, Daggett WM, Jr, Buckley MJ, Akins CW, Hilgenberg AD, Austen WG. Postinfarct ventricular septal defect repair: effect of coronary artery bypass grafting. Ann Thorac Surg. 1992;54(5):876–882. doi: 10.1016/0003-4975(92)90640-p. discussion 82–3. [DOI] [PubMed] [Google Scholar]
- 5.Labrousse L, Choukroun E, Chevalier JM, et al. Surgery for post infarction ventricular septal defect (VSD): risk factors for hospital death and long term results. Eur J Cardiothorac Surg. 2002;21(4):725–731. doi: 10.1016/s1010-7940(02)00054-4. discussion 31–2. [DOI] [PubMed] [Google Scholar]
- 6.Barker TA, Ramnarine IR, Woo EB, et al. Repair of post-infarct ventricular septal defect with or without coronary artery bypass grafting in the northwest of England: a 5-year multi-institutional experience. Eur J Cardiothorac Surg. 2003;24(6):940–946. doi: 10.1016/s1010-7940(03)00465-2. [DOI] [PubMed] [Google Scholar]
- 7.Cerin G, Di Donato M, Dimulescu D, et al. Surgical treatment of ventricular septal defect complicating acute myocardial infarction. Experience of a north Italian referral hospital. Cardiovasc Surg. 2003;11(2):149–154. doi: 10.1016/s0967-2109(02)00190-4. [DOI] [PubMed] [Google Scholar]
- 8.Holzer R, Balzer D, Amin Z, et al. Transcatheter closure of postinfarction ventricular septal defects using the new Amplatzer muscular VSD occluder: Results of a U.S. Registry. Catheter Cardiovasc Interv. 2004;61(2):196–201. doi: 10.1002/ccd.10784. [DOI] [PubMed] [Google Scholar]
- 9.Lee MS, Kozitza R, Mudrick D, et al. Intraoperative device closure of postinfarction ventricular septal defects. Ann Thorac Surg. 2010;89(6):e48–e50. doi: 10.1016/j.athoracsur.2010.03.081. [DOI] [PubMed] [Google Scholar]
- 10.Maltais S, Ibrahim R, Basmadjian AJ, et al. Postinfarction ventricular septal defects: towards a new treatment algorithm? Ann Thorac Surg. 2009;87(3):687–692. doi: 10.1016/j.athoracsur.2008.11.052. [DOI] [PubMed] [Google Scholar]
- 11.Thiele H, Kaulfersch C, Daehnert I, et al. Immediate primary transcatheter closure of postinfarction ventricular septal defects. Eur Heart J. 2009;30(1):81–88. doi: 10.1093/eurheartj/ehn524. [DOI] [PubMed] [Google Scholar]
- 12.Shahian DM, O'Brien SM, Filardo G, et al. The Society of Thoracic Surgeons 2008 cardiac surgery risk models: part 1--coronary artery bypass grafting surgery. Ann Thorac Surg. 2009;88(1 Suppl):S2–S22. doi: 10.1016/j.athoracsur.2009.05.053. [DOI] [PubMed] [Google Scholar]
- 13.van Buuren S. Multiple imputation of discrete and continuous data by fully conditional specification. Stat Methods Med Res. 2007;16(3):219–242. doi: 10.1177/0962280206074463. [DOI] [PubMed] [Google Scholar]
- 14.Figueras J, Alcalde O, Barrabes JA, et al. Changes in hospital mortality rates in 425 patients with acute ST-elevation myocardial infarction and cardiac rupture over a 30-year period. Circulation. 2008;118(25):2783–2789. doi: 10.1161/CIRCULATIONAHA.108.776690. [DOI] [PubMed] [Google Scholar]
- 15.Smith CR, Leon MB, Mack MJ, et al. Transcatheter versus surgical aortic-valve replacement in high-risk patients. N Engl J Med. 2011;364(23):2187–2198. doi: 10.1056/NEJMoa1103510. [DOI] [PubMed] [Google Scholar]
- 16.Conradi L, Treede H, Brickwedel J, Reichenspurner H. Use of initial biventricular mechanical support in a case of postinfarction ventricular septal rupture as a bridge to surgery. Ann Thorac Surg. 2009;87(5):e37–e39. doi: 10.1016/j.athoracsur.2009.01.046. [DOI] [PubMed] [Google Scholar]
- 17.Arnaoutakis GJ, George TJ, Allen JG, et al. Institutional volume and the effect of recipient risk on short-term mortality after orthotopic heart transplant. J Thorac Cardiovasc Surg. 2012;143(1):157–167. e1. doi: 10.1016/j.jtcvs.2011.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Russo MJ, Iribarne A, Easterwood R, et al. Post-heart transplant survival is inferior at low-volume centers across all risk strata. Circulation. 2010;122(11 Suppl):S85–S91. doi: 10.1161/CIRCULATIONAHA.109.926659. [DOI] [PubMed] [Google Scholar]
- 19.Lemery R, Smith HC, Giuliani ER, Gersh BJ. Prognosis in rupture of the ventricular septum after acute myocardial infarction and role of early surgical intervention. Am J Cardiol. 1992;70(2):147–151. doi: 10.1016/0002-9149(92)91266-7. [DOI] [PubMed] [Google Scholar]
- 20.Jacobs JP, Edwards FH, Shahian DM, et al. Successful linking of the Society of Thoracic Surgeons adult cardiac surgery database to Centers for Medicare and Medicaid Services Medicare data. Ann Thorac Surg. 2010;90(4):1150–1156. doi: 10.1016/j.athoracsur.2010.05.042. discussion 6–7. [DOI] [PubMed] [Google Scholar]