Abstract
IFN-alpha has been found to inhibit glucocorticoid receptor (GR) function by activating janus kinase-signal transducer and activator of transcription (JAK-STAT) inflammatory signaling pathways. In contrast, through stimulation of protein kinase A (PKA), cAMP has been shown to enhance GR function and can inhibit inflammatory signaling. We therefore examined whether increased cAMP-PKA pathway activation could reverse IFN-alpha-induced inhibition of GR function and whether decreased cAMP-PKA activity might exacerbate IFN-alpha effects on the GR. Activation of cAMP by forskolin (10uM) reversed the inhibitory effects of mIFN-alpha (1000U/ml) on dexamethasone (DEX)-induced MMTV-luciferase activity in hippocampal HT22 cells. Forskolin treatment also blocked both IFN-alpha-induced activation of phosphorylated STAT5 (pSTAT5) and inhibitory protein-protein interactions between pSTAT5 and GR in the nucleus of HT22 cells treated with IFN-alpha and DEX. These effects of forskolin were reversed by co-administration of the PKA inhibitor, H89. Conversely, the combination of IFN-alpha and treatment with either H89 or siRNA directed against the alpha and beta catalytic subunit isoforms of PKA led to an additive inhibitory effect on DEX-induced GR activity in HT22 cells. Taken together, these findings suggest that inhibition of GR signaling by mIFN-alpha and STAT5 can be reversed by activation of cAMP-PKA pathways, whereas decreased PKA activity increases the inhibitory effect of IFN-alpha on GR function. Given decreased PKA activity found in patients with major depression, these data suggest that depressed patients may be vulnerable to cytokine effects on GR, and cAMP-PKA agonists may serve to reverse glucocorticoid resistance in patients with depression and increased inflammation.
Keywords: Jak-STAT, interferon-alpha, STAT5, PKA, beta-adrenergic receptor, glucocorticoid receptor, major depression, cytokine
Introduction
A burgeoning literature suggests that inflammation and inflammatory cytokines may be involved in the pathophysiology of major depression (e.g. Irwin and Miller, 2007; Raison et al., 2006). Recent meta analyses have found that patients with major depression exhibit increased peripheral blood concentrations of inflammatory cytokines including interleukin (IL)-6 and tumor necrosis factor (TNF)-alpha, as well as increased peripheral blood concentrations of acute phase reactants such as C-reactive protein (Dowlati et al., 2011; Howren et al., 2009). Supportive of a direct relationship between inflammation and major depression, administration of inflammatory cytokines such as IFN-alpha to patients with cancer or hepatitis C has been shown to induce behavioral changes that meet symptom criteria for major depression in up to 50% of patients depending on the dose (Raison et al., 2006; Raison et al., 2005).
Regarding the mechanism by which cytokines such as IFN-alpha change behavior, one possibility includes effects on the hypothalamic-pituitary-adrenal (HPA) axis. For example, IFN-alpha administration has also been shown to lead to a flattening of the diurnal cortisol slope as well as increased evening plasma cortisol concentrations, both of which were correlated with depressive symptom severity (Raison et al., 2010). Flattening of the diurnal cortisol slope has been associated with non-suppression of cortisol following dexamethasone (DEX) administration in cancer patients (Bower et al., 2005), an effect believed to be mediated by impaired glucocorticoid receptor (GR) function. These data suggest that IFN-alpha may impair glucocorticoid negative feedback regulation of the HPA axis by inhibiting GR signaling. Consistent with this notion, previous studies have shown that IFN-alpha reduces GR-mediated gene transcription by activating the janus kinase-signal transducer and activator of transcription (JAK-STAT) pathway, in particular STAT5 (Hu et al., 2009). STAT5 and GR engage in inhibitory nuclear protein-protein interactions, which block GR-DNA binding and GR-mediated gene transcription. Other inflammatory cytokines have also been shown to disrupt GR function (Pace et al., 2007). Thus, cytokine-induced inhibition of GR may play an important role in the broader pathophysiology of major depression, a disorder well-known to be characterized by increased inflammation and impaired GR function as manifested both in vivo and in vitro (Pace et al., 2007; Pariante and Miller, 2001). Accordingly, strategies to reverse cytokine-induced dysfunction of GR warrant further exploration.
While cytokines and inflammatory signaling may disrupt GR function, several studies suggest that activity of cyclic adenosine monophosphate (cAMP)-dependent signaling and the protein kinase A (PKA) pathway can enhance GR function. For example, treatment of cells with forskolin (Dong et al., 1989), beta2-adrenergic receptor agonists (Eickelberg et al., 1999), and phosphodiesterase inhibitors (Miller et al., 2002), all of which increase cAMP-PKA signaling, have been shown to increase expression and stability of GR mRNA, or enhance GR-mediated gene transcription. In addition, constitutive cAMP and PKA activity appear to be required for normal GR function (Eickelberg et al., 1999). Of note, cAMP and PKA are of particular interest in the context of major depression as a number of different monoamine neurotransmitters act through G protein-coupled receptors, and evidence of decreased PKA activity has been reported in skin fibroblasts (Shelton et al., 1996; Shelton et al., 1999) and the prefrontal cortex of depressed patients (Shelton et al., 2009).
Interestingly, PKA has also been shown to inhibit a number of inflammatory signaling pathways including nuclear factor-κB (NF-κB)(Gao et al., 2010; Takahashi et al., 2002; Zhang et al., 2010) and mitogen activated protein kinase (MAPK)(e.g. Park et al., 2010; Saxena et al., 1999). Thus, the impact of cytokines on GR function may depend in part on the relative activation of cAMP-PKA, and GR dysfunction may result from impaired cAMP-PKA pathway activation, especially in the context of cytokine exposure. In addition, it may be possible to reverse GR dysfunction by enhancing signaling through cAMP-PKA (Pace et al., 2007). Accordingly, we sought to determine the extent to which activation of the cAMP-PKA pathway could reverse GR dysfunction induced by IFN-alpha. We also determined what effect disruption of constitutive cAMP-PKA pathway activity would have on GR function in the presence of IFN-alpha. Experiments utilized mouse HT22 cells, a hippocampus-derived cell line. Hippocampal GR have been shown to regulate glucocorticoid-mediated negative feedback of HPA axis function (De Kloet et al., 1998), and, as noted above, species-specific murine IFN (mIFNA)-alpha has been shown to inhibit GR function in HT22 cells (Hu et al., 2009). HT22 cells were also treated with forskolin and either H89 (a selective PKA antagonist) or siRNA directed against PKA. H89 allowed exploration of short-term effects of PKA pathway disruption, while siRNA directed against PKA induced a situation in which cells were more chronically deficient in PKA, as may be the case in major depression (Shelton et al., 1996). Given prior studies demonstrating synergies between GR and PKA (Dong et al., 1989; Doucas et al., 2000; Eickelberg et al., 1999; Gruol et al., 1986; Haske et al., 1994; Medh et al., 1998; Miller et al., 2002; Penuelas et al., 1998), we hypothesized that treatment of mouse HT22 cells with the cAMP agonist forskolin would reverse the inhibitory effects of IFN-alpha on GR-mediated gene transcription. Moreover, we predicted that activation of the PKA signaling pathway would disrupt normal activation of the JAK-STAT pathway by IFN-alpha. Finally, we hypothesized that disruption of constitutive cAMP/PKA activity would exacerbate the inhibitory effects of mIFN-alpha on GR.
Materials and Methods
Cells and Reagents
Mouse hippocampal HT22 cells were kindly provided by Dr. Y. Sagara (University of California, San Diego, CA) and were grown at 37°C with 5% CO2 in DMEM supplemented with 10% heat-inactivated (56°C, 30 minutes) fetal bovine serum (Hyclone, Logan, UT), 50 U/ml penicillin, and 50 mg/ml streptomycin. HT22 cells were stably or transiently transfected with the MMTV-luciferase reporter using Lipofectamine 2000 (Invitrogen Life Technologies, Carlsbad, CA). Stably transfected HT22 cells were produced by treatment for 2 weeks with G418 and cloned by limiting dilution. Clones were then screened for DEX-induced MMTV-luciferase activity. Transient transfection with the MMTV-luciferase reporter was performed using pAH-Luc plasmid in serum-free medium. Stripped fetal bovine serum (FBS) was added to wells 5 hours after transfection. Pharmacologic reagents included recombinant type-I mouse IFN-alpha (PBL Biomedical Laboratories, Piscataway, NJ), forskolin and DEX (Sigma Aldrich, St Louis, MO).
siRNA
Synthetic small interfering RNA (siRNA) probes were obtained from Dharmacon (Lafayette, Colorado). The following sequences were used: PKA Cα (5′-AAGTGGTTTGCCACGACTGAC); PKA Cβ (5′-AAGAGTTTCTAGCCAAAGCCA); and scrambled (5′-AAGGAGGCTGAACATTCCGTC). Exponentially growing cells were transfected with 50nM of PKA Cα plus 50nM PKA Cβ siRNA or 100nM of the scrambled control. All siRNA duplexes were transfected with Lipofectamine 2000 for 48 hours using 2μL Lipofectamine 2000 reagent/20pmol of siRNA. After 48 hours, cells were co-transfected with MMTV-luciferase reporter (pAH-Luc) and renilla plasmid. After 4 hours, the transfection complex was replaced with stripped serum media containing the indicated treatments. Cells lysates were harvested following treatment and analyzed for luciferase and renilla activities by using Dual-Luciferase reporter assay system (Promega, Madison, WI). Luciferase values were corrected for transfection efficiency using renilla activity. “Relative activity” was calculated as the mean of at least three independent experiments, represented as fold change relative to the vehicle control.
Luciferase assay
HT22 cells were seeded into 12-well plates or 100 mm culture dishes and grown for 20– 24 hours until 70–80% confluent. In transiently transfected cells, drug treatments were carried out 24 hours after transfection, and all samples were run in triplicate. After treatment, HT22 cells were washed once with cold 1× phosphate buffered saline (PBS), and lysed using a passive lysis buffer (see below). Cells were then centrifuged at 10,000 rpm for 15 seconds at room temperature (RT) to remove cellular debris. Luciferase activity was measured using a microplate luminometer (Luminoscan Ascent, Thermo Labsystems, Helsinki, Finland) and luciferase substrate (Promega, Madison, WI).
Nuclear, cytosolic and whole cell extracts
Cell monolayers were rinsed with 1× PBS and then harvested in a nuclear homogenization buffer (NHB) containing 20 mM Tris (pH 7.4), 10 mM NaCl, 3 mM MgCl2, 5 uM dithiothreitol, 1 uM phenylmethylsulfonyl fluoride, 1 uM pepstatin, 50 trypsin-inhibitory mU of aprotinin, 10 uM leupeptin, and 2 mM sodium vanadate. Igepal CA-630 (Nonidet P-40) was added to a final concentration of 0.15%, and cells were homogenized with 16 strokes in a Dounce homogenizer. The homogenates were centrifuged at 3500 rpm for 5 minutes. Supernatants were saved as cytosolic extract, and the nuclear pellets were resuspended in 0.5 volumes of NHB and were centrifuged as before. The pellet of intact nuclei was resuspended again in one-half of the original volume of NHB and centrifuged again. The majority of the pellet (intact nuclei) was resuspended in immunoprecipitation buffer (IP) buffer containing 10 mM Tris (pH 7.4), 150 mM NaCl, 1 mM ethylene glycol tetraacetic acid (EGTA), 1 mM ethylenediaminetetraacetic acid (EDTA), 1% Triton X-100, 1 uM phenylmethylsulfonyl fluoride, 1 uM pepstatin, 50 trypsin-inhibitory mU of aprotinin, 10 uM leupeptin, and 2 mM sodium vanadate. Nuclei were extracted for 30 minutes on ice. The samples were subjected to centrifugation at 10,000 rpm at 4 °C for 10 minutes. Supernatants were considered to contain nuclear protein. To generate whole cell extracts, cells were harvested in 1× PBS and lysed in RIPA buffer (50 mM Tris-HCl (pH 7.4), 1 mM EDTA, 150 mM NaCl, and 1% NP-40 containing protease inhibitors (1 mM PMSF, 1 μg /ml aprotinin, 1 μg /ml pepstatin, and 1 μg /ml leupeptin). After 30 minutes incubation on ice, cell lysates were centrifuged at 13,000 × g for 10 minutes at 4°C, and the supernatant was collected. For normalization of sample loading for relevant assays, protein concentrations were determined using a commercial bicinchoninic acid (BCA) assay (Pierce, Rockford, IL) with bovine serum albumin as the protein standard.
Western blot analysis
For western blot analysis, 50 μg total protein was mixed with sodium dodecyl sulfate (SDS) buffer before SDS-PAGE. Separated proteins were then electrophoretically transferred onto a nitrocellulose membrane. The membrane was blocked for 1 hour in a 5% milk/ tris-buffer saline tween 20 solution, and then incubated overnight in the presence of the primary antibody (1:1000 dilution) raised against STAT5 pY(Tyr694; Cell Signaling, Danvers, MA), GR (M-20) or PKA Cα (sc-903) or PKA Cβ (sc-904)(Santa Cruz Biotechnology, Santa Cruz, CA), The washed membrane was subsequently incubated with the secondary antibody (1:2000 dilution) for 1 hour. The membrane was washed again and visualized using a commercially available chemoluminescence kit from Amersham Biosciences Corp. (Piscataway, NJ) and autoradiography.
Immunoprecipitation
Nuclear protein (300 μg) were preincubated with protein A agarose, and the resulting supernatant was then incubated with 5 ug of the polyclonal anti-GR antibody (M-20; Santa Cruz Biotechnology) for 2 hours at 4 °C. Protein-A agarose (Santa Cruz Biotechnology, Santa Cruz, CA) was added to the mixture, and the sample was rotated for an additional 1 hour. Bound GR and any associated proteins were isolated by pelleting this mixture. The pellets were rinsed twice with 1× PBS, and bound proteins were eluted from the agarose by incubation at 100 °C for 10 minutes after the addition of Laemmli sample buffer. These samples were separated by SDS-polyacrylamide gel electrophoresis and analyzed by Western blotting with either STAT5 pY or GR antibodies.
Data analysis and statistics
Descriptive statistics, including the mean and standard error of the mean (SEM), were used to characterize dependent measures in all experiments. The effects of increasing concentrations of forskolin (or H89) on DEX-induced MMTV-luciferase were analyzed with a one-way analysis of variance (ANOVA). Results of experiments exploring the effect of forskolin and H89 on mIFN-alpha inhibition of DEX-induced MMTV-luciferase activity were analyzed in a two-step process. First, main effects of DEX and mIFN-alpha, as well as their interaction, were determined in conditions not involving forskolin or H89 treatment. This analysis used a two-way ANOVA to confirm the effect of mIFN-alpha on DEX-induced MMTV-luciferase activity (Hu et al., 2009). Effects of forskolin and H89 were then explored using separate ANOVAs including DEX and mIFN-alpha treatment variables. To determine the effect of siRNA directed against PKA on DEX-induced MMTV-luciferase activity by mIFN-alpha, results were analyzed with a 3-way ANOVA including the factors of DEX, mIFN-alpha, and siRNA.
For studies examining the effects of forskolin and H89 on mIFN-alpha-induced pSTAT5 activity, the effect of mIFN-alpha on pSTAT5 was first explored in conditions involving only mIFN-alpha (and not forskolin or H89 treatment) using a 2-way ANOVA with factors of mIFN-alpha and time. To determine the effects of forskolin on mIFN-alpha-induced pSTAT5, results from all conditions except those including H89 were analyzed using a 2-way ANOVA with factors of time and forskolin treatment. To explore the effect of H89, results from all conditions involving forskolin were analyzed using a 2-way ANOVA with factors of time and H89 treatment.
For all analyses, significant main effects were followed by Fisher's Least Significant Difference Tests to explore relevant post hoc comparisons. The level of significance was set at p<0.05, and all tests of significance were two-tailed.
Results
DEX-induced MMTV-luciferase activity is enhanced by forskolin and reduced by a PKA antagonist
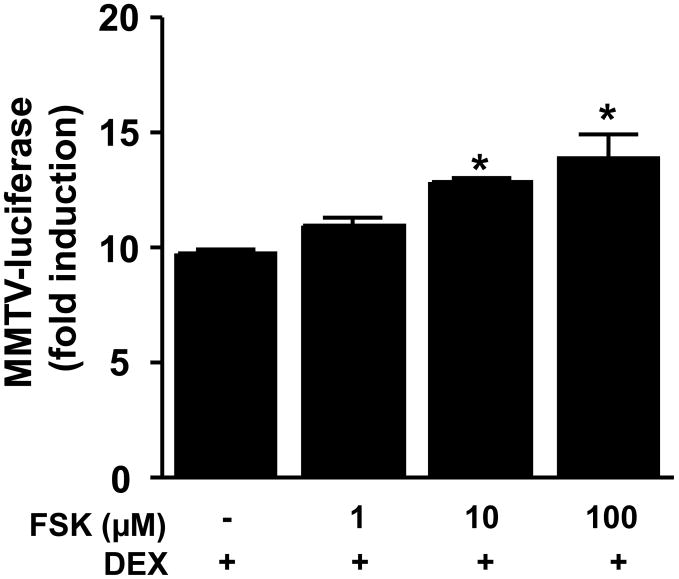
To explore the effect of cAMP-PKA pathway activation on DEX-induced GR function, mouse HT22 cells stably transfected with the MMTV-luciferase reporter were treated with either forskolin (an adenylyl cyclase agonist) for 22 hours before treatment with DEX (50 nM) for 2 hours. There was a significant main effect of forskolin treatment on DEX-induced MMTV-luciferase activity (F [3, 8]=11.96, p=0.003). Post hoc analysis revealed that both 10 μM and 100 μM forskolin significantly enhanced DEX-induced MMTV-luciferase activity over and above that seen in the control condition (Figure 1A).
Figure 1. Dexamethasone (DEX)-induced luciferase activity in HT22 cells is enhanced by forskolin treatment.
HT22 cells stably transfected with the MMTV-luciferase reporter gene construct were grown in 12-well culture plates until 80% confluent. Cells were treated with vehicle or forskolin (1, 10, or 100 μM) for 22 hours followed by 2 hours treatment with dexamethasone (DEX). Luciferase activity was then measured as described in Methods. All conditions were run in triplicate, and values shown are means (±SEM). Results are representative of three independent experiments. * p < 0.005 vs DEX only treatment condition.
Activation of the cAMP-PKA reverses inhibition of DEX-induced MMTV-luciferase activity by IFN-alpha
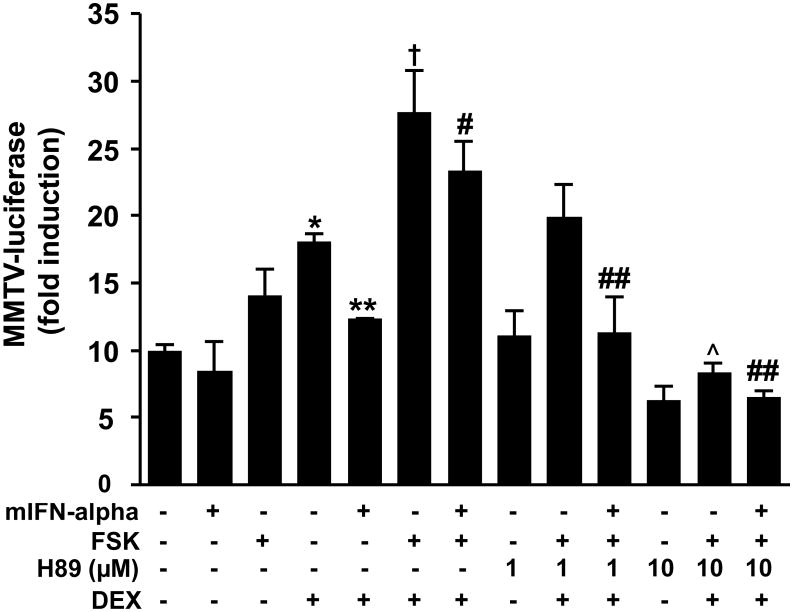
Prior studies as well as data presented in Figure 1 suggest that GR signaling is enhanced by stimulation of cAMP via PKA. In contrast, GR function is inhibited by IFN-alpha. Accordingly, we explored the degree to which the inhibitory effect of IFN-alpha on GR function is reversed by activation of the PKA pathway. Mouse HT22 cells stably transfected with the MMTV-luciferase reporter were first treated with mIFN-alpha, forskolin, H89, or a combination thereof (Figure 2) for 22 hours before treatment with DEX for 2 hours. As in prior experiments (Hu et al., 2009), there was a main effect of DEX (F [1,8] = 27.47, p = 0.001) as well as IFN-alpha treatment (F [1,8] =10.34, p = 0.01) on MMTV-luciferase activity. There was also a strong trend for an interaction between DEX and IFN-alpha treatments (F [1,8] = 3.44, p = 0.10). Post hoc analysis revealed that DEX treatment significantly increased MMTV-luciferase activity compared to vehicle treatment, and that DEX combined with IFN-alpha treatment significantly attenuated MMTV-luciferase activity compared to DEX treatment alone (Figure 2). Across all treatment conditions excluding those receiving H89, there was a main effect of forskolin (F [1,14] = 26.17, p < 0.001) on MMTV-luciferase activity. Post hoc analysis revealed that forskolin treatment combined with DEX increased MMTV-luciferase activity compared to DEX treatment alone (Figure 2). Interestingly, treatment with forskolin also reversed the inhibitory effects of IFN-alpha on DEX-induced MMTV luciferase activity. Finally, a main effect of H89 treatment was identified across all treatment conditions (F[2,26] = 32.44, p < 0.001). Post hoc analysis indicated that H89 (10 μM) dose dependently reversed the effects of forskolin on HT22 cells treated with either DEX alone or DEX plus IFN-alpha (Figure 2).
Figure 2. Inhibition of dexamethasone (DEX)-induced luciferase activity by mouse interferon (mIFN)-alpha is attenuated by cAMP-protein kinase A (PKA) pathway activation in HT22 cells.
HT22 cells stably transfected with the MMTV-luciferase reporter gene construct were grown in 12-well culture plates until 80% confluent. Cells were treated with a combination of mIFN-alpha (1000 U/ml), forskolin (FSK)(10 μM), and/or the selective PKA inhibitor H89 (1 or 10 μM) for 22 hours followed by continued treatment with the same respective combinations plus DEX (50 nM) or vehicle for 2 hours. Luciferase activity was then measured as described in Methods. All conditions were run in triplicate, and values shown are means (±SEM). Results are representative of three independent experiments. * p < 0.05 vs no treatment (all vehicle) condition; † p < 0.05 vs DEX only treatment condition; # p < 0.05 vs mIFN-alpha plus DEX condition; ## p < 0.05 vs mIFN-alpha plus DEX plus forskolin condition; (x0005E;) p < 0.05 vs DEX plus forskolin condition.
Of note, the effects of forskolin or IFN-alpha were not due to changes in whole cell GR protein expression, as has been reported in other cell types (Dong et al., 1989). Indeed, treatment of HT22 cells for 24 hours with either forskolin or IFN-alpha alone or in combination was not associated with any changes in GR protein expression (see Supplementary Figures 1 and 2).
PKA activation blocks IFN-alpha-induced phosphorylated STAT5
We have previously shown that inhibition of DEX-induced GR activation by IFN-alpha is mediated by the JAK-STAT pathway, and in particular STAT5 (Hu et al., 2009). In this experiment we explored the extent to which PKA pathway activation blocks STAT5 phosphorylation (activation) induced by IFN-alpha. Mouse HT22 cells were treated with either IFN-alpha alone, IFN-alpha plus forskolin, or IFN-alpha plus forskolin plus H89 for 24 hours before collection of whole cell extracts for analysis with western blot. Densitometric analysis of western blots from three independent experiment revealed that cells treated with mIFN-alpha alone showed a significant increase in phosphorylated (p)STAT5 with increasing durations of exposure (F[4, 10]=11.59, p=0.001). Post hoc analysis of differences between these groups indicated all time points 5 minutes and beyond showed increased pSTAT5 compared to the 0 minute condition (see Figure 3). Across all time points, forskolin treatment significantly attenuated pSTAT5 induction by IFN-alpha (F[1, 20]=24.51, p<0.001), and the attenuating effects of forskolin on mIFN-induced pSTAT5 depended on time point (F[4, 20]=2.85, p=0.05). Post hoc analysis revealed that forskolin treatment significantly attenuated pSTAT5 activation at 15 min and 60 minutes, with similar effects at 30 minutes (Figure 3). Finally, across all time points, cells treated with mIFN-alpha, forskolin, and H89 showed increased pSTAT5 compared to cells treated with mIFN-alpha and forskolin alone (F[1, 20]=18.70, p<0.001). Post hoc analysis indicated that H89 significantly attenuated the inhibitory effect of forskolin treatment on mIFN-alpha-induced pSTAT5 after 15 and 60 minutes of mIFN-alpha treatment, with similar effects after 30 minutes.
Figure 3. Activation of protein kinase A (PKA) attenuates mouse interferon (mIFN)-alpha-induced phosphorylated signal transducer and activator of transcription (pSTAT)5.
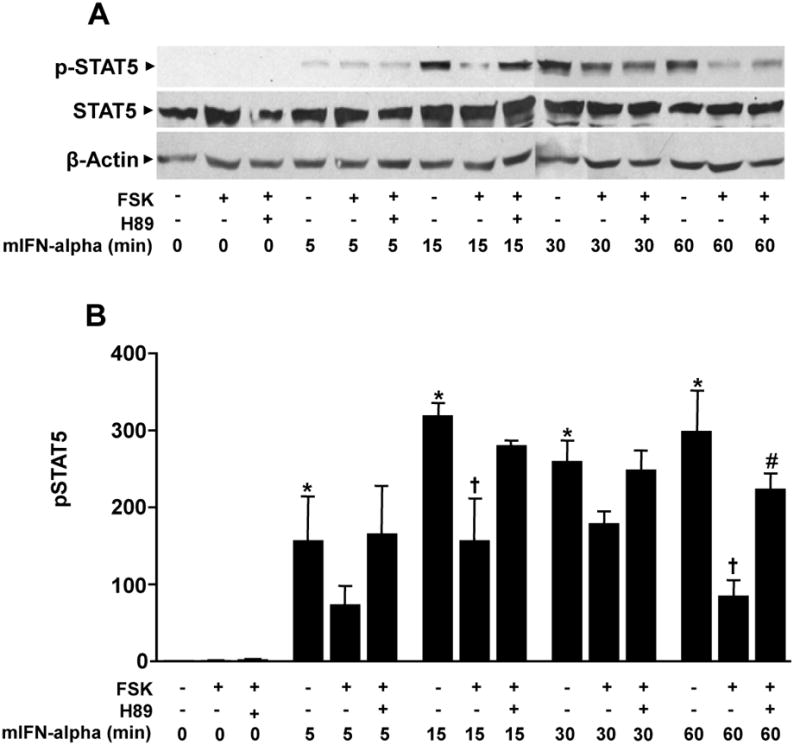
Wild type HT22 cells were grown in 100mm culture dishes until 80% confluent before treatment with a combination of forskolin (FSK)(10 μM) and/or the selective PKA antagonist H89 (10 μM) for 24 hours before treatment with mIFN-alpha for either 0, 5, 15, 30, or 60 minutes. At the end of these time periods cells were processed to obtain whole-cell extracts as described in methods. (A) Representative western blot using an antibody raised against pSTAT5. (B) Optical density analysis of pSTAT5 protein from three independent experiments. * p < 0.05 vs 0 min mIFN-alpha only group; † p < 0.05 vs mIFN-alpha only group at same time point; # p < 0.05 vs mIFN-alpha plus forskolin group at same time point.
PKA activation blocks co-immunoprecipitation of GR and pSTAT5 in HT22 cells treated with DEX and mIFN-alpha
We used immunoprecipitation to examine nuclear protein-protein interactions between pSTAT5 and GR in the context of cAMP-PKA activation with forskolin. Nuclear protein was extracted from cells treated for 30 minutes with DEX (50 nM), DEX plus mIFN-alpha (1000 U/ml), DEX plus mIFN-alpha plus forskolin (10 μM), or DEX plus mIFN-alpha plus forskolin plus H89 (10 μM) and then incubated with anti-GR antibodies for immunoprecipitation. Western blot analysis was conducted using an anti-pSTAT5 antibody. We selected a 30 minute treatment time based on earlier experiments (Hu et al., 2009) as well as a study by Biola and colleagues examining GR-STAT5 interactions in the context of IL-2 (Biola et al., 2001). As we have shown previously (Hu et al., 2009), DEX plus mIFN-alpha treatment resulted in co-immunoprecipitation of GR and pSTAT5, indicating nuclear protein-protein interactions. Interestingly, the addition of forskolin to DEX and mIFN-alpha treatment attenuated this interaction, while the addition of H89 produced immunoprecipitation results comparable to that seen in cells treated with DEX and mIFN-alpha alone. These data indicate that forskolin impaired the nuclear protein-protein interaction of GR and pSTAT5 in a PKA-dependent manner.
Pharmacological inhibition of PKA activity has an additive inhibitory effect on the impact of IFN-alpha on DEX-induced MMTV-luciferase activity
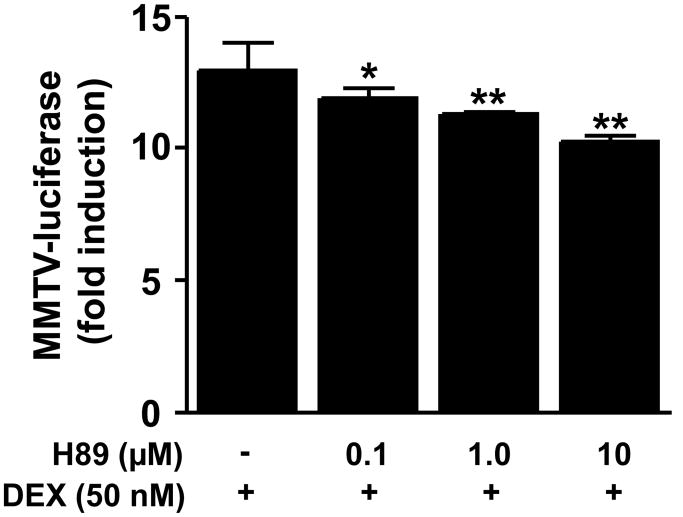
To investigate how disruption of constitutive PKA activity influences DEX-induced GR signaling, HT22 cells were treated with H89 (0.1, 1, and 10 μM) for 22 hours before co- treatment with H89 and DEX for 2 hours. A significant main effect of H89 on DEX-induced MMTV-luciferase activity was noted (F [3,8] = 8.49, p = 0.007). Post hoc testing revealed that all three doses of H89 significantly attenuated DEX-induced MMTV-luciferase activity (Figure 5).
Figure 5. Dexamethasone (DEX)-induced luciferase activity in HT22 cells is attenuated by pharmacological antagonism of PKA.
HT22 cells stably transfected with the MMTV-luciferase reporter gene construct were grown in 12-well culture plates until 80% confluent. Cells were then treated with the selective PKA antagonist H89 (0.1, 1, or 10 μM) or vehicle for 22 hours followed by 2 hours treatment with dexamethasone (DEX; 50 nM). Luciferase activity was then measured as described in methods. All conditions were run in triplicate, and values shown are means (±SEM). Results are representative of results obtained in three independent experiments. * p < 0.05 vs DEX only condition; ** p < 0.01 vs DEX only condition.
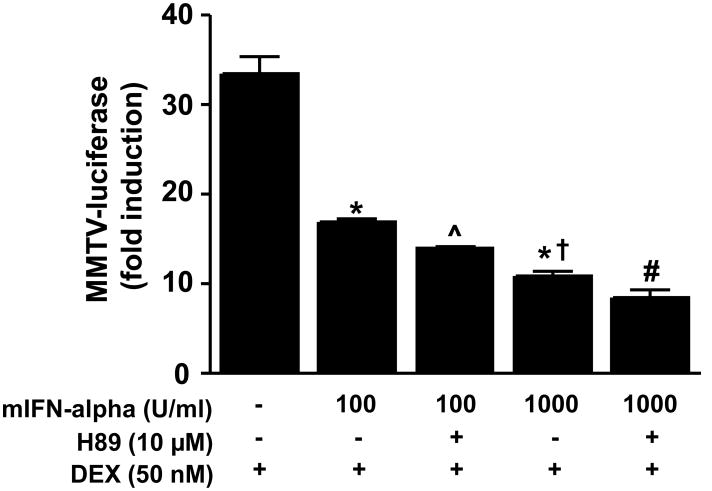
To explore how disruption of PKA activity alters IFN-alpha-induced inhibition of DEX-induced GR activity in HT22 cells, HT22 cells were treated for 22 hours with mIFN-alpha (100 or 1000 U/ml) with or without H89 (10 μM), followed by co-treatment with DEX (50 nM). We used two concentrations of mIFN-alpha in this experiment because preliminary studies suggested that antagonism of PKA activity in combination with lower versus higher concentrations of mIFN-alpha may better reveal disruption of DEX-induced GR activity. As noted above, treatment with IFN-alpha attenuated DEX-induced MMTV-luciferase activity (F[2,6] = 101.99, p < 0.001). Post hoc analysis indicated that treatment with 1000 U/ml IFN-alpha resulted in a significantly greater reduction in DEX-induced MMTV-luciferase activity compared to treatment with IFN-alpha 100 U/ml alone. Within groups of cells treated with H89, there was a main effect of IFN-alpha (F[1,8] = 134.91, p<.001) and H89 (F[1,8] = 27.57, p = 0.001) on DEX-induced MMTV-luciferase activity. Post hoc analysis indicated that cells treated with H89 plus 100 U/ml IFN-alpha showed significantly lower DEX-induced MMTV-luciferase activity compared to cells treated with 100 U/ml mIFN-alpha alone. Although cells treated with H89 plus 1000 U/ml mIFN-alpha showed reduced DEX-induced MMTV-luciferase activity compared to DEX-induced MMTV-luciferase activity in cells treated with 1000 U/ml mIFN-alpha alone, the difference did not reach statistical significance (p = 0.06) (Figure 6).
Figure 6. Antagonism of PKA enhances mouse interferon (mIFN)-alpha inhibition of dexamethasone (DEX)-induced luciferase activity by in HT22 cells.
HT22 cells stably transfected with the MMTV-luciferase reporter gene construct were grown in 12-well culture plates until 80% confluent. Cells were then treated with vehicle or the selective PKA antagonist H89 (10 μM) for 22 hours followed by 2 hours treatment with dexamethasone (DEX). Luciferase activity was measured as described in Methods. All conditions were run in triplicate, and values shown are means (±SEM). Results are representative of three independent experiments. * p < 0.05 vs DEX only condition; (x0005E;) p < 0.05 vs DEX plus IFN-alpha alone; † p < 0.05 vs DEX plus IFN-alpha (100 U/ml); # p < 0.05 vs DEX plus IFN-alpha (100 U/ml) plus H89;.
Blockade of constitutive PKA expression with siRNA has an additive inhibitory effect on IFN-alpha-induced reduction of DEX-induced MMTV-luciferase activity
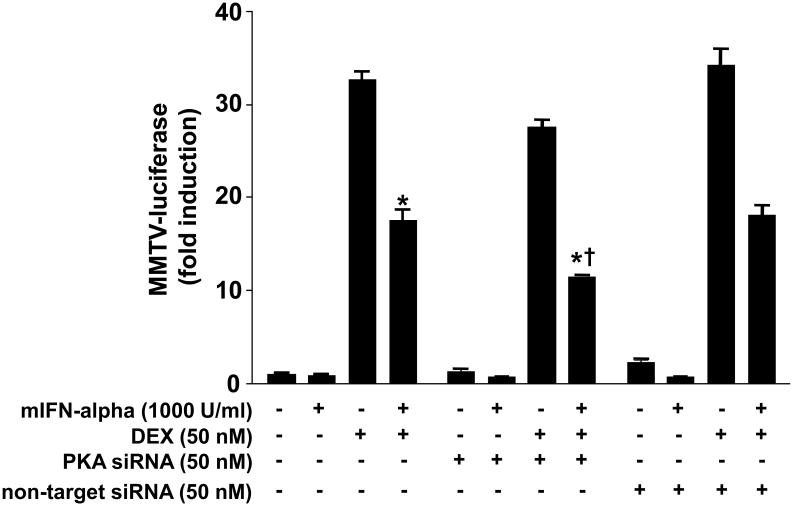
To further explore how constitutive activity of PKA influences the inhibitory effects of IFN-alpha on DEX-induced GR function, we first treated HT22 cells with siRNA directed against PKA (see Supplementary Figure 3) for 48 hours. Cells were then treated with IFN-alpha for 22 hours and then DEX for 2 hours before assessment of GR activity using the MMTV luciferase reporter. Across all non-siRNA treated groups, main effects of DEX and mIFN-alpha on MMTV-luciferase activity were observed (F[1,8] = 1060.38, p < 0.001 and F[1,8] = 104.67, p < 0.001, respectively) confirming previous observations (Figure 7). An interaction was also noted between DEX and mIFN-alpha (F[1,8] = 102.31, p<0.001). When examining all treatment conditions, a main effect of siRNA on MMTV-luciferase activity was found (F[1,16] = 44.00, p<0.001). In addition, a significant interaction between siRNA and DEX treatments was noted (F[1,16] = 45.47, p < 0.001). Post hoc analysis indicated that treatment with siRNA directed against PKA significantly reduced MMTV-luciferase activity when cells were treated with both mIFN-alpha and DEX (Figure 7). No effects of non-targeted siRNA were observed.
Figure 7. siRNA directed against PKA enhances inhibition of dexamethasone (DEX)-induced luciferase activity by mouse interferon (mIFN)-alpha in HT22 cells.
HT22 cells stably transfected with the MMTV-luciferase reporter gene construct were grown in 12-well culture plates until 80% confluent. Cells were then transiently transfected for 48 hours with 100 nM siRNA directed against PKA alpha and beta catalytic subunit isoforms. Following transfection, cells were treated with mIFN-alpha (1000 U/ml) for 22 hours followed by DEX (50 nM) for 2 hours. All conditions were run in triplicate, and values shown are means (±SEM). Results are representative of results obtained in three independent experiments. * p < 0.05 vs DEX only condition; † p < 0.05 vs DEX plus mIFN-alpha (no siRNA) condition.
Discussion
Experiments in the current report were designed to explore whether activation of the cAMP-PKA pathway can reverse inhibition of GR function by IFN-alpha. Prior studies have shown that cAMP-PKA signaling enhances GR function (Dong et al., 1989; Doucas et al., 2000; Eickelberg et al., 1999; Gruol et al., 1986; Haske et al., 1994; Medh et al., 1998; Miller et al., 2002; Penuelas et al., 1998), and we and others have found that inflammatory cytokines (Pariante et al., 1999) and their signaling pathways (Biola et al., 2001; Goleva et al., 2002; Hu et al., 2009; Wang et al., 2005; Wang et al., 2004), including IFN-alpha and the JAK-STAT pathway, impair activity of GR. The results shown here confirm that activation of cAMP-PKA signaling not only enhances DEX-induced GR function, but also abrogates IFN-alpha inhibition of DEX-induced GR-mediated gene transcription. Regarding the mechanism of this effect, we show that activation of cAMP-PKA signaling blocks induction of pSTAT5 by IFN-alpha and thereby disrupts the inhibitory nuclear protein-protein interactions between pSTAT5 and GR, allowing GR to act as a nuclear transcription factor. These results were not a function of cAMP-PKA effects on GR expression, because no increase in GR protein was found in HT22 cells treated with forskolin for up to 24 hours. The data also indicate that constitutive PKA activity is important for normal GR function, especially when cells are challenged with cytokines such as IFN-alpha. Indeed, disruption of cAMP-PKA signaling activity with the PKA antagonist H89 or siRNA directed against PKA led to significantly greater inhibition of DEX-induced GR activity than was found with IFN-alpha alone, suggesting that reduced PKA activity may have an additive inhibitory effect on GR function in the presence of inflammatory cytokines such as IFN-alpha.
Impaired activity of cAMP-PKA signaling has been indentified in several different tissues and cell types obtained from psychiatric patients, including those with mood disorders such as major depression (Akin et al., 2005; Dwivedi et al., 2002; Dwivedi et al., 2004; Manier et al., 1996a, b; Pandey et al., 2005; Shelton et al., 1996; Shelton et al., 2009). Reduced PKA activity is of interest in the context of major depression because mood disorders are widely believe to involve imbalances in key monoamine neurotransmitter systems (e.g. serotonin and norepinephrine) (Leonard, 2007; Willner, 1985). Activation of a variety of neurotransmitter receptors, including serotonin receptors and beta adrenergic receptors, induces conformational changes in G proteins that stimulate adenyl cyclase. Adenyl cyclase then catalyzes adenosine triphosphate to cAMP, which activates PKA. Activated PKA is then able to phosphorylate the transcription factor cAMP response element-binding protein (CREB) (Nibuya et al., 1996; Wilcox et al., 1998). Depressed patients have been found to exhibit reduced G protein function in mononuclear cells (Avissar et al., 1997), and reduced cAMP binding has been identified in the cytoplasmic fraction of cells obtained from bipolar depressed patients (Rahman et al., 1997). In addition, depressed patients have been found to have attenuated expression of specific PKA catalytic subunit isoforms in post-mortem brain tissue (Akin et al., 2005), as well as reduced PKA activity in fibroblasts (Shelton et al., 1999). Interestingly, antidepressant drugs such as imipramine and tranylcypromine have been shown to increase the activity of cAMP-dependent protein kinases in nuclear but not synaptosome fractions of rat prefrontal cortex (Nestler et al., 1989). Taken together, these findings suggest that the pathophysiology of major depression may involve abnormalities within critical effector systems responsible for neurotransmitter effects on cells, including PKA, and conventional antidepressants may ameliorate depression in part thorough effects on the cAMP-PKA pathway.
Studies by our group and others have found that antidepressants also enhance GR function (Pariante and Miller, 2001; Pariante et al., 1997). In the absence of ligand, GR exists in the cytoplasm and is associated with an assembly of chaperone molecules, notably heat shock proteins. Upon binding to hormone, GR dissociates from the chaperone complex, allowing a conformational change that includes exposure of a nuclear localization signal (Pace et al., 2007). GR then associates with the cytoskeleton (Galigniana et al., 1998) and translocates to the nucleus through nuclear pores. Once inside the nucleus, GR forms homodimers and binds to GREs on relevant glucocorticoid-sensitive genes or associates with other transcription factors through direct protein-protein interactions (Guiochon-Mantel et al., 1996). Our group and others have reported that the antidepressant drug desipramine (Pariante et al., 1997) as well as clomipramine, fluoxetine, citalopram, and paroxetine (Pariante and Miller, 2001) induce nuclear translocation of GR from the cytoplasm to the nucleus, even in the absence of glucocorticoids. The same antidepressants have been shown to enhance DEX-induced expression of genes regulated by GR (Pariante et al., 1997) (Pariante and Miller, 2001). Interestingly, evidence suggests that the effects of antidepressants on GR involve cAMP-PKA signaling. Our group has shown that the phosphodiesterase type 4 (PDE4)-inhibitor rolipram, which antagonizes the breakdown of cAMP, enhances DEX-induced gene expression (Miller et al., 2002). Rolipram was also found to facilitate the effects of desipramine on GR function. This finding agrees with reports by others indicating that antidepressants enhance GR function, at least in part by modulating cAMP-PKA function (Blendy, 2006; Eickelberg et al., 1999; Funato et al., 2006; Pariante et al., 2003; Rangarajan et al., 1992).
Antidepressants have also been found to promote neurogenesis, and this effect may involve both PKA and GR. A recent study by Anacker and colleagues demonstrated that neuronal cultures treated with sertraline for 3 days displayed increased neurogenesis that was blocked by the selective GR antagonist, RU38486 (Anacker et al., 2011) . Treatment with rolipram enhanced the effects of sertraline on neurogenesis, while H89 blocked the effect of sertraline. Indeed, Pariante and colleagues also reported that sertraline increased GR transactivation and modified GR phosphorylation in a manner consistent with the idea that cAMP-PKA signaling enhances GR function (Anacker et al., 2011). A number of studies have shown that inflammatory cytokines can inhibit neurogenesis both in vivo and in vitro (Koo and Duman, 2008; Koo et al.; Yirmiya and Goshen, 2011). However, it remains to be determined whether these effects are mediated in part through effects of cytokines on the GR and whether activation of cAMP-PKA pathways may reverse these effects. As noted previously, medically healthy individuals with major depression have been found to exhibit increased circulating concentrations of inflammatory cytokines. Increased inflammatory cytokines in depressed patients may in turn conspire with reduced cAMP-PKA pathway activity to inhibit GR function and reduce neurogenesis thereby promoting development of major depression. Likewise, normalization of cAMP-PKA activity by antidepressants may enhance GR function, in part by attenuating inflammatory signaling pathways (Pace et al., 2007).
Besides JAK-STAT, a number of inflammatory pathways that are known to be inhibited by PKA have been shown to inhibit GR function (Pace et al., 2007). For example, our group and other have shown that activation of MAPK pathways, including p38 MAPK and c-Jun N-terminal kinase (JNK), disrupts nuclear translocation of GR (Biola et al., 2001; Wang et al., 2005; Wang et al., 2004), and activation of PKA by forskolin has been shown to inhibit MAPK-induced raf-1 translocation (Melck et al., 1999). PKA has also been found to inhibit MAPK pathways by phosphorylating serine residues on raf-1, leading to reduced affinity of raf for Ras (Hafner et al., 1994). In addition, forskolin has been found to down-regulate extracellular signaling-related kinase (ERK) and JNK, both resulting in reduced T cell activation (Tamir et al., 1996). Like STAT5, NF-κB has also been found to inhibit GR signaling through nuclear protein-protein interactions (Smoak and Cidlowski, 2004), and PKA has been shown to inhibit NF-κB transcriptional activity through an interaction between p65 (a subunit of NF-κB) and the catalytic subunit of PKA (Takahashi et al., 2002). Thus, it is possible that induction of cAMP-PKA signaling by antidepressants and other drugs may block a number of inflammatory signaling pathways, ultimately obviating cytokine-induced inhibitory effects on GR function. Drugs that activate cAMP-PKA signaling, such as antidepressants or PDE-4 inhibitors, may therefore represent a worthwhile strategy to enhance GR function and limit glucocorticoid resistance in depressed patients with evidence of increased inflammation (Miller et al., 2002; Pace et al., 2007).
Several limitations regarding these experiments should be noted. Not addressed in the current set of studies is the possibility that constitutive or stimulated PKA activity may enhance GR phosphorylation, thereby augmenting GR function even in the presence of inflammatory cytokines. PKA has been shown to directly phosphorylate GR (Haske et al., 1994), and treatment with forskolin as well as other PKA agonists such as 8-Br-cAMP can increase the stability and expression of GR mRNA (Dong et al., 1989; Penuelas et al., 1998). Thus, in the current experiments it is possible that treatment with forskolin and therefore activation of PKA may have directly enhanced GR function in addition to independently inhibiting mIFN-alpha-induced STAT5 activity. Future studies should therefore examine how enhanced phosphorylation of GR by forskolin-induced PKA activation may “buffer” DEX-induced GR activity from the inhibitory effects of IFN-alpha. Future studies should also determine the extent to which interactions between STAT5, GR and cAMP-PKA signaling reported here also take place in other cell and tissue types. It would also be of benefit if future experiments on this topic explore additional endpoint measures known to be dependent on GR function, including cell proliferation.
In conclusion, forskolin treatment reverses attenuation of DEX-induced GR activity by mIFN-alpha in mouse hippocampal cells. In addition to showing a connection between cAMP-PKA and JAK-STAT signaling, these findings provide important insights into the possible interactions between cAMP-PKA pathway activity and inflammatory signaling in general. Activation of PKA by conventional antidepressants or other drugs may enhance GR function by directly phosphorylating the GR and blocking the activity of inflammatory signaling pathways. Thus, pharmacotherapies that influence PKA likely represent a “double hit” on molecular mechanisms related to GR dysfunction and glucocorticoid resistance. New approaches that more specifically target cAMP-PKA signaling may therefore be effective in treating disorders involving glucocorticoid resistance in the context of inflammation, including major depression.
Supplementary Material
Wild type HT22 cells were grown in 100mm culture dishes until 80% confluent before treatment with forskolin (10 μM) for 0, 1, 2, 3, 4, or 24 hours. After end of these time points whole cell extracts were generated, and western blot analyses were conducted using an antibody raised against GR (described in Methods). (A) Representative western blot. (B) Optical density analysis of GR protein from three independent experiments. One-way analysis of variance revealed no main effect of forskolin treatment over the six time points on GR protein (F[5,12] = 0.76, p = 0.60).
Wild type HT22 cells were grown in 100mm culture dishes until 80% confluent before treatment with H89 (10 μM), forskolin (10 μM), or mIFN-alpha (1000 U/ml) for 24 hours. Whole cell extracts were then generated, and western blot analyses were conducted using an antibody raised against GR (described in Methods). (A) Representative western blot. (B) Optical density analysis of GR protein from three independent experiments. Three-way analysis of variance revealed no significant main effects of H89 (F [1,16] = 0.76, p = 0.21), forskolin (F [1,16] = 0.76, p = 0.06), mIFN-alpha (F [1,16] = 0.76, p = 0.93) or interaction effects between these variables on GR protein.
Wild type HT22 cells were grown in 6-well culture plates until 80% confluent. Cells were then transiently transfected for 48 hours with 100 nM siRNA directed against PKA alpha and beta catalytic subunit isoforms before production of whole cell extracts for analysis of PKA protein by western blot. Results are representative of results obtained in three independent experiments.
Figure 4. Activation of PKA blocks nuclear protein-protein interactions between STAT5 and GR in HT22 cells treated with mouse interferon (mIFN)-alpha and dexamethasone (DEX).
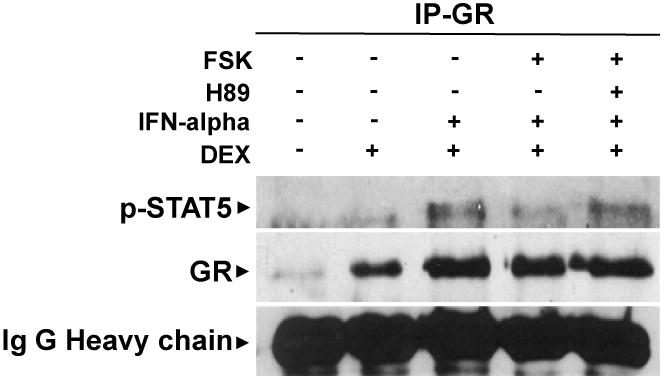
Nuclear extracts were obtained from cells treated with forskolin (FSK) and/ or the selective PKA antagonist H89 for 22 hours before treatment with mIFN-alpha and/ or DEX for 30 minutes. Immunoprecipitation was conducted using G-agarose beads with an anti-GR antibody. Proteins were resolved on 1-% Tris-HCl gels, electrophoretically transferred to nitrocellulos membranes, and blotted with anti-pSTAT5 or anti-GR antibodies. Results are representative of results obtained in three independent experiments.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akin D, Manier DH, Sanders-Bush E, Shelton RC. Signal transduction abnormalities in melancholic depression. Int J Neuropsychopharmacol. 2005;8:5–16. doi: 10.1017/S146114570400478X. [DOI] [PubMed] [Google Scholar]
- Anacker C, Zunszain PA, Cattaneo A, Carvalho LA, Garabedian MJ, Thuret S, Price J, Pariante CM. Antidepressants increase human hippocampal neurogenesis by activating the glucocorticoid receptor. Mol Psychiatry. 2011 doi: 10.1038/mp.2011.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avissar S, Nechamkin Y, Roitman G, Schreiber G. Reduced G protein functions and immunoreactive levels in mononuclear leukocytes of patients with depression. Am J Psychiatry. 1997;154:211–217. doi: 10.1176/ajp.154.2.211. [DOI] [PubMed] [Google Scholar]
- Biola A, Lefebvre P, Perrin-Wolff M, Sturm M, Bertoglio J, Pallardy M. Interleukin-2 inhibits glucocorticoid receptor transcriptional activity through a mechanism involving STAT5 (signal transducer and activator of transcription 5) but not AP-1. Molecular Endocrinology. 2001;15:1062–1076. doi: 10.1210/mend.15.7.0657. [DOI] [PubMed] [Google Scholar]
- Blendy JA. The role of CREB in depression and antidepressant treatment. Biol Psychiatry. 2006;59:1144–1150. doi: 10.1016/j.biopsych.2005.11.003. [DOI] [PubMed] [Google Scholar]
- Bower JE, Ganz PA, Dickerson SS, Petersen L, Aziz N, Fahey JL. Diurnal cortisol rhythm and fatigue in breast cancer survivors. Psychoneuroendocrinology. 2005;30:92–100. doi: 10.1016/j.psyneuen.2004.06.003. [DOI] [PubMed] [Google Scholar]
- De Kloet ER, Vreugdenhil E, Oitzl MS, Joels M. Brain corticosteroid receptor balance in health and disease. Endocr Rev. 1998;19:269–301. doi: 10.1210/edrv.19.3.0331. [DOI] [PubMed] [Google Scholar]
- Dong Y, Aronsson M, Gustafsson JA, Okret S. The mechanism of cAMP-induced glucocorticoid receptor expression. Correlation to cellular glucocorticoid response. J Biol Chem. 1989;264:13679–13683. [PubMed] [Google Scholar]
- Doucas V, Shi Y, Miyamoto S, West A, Verma I, Evans RM. Cytoplasmic catalytic subunit of protein kinase A mediates cross-repression by NF-kappa B and the glucocorticoid receptor. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:11893–11898. doi: 10.1073/pnas.220413297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowlati Y, Herrmann N, Swardfager W, Liu H, Sham L, Reim EK, Lanctot KL. A meta-analysis of cytokines in major depression. Biol Psychiatry. 2011;67:446–457. doi: 10.1016/j.biopsych.2009.09.033. [DOI] [PubMed] [Google Scholar]
- Dwivedi Y, Conley RR, Roberts RC, Tamminga CA, Pandey GN. [(3)H]cAMP binding sites and protein kinase a activity in the prefrontal cortex of suicide victims. Am J Psychiatry. 2002;159:66–73. doi: 10.1176/appi.ajp.159.1.66. [DOI] [PubMed] [Google Scholar]
- Dwivedi Y, Rizavi HS, Shukla PK, Lyons J, Faludi G, Palkovits M, Sarosi A, Conley RR, Roberts RC, Tamminga CA, Pandey GN. Protein kinase A in postmortem brain of depressed suicide victims: altered expression of specific regulatory and catalytic subunits. Biol Psychiatry. 2004;55:234–243. doi: 10.1016/j.biopsych.2003.11.003. [DOI] [PubMed] [Google Scholar]
- Eickelberg O, Roth M, Lorx R, Bruce V, Rudiger J, Johnson M, Block LH. Ligand-independent activation of the glucocorticoid receptor by beta2-adrenergic receptor agonists in primary human lung fibroblasts and vascular smooth muscle cells. J Biol Chem. 1999;274:1005–1010. doi: 10.1074/jbc.274.2.1005. [DOI] [PubMed] [Google Scholar]
- Funato H, Kobayashi A, Watanabe Y. Differential effects of antidepressants on dexamethasone-induced nuclear translocation and expression of glucocorticoid receptor. Brain Res. 2006;1117:125–134. doi: 10.1016/j.brainres.2006.08.029. [DOI] [PubMed] [Google Scholar]
- Galigniana MD, Scruggs JL, Herrington J, Welsh MJ, Carter-Su C, Housley PR, Pratt WB. Heat shock protein 90-dependent (geldanamycin-inhibited) movement of the glucocorticoid receptor through the cytoplasm to the nucleus requires intact cytoskeleton. Molecular Endocrinology. 1998;12:1903–1913. doi: 10.1210/mend.12.12.0204. [DOI] [PubMed] [Google Scholar]
- Gao N, Hibi Y, Cueno M, Asamitsu K, Okamoto T. A-kinase-interacting protein 1 (AKIP1) acts as a molecular determinant of PKA in NF-kappaB signaling. J Biol Chem. 2010;285:28097–28104. doi: 10.1074/jbc.M110.116566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goleva E, Kisich KO, Leung DY. A role for STAT5 in the pathogenesis of IL-2-induced glucocorticoid resistance. J Immunol. 2002;169:5934–5940. doi: 10.4049/jimmunol.169.10.5934. [DOI] [PubMed] [Google Scholar]
- Gruol DJ, Campbell NF, Bourgeois S. Cyclic AMP-dependent protein kinase promotes glucocorticoid receptor function. J Biol Chem. 1986;261:4909–4914. [PubMed] [Google Scholar]
- Guiochon-Mantel A, Delabre K, Lescop P, Milgrom E. The Ernst Schering Poster Award. Intracellular traffic of steroid hormone receptors. Journal of Steroid Biochemistry & Molecular Biology. 1996;56:3–9. doi: 10.1016/0960-0760(95)00268-5. [DOI] [PubMed] [Google Scholar]
- Hafner S, Adler HS, Mischak H, Janosch P, Heidecker G, Wolfman A, Pippig S, Lohse M, Ueffing M, Kolch W. Mechanism of inhibition of Raf-1 by protein kinase A. Mol Cell Biol. 1994;14:6696–6703. doi: 10.1128/mcb.14.10.6696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haske T, Nakao M, Moudgil VK. Phosphorylation of immunopurified rat liver glucocorticoid receptor by the catalytic subunit of cAMP-dependent protein kinase. Molecular & Cellular Biochemistry. 1994;132:163–171. doi: 10.1007/BF00926925. [DOI] [PubMed] [Google Scholar]
- Howren MB, Lamkin DM, Suls J. Associations of depression with C-reactive protein, IL-1, and IL-6: a meta-analysis. Psychosom Med. 2009;71:171–186. doi: 10.1097/PSY.0b013e3181907c1b. [DOI] [PubMed] [Google Scholar]
- Hu F, Pace TW, Miller AH. Interferon-alpha inhibits glucocorticoid receptor-mediated gene transcription via STAT5 activation in mouse HT22 cells. Brain Behav Immun. 2009;23:455–463. doi: 10.1016/j.bbi.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irwin MR, Miller AH. Depressive disorders and immunity: 20 years of progress and discovery. Brain Behav Immun. 2007;21:374–383. doi: 10.1016/j.bbi.2007.01.010. [DOI] [PubMed] [Google Scholar]
- Koo JW, Duman RS. IL-1beta is an essential mediator of the antineurogenic and anhedonic effects of stress. Proc Natl Acad Sci U S A. 2008;105:751–756. doi: 10.1073/pnas.0708092105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koo JW, Russo SJ, Ferguson D, Nestler EJ, Duman RS. Nuclear factor-kappaB is a critical mediator of stress-impaired neurogenesis and depressive behavior. Proc Natl Acad Sci U S A. 107:2669–2674. doi: 10.1073/pnas.0910658107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard BE. Psychopathology of depression. Drugs Today (Barc) 2007;43:705–716. doi: 10.1358/dot.2007.43.10.1122223. [DOI] [PubMed] [Google Scholar]
- Manier DH, Eiring A, Shelton RC, Sulser F. Beta-adrenoceptor-linked protein kinase A (PKA) activity in human fibroblasts from normal subjects and from patients with major depression. Neuropsychopharmacology. 1996a;15:555–561. doi: 10.1016/S0893-133X(96)00099-1. [DOI] [PubMed] [Google Scholar]
- Manier DH, Eiring A, Shelton RC, Sulser F. Beta-adrenoceptor-linked protein kinase A (PKA) activity in human fibroblasts from normal subjects and from patients with major depression. Neuropsychopharmacology. 1996b;15:555–561. doi: 10.1016/S0893-133X(96)00099-1. [DOI] [PubMed] [Google Scholar]
- Medh RD, Saeed MF, Johnson BH, Thompson EB. Resistance of human leukemic CEM-C1 cells is overcome by synergism between glucocorticoid and protein kinase A pathways: correlation with c-Myc suppression. Cancer Research. 1998;58:3684–3693. [PubMed] [Google Scholar]
- Melck D, Rueda D, Galve-Roperh I, De Petrocellis L, Guzman M, Di Marzo V. Involvement of the cAMP/protein kinase A pathway and of mitogen-activated protein kinase in the anti-proliferative effects of anandamide in human breast cancer cells. FEBS Letters. 1999;463:235–240. doi: 10.1016/s0014-5793(99)01639-7. [DOI] [PubMed] [Google Scholar]
- Miller AH, Vogt GJ, Pearce BD. The phosphodiesterase type 4 inhibitor, rolipram, enhances glucocorticoid receptor function. Neuropsychopharmacology. 2002;27:939–948. doi: 10.1016/S0893-133X(02)00381-0. [DOI] [PubMed] [Google Scholar]
- Nestler EJ, Terwilliger RZ, Duman RS. Chronic antidepressant administration alters the subcellular distribution of cyclic AMP-dependent protein kinase in rat frontal cortex. J Neurochem. 1989;53:1644–1647. doi: 10.1111/j.1471-4159.1989.tb08564.x. [DOI] [PubMed] [Google Scholar]
- Nibuya M, Nestler EJ, Duman RS. Chronic antidepressant administration increases the expression of cAMP response element binding protein (CREB) in rat hippocampus. J Neurosci. 1996;16:2365–2372. doi: 10.1523/JNEUROSCI.16-07-02365.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pace TW, Hu F, Miller AH. Cytokine-effects on glucocorticoid receptor function: Relevance to glucocorticoid resistance and the pathophysiology and treatment of major depression. Brain Behav Immun. 2007;21:9–19. doi: 10.1016/j.bbi.2006.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey GN, Dwivedi Y, Ren X, Rizavi HS, Mondal AC, Shukla PK, Conley RR. Brain region specific alterations in the protein and mRNA levels of protein kinase A subunits in the post-mortem brain of teenage suicide victims. Neuropsychopharmacology. 2005;30:1548–1556. doi: 10.1038/sj.npp.1300765. [DOI] [PubMed] [Google Scholar]
- Pariante CM, Hye A, Williamson R, Makoff A, Lovestone S, Kerwin RW. The antidepressant clomipramine regulates cortisol intracellular concentrations and glucocorticoid receptor expression in fibroblasts and rat primary neurones. Neuropsychopharmacology. 2003;28:1553–1561. doi: 10.1038/sj.npp.1300195. [DOI] [PubMed] [Google Scholar]
- Pariante CM, Miller AH. Glucocorticoid receptors in major depression: relevance to pathophysiology and treatment. Biol Psychiatry. 2001;49:391–404. doi: 10.1016/s0006-3223(00)01088-x. [DOI] [PubMed] [Google Scholar]
- Pariante CM, Pearce BD, Pisell TL, Owens MJ, Miller AH. Steroid-independent translocation of the glucocorticoid receptor by the antidepressant desipramine. Mol Pharmacol. 1997;52:571–581. doi: 10.1124/mol.52.4.571. [DOI] [PubMed] [Google Scholar]
- Pariante CM, Pearce BD, Pisell TL, Sanchez CI, Po C, Su C, Miller AH. The proinflammatory cytokine, interleukin-1alpha, reduces glucocorticoid receptor translocation and function. Endocrinology. 1999;140:4359–4366. doi: 10.1210/endo.140.9.6986. [DOI] [PubMed] [Google Scholar]
- Park CH, Moon Y, Shin CM, Chung JH. Cyclic AMP suppresses matrix metalloproteinase-1 expression through inhibition of MAPK and GSK-3beta. J Invest Dermatol. 2010;130:2049–2056. doi: 10.1038/jid.2010.62. [DOI] [PubMed] [Google Scholar]
- Penuelas I, Encio IJ, Lopez-Moratalla N, Santiago E. cAMP activates transcription of the human glucocorticoid receptor gene promoter. J Steroid Biochem Mol Biol. 1998;67:89–94. doi: 10.1016/s0960-0760(98)00097-1. [DOI] [PubMed] [Google Scholar]
- Rahman S, Li PP, Young LT, Kofman O, Kish SJ, Warsh JJ. Reduced [3H]cyclic AMP binding in postmortem brain from subjects with bipolar affective disorder. J Neurochem. 1997;68:297–304. doi: 10.1046/j.1471-4159.1997.68010297.x. [DOI] [PubMed] [Google Scholar]
- Raison CL, Borisov AS, Woolwine BJ, Massung B, Vogt G, Miller AH. Interferon-alpha effects on diurnal hypothalamic-pituitary-adrenal axis activity: relationship with proinflammatory cytokines and behavior. Mol Psychiatry. 2010;15:535–547. doi: 10.1038/mp.2008.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raison CL, Capuron L, Miller AH. Cytokines sing the blues: inflammation and the pathogenesis of depression. Trends Immunol. 2006;27:24–31. doi: 10.1016/j.it.2005.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raison CL, Demetrashvili M, Capuron L, Miller AH. Neuropsychiatric adverse effects of interferon-alpha: recognition and management. CNS Drugs. 2005;19:105–123. doi: 10.2165/00023210-200519020-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rangarajan PN, Umesono K, Evans RM. Modulation of glucocorticoid receptor function by protein kinase A. Mol Endocrinol. 1992;6:1451–1457. doi: 10.1210/mend.6.9.1435789. [DOI] [PubMed] [Google Scholar]
- Saxena M, Williams S, Tasken K, Mustelin T. Crosstalk between cAMP-dependent kinase and MAP kinase through a protein tyrosine phosphatase. Nature Cell Biology. 1999;1:305–311. doi: 10.1038/13024. [DOI] [PubMed] [Google Scholar]
- Shelton RC, Mainer DH, Sulser F. cAMP-dependent protein kinase activity in major depression. Am J Psychiatry. 1996;153:1037–1042. doi: 10.1176/ajp.153.8.1037. [DOI] [PubMed] [Google Scholar]
- Shelton RC, Manier DH, Peterson CS, Ellis TC, Sulser F. Cyclic AMP-dependent protein kinase in subtypes of major depression and normal volunteers. Int J Neuropsychopharmcol. 1999;2:187–192. doi: 10.1017/S1461145799001509. [DOI] [PubMed] [Google Scholar]
- Shelton RC, Sanders-Bush E, Manier DH, Lewis DA. Elevated 5-HT 2A receptors in postmortem prefrontal cortex in major depression is associated with reduced activity of protein kinase A. Neuroscience. 2009;158:1406–1415. doi: 10.1016/j.neuroscience.2008.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smoak KA, Cidlowski JA. Mechanisms of glucocorticoid receptor signaling during inflammation. Mech Ageing Dev. 2004;125:697–706. doi: 10.1016/j.mad.2004.06.010. [DOI] [PubMed] [Google Scholar]
- Takahashi E, Onda K, Hirano T, Oka K, Maruoka N, Tsuyuguchi M, Matsumura Y, Niitsuma T, Hayashi T. Expression of c-fos, rather than c-jun or glucocorticoid-receptor mRNA, correlates with decreased glucocorticoid response of peripheral blood mononuclear cells in asthma. Int Immunopharmacol. 2002;2:1419–1427. doi: 10.1016/s1567-5769(02)00083-8. [DOI] [PubMed] [Google Scholar]
- Tamir A, Granot Y, Isakov N. Inhibition of T lymphocyte activation by cAMP is associated with down-regulation of two parallel mitogen-activated protein kinase pathways, the extracellular signal-related kinase and c-Jun N-terminal kinase. Journal of Immunology. 1996;157:1514–1522. [PubMed] [Google Scholar]
- Wang X, Wu H, Lakdawala VS, Hu F, Hanson ND, Miller AH. Inhibition of Jun N-terminal kinase (JNK) enhances glucocorticoid receptor-mediated function in mouse hippocampal HT22 cells. Neuropsychopharmacology. 2005;30:242–249. doi: 10.1038/sj.npp.1300606. [DOI] [PubMed] [Google Scholar]
- Wang X, Wu H, Miller AH. Interleukin-1 alpha-induced activation of p38 mitogen-activated protein kinase inhibits glucocorticoid receptor function. Molecular Psychiatry. 2004;9:65–75. doi: 10.1038/sj.mp.4001339. [DOI] [PubMed] [Google Scholar]
- Wilcox RE, Gonzales RA, Miller JD. Introduction to neurotransmitters, receptors, signal transduction, and second messan- gers. In: Schatzberg AF, Nemeroff CB, editors. Textbook of psychopharmacology. American Psychiatric Publishing; Arlington, VA: 1998. [Google Scholar]
- Willner P. Antidepressants and serotonergic neurotransmission: an integrative review. Psychopharmacology (Berl) 1985;85:387–404. doi: 10.1007/BF00429653. [DOI] [PubMed] [Google Scholar]
- Yirmiya R, Goshen I. Immune modulation of learning, memory, neural plasticity and neurogenesis. Brain Behav Immun. 2011;25:181–213. doi: 10.1016/j.bbi.2010.10.015. [DOI] [PubMed] [Google Scholar]
- Zhang D, Ma QY, Hu HT, Zhang M. beta2-adrenergic antagonists suppress pancreatic cancer cell invasion by inhibiting CREB, NFkappaB and AP-1. Cancer Biol Ther. 2010;10:19–29. doi: 10.4161/cbt.10.1.11944. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Wild type HT22 cells were grown in 100mm culture dishes until 80% confluent before treatment with forskolin (10 μM) for 0, 1, 2, 3, 4, or 24 hours. After end of these time points whole cell extracts were generated, and western blot analyses were conducted using an antibody raised against GR (described in Methods). (A) Representative western blot. (B) Optical density analysis of GR protein from three independent experiments. One-way analysis of variance revealed no main effect of forskolin treatment over the six time points on GR protein (F[5,12] = 0.76, p = 0.60).
Wild type HT22 cells were grown in 100mm culture dishes until 80% confluent before treatment with H89 (10 μM), forskolin (10 μM), or mIFN-alpha (1000 U/ml) for 24 hours. Whole cell extracts were then generated, and western blot analyses were conducted using an antibody raised against GR (described in Methods). (A) Representative western blot. (B) Optical density analysis of GR protein from three independent experiments. Three-way analysis of variance revealed no significant main effects of H89 (F [1,16] = 0.76, p = 0.21), forskolin (F [1,16] = 0.76, p = 0.06), mIFN-alpha (F [1,16] = 0.76, p = 0.93) or interaction effects between these variables on GR protein.
Wild type HT22 cells were grown in 6-well culture plates until 80% confluent. Cells were then transiently transfected for 48 hours with 100 nM siRNA directed against PKA alpha and beta catalytic subunit isoforms before production of whole cell extracts for analysis of PKA protein by western blot. Results are representative of results obtained in three independent experiments.