Abstract
No therapeutic agent has yet been established as the definitive therapy for adenovirus infections. We describe the clinical experience of 13 immunocompromised patients who received CMX001 (hexadecyloxypropyl cidofovir), an orally bioavailable lipid conjugate of cidofovir, for adenovirus disease. We retrospectively analyzed 13 patients with adenovirus disease and viremia treated with CMX001; data were available for ≥4 weeks after initiation of CMX001 therapy. Virologic response (VR) was defined as a 99% drop from baseline or undetectable adenovirus DNA in serum. The median age of the group was 6 years (range, 0.92-66 years). One patient had severe combined immunodeficiency, 1 patient was a small bowel transplant recipient, and 11 were allogeneic stem cell transplant recipients. Adenovirus disease was diagnosed at a median of 75 days (range, 15-720 days) after transplantation. All patients received i.v. cidofovir for a median of 21 days (range, 5-90 days) before CMX001 therapy. The median absolute lymphocyte count at CMX001 initiation was 300 cells/μL (range, 7-1500 cells/μL). Eight patients (61.5%) had a ≥1 log10 drop in viral load after the first week of therapy. By week 8, 9 patients (69.2%) demonstrated a VR, with a median time to achieve VR of 7 days (range, 3-35 days). The change in absolute lymphocyte count was inversely correlated with the change in log10 viral load only at week 6 (r = −0.74; P = .03). Patients with VR had longer survival than those without VR (median 196 days versus 54.5 days; P = .04). No serious adverse events were attributed to CMX001 during therapy. CMX001 may be a promising therapeutic option for the treatment of severe adenovirus disease in immunocompromised patients.
Keywords: Virologic response, Nephrotoxicity, Therapeutic option, Small bowel transplant, Allogeneic stem cell transplant, Treatment
INTRODUCTION
Adenovirus infections are endemic, causing mainly respiratory and gastrointestinal infections, particularly in the pediatric population and military recruits [1]. Adenovirus can establish latency in lymphocytes after primary infection [2,3]. Either primary infection or reactivation can lead to severe and prolonged invasive disease that is associated with a high rate of mortality in immunocompromised patients [1,4,5].
The incidence of adenovirus infections in hematopoietic stem cell transplantation (HCT) and solid organ transplantation recipients is increasing, likely due to a combination of more potent immunosuppression, improved diagnostic sensitivity (eg, polymerase chain reaction [PCR]), systematic screening, and an increasing pool of high-risk recipients with improved survival [1,6]. Estimates of the incidence of adenovirus infection in HCT recipients range from 5% to 47%, with the highest rates reported in the first 100 days posttransplantation [4,5,7-22]. In solid organ transplantation recipients, rates of adenovirus infection vary by the organ transplanted, ranging from 4% to 10% in pediatric liver transplant recipients [23] to as high as 57% in small bowel transplant recipients [24-26]. Reported mortality rates in HCT recipients are up to 70% [4,5,7-22], whereas in solid organ transplantation recipients, mortality depends on the type of allograft (ranging from 18% in kidney recipients to 53% in liver recipients) [6].
No currently licensed drug has been established as the definitive therapy for adenovirus infections. However, in most transplantation centers, i.v. cidofovir is used to treat adenovirus disease, albeit without supportive data from randomized controlled clinical trials. Cidofovir is a nucleoside phosphonate analogue [27] with significant adverse effects [28]. CMX001 (hexadecyloxypropyl cidofovir), a lipid conjugate of cidofovir, has good oral bioavailability and achieves higher intra-cellular levels of active drug compared with cidofovir [29,30]. In contrast to cidofovir, which is inefficiently taken up by cells, CMX001 resembles natural lipids and uses lipid uptake pathways in enterocyte and target-cell membranes to achieve high intracellular concentrations [29-31]. Inside target cells, the lipid side chain of CMX001 is cleaved to yield free cidofovir that is then converted to the active antiviral agent, cidofovir-diphosphate, by a 2-step phosphorylation process catalyzed by intracellular anabolic kinases [30]. Cidofovir-diphosphate is a potent inhibitor of viral DNA synthesis. A potential advantage of CMX001 may be a better safety profile relative to cidofovir [32,33].
CMX001 is currently in clinical trials. The manufacturer (Chimerix, Inc.) and the U.S. Food and Drug Administration (FDA) have made CMX001 available for compassionate use through an emergency investigational new drug (EIND) mechanism for patients with severe dsDNA viral infections [34]. In this report, we describe the clinical experience with CMX001 in treating immunocompromised patients with adenovirus disease, focusing on clinical efficacy and safety.
METHODS
We retrospectively analyzed the clinical and electronic records of all pediatric and adult patients who were granted FDA EIND approval for CMX001 to treat adenovirus disease between October 2009 and April 2010. All patients were immunocompromised, had life-threatening adenovirus disease, and were refractory or intolerant to standard cidofovir treatment. Informed consent to use CMX001 was obtained from the patients or proxy at each site. Local Institutional Review Boards provided approval in accordance with applicable regulations.
Our goal was to assess viral responses to treatment. We included only patients with adenovirus disease and viremia for whom data on adenovirus viral load (VL) were available for at least 4 weeks after initiation of CMX001, to quantify the response to CMX001 and compare it with the response to cidofovir. Patients with adenovirus disease without posttreatment VL data and patients with end-organ disease without viremia were excluded from our analysis.
Data collected from patients’ medical records included age, sex, type of graft and transplantation, immunocompromised state (conditioning regimen, graft-versus-host disease [GVHD] prophylaxis, induction therapy, maintenance immunosuppression), previous rejection and/or GVHD, sites of adenovirus infection, absolute lymphocyte count (ALC), adenovirus VL, treatment with cidofovir and CMX001, and other viral coinfections. Adverse events documented by local investigators were reviewed, with a special focus on renal and hematologic side effects. Creatinine clearance was calculated using the Schwartz formula for children and the Cockroft-Gault formula for adults.
Definitions
Adenovirus disease was defined as symptoms and signs suggestive of infection with no other attributable cause combined with histopathological documentation of adenovirus (eg, immunohistochemical staining, adenovirus inclusion) and/or adenovirus detection by culture, antigen test, or nucleic acid test from biopsy specimens, bronchoalveolar lavage fluid, or cerebro-spinal fluid [26,35]. Disseminated adenovirus disease was defined as documented disease in 2 or more organs [1]. Virologic response (VR) was defined as achievement of ≥99% decrease in plasma VL from baseline or undetectable VL by the end of treatment or follow-up period. Patients with VR were considered responders, and those who did not achieve VR were considered nonresponders. Common Terminology Criteria for Adverse Events, version 4.0 was used to define the adverse events. Primary outcome measurements were the proportion with VR and a 1 log10 drop in VL from baseline to the corresponding time point or last observation carried forward. Secondary outcomes included safety evaluations, with an emphasis on renal and hematologic outcomes.
Sample Collection and Analysis
After primary diagnosis, serial serum, stool, and other bodily fluids were collected once or twice weekly for adenovirus detection. Quantitative real-time adenovirus PCR was performed locally using center-specific methods to detect common serotypes of human adenovirus. All results were reported as viral copies/mL; all tissue biopsy specimens were routinely sent for histopathological examination and viral identification by immunohistochemical staining, culture, and PCR.
CMX001 Dosing
CMX001 dosing varied in the cohort. The first group of 4 patients received 1 mg/kg/week. Based on pharmacokinetic and virology results, and in the absence of safety concerns, the dose was adjusted to 2 mg/kg/wk, then to 3 mg/kg/week as the cohort progressed. Therapy was adjusted in frequency from weekly to biweekly on a case-by-case basis by local investigators, guided by therapeutic drug levels available from the manufacturing company when available. All patients received the drug orally or through a nasogastric tube while in a fasting state (a minimum of 4 hours predose and 1 hour postdose). No patient received probenecid. The dose was not adjusted in patients with renal dysfunction. Patients undergoing dialysis received the drug after dialysis. Treatment was continued until VR was achieved or to a maximum of 6 months, at which time the study drug was discontinued. Patients were monitored by each center for previously described cidofovir-related toxicities by assessing weekly safety laboratory results; medical records were thoroughly reviewed to ensure detection of all potential side effects. Any side effects considered associated with CMX001 were reported directly to the FDA through MedWatch, as well as to the manufacturer.
Statistical Analysis
VL and ALC were recorded at weeks 0, 1, 2, 4, 6, and 8 after initiation of CMX001; VL was log10-transformed before analysis. Changes in log10 VL and ALC measures were defined as the follow-up measures minus the baseline values. The primary outcomes of the study—VR and log10 change in VL from baseline to week 8—were calculated using a last-observation-carried-forward approach [36]. Distributions of log10 VL and ALC levels at various time points were examined using boxplots, and Wilcoxon’s signed-rank test was used to compare log10 VL, ALC, and creatinine clearance at baseline and specific time points during follow-up. Wilcoxon’s signed rank test was also used to compare change in log10 VL measures under cidofovir treatment and under CMX001 treatment after the same duration of exposure to both drugs. Spearman’s correlation coefficient was used to examine the relationship between the change in log10 VL and the change in ALC at each time point. Continuous variables were compared between responder and nonresponder groups using the nonparametric exact Wilcoxon rank sum test, and categorical variables were compared using the Fisher exact test. Overall survival was defined as the time interval from the initiation of CMX001 to death from any cause or to last follow-up. Kaplan-Meier survival analysis was performed to estimate overall survival, and the exact log-rank test was used to compare overall survival distributions in the responder and nonresponder groups.
RESULTS
Patients
Of the 17 FDA EIND patients who received CMX001, 13 (8 children and 5 adults) were included in this analysis. Three patients were excluded because they died before the first posttreatment VL measurement, and 1 patient was excluded because he did not have viremia. Among the 13 patients included in the analysis, 1 patient had severe combined immunodeficiency, 1 patient had received a small bowel transplant, and 11 patients had undergone HCT. Baseline and posttransplantation demographic data for this cohort are presented in Table 1.
Table 1.
Data for Patients with Disseminated Adenovirus Treated with CMX001 (n = 13)
| Variable | n (%) |
|---|---|
| Age, years, median (range) | 6 (0.92-66) |
| Pediatric (≤18 years), n (%) | 8 (62) |
| Adult (>18 years), n (%) | 5 (38) |
| Male sex, n (%) | 5 (38) |
| Underlying risk factors for adenovirus, n (%) | |
| HCT | |
| AML/ALL | 5 (38) |
| CML/CLL | 2 (15) |
| Aplastic anemia | 2 (15) |
| SCID | 1 (7) |
| Non-Hodgkin lymphoma | 1 (7) |
| Other conditions | |
| Solid organ transplantation* | 1 (7) |
| SCID† | 1 (7) |
| Donor stem cell source, n (%)‡ | |
| Cord blood | 5 (45) |
| Peripheral blood stem cells | 4 (36) |
| Bone marrow | 2 (18) |
| Conditioning regimen, n (%)‡ | |
| Myeloablative | 5 (50) |
| Nonmyeloablative | 5 (50) |
| Receipt of lymphocyte-depleting agents, n (%)§ | |
| Rabbit antithymocyte globulin | 4 (31) |
| Alemtuzumab | 3 (23) |
| GVHD grade, n (%)‡ | |
| None | 3 (27) |
| I-II | 2 (18) |
| III-IV | 5 (45) |
| Unknown | 1 (9) |
AML indicates acute myelogenous leukemia; ALL, acute lymphocytic leukemia; CML, chronic myelogenous leukemia; CLL, chronic lymphocytic leukemia; SCID, severe combined immunodeficiency disorder.
Percentages reported might not equal 100% because of rounding.
Small bowel transplant.
Underwent HCT 7 weeks after initiation of CMX001.
Only for HCT recipients.
Agent received during conditioning or for treatment of GVHD, but all given before development of adenovirus.
In addition to viremia, 6 patients (46%) had disseminated adenovirus disease. For the 12 transplant recipients (small bowel and hematopoietic cell), the disease was diagnosed at a median of 75 days (range, 15-720 days) after transplantation. The gastrointestinal tract was the most common site involved, affecting 7 patients (53.8%), followed by the genitourinary tract (n = 4; 30.8%), respiratory (n = 3; 23.1%), and central nervous system and bone marrow (each n = 1; 7.69%). Ten patients (76.9%) developed other viral infections, including 6 with BK virus, 3 with cytomegalovirus, 2 with respiratory syncytial virus, 2 with Epstein-Barr virus, and 1 each with herpes simplex virus, human herpesvirus-6, and parainfluenza.
All patients had received cidofovir previously and had been switched to CMX001 after a median of 21 days (range, 5-90 days). Median adenovirus log10 VL was 4.72 (range, 2-8.04) at cidofovir initiation and 5.06 (range, 2-7.81) at CMX001 initiation. Median ALC at CMX001 initiation was 300 cells/μL (range, 7-1500 cells/μL), reflecting the severity of immunosuppression in these patients.
Virologic Efficacy
Using matched therapy time (ie, the number of weeks on cidofovir matched with the number of weeks on CMX001), the median change in log10 VL on cidofovir treatment before initiation of CMX001 was 0.08 (range, −2.14 to 4.66) (P = .95 for difference from 0), whereas the median change in log10 VL on CMX001 treatment was −2.71 (range, −5.63 to 1.1) (P = .001). There also was a significant difference in the decrease in median change in log10 VL between cidofovir treatment and CMX001 treatment (−2.21; range, −6.9 to 2.15) (P = .003) (Figure 1).
Figure 1.

Change in adenovirus VL between cidofovir and CMX001 treatment. The median change in log10 VL on cidofovir treatment (before receipt of CMX001) was 0.08 (range, −2.14 to 4.66) (P = .95). The median change in log10 VL for CMX001 treatment was −2.71 (range, −5.63 to 1.1) (P = .001). The median was connected over time.
Nine of the 13 patients (69.2%) achieved a VR at week 8, with a median time to VR of 7 days (range, 3-35 days). At least a 1 log10 drop in VL was seen in 8 patients (61.5%) at week 1, in 9 patients (69.2%) at week 2, and in 10 patients (76.9%) at week 4. A statistically significant decrease in the median log10 VL after initiation of CMX001 therapy was seen at all time points compared with week 0. The median change was −1.78 (range, −2.91 to 0.46) at week 1 (P = .002), −1.77 (range, −4.65 to 0.36) at week 2 (P = .0007), −1.89 (range, −4.65 to 1.42) at week 4 (P = .002), −2.6 (range, −5.81 to 1.34) at week 6 (P = .006), and −3.06 (range, −7.81 to −1.54) at week 8 (P = .004) (Figure 2).
Figure 2.

Change in adenovirus VL (log10) and change in ALC after initiation of CMX001 to week 8 of therapy. The median was connected over time. The median change in log10 VL (upper part of the graph) was −1.78 (range, −2.91 to 0.46) from week 0 to week 1 (P = .002), −1.77 (range, −4.65 to 0.36) from week 0 to week 2 (P = .0007), −1.89 (range, −4.65 to 1.42) from week 0 to week 4 (P = .002), −2.6 (range, −5.81 to 1.34) from week 0 to week 6 (P = .006), and −3.06 (range, −7.81 to −1.54) from week 0 to week 8 (P = .004). The median change in ALC (lower part of the graph) was 40 (range, −100 to 4100) from week 0 to week 1 (P = .01), 100 (range, −100 to 5300) from week 0 to week 2 (P = .02), 300 (range, −300 to 5100) from week 0 to week 4 (P = .059), 164.5 (range, −300 to 4700) from week 0 to week 6 (P = .094), and 577 (range, −300 to 1300) from week 0 to week 8 (P = .094).
As a possible confounder of the virologic efficacy of CMX001, the difference in the median ALC value between week 0 and several time points was assessed as well. The median change was −40 (range, −100 to 4100) at week 1 (P = .01), 100 (range, −100 to 5300) at week 2 (P = .02), 300 (range, −300 to 5100) at week 4 (P = .059), 164.5 (range, −300 to 4700) at week 6 (P = .094), and 577 (range, −300 to 1300) at week 8 (P = .094) (Figure 2). The change in ALC was inversely correlated with the change in log10 VL only at week 6 (r = −0.18, P = .56 at week 1; r = 0.39, P = .19 at week 2; r = −0.24, P = .44 at week 4; r = −0.74, P = .03 at week 6; and r = −0.49, P = .33 at week 8).
Responders versus Nonresponders
There were no statistical differences between the responders and nonresponders with regard to age, sex, GVHD, median time to infection, adenovirus log10 VL at cidofovir or CMX001 initiation, days on cidofovir treatment, total CMX001 exposure, or ALC at CMX001 initiation (Table 2). Only a trend toward differences was seen in median adenovirus log10 VL at week 4 after initiation of CMX001 (P = .07).
Table 2.
Comparison of Variables in Responders and Nonresponders
| Variable | Responders (n = 9) | Nonresponders (n = 4) | P Value |
|---|---|---|---|
| Demographic factors | |||
| Age, years, median (range) | 6 (1.42-43) | 24.7 (0.92-66) | .99 |
| Pediatric, n (%) | 6 (66.66) | 2 (50.0) | .99 |
| GVHD, n (%) | 7 (77.78) | 3 (75) | .99 |
| Acute renal failure before CMX001 initiation, n (%) | 4 (44.4) | 2 (50) | .99 |
| Time to infection after HCT, days, median (range) | 105 (17-720) | 45 (15-161) | .70 |
| Pretreatment factors | |||
| Days on cidofovir before CMX001 initiation, median (range) | 34 (7-90) | 21 (5-21) | .18 |
| Adenovirus, log10 VL at cidofovir initiation, median (range) | 4.57 (2.00-8.04) | 5.5 (4.14-6.19) | .50 |
| Adenovirus, log10 VL at CMX001 initiation, median (range) | 5.06 (2.00-7.81) | 5.1 (4.33-6.65) | .99 |
| ALC at CMX001 initiation, median (range) | 300 (7-1500) | 200 (90-400) | .70 |
| ALC <300 at CMX001 initiation, n (%) | 4 (44.4) | 2 (50) | .99 |
| Response to treatment | |||
| Total CMX001 exposure, days, median (range) | 14 (6-72) | 23.5 (15-34.7) | .70 |
| Adenovirus, log10 VL after 4 weeks of CMX001, median (range) | 2.85 (0-4.77) | 5.29 (2.48-6.78) | .07 |
Survival
There were no deaths during the first 4 weeks of treatment, but 3 patients died during the 8-week follow-up period. The corresponding 8-week survival rate was 76.9% (95% CI, 44.2%-92%). The median overall survival time was 101 days (range, 54-213 days). Only 3 patients (23%) remained alive at the last follow-up; the median follow-up time for the 3 living patients was 274 days (range, 222-278 days). Compared with nonresponders, complete responders had longer survival (median, 196 days versus 54.5 days; P = .04) (Figure 3). The 8-week survival rate after CMX001 treatment was 88.9% (95% CI, 43.3%-98.4%) for responders and 50% (95% CI, 5.78%-84.5%) for nonresponders. Adenovirus pneumonia was the cause of death in 1 patient.
Figure 3.
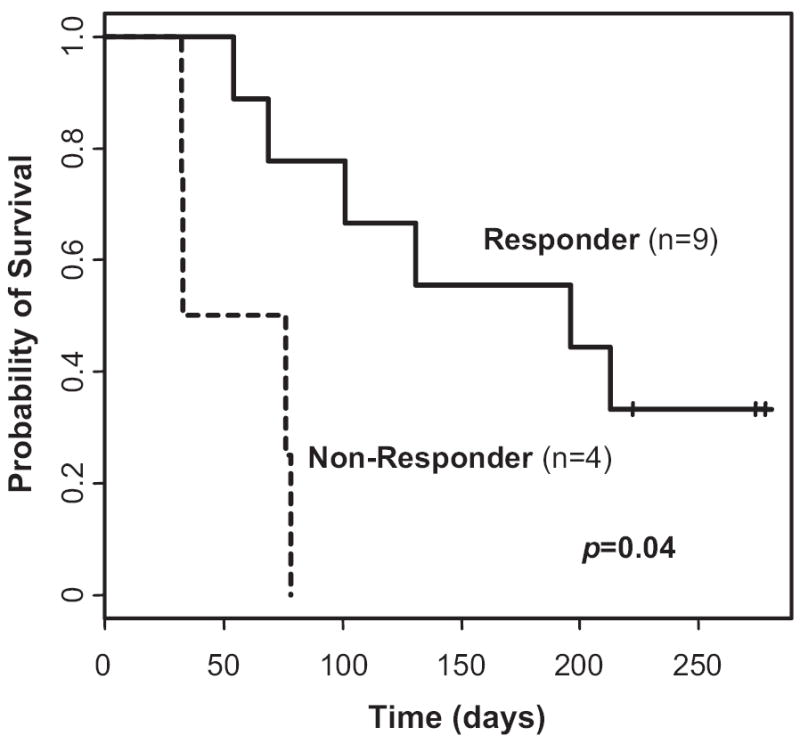
Overall survival after starting therapy for disseminated adenovirus in responders and nonresponders to CMX001 (n = 13). Median survival was 196 days in the complete responders and 54.5 days in the nonresponders (P = .04).
Drug Safety
Five patients (38.46%) were receiving renal replacement therapy at the time of CMX001 initiation; 2 of these 5 patients (40%) recovered renal function by the end of the follow-up period. There was no significant change in the median creatinine clearance between week 0 and week 8 of CMX001 therapy in the children (68.63 mL/min [range, 10-306 mL/min] to 92.03 mL/min [range, 10-287.8 mL/min]; P = .58) or the adults (34 mL/min [range, 14-165.15 mL/min] to 39.22 mL/min [range, 13-122.8 mL/min]; P = .38). No patient discontinued therapy before the end of the study specifically due to CMX001-related side effects. No other serious adverse events (grade 3 and 4 Common Terminology Criteria for Adverse Events, version 4.0), including renal, gastrointestinal, or marrow toxicity, were attributed to CMX001 during the treatment phase or follow-up.
DISCUSSION
This retrospective study examined initial outcomes in a cohort of patients receiving salvage CMX001 for adenoviral disease. All patients were immunocompromised and had failed previous cidofovir therapy. In these patients, the effects of CMX001 on adenovirus replication became evident during the first week of treatment and were sustained throughout the follow-up period. Nearly two-thirds of patients treated with CMX001 had a ≥10-fold drop in VL after just 1 week of therapy, and up to 70% patients by 2 months of treatment. Responders sustained at least a 100-fold drop from baseline or had an undetectable VL at the end of the follow-up period.
Infection occurred preferentially in younger patients in this cohort, corresponding to other studies showing a higher prevalence in pediatric populations [1]. Although the timing of adenovirus infections varied, most infections occurred relatively early after transplantation. This period is associated with the highest degree of immunosuppression and the lowest ALC and is consistent with previously reported epidemiologic studies of adenovirus in transplantation [4,11,26,37,38].
Delayed recovery of T cell immunity has been considered a risk factor for posttransplantation adenovirus infection; conversely, recovery of T cell immunity has been shown to be essential for the clearance of adenovirus infection [39-41]. To assess whether lymphocyte recovery was a confounding factor in the response to CMX001 treatment, we simultaneously measured ALC and adenovirus VL at each time point. The ALC increased significantly in the first 2 weeks after initiation of CMX001, and largely plateaued thereafter. The change in ALC from baseline was inversely correlated with VL only at week 6, and was not believed to significantly confound this uncontrolled estimation of the response to CMX001 therapy over the relatively short follow-up period.
Unlike cidofovir, for which strict monitoring is necessary, with nephrotoxicity seen in up to 50% of patients and neutropenia in up to 20% [27,42-44], CMX001 was well tolerated. Although assessing toxicity is difficult in retrospective studies, especially in high-risk patients with baseline abnormalities and baseline risk factors for myelosuppression, attributable toxicities were not reported during the follow-up period. Consistent with previously reported data [33], renal function improved or remained unchanged during CMX001 therapy, suggesting a lack of nephrotoxicity. Although encouraging, these safety data must be interpreted with caution.
In previous studies, disseminated adenovirus disease in immunocompromised patients has been associated with up to 80% mortality [11,26,37,38,45]. The high mortality in our medically complex patient population was influenced by the presence of other pathogens, GVHD, and progression of the underlying disease. Survival was improved in the patients with VR compared with those who failed to respond to CMX001. Based on these data, the virologic efficacy of CMX001 was approximately 70%. These nonrandomized results must be interpreted with caution, Prospective comparative trials are needed to estimate the true benefit of this drug.
This study was limited by its retrospective nature and the heterogeneity of the study population. We specifically limited our analysis to patients with virologic data sufficient for comparative analysis. By selecting a population that had survived for at least 4 weeks after starting the drug, we likely biased our population to one more likely to have a better outcome. It is important to put this in context, however, particularly because these patients were extremely high-risk, having failed primary cidofovir therapy and being burdened with multiple comorbidities. Because of our small sample size, statistical effects must be interpreted with caution. In particular, the survival of our patients might have been affected by variances in treatment, severity of primary disease, CMX001 dosage, and underlying disease. Although this is the largest multicenter study to date evaluating the effectiveness of this new agent administered through the FDA EIND process, the small number of patients, center-specific treatment variations, and dosing differences between patients limit broader conclusions. We also appreciate that, as with any oral drug, absorption could be a factor affecting response. Unfortunately, differences in drug absorption could not be assessed in this study, because the sampling was not sufficient to specifically characterize absorption patterns. Finally, although there were no major complications from therapy, we had a limited ability to examine the potential side effects or drug toxicities associated with CMX001.
CONCLUSIONS
This is the first multicenter cohort to provide clinical data on the safety and efficacy of CMX001 as salvage therapy for highly immunocompromised patients with adenovirus disease. Although the number of subjects in this cohort was small, the sustained VR in patients who had failed cidofovir treatment suggests that CMX001 may be an effective treatment for severe adenovirus disease. Adenovirus can be a major cause of morbidity and mortality in immunocompromised patients, and a safe and effective antiviral agent would be an important therapeutic advance in the field of transplantation. Prospective trials of CMX001 as treatment for adenovirus disease in immunocompromised patients are warranted.
Acknowledgments
We thank Ashley Calhoon for preparing the manuscript. We also thank our colleagues for helping to manage these complex patients: A. C. Kalil, MD, University of Nebraska Medical Center; S. Johnson, University of Washington; Vinod Prasad, MD, Duke University Medical Center; J. Serody, D. Gabriel, K. V. Rao, UNC Hospitals; and Jill Hoffman, Children’s Hospital Los Angeles.
Footnotes
Financial disclosure: D.F. Florescu: support from Chimerix for travel to collect data for the manuscript; grant from Chimerix for study CMX001-350. S.A. Pergam: consultancy for ViroPharma; grants from ViroPharma, Seattle Genetics, Merck Sharpe & Dohme. M.N. Neely: advisory board Chimerix. F. Qiu: no conflict of interest. C. Johnston: grants from Aicuris GmB & Co. KG and GlaxoSmithKline. S.S. Way: no conflict of interest. J. Sande: no conflict of interest. D.A. Lewinsohn: grant from Chimerix. J.A. Guzman-Cottrill: grant from Chimerix. M.L. Graham: no conflict of interest. G. Papanicolaou: advisory board honorarium from Chimerix. J. Kurtzberg: FACT board member; consultancy: Stemcyte - Scientific Advisor, CORD:USE – Medical Director, NMDP - Medical Advisor; grant from Chimerix. J. Rigdon: no conflict of interest W. Painter: Chimerix employee and stocks. H. Mommeja-Marin: Chimerix employee and stocks. R. Lanier: Chimerix employee and stocks. M. Anderson: Chimerix employee and stocks. C. van der Horst: grants from NIAID (P30-AI-50410), GlaxsoSmithKline.
References
- 1.Echavarria M. Adenoviruses in immunocompromised hosts. Clin Microbiol Rev. 2008;21:704–715. doi: 10.1128/CMR.00052-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tang Q, Lundholm-Beauchamp U, Ginsberg HS. Spontaneous occurrence of early region 1A reiteration mutants of type 5 adenovirus in persistently infected human T-lymphocytes. Virology. 1997;230:281–291. doi: 10.1006/viro.1997.8482. [DOI] [PubMed] [Google Scholar]
- 3.Garnett CT, Erdman D, Xu W, et al. Prevalence and quantitation of species C adenovirus DNA in human mucosal lymphocytes. J Virol. 2002;76:10608–10616. doi: 10.1128/JVI.76.21.10608-10616.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hale GA, Heslop HE, Krance RA, et al. Adenovirus infection after pediatric bone marrow transplantation. Bone Marrow Transplant. 1999;23:277–282. doi: 10.1038/sj.bmt.1701563. [DOI] [PubMed] [Google Scholar]
- 5.La Rosa AM, Champlin RE, Mirza N, et al. Adenovirus infections in adult recipients of blood and marrow transplants. Clin Infect Dis. 2001;32:871–876. doi: 10.1086/319352. [DOI] [PubMed] [Google Scholar]
- 6.Kojaoghlanian T, Flomenberg P, Horwitz MS. The impact of adenovirus infection on the immunocompromised host. Rev Med Virol. 2003;13:155–171. doi: 10.1002/rmv.386. [DOI] [PubMed] [Google Scholar]
- 7.van Tol MJ, Claas EC, Heemskerk B, et al. Adenovirus infection in children after allogeneic stem cell transplantation: diagnosis, treatment and immunity. Bone Marrow Transplant. 2005;35(Suppl 1):S73–S76. doi: 10.1038/sj.bmt.1704852. [DOI] [PubMed] [Google Scholar]
- 8.Shields AF, Hackman RC, Fife KH, et al. Adenovirus infections in patients undergoing bone-marrow transplantation. N Engl J Med. 1985;312:529–533. doi: 10.1056/NEJM198502283120901. [DOI] [PubMed] [Google Scholar]
- 9.Wasserman R, August CS, Plotkin SA. Viral infections in pediatric bone marrow transplant patients. Pediatr Infect Dis J. 1988;7:109–115. doi: 10.1097/00006454-198802000-00009. [DOI] [PubMed] [Google Scholar]
- 10.Ljungman P, Gleaves CA, Meyers JD. Respiratory virus infection in immunocompromised patients. Bone Marrow Transplant. 1989;4:35–40. [PubMed] [Google Scholar]
- 11.Flomenberg P, Babbitt J, Drobyski WR, et al. Increasing incidence of adenovirus disease in bone marrow transplant recipients. J Infect Dis. 1994;169:775–781. doi: 10.1093/infdis/169.4.775. [DOI] [PubMed] [Google Scholar]
- 12.Blanke C, Clark C, Broun ER, et al. Evolving pathogens in allogeneic bone marrow transplantation: increased fatal adenoviral infections. Am J Med. 1995;99:326–328. doi: 10.1016/s0002-9343(99)80169-7. [DOI] [PubMed] [Google Scholar]
- 13.Howard DS, Phillips GL, II, Reece DE, et al. Adenovirus infections in hematopoietic stem cell transplant recipients. Clin Infect Dis. 1999;29:1494–1501. doi: 10.1086/313514. [DOI] [PubMed] [Google Scholar]
- 14.Venard V, Carret A, Corsaro D, et al. Genotyping of adenoviruses isolated in an outbreak in a bone marrow transplant unit shows that diverse strains are involved. J Hosp Infect. 2000;44:71–74. doi: 10.1053/jhin.1999.0656. [DOI] [PubMed] [Google Scholar]
- 15.Hoffman JA, Shah AJ, Ross LA, et al. Adenoviral infections and a prospective trial of cidofovir in pediatric hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2001;7:388–394. doi: 10.1053/bbmt.2001.v7.pm11529489. [DOI] [PubMed] [Google Scholar]
- 16.Bordigoni P, Carret AS, Venard V, et al. Treatment of adenovirus infections in patients undergoing allogeneic hematopoietic stem cell transplantation. Clin Infect Dis. 2001;32:1290–1297. doi: 10.1086/319984. [DOI] [PubMed] [Google Scholar]
- 17.Echavarria M, Forman M, van Tol MJ, et al. Prediction of severe disseminated adenovirus infection by serum PCR. Lancet. 2001;358:384–385. doi: 10.1016/S0140-6736(01)05580-5. [DOI] [PubMed] [Google Scholar]
- 18.Leruez-Ville M, Minard V, Lacaille F, et al. Real-time blood plasma polymerase chain reaction for management of disseminated adenovirus infection. Clin Infect Dis. 2004;38:45–52. doi: 10.1086/380450. [DOI] [PubMed] [Google Scholar]
- 19.Kampmann B, Cubitt D, Walls T, et al. Improved outcome for children with disseminated adenoviral infection following allogeneic stem cell transplantation. Br J Haematol. 2005;130:595–603. doi: 10.1111/j.1365-2141.2005.05649.x. [DOI] [PubMed] [Google Scholar]
- 20.Yusuf U, Hale GA, Carr J, et al. Cidofovir for the treatment of adenoviral infection in pediatric hematopoietic stem cell transplant patients. Transplantation. 2006;81:1398–1404. doi: 10.1097/01.tp.0000209195.95115.8e. [DOI] [PubMed] [Google Scholar]
- 21.Kroes AC, de Klerk EP, Lankester AC, et al. Sequential emergence of multiple adenovirus serotypes after pediatric stem cell transplantation. J Clin Virol. 2007;38:341–347. doi: 10.1016/j.jcv.2007.01.001. [DOI] [PubMed] [Google Scholar]
- 22.Kalpoe JS, van der Heiden PL, Barge RM, et al. Assessment of disseminated adenovirus infections using quantitative plasma PCR in adult allogeneic stem cell transplant recipients receiving reduced intensity or myeloablative conditioning. Eur J Haematol. 2007;78:314–321. doi: 10.1111/j.1600-0609.2007.00821.x. [DOI] [PubMed] [Google Scholar]
- 23.Humar A. Reactivation of viruses in solid organ transplant patients receiving cytomegalovirus prophylaxis. Transplantation. 2006;82:S9–S14. doi: 10.1097/01.tp.0000230432.39447.8b. [DOI] [PubMed] [Google Scholar]
- 24.Pinchoff RJ, Kaufman SS, Magid MS, et al. Adenovirus infection in pediatric small bowel transplantation recipients. Transplantation. 2003;76:183–189. doi: 10.1097/01.TP.0000072808.93060.0F. [DOI] [PubMed] [Google Scholar]
- 25.McLaughlin GE, Delis S, Kashimawo L, et al. Adenovirus infection in pediatric liver and intestinal transplant recipients: utility of DNA detection by PCR. Am J Transplant. 2003;3:224–228. doi: 10.1034/j.1600-6143.2003.00007.x. [DOI] [PubMed] [Google Scholar]
- 26.Florescu DF, Islam MK, Mercer DF, et al. Adenovirus infections in pediatric small bowel transplant recipients. Transplantation. 2010;90:198–204. doi: 10.1097/TP.0b013e3181e0de97. [DOI] [PubMed] [Google Scholar]
- 27.Jacobson MA. Treatment of cytomegalovirus retinitis in patients with the acquired immunodeficiency syndrome. N Engl J Med. 1997;337:105–114. doi: 10.1056/NEJM199707103370207. [DOI] [PubMed] [Google Scholar]
- 28.Gilead Sciences. [February 2, 2011];VISTIDE (cidofovir injection) Available from: http://www.gilead.com/pdf/vistide.pdf.
- 29.Ciesla SL, Trahan J, Wan WB, et al. Esterification of cidofovir with alkoxyalkanols increases oral bioavailability and diminishes drug accumulation in kidney. Antiviral Res. 2003;59:163–171. doi: 10.1016/s0166-3542(03)00110-4. [DOI] [PubMed] [Google Scholar]
- 30.Aldern KA, Ciesla SL, Winegarden KL, et al. Increased antiviral activity of 1-O-hexadecyloxypropyl-[2-(14)C]cidofovir in MRC-5 human lung fibroblasts is explained by unique cellular uptake and metabolism. Mol Pharmacol. 2003;63:678–681. doi: 10.1124/mol.63.3.678. [DOI] [PubMed] [Google Scholar]
- 31.Painter GR, Hostetler KY. Design and development of oral drugs for the prophylaxis and treatment of smallpox infection. Trends Biotechnol. 2004;22:423–427. doi: 10.1016/j.tibtech.2004.06.008. [DOI] [PubMed] [Google Scholar]
- 32.Hostetler KY. Alkoxyalkyl prodrugs of acyclic nucleoside phosphonates enhance oral antiviral activity and reduce toxicity: current state of the art. Antiviral Res. 2009;82:A84–98. doi: 10.1016/j.antiviral.2009.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Trost L, Tippin TK, Anderson MT, et al. Compromised renal function does not affect the pharmacokinetics of CMX001 in patients with severe double-stranded DNA virus infections. 50th ICAAC; Boston, MA. September 12-15, 2010; A1-2017a. [Google Scholar]
- 34.US Food and Drug Administration. [February 2, 2011];Emergency investigational new drug (EIND) applications for antimicrobial products. Available from: http://www.fda.gov/Drugs/DevelopmentApprovalProcess/HowDrugsareDevelopedandApproved/ApprovalApplications/InvestigationalNewDrugINDApplication/ucm090039.htm.
- 35.Ljungman P, Ribaud P, Eyrich M, et al. Cidofovir for adenovirus infections after allogeneic hematopoietic stem cell transplantation: a survey by the Infectious Diseases Working Party of the European Group for Blood and Marrow Transplantation. Bone Marrow Transplant. 2003;31:481–486. doi: 10.1038/sj.bmt.1703798. [DOI] [PubMed] [Google Scholar]
- 36.Unnebrink K, Windeler J. Intention-to-treat: methods for dealing with missing values in clinical trials of progressively deteriorating diseases. Stat Med. 2001;20:3931–3946. doi: 10.1002/sim.1149. [DOI] [PubMed] [Google Scholar]
- 37.Baldwin A, Kingman H, Darville M, et al. Outcome and clinical course of 100 patients with adenovirus infection following bone marrow transplantation. Bone Marrow Transplant. 2000;26:1333–1338. doi: 10.1038/sj.bmt.1702716. [DOI] [PubMed] [Google Scholar]
- 38.Lion T, Baumgartinger R, Watzinger F, et al. Molecular monitoring of adenovirus in peripheral blood after allogeneic bone marrow transplantation permits early diagnosis of disseminated disease. Blood. 2003;102:1114–1120. doi: 10.1182/blood-2002-07-2152. [DOI] [PubMed] [Google Scholar]
- 39.Chakrabarti S, Mautner V, Osman H, et al. Adenovirus infections following allogeneic stem cell transplantation: incidence and outcome in relation to graft manipulation, immunosuppression, and immune recovery. Blood. 2002;100:1619–1627. doi: 10.1182/blood-2002-02-0377. [DOI] [PubMed] [Google Scholar]
- 40.Feuchtinger T, Lucke J, Hamprecht K, et al. Detection of adenovirus-specific T cells in children with adenovirus infection after allogeneic stem cell transplantation. Br J Haematol. 2005;128:503–509. doi: 10.1111/j.1365-2141.2004.05331.x. [DOI] [PubMed] [Google Scholar]
- 41.Feuchtinger T, Richard C, Pfeiffer M, et al. Adenoviral infections after transplantation of positive selected stem cells from haploidentical donors in children: an update. Clin Pediatr. 2006;217:339–344. doi: 10.1055/s-2005-872530. [DOI] [PubMed] [Google Scholar]
- 42.Ljungman P. Treatment of adenovirus infections in the immunocompromised host. Eur J Clin Microbiol Infect Dis. 2004;23:583–588. doi: 10.1007/s10096-004-1165-x. [DOI] [PubMed] [Google Scholar]
- 43.Nagafuji K, Aoki K, Henzan H, et al. Cidofovir for treating adenoviral hemorrhagic cystitis in hematopoietic stem cell transplant recipients. Bone Marrow Transplant. 2004;34:909–914. doi: 10.1038/sj.bmt.1704682. [DOI] [PubMed] [Google Scholar]
- 44.VISTIDE prescribing information. Foster City, CA: Gilead Sciences; 2002. [Google Scholar]
- 45.Symeonidis N, Jakubowski A, Pierre-Louis S, et al. Invasive adenoviral infections in T cell–depleted allogeneic hematopoietic stem cell transplantation: high mortality in the era of cidofovir. Transpl Infect Dis. 2007;9:108–113. doi: 10.1111/j.1399-3062.2006.00184.x. [DOI] [PubMed] [Google Scholar]


