Abstract
Ancient philosophers and theologians believed that altered consciousness freed the mind to prophesy the future, equating sleep with seizures. Only recently has the bidirectional influences of epilepsy and sleep upon one another received more substantive analysis. This article reviews the complex and increasingly recognized interrelationships between sleep and epilepsy. NREM sleep differentially activates interictal epileptiform discharges during slow wave (N3) sleep, while ictal seizure events occur more frequently during light NREM stages N1 and N2. The most commonly encountered types of sleep-related epilepsies (those with preferential occurrence during sleep or following arousal) include frontal and temporal lobe partial epilepsies in adults, and benign epilepsy of childhood with centrotemporal spikes (benign rolandic epilepsy) and juvenile myoclonic epilepsy in children and adolescents. Comorbid sleep disorders are frequent in patients with epilepsy, particularly obstructive sleep apnea in refractory epilepsy patients which may aggravate seizure burden, while treatment with nasal continuous positive airway pressure often improves seizure frequency. Distinguishing nocturnal events such as NREM parasomnias (confusional arousals, sleep walking, and night terrors), REM parasomnias including REM sleep behavior disorder, and nocturnal seizures if frequently difficult and benefits from careful history taking and video-EEG-polysomnography in selected cases. Differentiating nocturnal seizures from primary sleep disorders is essential for determining appropriate therapy, and recognizing co-existent sleep disorders in patients with epilepsy may improve their seizure burden and quality of life.
Keywords: EEG, epilepsy, parasomnia, pathophysiology, sleep, therapy
Parallels between sleep and epilepsy have been recognized since antiquity. Philosophers and theologians from Aristotle to Aquinas believed that states of altered consciousness allowed dissociation of the soul and the body, freeing the mind to prophesy the future, including sleep and seizures.1 While others have speculated on the similarities and contrasts between sleep and epilepsy, no objective scrutiny of their interwoven nature unfolded until the later nineteenth century. Gowers found that approximately twenty percent of those with epilepsy experienced seizures solely during sleep, while over one-third of epilepsy patients had diurnal seizures.2 During the dawn of EEG, Gibbs and Gibbs recognized that epileptiform activity increased significantly during sleep.3 Sleep and sleep deprivation have since become standard laboratory activating techniques for EEG recordings. Dieter Janz noted that 45% of patients with generalized tonic-clonic seizures had nocturnal predominance.4 Aside from these tantalizing earlier clinical observations, more substantive study of epilepsy and sleep has evolved predominantly over the last two decades.
This article reviews the increasingly recognized interrelationships between sleep and epilepsy, emphasizing activation of spikes and seizures during sleep, the sleep-related epilepsies, and several sleep disorders that may mimic or complicate epilepsy. Differentiating nocturnal seizures from primary sleep disorders is essential for determining appropriate therapy, and recognizing co-existent sleep disorders in patients with epilepsy may improve seizure burden and quality of life.
Of Seizures, Spikes, and Sleep
An epileptic seizure is caused by paroxysmal hyperexcitability of a population of neurons, resulting in an alteration of either subjective or objective behavior. Seizures may either be provoked or spontaneous. Epilepsy is the tendency toward recurrent, unprovoked seizures. Epilepsies are further classified as either generalized when they arise synchronously from the bilateral cerebral cortices, or partial when they begin from a particular region of the brain. Partial seizures begin locally but may spread beyond the original seizure focus, and when a partial seizure spreads bilaterally, it becomes a secondary generalized seizure. Electroencephalography (EEG) is an electrophysiologic measure of brain function, and is most often utilized to evaluate patients with epilepsy or altered consciousness, and is an integral part of polysomnography (PSG) in the evaluation of patients with sleep disorders, in which EEG together with chin electromyography (EMG) and electrooculography (EOG) is useful for staging human sleep. EEG is useful for the detection of interictal epileptiform discharges (IEDs) such as spike discharges (<70 msec duration) or sharp waves (70–200 msec duration). IEDs disrupt the normal interictal (between seizure episodes) EEG background, and are usually although not invariably correlated with underlying epileptogenicity, the capacity to generate epileptic seizures. IEDs may be focally distributed over one brain region in partial epilepsies, or generalized in primary generalized epilepsies. No clinical accompaniment is usually evident during brief IEDs. Ictal (during seizure) EEG changes are heterogeneous, and vary from rhythmic waveforms, to background attenuation, to repetitive generalized spike-wave discharges depending on the epileptic syndrome.
Sleep clearly results in significant activation of both IEDs and seizures. In general, IEDs and seizures are facilitated during non-REM (NREM) sleep and relatively inhibited during REM sleep.5–9 (See Figure 1 and Figure 2) Thalamocortical circuitry responsible for the neuronal hypersynchronization of NREM sleep, which normally generates sleep spindles and high amplitude delta waves, also may activate epileptic foci. In temporal lobe epilepsy, IEDs predominate during slow-wave sleep.6,8 The density of IEDs increases directly with descending sleep depth as measured by log depth power (quantity of delta activity in 30-second epochs), but IEDs do not increase further above baseline frequency in the hour before seizure onset.10 Depth electrode single neuron recordings demonstrate clear state-dependent increases in firing rates and hypersynchronous burst-firing during both slow-wave sleep and REM in the epileptic focus as compared to non-seizure generating regions, while there is no such difference in firing during the waking state.11–12 NREM sleep microarchitecture, a method for NREM sleep staging that analyzes spindle and K complex regularity, has been correlated to IED facilitation. Generalized IEDs occur most commonly during descending depth and increasing synchronization during NREM sleep, especially during cyclic alternating pattern (CAP) type A1, and decrease in frequency during the ascending (lightening and relatively desynchronizing) limb of NREM sleep.13 NREM CAP microarchitecture rhythms have been suggested to represent the scalp EEG correlate of underlying infraslow cortical oscillations, intrinsic cortical rhythms within the frequency range of 0.02 – 0.2 hz which have been shown to underlie both the physiological sleep paroxysms of K-complexes and pathophysiologic IEDs.14–15 Conversely, REM IEDs are more restricted in electrical field and thus more localizing to the epileptic focus.8 Despite the usual limitation of IEDs and seizures during REM, REM sleep-onset seizures may be rarely seen.16
Figure 1.
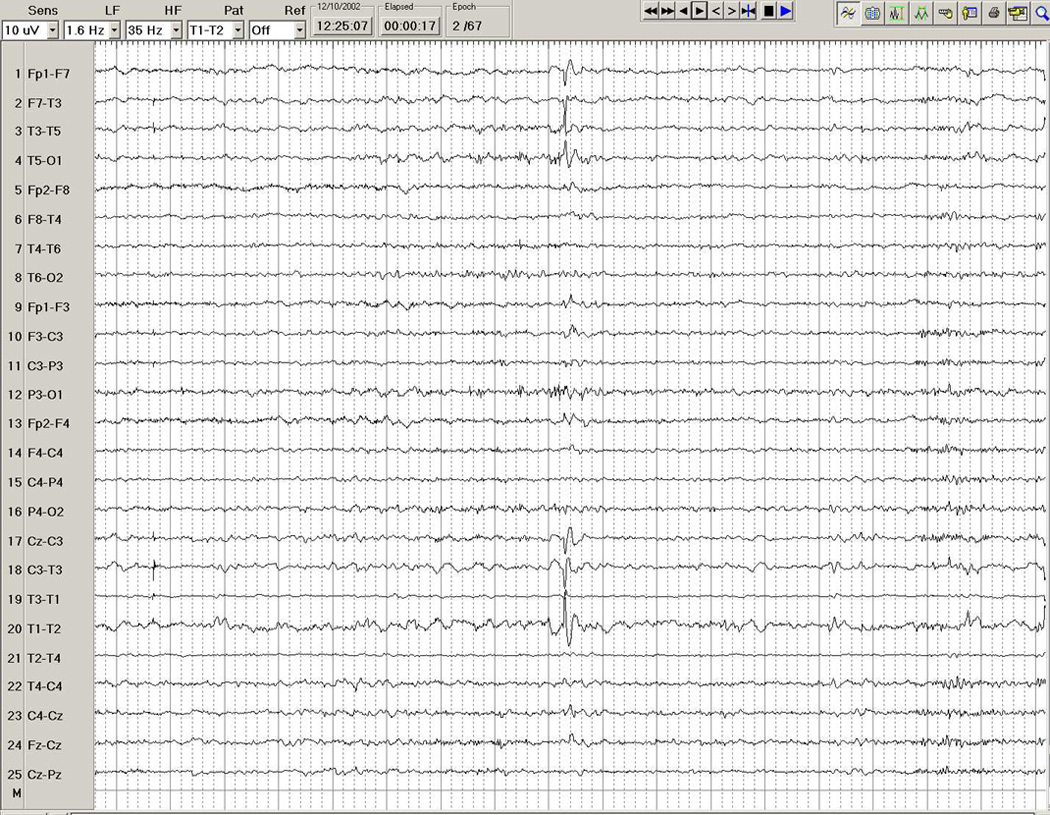
EEG in a 24-year-old woman, showing a left temporal interictal epileptiform discharge during stage 2 sleep, in the form of a spike with maximal electronegativity at T1-T3.
Figure 2.
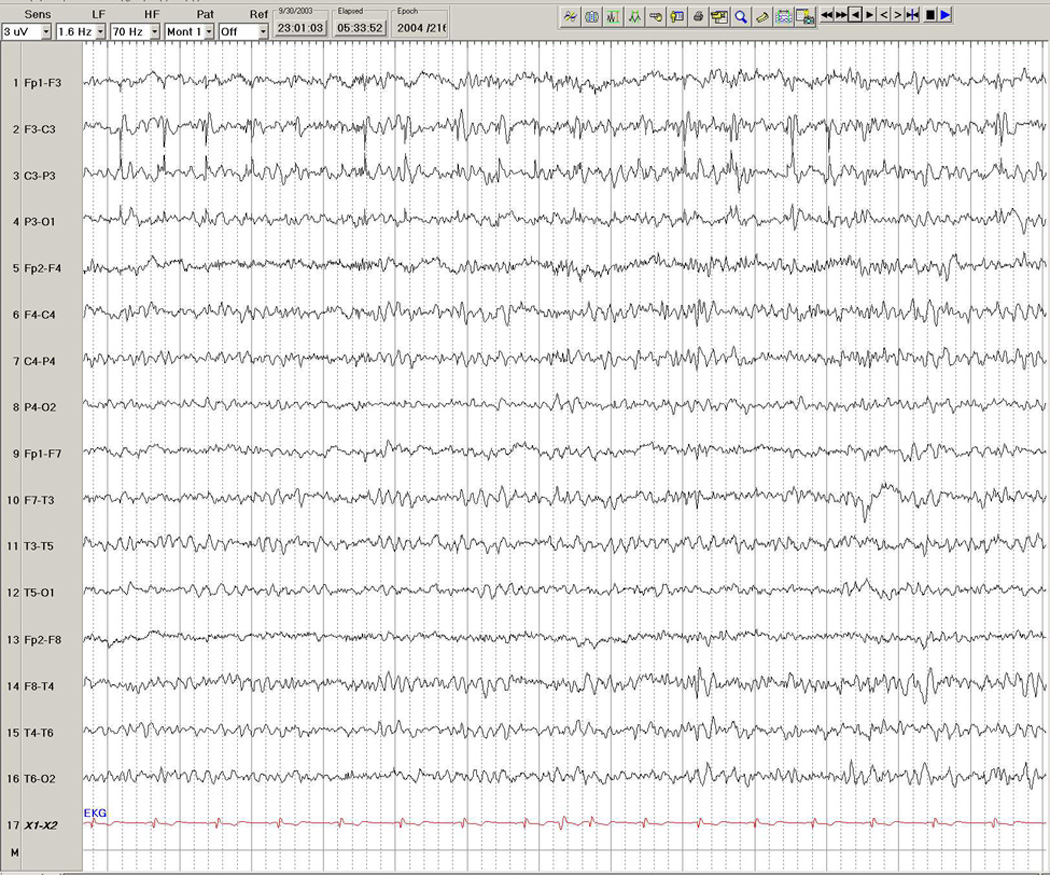
EEG in a 46-year-old man with partial seizures characterized by a tingling feeling in the right hand, progressing to a partial motor seizure affecting the right body. Note the prolonged train of interictal epileptiform discharges arising from the left central region, with maximal electronegativity at C3, occurring during stage 1 sleep.
The peak prevalence of IEDs and seizures during sleep differs. IEDs are most prevalent in slow wave sleep, while seizures occur more frequently in lighter stages of NREM sleep.5,17–18 Over one-third of partial seizures arise from sleep (See Figure 3a, Figure 3b, Figure 3c and Figure 3d), most frequently during stages 1 or 2, and nocturnal complex partial seizures are more likely than awake complex partial seizures to propagate and undergo secondary generalization.17–18 Seizures from slow wave sleep tend to last longer.5 The anatomy of the epileptic focus correlates with sleep seizure occurrence, since frontal lobe seizures are more likely to arise from sleep than are temporal lobe seizures.17,19
Figure 3.
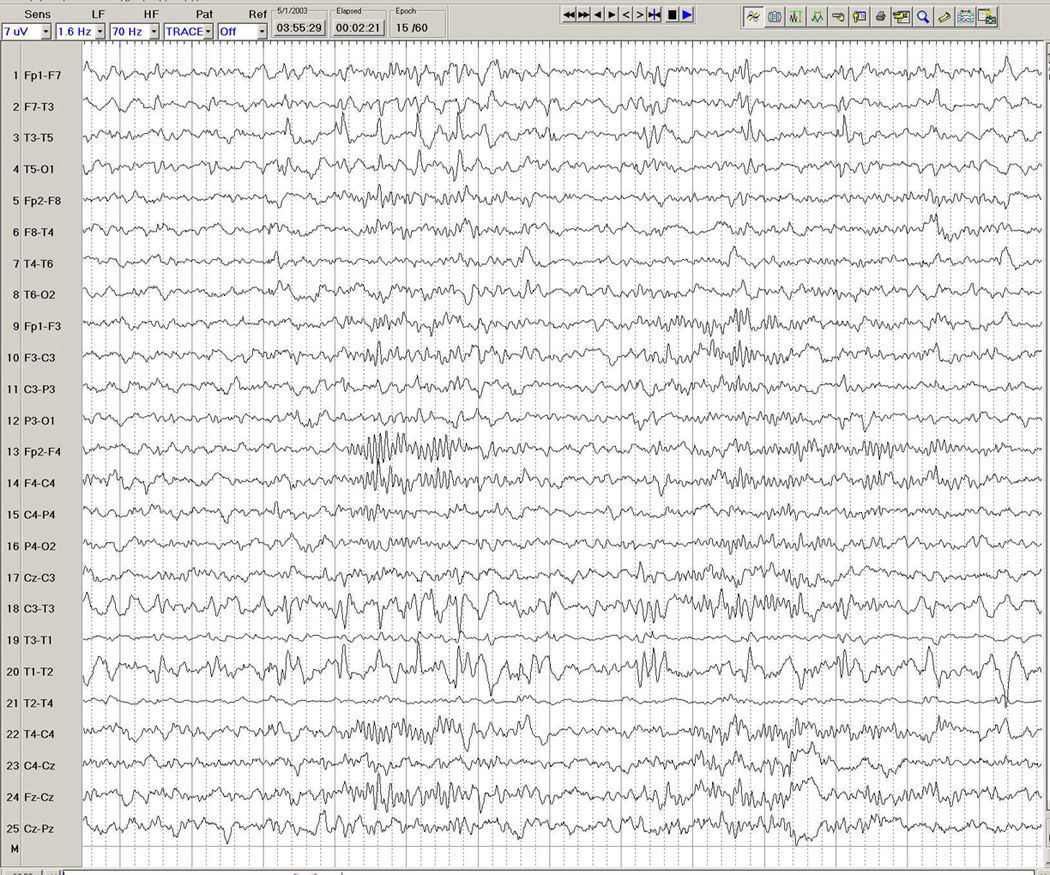
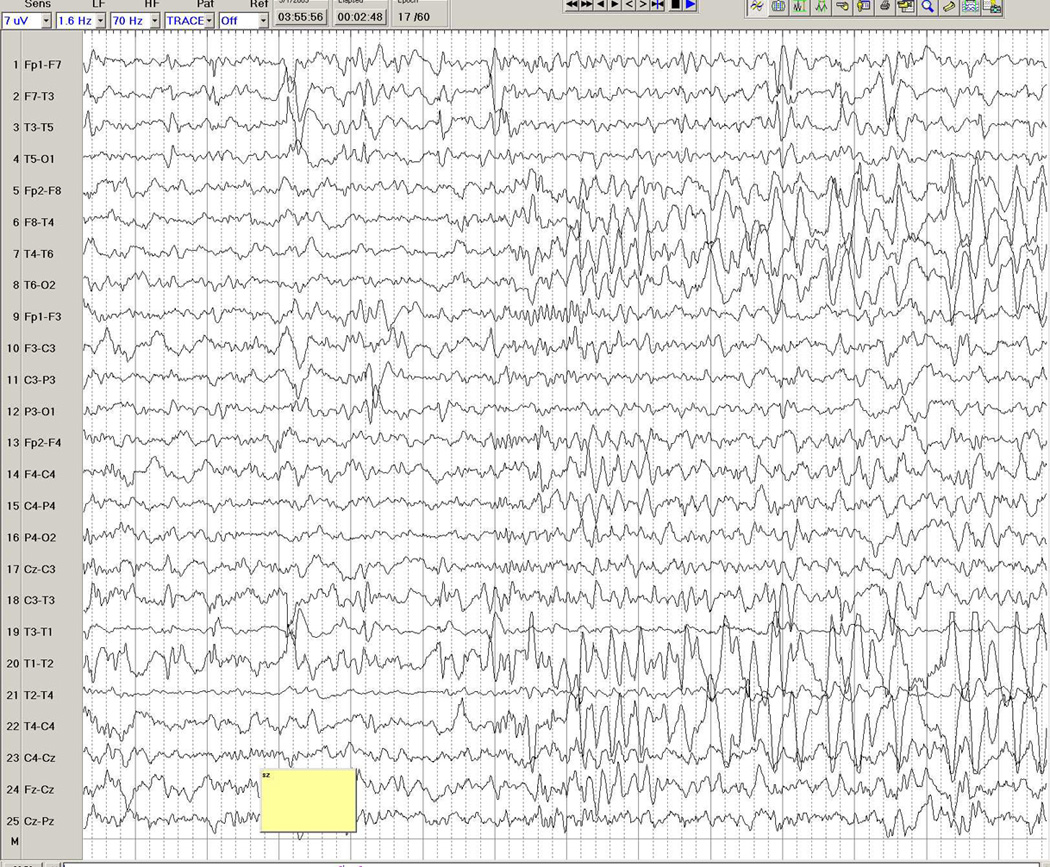
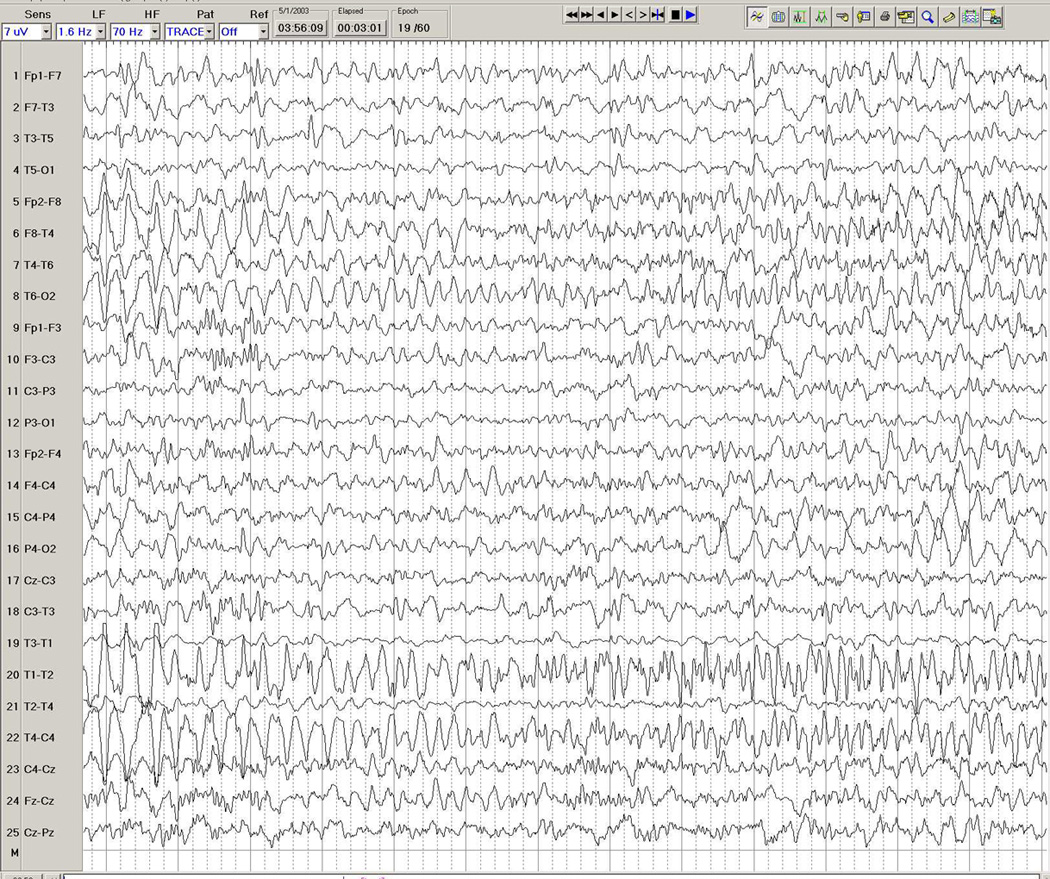
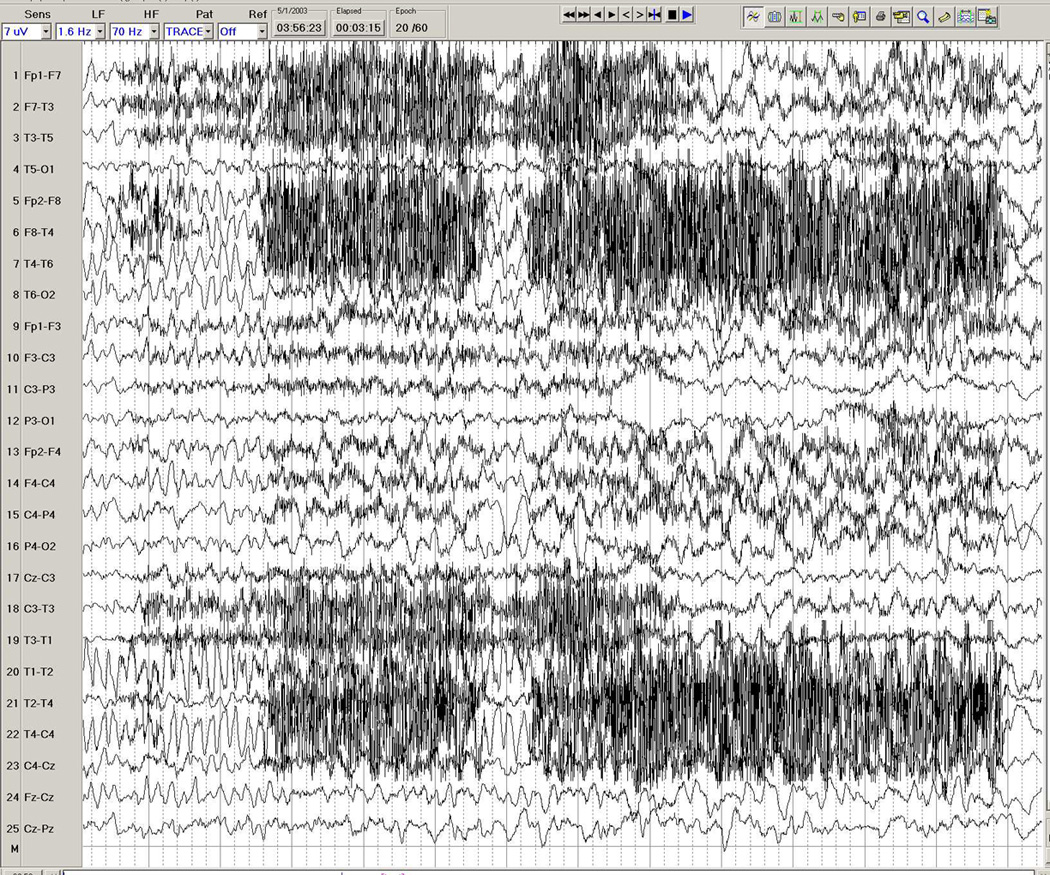
a–d. Ictal EEG in a 26-year-old man with medically intractable partial epilepsy. Figure 3a shows the patient's interictal background immediately before the seizure, with frequent interictal left temporal spike discharges. Figure 3b shows onset of a rhythmic theta-delta discharge in the right fronto-temporal region (seventh second), with further spatio-temporal evolution and maximal seizure discharge over the right temporal region in Figure 3c, and eventual propagation more diffusely to the left hemisphere in Figure 3d.
Some patients experience seizures only during sleep. Most pure sleep-onset seizures are partial onset.20 Nocturnal partial seizures correlate with medical intractability, while generalized tonic-clonic seizures without focality carry a more benign prognosis.21 Normal sleep or its disruption may significantly impact occurrence of both IEDs and seizures. Primary sleep disorders may worsen seizure control by increasing seizure frequency or severity. Sleep deprivation activates IEDs but recently was found not to promote seizures in epilepsy patients undergoing inpatient video-EEG monitoring.22
Some seizures appear to be activated by arousal. The prototypical idiopathic primary generalized epilepsy syndromes of adolescent onset, such as juvenile myoclonic epilepsy or generalized tonic-clonic seizures upon awakening, often produce myoclonic or generalized tonic-clonic seizures within the first hour after awakening.4 Exceptional patients selectively activate their sleep-related partial seizures following arousal rather than directly during sleep.7
While many studies have presented compelling evidence for sleep facilitation of IEDs and seizures, circadian mechanisms may be an even more important determinant of seizure occurrence rather than any specific effect of the sleep-wake cycle itself. In one pivotal study examining limbic onset seizure frequency in predominantly nocturnal rats and diurnal humans, peak seizure frequency was found to be in-phase for both species, occurring during daytime hours maximally in the mid-afternoon, suggesting circadian rhythms rather than sleep cycles played the major role in determining timing of seizure occurrence.23 Several other recent studies have also confirmed day or night patterns for seizure periodicity, varying by lobe of onset in the partial epilepsies with most studies suggesting either an afternoon or bimodal morning and afternoon peak for temporal lobe seizures and an evening peak for frontal lobe seizures.24–29 Thus, circadian factors appear to also play a significant role in the timing of seizure occurrence.
The Common Sleep-related Epilepsies
Representative epilepsy syndromes that have an intimate relationship to the sleep cycle are summarized in Table 1. Nocturnal epileptic seizures frequently arise outside the temporal lobe and initially may be misdiagnosed as psychogenic given lack of obvious EEG change, variably preserved consciousness, bizarre movements, and lack of a postictal state. However, stereotypic spells arising directly out of sleep occurring multiple times per night should be considered epileptic until proven otherwise.
Table 1.
The Common Sleep-Related Epilepsies
| Epilepsy Syndrome |
Classification | Age of Onset |
Waking Seizures |
Seizures Types |
EEG Findings |
Imaging Findings |
Treatment Options |
|---|---|---|---|---|---|---|---|
|
Benign Rolandic Epilepsy |
Partial | Children | Seen in 25 percent |
SP, CP, GTC |
Centro- temporal (Rolandic) Spikes |
Normal | CBZ, OXC, GBP |
|
Landau Kleffner Syndrome |
Unknown | Children | Yes | SP, CP, At Ab, Ast, GTC |
Continuous Spike-wave in SWS |
Normal,
less commonly symptomatic lesional cause |
CBZ, OXC,
etc. Steroids/IVIg MSPT |
|
Lennox Gastaut Syndrome |
Symptomatic Generalized |
Children | Yes | AtAb, T, Ast, M, GTC |
Slow Spike and Wave |
Normal
or symptomatic lesion(s) |
VPA, LTG, TPM; FBM, etc. VNS |
|
Juvenile Myoclonic Epilepsy |
Primary Generalized |
Adolescence | Yes (typically a.m. hours) |
A, M, GTC | 4–6 hz Gen. S/W |
Normal | VPA, LTG, TPM, ZNS |
|
Autosomal Dominant Nocturnal Frontal Lobe Epilepsy |
Partial | Children or Adults |
Possible, but usually not |
SP, CP, GTC |
Normal, or Frontal Spikes |
Normal | CBZ, OXC, etc. VNS Epilepsy Surgery |
|
Nocturnal Temporal Lobe Epilepsy |
Partial | Adults | Possible, but usually not |
SP, CP, GTC |
Temporal Spikes |
Normal | CBZ, OXC, etc. Epilepsy Surgery |
Key to abbreviations used: SP=simple partial; CP=complex partial; GTC=generalized tonic-clonic; AtAb=a typical absence; Ast=astatic; T=tonic; M=myoclonic; A=absence; CBZ=carbamazepine; OXC=oxcarbazepine; GBP=gabapentin; IVIg= intravenous immunoglobulin; MSPT=multiple subpial transections; VPA=valproic acid; LTG=lamotrigine; TPM=topiramate; FBM=felbamate; ZNS=zonisamide; VNS=vagus nerve stimulator.
The spectrum of pediatric sleep-related epilepsy syndromes spans both clinically indolent and malignant epilepsies. The most common is benign epilepsy of childhood with centrotemporal spikes (BECTS), or benign rolandic epilepsy (BRE). BRE features simple partial seizures with hypersalivation, hemifacial focal motor clonic and secondary generalized tonic-clonic seizure activity, often occurring exclusively during sleep. Family history is common. Mean age of onset is approximately age seven, ranging from 3 to 13 years, with recovery by mid- adolescence and males more commonly affected.30 Seizures are confined to sleep in approximately three-quarters of patients. In its typical presentation, children enjoy normal development and may have only infrequent seizures, but occasionally treatment is necessary if seizures become frequent enough to disrupt the patient's or family's sleep. Treatment is usually with antiepileptic drugs (AEDs) effective in partial epilepsy syndromes, such as carbamazepine, oxcarbazepine, gabapentin, or levetiracetam. EEG shows high-voltage spike-wave discharges over the ipsilateral, centrotemporal region, although discharges may occur contralaterally or bilaterally. These discharges increase in frequency and complexity during sleep.
The Landau-Kleffner syndrome (LKS) typically presents with subacutely progressive language regression following previously normal language development, accompanied by CSWS with or without clinical seizures. LKS usually presents within the middle of the first decade, most commonly in children 2–10 years of age. Which patients with LKS are at greatest risk for a benign outcome or autistic regression remains unclear.31 Treatment is with AEDs, courses of immunosuppressive therapy, or in dire medically refractory cases, epilepsy surgery utilizing multiple subpial transections in the eloquent neocortical language regions.32
The Lennox-Gastaut syndrome typically begins in the first decade of life, often preceded by a history of infantile spasms with hypsarrhythmic EEG findings (West syndrome). Multiple primary generalized seizure types, including prominent nocturnal tonic, astatic/atonic, atypical absence, myoclonic, and generalized tonic-clonic seizures with accompanying psychomotor and cognitive maldevelopment are usual. EEG typically demonstrates slow spike and wave complexes at 1.5–2.5 Hz, multifocal epileptiform abnormalities, and generalized background slowing.
Most nocturnal epileptic phenomena with prominent motor features are extratemporal partial onset seizures, of which frontal lobe onset is particularly common. Episodic nocturnal wanderings, an unusual parasomnia involving ambulation, unintelligible speech, screaming, and complex and variably violent behavior, are also often ultimately found to be an expression of nocturnal partial epilepsy.33 Nocturnal paroxysmal dystonia (NPD) was previously thought to be a distinctive non-epileptic parasomnia, but is now in most instances found to actually represent extratemporal partial epilepsy, although a few cases of particularly prolonged spells may represent nonepileptic, neurological movement disorders.33
Nocturnal frontal lobe epilepsy (NFLE) may be characterized by varying phenotypes: paroxysmal arousals with brief hypermotor movements, motor attacks with complex dystonic and dyskinetic features, or episodic nocturnal wandering often mimicking sleepwalking.34–37 NFLE predominates in males, typically with onset in infancy through adolescence, and 6–40% of cases are familial. Autosomal dominant nocturnal frontal lobe epilepsy (ADNFLE) is associated with genetically heterogeneous mutations in the neuronal nicotinic acetylcholine receptor in several international kindreds.38 Video-EEG polysomnography is necessary for definitive diagnosis, confirming abrupt awakening, and stereotypical motor behavior with vocalization and violent or dystonic-dyskinetic movements. There are often multiple attacks per night. As is frequently the case in extratemporal partial seizures, approximately 50% of patients have normal accompanying ictal and interictal EEGs. Response to carbamazepine or other antiepileptic drugs is usually excellent, although up to one-third of patients may prove medically intractable.34–38
Supplementary sensorimotor area (SSMA) epilepsy is another distinctive subtype of frontal lobe epilepsy. SSMA seizures typically begin with somatosensory auras, then progress to abrupt assumption of a "fencer" posture (arm contralateral to seizure focus relatively extended, ipsilateral arm abducted and flexed), speech arrest or vocalization, and flailing or thrashing limb movements.
Sleep-related temporal lobe seizures are quite frequent, representing approximately one-third of overall temporal lobe seizures recorded in epilepsy monitoring units.18 Nocturnal temporal lobe epilepsy (NTLE) is a subtype of medically refractory temporal lobe epilepsy with nearly exclusive seizures at nighttime.39 Approximately 70% of these patients may awaken from sleep with an aura, then progress to a complex partial seizure involving amnesia and automatisms. Most also have secondary generalized tonic-clonic seizures. Compared to age-matched, non-lesional, temporal lobe subjects with diurnal seizure patterns, the nocturnal subgroup has less frequent seizures that do not cluster, rarer family history of seizures, and less frequent history of childhood febrile convulsions. A subset of patients undergoing epilepsy surgery enjoyed a favorable seizure-free outcome postoperatively.
Juvenile myoclonic epilepsy is an idiopathic primary generalized epilepsy syndrome characterized by myoclonic, absence, and generalized tonic-clonic seizures, usually occurring shortly after arousal.4,39 Seizures may also occur during sleep or throughout the daytime hours. Mean age of onset is in mid-adolescence. EEG typically demonstrates primary generalized epileptiform discharges. Valproic acid or other newer broad spectrum AEDs such as levetiracetam, lamotrigine, topiramate, or zonisamide may lead to excellent seizure control in patients who adhere to rigid compliance and avoid seizure precipitant such as sleep deprivation and alcohol binges. Medication requirement typically endures throughout life, with few patients being successfully weaned from therapy long-term in later adulthood. A closely related primary generalized epilepsy syndrome, generalized tonic-clonic seizures upon awakening (GTCOA), has a similar pattern of occurrence of convulsions but lacks myoclonic seizures.
Co-morbid Sleep Disorders
Causes of excessive daytime sleepiness (EDS) in patients with epilepsy may include nocturnal seizures, sedative effects of AEDs, poor sleep hygiene, and co-morbid primary sleep disorders.41 Patients with epilepsy that have EDS should be evaluated for a concurrent primary sleep disorder, which may worsen a patient's seizure burden.
EDS is common in patients with intractable epilepsy and is frequently mistakenly attributed to AED toxicity, when oftentimes an underlying primary sleep disorder is the true culprit. One study found epilepsy patients were significantly sleepier than neurology outpatients without epilepsy, while perhaps surprisingly, the number and type of AEDs, seizure frequency, epilepsy syndrome, and nocturnal seizures were not significantly associated with EDS.42 Co-morbid primary sleep disorders should be aggressively sought and treated to achieve optimal management in patients with epilepsy and EDS.43–46
Sleep-disordered breathing may exacerbate seizure burden in as many as one-third of patients with medically intractable epilepsy undergoing presurgical evaluation, and may be a particularly important and treatable problem in elderly with refractory seizure disorders.45–46 OSA is the most common cause of sleep-disordered breathing. EDS and heavy snoring with or without witnessed apneas or nocturnal gasping are the cardinal clinical features. Predisposing factors include older age, male gender, obese body habitus, and oropharyngeal airspace narrowing; an independent predictor for OSA in epilepsy shown in one recent study was thickened neck circumference.47 OSA is particularly important to identify given the risk of injury while driving with EDS, as well as potential cardiovascular and general health complications. When there is a high index of suspicion for sleep-disordered breathing, polysomnography is the test of choice. Several retrospective and prospective studies have demonstrated the benefit of nasal continuous positive airway pressure (CPAP) therapy for seizure reduction in patients with refractory epilepsy and co-morbid OSA.48–50 One recent study suggests that the level of benefit toward seizure reduction provided by nasal CPAP treatment in epilepsy patients with co-morbid OSA is comparable to the effect of adding on an adjunctive antiepileptic drug for seizure treatment; approximately 50–60% of patients experienced a 50% or greater seizure reduction.50
Seizures and Antiepileptic Drug Therapies Fragment Sleep
Seizures themselves also may interrupt sleep, leading to poorly restorative sleep and EDS. The limbic system participates in the neural networks underlying sleep organization, sleep induction, and arousal. Temporal lobe pathology therefore may be an important cause of disturbed sleep. In an animal model of epilepsy known as amygdala kindling, disturbed sleep patterns with sleep fragmentation and a shift toward lighter sleep are seen.51 In epilepsy patients, formal sleep evaluation demonstrates significantly reduced REM and stages 2 and 4 sleep, and objective EDS on a modified maintenance of wakefulness test (MWT). Patients having nocturnal seizures show reduced sleep efficiency, increased time to first REM period, and increased drowsiness on the MWT.52
Many epilepsy therapies appear to have additional independent effects on sleep. All of the older and some newer AEDs have important modulatory effects on sleep physiology. Most of the older AEDs reduce REM and slow wave sleep, shorten sleep latency, and increase the percentage of Stage 1 and 2 NREM sleep.53 Following initiation, carbamazepine transiently reduces REM sleep but otherwise has little effect on sleep architecture.54 Lamotrigine reduces slow wave sleep while increasing stage 2 sleep, but is also associated with reduced arousals and stage shifts and an increase in REM periods without significant subjective insomnia.55 Whether AEDs have any specific or independent effects on sleep-disordered breathing hasn't yet been explored, but is in need of study.
Parasomnias Mimic Epilepsy
Video-EEG polysomnography combines the seizure-localizing properties of video-EEG monitoring with sleep staging by polysomnography, allowing for confident specific diagnosis of epileptic seizures and the ability to distinguish these from non-epileptic nocturnal events, such as parasomnias, which may share similar clinical features.
An expanded EEG montage with particular coverage of the fronto-temporal regions and wide bandpass filter settings is essential to allow appropriate capture of electrophysiologic features, and time-synchronized video telemetry should be employed.56–57 Review of the clinical behavior by video analysis is critical in all patients with nocturnal events. Withdrawing anti-epileptic drugs (AEDs) may increase the yield of capturing seizures, while sleep deprivation increases the yield for parasomnias.58 The effect of sleep deprivation on seizure activation remains controversial, although a recent study suggested that sleep deprivation had little impact on precipitating seizures in an inpatient epilepsy monitoring unit.59 Medication should not be withdrawn in outpatient settings in an attempt to provoke seizure or spell occurrence, and physicians should only consider careful antiepileptic drug withdrawal in a safe, continuously supervised, inpatient epilepsy monitoring unit setting given the risks of precipitating severe seizures including status epilepticus.
The differentiation of nocturnal epilepsy from other nonepileptic dissociated states of wakefulness and sleep is of extreme importance. Many parasomnias are easily confused with seizures by their similar clinical phenomenology of episodic confusion and movement. (See Table 2.) Clinical features of multiple recurrences within a single sleep episode, relative stereotypy, postictal behavior and rhythmic EEG abnormalities are more commonly seen with epileptic events.
Table 2.
Distinguishing features of Nocturnal Events.
| NREM Parasomnias |
Premonitory Symptoms |
Behavioral Characteristics |
Duration | Frequency | EEG/PSG Findings |
|---|---|---|---|---|---|
| Night Terrors | None | Inconsolable screaming |
Minutes | 1 or less nightly |
Arousal from N2-N3 |
|
Confusional Arousals |
None | Confused, amnestic |
Seconds- Minutes |
1 or less nightly |
Arousal from N3>N2 |
| Sleepwalking | None | Ambulation, Amnesia |
Minutes | 1 or less nightly |
Arousal from N3≫N2 |
|
REM Parasomnias | |||||
| Nightmares | Dream Recall |
Arousal, frightened, palpitations |
Seconds | Generally 1/nightly |
Arousal from REM |
| RBD | Variable Dream Recall |
Thrashing, complex motor behavior |
Seconds to minutes |
>1/night, second>first half |
REM sleep without atonia |
|
Non-State
Dependent Parasomnias | |||||
| Rhythmic Movement Disorder |
None | head banging, body rocking, bruxism |
Seconds to minutes |
Several times nightly |
Any sleep stage, movement artifact |
|
Sleep-related
Epilepsies | |||||
| BECTS | Facial twitching, hypersalivation |
Focal motor or GTC, postictal |
Seconds to minutes |
>1/night | Arousal from NREM, IEDs, ictal EEG pattern |
| ADNFLE | Bizarre stereotyped motor behavior |
Focal motor, bizarre motor |
Seconds, <1 minute |
1 or multiple attacks/night |
Arousal from N2 |
| TLE | Aura variable | CPS, Postictal |
1–2minutes | 1 or multiple attacks/night |
Arousal from N2 |
|
Physiologic/Psychogenic
Nocturnal Events | |||||
|
Physiologic/ “Hypnic” Myoclonus |
None | brief body/ limb jerks |
Seconds | 1 or multiple/night at sleep onset or awakening |
Sleep-wake transition movement artifact |
|
Periodic
leg Movements |
None, variable restless legs while awake |
leg/arm movements |
Seconds, recurrent |
Multiple/night | Typically NREM sleep predominance |
|
Psychogenic Spells |
Variable | Variable | often >5 minutes | 1 or multiple attacks/night |
Normal awake EEG |
| Nocturnal Panic | None | Arousal, Feeling of panic/ anxiousness, fear doom, diaphoresis, palpitations |
Seconds to 1 minute |
Generally 1/nightly |
Arousal from N1-N2 |
Abbreviations: NREM=non-rapid eye movement sleep; N1=Stage 1 NREM sleep; N2=Stage 2 NREM sleep; N3=Stage 3 NREM sleep; REM=rapid eye movement sleep; IEDs=interictal epileptiform discharges; EEG=electroencephalogram; PSG=polysomnogram; BECTs=Benign epilepsy of childhood with centro-temporal spikes; ADNFLE=autosomal dominant nocturnal frontal lobe epilepsy; TLE=temporal lobe epilepsy; RBD=REM sleep behavior disorder.
Video-EEG polysomnography allows for confident specific diagnosis in most instances when clinical history is inconclusive, and it rarely reveals evidence for partial epilepsy when the history is otherwise suggestive of nonepileptic parasomnias.60–62 Formal diagnostic assessment should be pursued for any nocturnal spell involving potentially injurious behavior, disturbance of the patient or partner's sleep, or when the diagnosis is unclear on clinical grounds.
Nonepileptic parasomnias in the differential diagnosis of nocturnal events include REM sleep behavior disorder, NREM parasomnias, non-state dependent parasomnias, periodic limb movements of sleep, and nocturnal panic attacks. Formal diagnostic assessment should be considered for any nocturnal spell that may involve potentially injurious or dangerous behavior, disturbance of the patient or partner's sleep, or when the diagnosis is unclear from clinical grounds.
Disorders arising from REM sleep include nightmares and REM sleep behavior disorders. Nightmares usually occur during REM sleep, and may awaken the sleeper. Dream recall is a common feature, and patients are alert, coherent, and well oriented. Night terrors of NREM sleep, in contrast, involve a confusional arousal characterized by prominent vocalization and movement.
REM sleep behavioral disorder (RBD) is characterized by complex, often violent behavior occurring most commonly in the second half of the night when REM sleep is most prevalent.64 Behavior may be dramatic and extreme, leading clinicians to initially suspect a psychogenic cause. Making the diagnosis of RBD is imperative given risk for severe injury to the patient (which may include cervical fractures or concussion) or sleeping partner.65 RBD results from acting out of dreams enabled by loss of the normal skeletal muscle atonia of REM sleep. Frequently, RBD may herald or accompany parkinsonism and most commonly presents in elderly men. RBD may also present in younger individuals with narcolepsy or become provoked by use of selective serotonin reuptake inhibitor (SSRI) antidepressants. Video-EEG polysomnography is useful for making the diagnosis, and may support a clinical diagnosis of RBD, even if clinical spells are not captured, by documenting features of REM sleep without atonia (Figure 4). When there is a precipitating agent, for example, SSRI antidepressants such as fluoxetine (Prozac), discontinuance of the offending agent is usually necessary. Treatment for RBD is with bedtime melatonin or clonazepam, and ensuring safety in the bedroom.
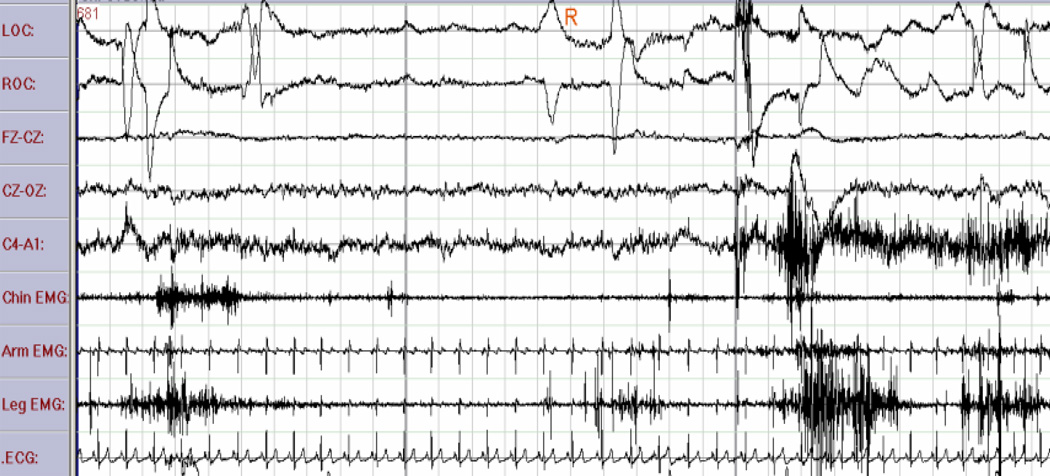
Figure 4.
REM sleep without atonia in a 75 year-old man with parkinsonism and dream enactment behavior. Note the elevated muscle tone in channels 6, 7, and 8 corresponding to the chin, arm, and leg EMG leads, respectively.
The etiology and pathophysiology of NREM parasomnias is poorly understood. The NREM parasomnias are a continuum of different behavioral disorders associated with abnormal arousal from NREM sleep, particularly slow-wave sleep. Whether the arousal disorders result from an excess of sleep maintenance or an insufficiency of arousal mechanisms in the brain remains unclear. Since NREM parasomnias are more common in children than adults, maturational factors are felt to be important, and hereditary factors likely play an important role since familial history is frequent. Arousal disorders tend to occur most commonly in the first third of the night, when most slow-wave sleep occurs.
A diverse range of paroxysmal behavior is possible, the common features being a sudden arousal from slow-wave sleep followed by poor reactivity and amnesia for the event, clinical features overlapping substantially with epilepsy. Early reports on the clinical neurophysiology of NREM parasomnias were conflicting and limited by daytime interictal recording without substantive polysomnographic data. More recent findings appear to confirm that NREM parasomnias are nonepileptic phenomena resulting from a dissociated state, as the patient awakens they are caught between sleep and arousal. This theory is supported by single photon emission computed tomography (SPECT) during a sleepwalking episode, which demonstrated activation of thalamocingulate pathways and deactivation of other thalamocortical arousal systems.65 Patients with NREM parasomnias usually manifest sudden arousals during slow-wave sleep, and show delta hypersychronization prior to slow-wave sleep arousals more commonly than age-matched controls.66
The range of behavior seen with NREM parasomnias include night terrors, confusional arousals, sleep walking and talking, nocturnal eating syndrome, nocturnal panic disorder, and complex nocturnal visual hallucinosis. Pavor nocturnus is usually due to a confusional arousal, but proven epileptic causes have been documented.67–68 Sleepwalking may be more easily confused with ictal or postictal behavior of nocturnal seizures; video-EEG polysomnography should be employed in most cases to differentiate epileptic and non-epileptic parasomnias. Superimposed complex motor behavior, stereotypy, post-ictal confusion, and ictal EEG manifestations are supportive of an epileptic etiology for episodic nocturnal wandering, a more extreme form of sleep walking with bizarre behavior and AED responsiveness.69–70 However, prolonged confusion suggestive of post-ictal confusion (of 20 to 45 minutes duration) has been also noted in exceptional patients with non-epileptic parasomnias, leading to added diagnostic confusion on clinical grounds alone.71 Stereotypy, multiple episodes per night, prolonged post-ictal confusion, or incontinence, and focally abnormal ictal EEG recording all favor an epileptic etiology.
Typically, NREM parasomnias represent arousals from slow-wave sleep, with three varying patterns seen on EEG following arousal: diffuse rhythmic delta, diffuse delta-theta with intermixed alpha and beta, or prominent alpha and beta activity.72 Commonly, rhythmic delta persists during a clinical arousal (Figure 5). Analysis of delta power prior to sleep walking episodes suggests increases of relative low-frequency delta power (in the 0.75–2 Hz bandwidth) immediately preceding confusional arousals.73 Sleepwalkers may have altered slow-wave power spectrum during NREM sleep, particularly in the early part of the night when most pathologic arousals from slow- wave sleep occur, in comparison with normal age-matched controls, suggesting they may harbor an abnormality in the neural mechanisms underlying regulation of slow-wave sleep.74
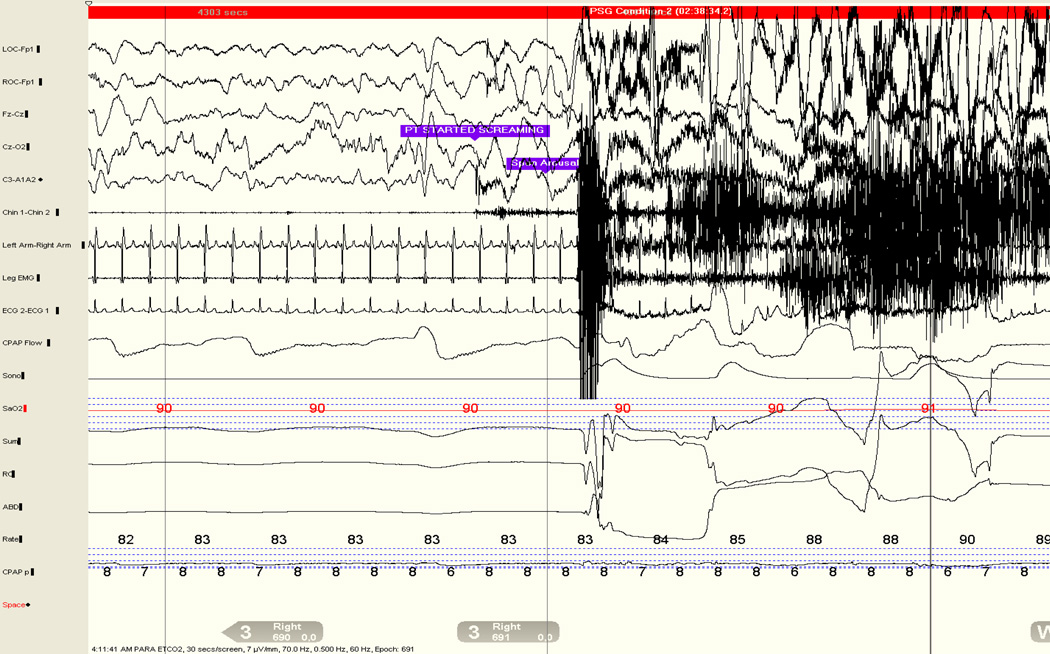
Figure 5.
Hypersynchronous delta activity accompanying a confusion arousal in a 34 year-old woman.
Pharmacotherapy and behavioral therapies are the mainstay of management options for the arousal disorders. Long-acting benzodiazepines such as clonazepam are typically most effective, and addressing environmental safety precautions is of vital importance. In particular, ensuring that access to doors and windows is secured and that injurious obstacles or stairs are isolated from the patient, and various behavioral measures such as tethering patients to bed with a belt, scheduled anticipatory awakenings, and avoidance of precipitants (sleep deprivation or sleep disorders, drugs and alcohol, or febrile illness) should be discussed. In selected patients, hypnosis may be beneficial.75
Rhythmic movement disorder (RMD) consists of stereotyped, semi-rhythmic movements of large muscle groups, such as head banging or body rocking. RMD may occur during any stage of sleep or wakefulness, is most frequently seen in cognitively disabled individuals, and is unaccompanied by a change in the EEG background other than muscle and movement artifact. Sometimes RMD is outgrown, but if violent, recommendation for protective bed padding or head gear is necessary, and benzodiazepines may be utilized. Bruxism is a similar phenomenon, involving tooth grinding. Dental appliances are helpful to reduce wear and protect the teeth and gums.
Enuresis is normal in childhood and typically outgrown by age five, although it may persist in up to 10% of boys and nearly 5% of girls to age 12. Various behavioral treatments are the mainstay of treatment, although nasal vasopressin (ddA VP) and imipramine are effective temporarily.
A very rare and unusual nocturnal parasomnia of children is benign nocturnal alternating hemiplegia of childhood (BNAHC). Clinical features include early life onset, typically before or shortly following age three, with episodic attacks of hemiplegia lasting 5 to 20 minutes and occurring numerous times per month.76 There is a frequent family history of migraine, and unlike classic alternating hemiplegia of childhood (AHC), which has diurnal attacks, no patient with exclusively nocturnal attacks has yet experienced developmental regression of motor or cognitive function. Treatment with anticonvulsants or flunarizine, a calcium channel blocker used with good effect in the classic diurnal form of AHC, has shown inconsistent results. BNAHC may represent a variant of hemiplegic migraine, but further clinical and genetics data will be necessary to clarify its nature.
Periodic limb movements of sleep (PLMS) may be normal if limited in number, but if associated with frequent arousals may present as a primary sleep disorder associated with excessive daytime sleepiness. Polysomnography demonstrates periodically recurring limb movements. The cause of PLMS remains unclear. Correlation between the degree of subjective daytime sleepiness and arousals is often poor. Treatment with dopaminergic agents or clonazepam is usual. Sleep starts, sensory starts, and hypnic myoclonic jerks may occur at the transition between sleep and wakefulness. These are brief, often singular motor or sensory phenomena such as an isolated limb jerk, or perception of a bright flash of light or loud noise. Hypnic jerks are a physiologic form of myoclonus. They are bilateral, sometimes asymmetrical and usually single, brief body jerks that coincide with sleep onset. On occasion, they can be confounding when occurring in epileptic subjects, especially in children, but they are still a benign finding of no clinical significance and should be recognized as such to avoid overzealous treatment or misdiagnosis as another parasomnia. Video-EEG polysomnography can be helpful to establish the diagnosis when it is unclear from clinical history alone.
Other sleep-related movements that may be rarely seen include abnormal motor phenomena associated with severe sleep disordered breathing. In this case, hypoxia becomes so extreme that it mediates brain anoxia with cortical "release" of subcortically generated movements, including tonic posturing or clonic-like movements, akin to what may be seen in convulsive syncope. Propriospinal myoclonus is a sleep-wake transition disorder characterized by single or briefly repetitive myoclonic jerks.77
Although it is unusual for psychogenic or functional disturbances to arise from sleep, nocturnal panic attacks or conversion disorders that may resemble partial seizures can occur rarely. Nocturnal panic attacks are common amongst those with diurnal panic disorder but may occur as a distinctive and isolated nighttime episode of arousal from NREM sleep.78 Cognitive-behavioral therapy and selective serotonin reuptake inhibitor (SSRI) antidepressants are effective. Psychogenic non-epileptic spells (PNES) may be difficult to distinguish from a true epileptic seizure; eye closure and non-physiologic spread of movements (i.e., from face to leg to opposite arm) are typical in PNES, and a helpful observation during video-EEG polysomnography allowing the distinction of psychogenic from epileptic seizures is “pre-ictal pseudosleep” (a waking EEG pattern of normal alpha activity, accompanied by a behavioral state of eye closure and apparent sleep that precedes behavior consistent with a psychogenic spell).79 Medical disorders such as nocturnal gastroesophageal reflux and nocturnal asthma or paroxysmal nocturnal dyspnea from congestive heart failure also enter the differential diagnosis, but are usually easily distinguished from seizures or the neurological parasomnias by associated clinically evident symptoms or medical co-morbidities. Nocturnal electrocardiographic telemetry and esophageal pH monitoring may be helpful in some instances.
Conclusions
Sleep and epilepsy are connected in several ways: (1) NREM sleep may activate spikes and seizures; (2) seizures and AEDs may lead to sleep fragmentation, (3) co-morbid primary sleep disorders may further impair quality of life and increase medical risk in patients with epilepsy, and (4) the parasomnias may mimic epileptic seizures, leading to inaccurate diagnosis and ineffective therapy.
Recognizing and effectively treating primary sleep disorders in patients with epilepsy frequently may improve seizure frequency and overall functioning. The diagnostic evaluation of nocturnal events benefits from a collaborative approach by epilepsy and sleep specialists and utilization of video-EEG polysomnography for confident and accurate diagnosis. Epilepsy and sleep are interwoven in many respects, leading to many fascinating intersections in the patient bedroom and physician office.
ACKNOWLEDGEMENT
This publication was made possible by Grant Number 1 UL1 RR024150 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH), and the NIH Roadmap for Medical Research. Its contents are solely the responsibility of the author and do not necessarily represent the official view of NCRR or NIH. Information on NCRR is available at . Information on Reengineering the Clinical Research Enterprise can be obtained from http://nihroadmap.nih.gov.
Biography
Dr. St. Louis is associate professor of neurology at the Mayo Clinic College of Medicine and a Consultant in Neurology and Sleep Medicine at Mayo Clinic and Foundation, Rochester, Minnesota, U.S.A. His research interests include sleep and epilepsy, cognitive neurophysiology, and the medical humanities.
References
- 1.Temkin O. The falling sickness: a history of epilepsy from the Greeks to the beginnings of modern neurology. Baltimore and London: Johns Hopkins University Press; 1971. [Google Scholar]
- 2.Gowers WR. Epilepsy and other chronic convulsive diseases. New York: William Wood and Company; 1885. [Google Scholar]
- 3.Lennox WG. Epilepsy and related disorders. Boston: Little Brown and Company; 1960. pp. 509–512. [Google Scholar]
- 4.Janz D. The grand mal epilepsies and the sleeping-waking cycle. Epilepsia. 1962;3:69–109. doi: 10.1111/j.1528-1157.1962.tb05235.x. [DOI] [PubMed] [Google Scholar]
- 5.Bazil CW, Walczak TS. Effects of sleep and sleep stage on epileptic and nonepileptic seizures. Epilepsia. 1997;38(1):56–62. doi: 10.1111/j.1528-1157.1997.tb01077.x. [DOI] [PubMed] [Google Scholar]
- 6.Malow BA, Lin X, Kushwaha R, Aldrich MS. Interictal spiking increases with sleep depth in temporal lobe epilepsy. Epilepsia. 1998;39:1309–1316. doi: 10.1111/j.1528-1157.1998.tb01329.x. [DOI] [PubMed] [Google Scholar]
- 7.Malow BA, Bowes RJ, Ross D. Relationship of temporal lobe seizures to sleep and arousal: a combined scalp-intracranial electrode study. Sleep. 2000;23(2):231–234. [PubMed] [Google Scholar]
- 8.Sammaritano M, Gigli GL, Gotman J. Interictal spiking during wakefulness and sleep and the localization of foci in temporal lobe epilepsy. Neurology. 1991;41:290–297. doi: 10.1212/wnl.41.2_part_1.290. [DOI] [PubMed] [Google Scholar]
- 9.Kumar P, Raju TR. Seizure susceptibility decreases with enhancement of rapid eye movement sleep. Brain Res. 2001;922(2):299–304. doi: 10.1016/s0006-8993(01)03174-2. [DOI] [PubMed] [Google Scholar]
- 10.Natarajan A, Marzec ML, Lin X, Minecan D, Malow BA. Interictal epileptiform discharges do not change before seizures during sleep. Epilepsia. 2002;43(1):46-SI. doi: 10.1046/j.1528-1157.2002.24301.x. [DOI] [PubMed] [Google Scholar]
- 11.Staba RJ, Wilson CL, Fried I, Engel J., Jr. Single neuron burst firing in the human hippocampus during sleep. Hippocampus. 2002;12(6):724–734. doi: 10.1002/hipo.10026. [DOI] [PubMed] [Google Scholar]
- 12.Staba RJ, Wilson CL, Bragin A, Fried T, Engel J., Jr Sleep states differentiate single neuron activity recorded from human epileptic hippocampus, entorihinal cortex, and subiculum. J Neurosci. 2002;22(13):5694–5704. doi: 10.1523/JNEUROSCI.22-13-05694.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Parrino L, Smerieri A, Terzano MG. Combined influence of cyclic arousability and EEG synchrony on generalized interictal discharges within the sleep cycle. Epilepsy Res. 2001;44(1):7–18. doi: 10.1016/s0920-1211(00)00192-3. [DOI] [PubMed] [Google Scholar]
- 14.Parrino L, Halasz P, Tassinari CA, Terzano MG. CAP, epilepsy and motor events during sleep: the unifying role of arousal. Sleep Med Rev. 2006;10(4):267–285. doi: 10.1016/j.smrv.2005.12.004. [DOI] [PubMed] [Google Scholar]
- 15.Vanhatalo S, Palva JM, Holmes MD, Miller JW, Voipio J, Kaila K. Infraslow oscillations modulate excitability and interictal epileptic activity in the human cortex during sleep. Proc Natl Acad Sci U S A. 2004;101(14):5053–5057. doi: 10.1073/pnas.0305375101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.D'Cruz OF, Vaughn BV. Nocturnal seizures mimic REM behavior disorder. Am J END Technol. 1997;37(4):258–264. [Google Scholar]
- 17.Herman ST, Walczak TS, Bazil CW. Distribution of partial seizures during the sleep-wake cycle: differences by seizure onset site. Neurology. 2001;56(11):1453–1459. doi: 10.1212/wnl.56.11.1453. [DOI] [PubMed] [Google Scholar]
- 18.St. Louis EK, Genilo P, Granner MA, Zimmerman B. Sleep-onset mesial temporal seizures arise from light NREM sleep. Epilepsia. 2004;45(Suppl. 7):86–87. [Google Scholar]
- 19.Crespel A, Baldy-Moulinier M, Coubes P. The relationship between sleep and epilepsy in frontal and temporal lobe epilepsies: practical and physiopathologic considerations. Epilepsia. 1998;39:150–157. doi: 10.1111/j.1528-1157.1998.tb01352.x. [DOI] [PubMed] [Google Scholar]
- 20.Yaqub BA, Waheed G, Kabiraj MM. Nocturnal epilepsies in adults. Seizure. 1997;6(2):145–149. doi: 10.1016/s1059-1311(97)80069-6. [DOI] [PubMed] [Google Scholar]
- 21.Park SA, Lee BI, Park SC, Lee SJ, Kim WJ, Lee JH, Kim JY. Clinical course of pure sleep epilepsies. Seizure. 1998;7(5):369–377. doi: 10.1016/s1059-1311(05)80005-6. [DOI] [PubMed] [Google Scholar]
- 22.Malow BA, Passaro E, Milling C, Minecan DN, Levy K. Sleep deprivation does not affect seizure frequency during inpatient video-EEG monitoring. Neurology. 2002;59(9):1371–1374. doi: 10.1212/01.wnl.0000031810.15811.9e. [DOI] [PubMed] [Google Scholar]
- 23.Quigg M, Straume M, Menaker M, Beltram EH., 3rd Temporal distribution of partial seizures: comparison of an animal model with human partial epilepsy. Ann Neurol. 1998;43:748-SS. doi: 10.1002/ana.410430609. [DOI] [PubMed] [Google Scholar]
- 24.Duckrow RB, Tcheng TK. Daily variation in an intracranial EEG feature in humans detected by a responsive neurostimulator system. Epilepsia. 2007;48(8):1614–1620. doi: 10.1111/j.1528-1167.2007.01091.x. [DOI] [PubMed] [Google Scholar]
- 25.Durazzo TS, Spencer SS, Duckrow RB, Novotny EJ, Spencer DD, Zaveri HP. Temporal distributions of seizure occurrence from various epileptogenic regions. Neurology. 2008;70(15):1265–1271. doi: 10.1212/01.wnl.0000308938.84918.3f. [DOI] [PubMed] [Google Scholar]
- 26.Hofstra WA, Gordijn MC, van der Palen J, van Regteren R, Grootemarsink BE, de Weerd AW. Timing of temporal and frontal seizures in relation to the circadian phase: A prospective pilot study. Epilepsy Res. 2011;94(3):158–162. doi: 10.1016/j.eplepsyres.2011.01.015. [DOI] [PubMed] [Google Scholar]
- 27.Karafin M, St Louis EK, Zimmerman MB, Sparks JD, Granner MA. Bimodal ultradian seizure periodicity in human mesial temporal lobe epilepsy. Seizure. 2010;19(6):347–351. doi: 10.1016/j.seizure.2010.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pavlova MK, Shea SA, Bromfield EB. Day/night patterns of focal seizures. Epilepsy Behav. 2004;5(1):44–49. doi: 10.1016/j.yebeh.2003.10.013. [DOI] [PubMed] [Google Scholar]
- 29.Zarowski M, Loddenkemper T, Vendrame M, Alexopoulos AV, Wyllie E, Kothare SV. Circadian distribution and sleep/wake patterns of generalized seizures in children. Epilepsia. 2011;52(6):1076–1083. doi: 10.1111/j.1528-1167.2011.03023.x. [DOI] [PubMed] [Google Scholar]
- 30.Bouma PAD, Bovenkerk AC, Westendorp RGJ, Brouwer OF. The course of benign partial epilepsy of childhood with centrotemporal spikes: a rneta-analysis. Neurology. 1997;48:430–437. doi: 10.1212/wnl.48.2.430. [DOI] [PubMed] [Google Scholar]
- 31.Camfield P, Camfield C. Epileptic syndromes in childhood: clinical features, outcomes, and treatment. Epilepsia. 2002;43(Suppl 2):27–32. doi: 10.1046/j.1528-1157.43.s.3.3.x. [DOI] [PubMed] [Google Scholar]
- 32.Morrell F, Whisler WW, Smith MC, Hoeppner TJ, de Toledo-Morrell L, Pierre-Louis SJ, Kanner AM, Buelow JM, Ristanovic R, Bergen D, et al. Landau-Kleffner syndrome: treatment with subpial intracortical transection. Brain. 1995;118:1529–1546. doi: 10.1093/brain/118.6.1529. [DOI] [PubMed] [Google Scholar]
- 33.Montagna P. Nocturnal paroxysmal dystonia and nocturnal wandering. Neurology. 1992;42(7 Suppl. 6):61–67. [PubMed] [Google Scholar]
- 34.Provini F, Plazzi G, Tinuper P, Vandi S, Lugaresi E, Montagna P. octurnal frontal lobe epilepsy: a clinical and polygraphic overview of 100 consecutive cases. Brain. 1999;122(PI. 6):1017–1031. doi: 10.1093/brain/122.6.1017. [DOI] [PubMed] [Google Scholar]
- 35.Provini F, Plazzi G, Lugaresi E. From nocturnal paroxysmal dystonia to nocturnal frontal lobe epilepsy. Clin Neurophysiol. 2000;(1 Suppl 2):S2–S8. doi: 10.1016/s1388-2457(00)00396-5. [DOI] [PubMed] [Google Scholar]
- 36.Oldani A, Zucconi M, Ferini-Strarnbi L, Bizzozero D, Smirne S. Autosomal dominant nocturnal frontal lobe epilepsy: electroclinical picture. Epilepsia. 1996;37(10):964–976. doi: 10.1111/j.1528-1157.1996.tb00534.x. [DOI] [PubMed] [Google Scholar]
- 37.Oldani A, Zucconi M, Asselta R, Modugno M, Bonati MT, Dalpra L, Malcovati M, Tenchini ML, Smirne S, Ferini-Strambi L. Autosomal dominant nocturnal frontal lobe epilepsy: a video- polysomnographic and genetic appraisal of 40 patients and delineation of the epileptic syndrome. Brain. 1998;121(PI. 2):20S–23S. doi: 10.1093/brain/121.2.205. [DOI] [PubMed] [Google Scholar]
- 38.di Corcia G, Blasetti A, De Simone M, Verrotti A, Chiarelli F. Recent advances on autosomal dominant nocturnal frontal lobe epilepsy: "understanding the nicotinic acetylcholine receptor (nAChR)". Eur J Paediatr Neurol. 2005;9(2):59–66. doi: 10.1016/j.ejpn.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 39.Bernasconi A, Andermann F, Cendes F, Dubeau F, Andermann E, Olivier A. Nocturnal temporal lobe epilepsy. Neurology. 1998;50:1772–1777. doi: 10.1212/wnl.50.6.1772. [DOI] [PubMed] [Google Scholar]
- 40.Alfradique I, Vasconcelos MM. Juvenile myoclonic epilepsy. Arq Neuropsiquiatr. 2007;65(4B):1266–1271. doi: 10.1590/s0004-282x2007000700036. [DOI] [PubMed] [Google Scholar]
- 41.Foldvary N. Sleep and epilepsy. Curr Treat Options Neurol. 2002;4(2):129–135. doi: 10.1007/s11940-002-0021-5. [DOI] [PubMed] [Google Scholar]
- 42.Malow BA, Bowes RJ, Lin X. Predictors of sleepiness in epilepsy patients. Sleep. 1997;20(12):1105–1110. doi: 10.1093/sleep/20.12.1105. [DOI] [PubMed] [Google Scholar]
- 43.Bassetti CL, Gugger M. Sleep disordered breathing in neurological disorders. Swiss Med Wkly. 2002;132(9–10):109–115. doi: 10.4414/smw.2002.09703. [DOI] [PubMed] [Google Scholar]
- 44.Vaughn BV, D'Cruz OF. Obstructive sleep apnea in epilepsy. Clin Chest Med. 2003 Jun;24(2):239–248. doi: 10.1016/s0272-5231(03)00023-6. [DOI] [PubMed] [Google Scholar]
- 45.Malow BA, Levy K, Maturen K, Bowes R. Obstructive sleep apnea is common in medically refractory epilepsy patients. Neurology. 2000;55(7):1002–1007. doi: 10.1212/wnl.55.7.1002. [DOI] [PubMed] [Google Scholar]
- 46.Chihorek AM, Abou-Khalil B, Malow BA. Obstructive sleep apnea is associated with seizure occurrence in older adults with epilepsy. Neurology. 2007;69(19):1823–1827. doi: 10.1212/01.wnl.0000279334.78298.d5. [DOI] [PubMed] [Google Scholar]
- 47.Berth W, St. Louis EK, Zimmerman MB, Granner MA, Dyken ME. Predictors of co-morbid obstructive sleep apnea in epilepsy. Neurology. 2011;76(Suppl. 4):A337. [Google Scholar]
- 48.Malow BA, Foldvary-Schaefer N, Vaughn BV, Selwa LM, Chervin RD, Weatherwax KJ, Wang L, Song Y. Treating obstructive sleep apnea in adults with epilepsy: a randomized pilot trial. Neurology. 2008;71(8):572–577. doi: 10.1212/01.wnl.0000323927.13250.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vendrame M, Auerbach S, Loddenkemper T, Kothare S, Montouris G. Effect of continuous positive airway pressure treatment on seizure control in patients with obstructive sleep apnea and epilepsy. Epilepsia. 2011 Aug 12; doi: 10.1111/j.1528-1167.2011.03214.x. doi: 10.1111/j.1528-1167.2011.03214.x. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 50.St. Louis EK, Berth W, Granner MA, Dyken ME, Zimmerman MB. Seizure reduction following nasal continuous positive airway pressure therapy for co-morbid obstructive sleep apnea in epilepsy. Neurology. 2011;76(Suppl. 4):A453. [Google Scholar]
- 51.Van Sweden B. Sleep and the temporal lobe. Acta Neurol Belg. 1996;96(1):19–30. [PubMed] [Google Scholar]
- 52.Bazil CW, Castro LH, Walczak TS. Reduction of rapid eye movement sleep by diurnal and nocturnal seizures in temporal lobe epilepsy. Arch Neurol. 2000;57(3):363–368. doi: 10.1001/archneur.57.3.363. [DOI] [PubMed] [Google Scholar]
- 53.Sammaritano MR, Sherwin AL. Effects of anticonvulsants on sleep. In: Bazil C, Malow B, Sammaritano M, editors. Sleep and epilepsy: the clinical spectrum. Amsterdam: Elsevier; 2002. pp. 187–194. [Google Scholar]
- 54.Gigli GL, Placidi F, Diomedi M, Maschio M, Silvestri G, Scalise A, Marciani MG. Nocturnal sleep and daytime somnolence in untreated patients with temporal lobe epilepsy: changes after treatment with controlled-released carbamazepine. Epilepsia. 1997;38(6):696–701. doi: 10.1111/j.1528-1157.1997.tb01239.x. [DOI] [PubMed] [Google Scholar]
- 55.Foldvary N, Perry M, Lee J, Dinner D, Morris HH. The effects of lamotrigine on sleep in patients with epilepsy. Epilepsia. 2001;42(12):1569–1573. doi: 10.1046/j.1528-1157.2001.46100.x. [DOI] [PubMed] [Google Scholar]
- 56.Foldvary N, Caruso AC, Mascha E, Perry M, Klem G, McCarthy V, Qureshi F, Dinner D. Identifying montages that best detect electrographic seizure activity during polysomnography. Sleep. 2000;23(2):221–229. [PubMed] [Google Scholar]
- 57.Geyer JD, Payne TA, Carney PR, Aldrich MS. Atlas of digital polysomnography. Philadelphia: Lippincott Williams and Wilkins; 2000. [Google Scholar]
- 58.Joncas S, Zadra A, Paquet J, Montplasir J. The value of sleep deprivation as a diagnostic tool in adult sleepwalkers. Neurology. 2002;58(6):936–940. doi: 10.1212/wnl.58.6.936. [DOI] [PubMed] [Google Scholar]
- 59.Malow BA, Passaro E, Milling C, Minecan DN, Levy K. Sleep deprivation does not affect seizure frequency during inpatient video-EEG monitoring. Neurology. 2002;59(9):1371–1374. doi: 10.1212/01.wnl.0000031810.15811.9e. [DOI] [PubMed] [Google Scholar]
- 60.Dyken ME, Yamada T, Lin-Dyken DC. Polysomnographic assessment of spells in sleep: nocturnal seizures versus parasomnias. Semin Neurol. 2001;21(4):377–390. doi: 10.1055/s-2001-19409. [DOI] [PubMed] [Google Scholar]
- 61.D'Cruz OF, Vaughn BV. Nocturnal seizures: mimic REM behavior disorder. Am J Electroneurodiagn Technol. 1997;37(4):258–64.61. [Google Scholar]
- 62.Aldrich MS, Jahnke B. Diagnostic value of video-EEG polysomnography. Neurology. 1991;41(7):1060–1066. doi: 10.1212/wnl.41.7.1060. [DOI] [PubMed] [Google Scholar]
- 63.Boeve BF. REM sleep behavior disorder: Updated review of the core features, the REM sleep behavior disorder-neurodegenerative disease association, evolving concepts, controversies, and future directions. Ann N Y Acad Sci. 2010;1184:15–54. doi: 10.1111/j.1749-6632.2009.05115.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Schenck CH, Mahowald MW. Injurious sleep behavior disorders (parasomnias) affecting patients on intensive care units. Intensive Care Med. 1991;17(4):219–224. doi: 10.1007/BF01709881. [DOI] [PubMed] [Google Scholar]
- 65.Bassetti C, Vella S, Donati F, Wielepp P, Weder B. SPECT during sleepwalking. Lancet. 2000;356(9228):484–485. doi: 10.1016/S0140-6736(00)02561-7. [DOI] [PubMed] [Google Scholar]
- 66.Halasz P, Ujszaszi J, Gadoros J. Are microarousals preceded by electroencephalographic slow wave synchronization precursors of confusional awakenings? Sleep. 1985;8(3):231–238. doi: 10.1093/sleep/8.3.231. [DOI] [PubMed] [Google Scholar]
- 67.Lombroso CT. Pavor nocturnus of proven epileptic origin. Epilepsia. 2000;41(9):1221–1226. doi: 10.1111/j.1528-1157.2000.tb00329.x. [DOI] [PubMed] [Google Scholar]
- 68.Huppertz HJ, Schulze-Bonhage A. Epileptic pavor nocturnus. Epilepsia. 2001;42(5):704. doi: 10.1046/j.1528-1157.2001.le425.x. [DOI] [PubMed] [Google Scholar]
- 69.Plazzi G, Tinuper P, Montagna P, Provini F, Lugaresi E. Epileptic nocturnal wanderings. Sleep. 1995;18(9):749-S6. doi: 10.1093/sleep/18.9.749. [DOI] [PubMed] [Google Scholar]
- 70.Huang YZ, Chu NS. Episodic nocturnal wandering and complex visual hallucination. A case with long-term follow-up. Seizure. 1998;7(1):67–71. doi: 10.1016/s1059-1311(98)90011-5. [DOI] [PubMed] [Google Scholar]
- 71.Kushida CA, Clerk AA, Kirsch CM, Hotson JR, Guilleminault C. Prolonged confusion with nocturnal wandering arising from NREM and REM sleep: a case report. Sleep. 1995;18(9):757–764. [PubMed] [Google Scholar]
- 72.Schenck CH, Pareja JA, Patterson AL, Mahowald MW. Analysis of polysomnographic events surrounding 252 slow-wave sleep arousals in thirty-eight adults with injurious sleepwalking and sleep terrors. J Clin Neurophysiol. 1998;15(2):159–166. doi: 10.1097/00004691-199803000-00010. [DOI] [PubMed] [Google Scholar]
- 73.Guilleminault C, Poyares D, Aftab FA, Palombini L, Abat F. Sleep and wakefulness in somnabulism: a spectral analysis study. J Psychosom Res. 2001;51(2):411–416. doi: 10.1016/s0022-3999(01)00187-8. [DOI] [PubMed] [Google Scholar]
- 74.Gaudreau J, Joncas S, Zadra A, Montplaisir J. Dynamics of slow-wave activity during the NREM sleep of sleepwalkers and normal subjects. Sleep. 2000;23(6):755–760. [PubMed] [Google Scholar]
- 75.Hurwitz TD, Mahowald MW, Schenck CH, Schluter JL, Bundlie SR. A retrospective outcome study and review of hypnosis as treatment of adults with sleepwalking and sleep terror. J Nerv Ment Dis. 1991;179(4):228–233. doi: 10.1097/00005053-199104000-00009. [DOI] [PubMed] [Google Scholar]
- 76.Chaves-Vischer V, Picard F, Andermann E, Dalla Bernardina B, Andermann F. Benign nocturnal alternating hemiplegia of childhood: six patients and long-term follow-up. Neurology. 2001 Oct 23;57(8):1491–1493. doi: 10.1212/wnl.57.8.1491. [DOI] [PubMed] [Google Scholar]
- 77.Roze E, Bounolleau P, Ducreux D, Cochen V, Leu-Semenescu S, Beaugendre Y, Lavallard-Rousseau MC, Blancher A, Bourdain F, Dupont P, Carluer L, Verdure L, Vidailhet M, Apartis E. Propriospinal myoclonus revisited: Clinical, neurophysiologic, and neuroradiologic findings. Neurology. 2009;72(15):1301–1309. doi: 10.1212/WNL.0b013e3181a0fd50. [DOI] [PubMed] [Google Scholar]
- 78.Craske MG, Tsao JC. Assessment and treatment of nocturnal panic attacks. Sleep Med Rev. 2005;9(3):173–184. doi: 10.1016/j.smrv.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 79.Benbadis SR, Lancman ME, King LM, Swanson SJ. Preictal pseudosleep: a new finding in psychogenic seizures. Neurology. 1996;47(1):63–67. doi: 10.1212/wnl.47.1.63. [DOI] [PubMed] [Google Scholar]