Abstract
Objective. To assess the efficacy and safety of Chinese medicinal herbs for Childhood Pneumonia. Methods. We included randomized controlled trials (RCTs). The searched electronic databases included PubMed, the Cochrane Central Register of Controlled Trials, EMBASE, CBM, CNKI, and VIP. All studies included were assessed for quality and risk bias. Review Manager 5.1.6 software was used for data analyses, and the GRADEprofiler software was applied to classify the systematic review results. Results. Fourteen studies were identified (n = 1.824). Chinese herbs may increase total effective rate (risk ratio (RR) 1.18; 95% confidence interval (CI), 1.11–1.26) and improve cough (total mean difference (MD), −2.18; 95% CI, (−2.66)–(−1.71)), fever (total MD, −1.85; 95% CI, (−2.29)–(−1.40)), rales (total MD, −1.53; 95% CI, (−1.84)–(−1.23)), and chest films (total MD, −3.10; 95% CI, (−4.11)–(−2.08)) in Childhood Pneumonia. Chinese herbs may shorten the length of hospital stay (total MD, −3.00; 95% CI, (−3.52)–(−2.48)), but no significant difference for adverse effects (RR, 0.39; 95% CI, 0.09–1.72) was identified. Conclusion. Chinese herbs may increase total effective rate and improve symptoms and signs. However, large, properly randomized, placebo-controlled, double-blind studies are required.
1. Introduction
Childhood Pneumonia is an acute virus, bacterial, or fungal respiratory infection that affects the lungs [1]. The symptoms and signs of Childhood Pneumonia include cough, fever, rapid or difficult breathing, loss of appetite, and lower chest wall indrawing [2]. Auscultation reveals rales. However, severe Childhood Pneumonia is defined as cough or difficult breathing combined with lower chest wall indrawing [3]. Childhood Pneumonia has been identified as a mixed viral-bacterial infection in 23%–33% of cases [4]. Respiratory syncytial virus is the most common viral cause of children pneumonia, and Streptococcus pneumoniae is the most common cause of bacterial pneumonia in children [5]. Each year, pneumonia kills an estimated 1.4 million children <5 years of age, accounting for 18% of all deaths worldwide [6]. Pneumonia is the most common reason for hospitalization in children <2 years of age [7]. The cost of antibiotic treatment for all children with pneumonia in 42 of the poorest countries is estimated to be about US $600 million per year. The estimated incidence rates are 0.29 and 0.05 episodes per child-year in low-income and high-income countries, respectively. Approximately 156 million new episodes occur each year, the majority in India (43 million), China (21 million), Pakistan (10 million), Bangladesh, Indonesia, and Nigeria (6 million each) [8].
Administering appropriate antibiotics at the early stage of pneumonia improves outcomes, particularly when the causative agent is bacterial [9]. However, antibiotic treatment of pneumonia in children remains mostly empirical because determining the etiologic pathogen is difficult in this age group [10]. According to the results of a questionnaire, the following agents have been used against Childhood Pneumonia: ampicillin, ampicillin/sulbactam, second/third generation cephalosporins, azithromycin, vancomycin, clindamycin, and linezolid. In subsequent analyses, we categorized ampicillin, ampicillin/sulbactam, and cephalosporins as beta-lactam antibiotics and vancomycin, clindamycin, and linezolid as antimethicillin-resistant Staphylococcus aureus antibiotics. Respondents were asked to select the duration of antibiotic therapy they would recommend for uncomplicated and parapneumonic empyema cases using the following categories: 3–5 days, 6-7 days, 8–10 days, 11–14 days, 15–21 days, and >21 days [11]. Empirical antibiotic administration is relied upon in most instances to meet the public health goal of reducing child mortality due to pneumonia. This is necessary in view of the inability of most commonly available laboratory tests to identify causative pathogens. Empirical antibiotic administration is the main treatment for Childhood Pneumonia. Multiple antibiotics are prescribed for treating pneumonia, so it is important to know which work best for pneumonia in children [12].
Traditional Chinese Medicine (TCM) follows a particular theoretical and methodological approach to estimate the cause of a disease, leading to diagnosis and treatment [13]. Pneumonia is equivalent to the TCM cough category. Ma Xing Shi Gan Tang, San Ao Tang, Zhi Sou San, and other self-developed TCM prescriptions are Chinese medicinal formulas that have been used to treat Childhood Pneumonia for many years. In recent years, preparations of Chinese herbal medicines, such as Tanreqing injection, Chuanhuning injection, and Reduning injection have been used to treat Childhood Pneumonia in China. Chinese herbal medicine formulas function to clear heat, resolve phlegm, ventilate the lungs, dissipate phlegm, relieve cough, and reduce sputum. The function of Chinese herbal medicine preparations is to clear heat and remove toxicity. A study showed that the pharmacological action of Ma Xing Shi Gan Tang includes antiasthmatic, antitussive, and antiviral effects as well as bacteriostatic and immunoregulatory functions [14].
Although these formulae and other Chinese herbal medicine preparations have been used widely to treat Childhood Pneumonia in China, their effects and safety have not been reviewed systematically.
2. Methods
2.1. Criteria for Considering Studies for this Paper
2.1.1. Types of Studies
Only randomized controlled trials (RCTs) were included.
2.1.2. Types of Participants
Studies that enrolled patients with pneumonia in children who had cough, fever >37.5°C, raised respiratory rate, lower chest wall indrawing, rales, and changes on chest films were included. Patients with Childhood Pneumonia of either gender, any ethnic group, and ages of 1 month to 18 years were included. Studies were excluded if they included children suffering from other debilitating diseases.
2.1.3. Types of Interventions
There were Chinese medicinal herbs versus other drugs, formulas, and placebo alone; Chinese medicinal herbs plus basic therapy versus basic therapy. Antibiotics were one of the main basic therapies for Childhood Pneumonia. Prohibited or suspended Chinese herbal preparations were excluded.
2.1.4. Types of Outcome Measures
Primary outcomes included mortality and total effective rate (e.g., ratio of signs and symptoms improvement or recovery); secondary outcomes included time to clinical recovery (e.g., cough, fever, rales, and chest films), relapse rate, length of hospital stay, and adverse effects (e.g., nausea, diarrhea, vomiting, and gastrointestinal bleeding). TCM outcomes such as tongue coat, pulse condition, and economic index were included.
2.2. Search Methods for Identification Studies
We searched for all relevant studies in the following electronic databases: PubMed (1966–July 2012), the Cochrane Central Register of Controlled Trials, EMBASE (1980–July 2012), the Chinese Biomedicine Database (CBM) (1976–July 2012), Chinese National Knowledge Internet (CNKI) (1979–July 2012), and Chinese Biomedical Journals (VIP) (1989–July 2012). All studies included were analyzed according to Cochrane Handbook criteria. The following search terms were used: (Chinese herbs OR Chinese traditional herbs OR Chinese medicinal herbs OR traditional Chinese herbs OR Chinese herbal medicines) AND (child pneumonia OR children pneumonia OR Childhood Pneumonia OR Pediatric Pneumonia OR Infantile Pneumonia). We conducted a manual search for the Journal of Guangzhou University of Traditional Chinese Medicine. We attempted to contact original authors to obtain the protocol for the studies.
2.3. Data Collection and Analysis
2.3.1. Study Selection
Two review authors independently browsed the titles and abstracts of all articles identified by the literature search. The same review authors independently estimated whether the trials met the inclusion criteria. Disagreements were resolved by discussion or consultation with a third author. We assessed abstracts from the initial search independently to identify studies that met the inclusion criteria. We telephone-interviewed authors of Chinese language articles and emailed the original authors of English articles to identify the randomization procedure and other methodological questions to ensure that the included studies were RCTs. If the required information was not available or if the required information did not meet the inclusion criteria, the article was excluded.
2.3.2. Data Extraction and Management
We extracted data including methodological details and data from publications using a data extraction form. We extracted data on study characteristics, including methods, participants, interventions, and outcomes. There were no disagreements among the authors.
We extracted the formulation contents of the included studies, and the names of the herbs are provided in three languages (e.g., Chinese, Latin, and English) in Table 1.
Table 1.
Contents of the formulations used and the three languages are included in the included studies.
| Study ID | Herbs (composition) in three languages | Method of administration |
|---|---|---|
| Wang et al., 2009 [15] | Modified Ma Xing Shi Gan Tang: Mahuang (Herba Ephedrae/Ephedra Herb), Xingren (Armeniacae Amarum/Bitter Apricot Seed), Shigao (Gypsum Fibrosum/Gypsum), Gancao (Radix Glycyrrhizae/LiquoriceRoot), Yuxingcao (Herba Houttuyniae/Heartleaf Houttuynia Herb), lianqiao (Fructus Forsythiae/WeepingForsythiaecapsule), Chanyi (Periostracum Cicadae/Cicada Slough), and Niupangzi (Fructus Arctii/Great Burdock Achene) | Oral administration |
|
| ||
| Zhao and Ji, 2009 [16] | Modified Ma Xing Shi Gan Tang: Mahuang (Herba Ephedrae/Ephedra Herb), Xingren (Armeniacae Amarum/Bitter Apricot Seed), Shigao (Gypsum Fibrosum/Gypsum), Gancao (Radix Glycyrrhizae/Liquorice Root), Yuxingcao (Herba Houttuyniae/Heartleaf Houttuynia Herb), and Huangqin (Radix Astragali Root) | Oral administration |
|
| ||
| Guo, 1999 [17] | Modified Ma Xing Shi Gan Tang: Mahuang (Herba Ephedrae/Ephedra Herb), Xingren (Armeniacae Amarum/Bitter Apricot Seed), Shigao (Gypsum Fibrosum/Gypsum), Gancao (Radix Glycyrrhizae/Liquorice Root), Yuxingcao (Herba Houttuyniae/Heartleaf Houttuynia Herb), and lianqiao (Fructus Forsythiae/WeepingForsythiaeCapsule) | Oral administration |
|
| ||
| Zhang, 2012 [18] | Modified Ma Xing Shi Gan Tang: Mahuang (Herba Ephedrae/Ephedra Herb), Xingren (Armeniacae Amarum/Bitter Apricot Seed), Shigao (Gypsum Fibrosum/Gypsum), Gancao (Radix Glycyrrhizae/Liquorice Root), Yuxingcao (Herba Houttuyniae/Heartleaf Houttuynia Herb), and Huangqin (Radix Astragali Root) | Oral administration |
|
| ||
| He, 2011 [19] | Modified Ma Xing Shi Gan Tang: Mahuang (Herba Ephedrae/Ephedra Herb), Xingren (Armeniacae Amarum/Bitter Apricot Seed), Jinhua (Flos Lonicerae/Honeysuckle Flower), Yinhua (Flos Lonicerae/Honeysuckle Flower), Shigao (Gypsum Fibrosum/Gypsum), Yuxingcao (Herba Houttuyniae/Heartleaf Houttuynia Herb), Banxia (Rhizome/Pinellia Tuberifera Tenora), and Gancao (Radix Glycyrrhizae/Liquorice Root) | Oral administration |
|
| ||
| He et al., 2011 [20] | Modified Ma Xing Shi Gan Tang: Mahuang (Herba Ephedrae/Ephedra Herb), Xingren (Armeniacae Amarum/Bitter Apricot Seed), Shigao (Gypsum Fibrosum/Gypsum), Suzi (Fructus Perillae/PerillaFruit), Shangbaipi (Cortex Mori/White Mulberry Root Bark), Kuandonghua (Flos Farfarae/Common Coltsfoot Flower), Banxia (Rhizome/Pinellia Tuberifera Tenora), Tinglizi (Semen Lepidii/SemenDescurainiae Pepperweed Seed/Tansymustard), Yuxingcao (Herba Houttuyniae/Heartleaf Houttuynia Herb), and Gancao (Radix Glycyrrhizae/Liquorice Root) | Oral administration |
|
| ||
| Zhang, 2012 [21] | San Ao Tang: Mahuang (Herba Ephedrae/Ephedra Herb), Xingren (Armeniacae Amarum/Bitter Apricot Seed), and Gancao (Radix Glycyrrhizae/Liquorice Root) | Oral administration |
|
| ||
| Wang et al., 2009 [22] | Modified Zhi Sou San: Jiegeng (Radix Platycodi/Platycodon Root), Gancao (Radix Glycyrrhizae/Liquorice Root), Ziwan (Radix Asteris/Tatarian Aster Root), Chenpi (Pericarpium Citri Reticulatae/Tangerine Peel), Xingren (Armeniacae Amarum/Bitter Apricot Seed), Baiguo (Semen Gingko/Ginkgo Seed), Huangqi (Radix Astragali Root), Chaomaiya (Fructus Hordei Germina/Malt), Yunling (Poria/Indian Buead), and Baiqian (Rhizoma Cynanchi Stauntonii/Willowleaf Swallowwort Rhizome/Glaucescent) | Oral administration |
|
| ||
| Lv et al., 2009 [23] | Self-Developed TCM Prescription: Mahuang (Herba Ephedrae/Ephedra Herb), Xingren (Armeniacae Amarum/Bitter Apricot Seed), Rengongniuhuang (Calculus Bovis/Bezoar), Baiqian (Rhizoma Cynanchi Stauntonii/Willowleaf Swallowwort Rhizome/Glaucescent), Shigao (Gypsum Fibrosum/Gypsum), Zhusha (Cinnabaris/Cinnabar), Chuanbeimu (Bulbus Fritillariae Unibracteatae/Unibract Fritillary Bulb), Huanglian (Rhizoma Coptidis/Golden Thread), Banxia (Rhizome/Pinellia Tuberifera Tenora), Dannanxing (Pinellia Pedatisecta/Arisaema with Bile), Shangbaipi (Cortex Mori/White Mulberry Root Bark), Huangqin (Radix Astragali Root), and Gancao (Radix Glycyrrhizae/Liquorice Root) | Oral administration |
|
| ||
| Lei, 2010 [24] | Tanreqing injection: no information provided about Tanreqing composition | Intravenous injection |
|
| ||
| Shi, 2009 [25] | Reduning injection: no information provided about Reduning composition | Intravenous injection |
|
| ||
| Pan, 2011 [26] | Reduning injection: no information provided about Reduning composition | Intravenous injection |
|
| ||
| Duan and Feng, 2011 [27] | Reduning injection: no information provided about Reduning composition | Intravenous injection |
|
| ||
| Wei and Feng, 2003 [28] | Chuanhuning injection: no information provided about Chuanhuning composition | Intravenous injection |
2.3.3. Assessment of Risk of Bias in Included Studies
The following items were independently assessed by our authors using the risk of bias assessment tool. (1) Was there adequate sequence generation (selection bias)? (2) Was allocation adequately concealed (selection bias)? (3) Was knowledge of the allocated interventions adequately prevented during the study (e.g., participants and personnel, outcome assessors) (detection bias)? (4) Were incomplete outcome data adequately addressed (attrition bias)? (5) Are reports of the study free of suggesting selective outcome reporting (reporting bias)? (6) Was the study apparently free of other problems that could put it at risk for bias?
2.3.4. Measures of Treatment Effect
Data analyses were performed using the Cochrane Collaboration's RevMan software, version 5.1.6. Results are expressed as risk ratios (RR) and 95% confidence intervals (CIs) for dichotomous outcomes (e.g., mortality, effective rate, adverse effects, and relapse rate) and as mean differences (MD) with 95% CIs for continuous outcomes (such as time to clinical recovery and length of hospital stay).
2.3.5. Assessment of Heterogeneity
Heterogeneity was analyzed using the chi-square test on n − 1 degrees of freedom, and an alpha of 0.05 was used for statistical significance with the I 2 test. I 2 values of 25, 50, and 75% corresponded to low, medium, and high levels of heterogeneity, respectively.
2.3.6. Data Synthesis
We used fixed-effects and random-effects models for the pooled data analysis. We performed a pooled analysis for the 14 studies.
2.3.7. Subgroup Analyses
Subgroup analyses were performed according to formula type and Chinese medicinal herb preparation type, using the same comparators (e.g., same types of antibiotics).
2.3.8. Sensitivity Analyses
Sensitivity analyses were conducted by excluding low-quality studies (based on descriptions of randomization, allocation concealment, blinded assessment of outcomes, and description/analyses of withdrawals and dropouts) and a comparison of the merger analysis results for the fixed- and random-effects models.
3. Results
3.1. Search Results
An initial search identified 2,502 potentially relevant articles. Of these, 15 were in the English database. A total of 891 articles were initially included after duplicate publications were removed and any obviously irrelevant were excluded; 800 articles were later excluded, because they did not meet the inclusion criteria. Of the 91 potentially eligible reports, 77 were excluded for further assessment because telephone interviews with the original authors revealed that they were not RCTs. Therefore, 14 studies (1,824 participants) were included in this paper. All 14 studies were published in Chinese (Figure 1).
Figure 1.
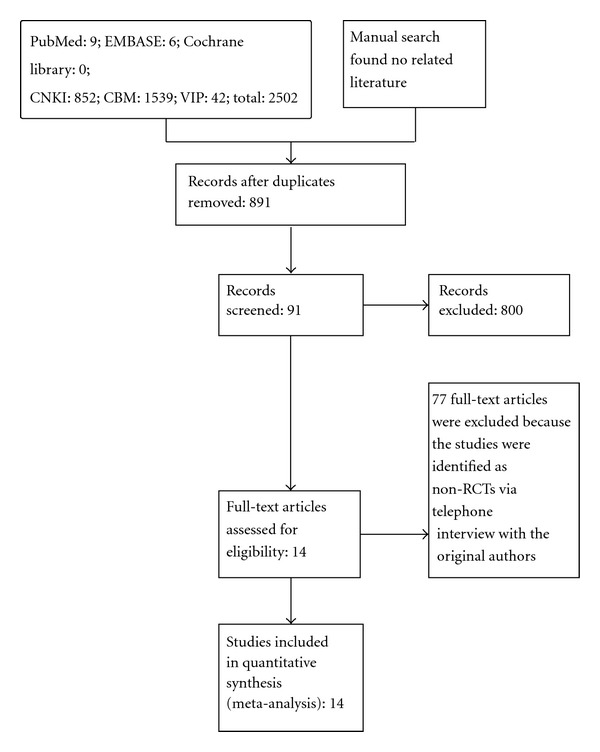
Summary of the search results in a flow diagram.
3.2. Included Studies
3.2.1. Participants
The ratio of male to female participants in the 14 studies was 879/615 [15–21, 23–28]. One study [22] did not report the number of males and females. In total, 1,824 children were included in the 14 studies, and all were from China [15–28]. The ages of the patients were 1 month–15 years. The average size of the trials was 130 participants (range 60–200 participants).
3.2.2. Inclusion Criteria
The diagnostic criteria for childhood pneumonia in all studies included fever >37.5°C, chest recession, increased respiratory rate, cough, rales, or difficulty breathing combined with fast breathing and a change on chest films.
3.2.3. Intervention
Chinese medicinal herbs interventions were given as oral decoctions or intravenous infusions. The longest therapy duration was 3 weeks, and the shortest was 5 days. Follow-up duration was not mentioned by any of the authors. All 14 studies compared Chinese medicinal herbs plus basic therapy versus basic therapies.
3.2.4. Outcomes
All 14 studies [15–28] reported the total effective rate; seven studies [15, 17, 18, 21, 23, 25, 28] reported clinical recovery of cough, fever, rales, and chest films; four [16, 22, 24, 26] reported clinical recovery of cough, fever, and rales; one [27] reported clinical recovery of fever; three [15, 21, 22] reported adverse effects (e.g., nausea, vomiting, diarrhea, and gastrointestinal bleeding); and one [28] reported the length of hospital stay. The description of studies is detailed in the characteristics of included studies in Table 2.
Table 2.
Characteristics of the included studies.
| Wang et al., 2009 [15] | |
|---|---|
| Randomized controlled trial (RCT): randomization mentioned, but not described in detail | |
| Allocation concealment: not mentioned | |
| Followup: not mentioned | |
| Methods | Study duration: 3 weeks |
| Parallel/crossover/factorial RCT: parallel | |
| Randomization method: we interviewed the author by telephone and learned that a random number table was used to generate the random sequence | |
| Blinding: no detailed information on blindness was offered. A telephone interview with the author revealed that single blinding was used | |
| ITT: not mentioned | |
| Setting: inpatients | |
| Participants | Country: China |
| Number: 106 patients with childhood pneumonia | |
| 54 boys (50.8%) and 52 girls (49.2%); age 3–14 years old; disease duration: not mentioned | |
| Interventions | Treatment group: modified Ma Xing Shi Gan Tang formula plus basic therapy: Mahuang 6 g, Xingren 8 g, Shigao 15 g, Gancao 5 g, Yuxingcao 20 g, lianqiao 15 g, Chanyi 10 g, and Niupangzi 15 g boiled in 3 L water and decocted to 300 mL. Orally twice daily (bid) for 3 weeks |
| Control group: basic therapy including intravenous infusion of azithromycin and azithromycin orally | |
| Outcomes | (1) Total effective rate |
| (2) Adverse effects (e.g., nausea, diarrhea, and vomit) | |
| Notes | (1) Duration of disease: not mentioned; (2) mortality: not mentioned; (3) relapse rate: not mentioned; (4) length of hospital stay: not mentioned; (5) clinical recovery (e.g., cough, fever, rales, and chest films): not mentioned; (6) TCM outcomes, such as tongue coat and pulse condition: not mentioned; (7) economic index: not mentioned; (8) withdrawal rates: not specified; (9) source of funding: none |
|
| |
| Zhao and Ji, 2009 [16] | |
|
| |
| RCT: randomization mentioned, but not described in detail | |
| Allocation concealment: not mentioned | |
| Followup was not mentioned | |
| Methods | Study duration: not mentioned |
| Parallel/crossover/factorial RCT: parallel | |
| Randomization method: a telephone interview with the author revealed that a random number table was used to generate the random sequence | |
| Blinding: no detailed information on blindness was offered. A telephone interview with the author revealed that single blinding was used | |
| ITT: not mentioned | |
| Setting: inpatients and outpatients | |
| Country: China | |
| Participants | Number: 60 patients with childhood pneumonia |
| Treatment group: 30 patients with childhood pneumonia: 16 boys (53%) and 14 girls (47%); age: 2 months–9 years (mean: 3.5 years); disease duration: 4.00 ± 1.55 years | |
| Control group: 30 patients with childhood pneumonia: 17 boys (56.6%) and 13 girls (43.4%); age: month –11 years (mean: 3.25 years); disease duration: 4.00 ± 1.75 years | |
| Interventions | Treatment group: modified Ma Xing Shi Gan Tang formula plus basic therapy: Mahuang 6 g, Xingren 10 g, Shigao 20 g, Gancao 3 g, Yuxingcao 10 g, and Huangqin 10 g boiled in 3 L water and decocted to 300 mL. Orally twice daily (bid) for 3 weeks |
| Control group: basic therapy including intravenous infusion of azithromycin and azithromycin orally | |
| Outcomes | (1) Total effective rate |
| (2) Clinical recovery (e.g., cough, fever, and rales) | |
| Notes | (1) Mortality: not mentioned; (2) relapse rate: not mentioned; (3) length of hospital stay: not mentioned; (4) TCM outcomes, such as tongue coat and pulse condition: not mentioned; (5) clinical recovery (e.g., chest films): not mentioned; (6) economic index: not mentioned; (7) withdrawal rates: not mentioned; (8) source of funding: none;(9)adverse effects (e.g., nausea, diarrhea, and vomiting): not mentioned |
|
| |
| Guo, 1999 [17] | |
|
| |
| RCT: randomization mentioned, but not described in detail | |
| Allocation concealment: not mentioned | |
| Followup: not mentioned | |
| Methods | Not mentioned |
| Parallel/crossover/factorial RCT: parallel | |
| Randomization method: a telephone interview with the author revealed that a random number table was used to generate the random sequence | |
| Blinding: no detailed information on blindness was offered. A telephone interview with the author revealed that single-blinding was used | |
| ITT: not mentioned | |
| Setting: not mentioned | |
| Country: China | |
| Participants | Number: 170 patients with childhood pneumonia |
| Treatment group: 86 patients with childhood pneumonia: 45 boys (52.3%) and 41 girls (48.7%); age: 2 months–12 years (mean: 3.8 years) | |
| Control group: 84 patients with childhood pneumonia: 44 boys (52.4%) and 40 girls (48.6%); age: 2 months–14 years (mean: 3.2 years) | |
| Interventions | Treatment group: modified Ma Xing Shi Gan Tang formula plus basic therapy. Mahuang 1.5 g, Xingren 3 g, Shigao 10 g, Gancao 1.5 g, Yuxingcao 9 g, and lianqiao 3 g, boiled in 3 L water and decocted to 300 mL. Taken orally, three times daily (tid) |
| Control group: basic therapy included penicillin, Xianfeng Meisu, and ribavirin. Intravenous infusion of azithromycin and azithromycin orally | |
| Outcomes | (1) Total effective rate |
| Notes | (1) Mortality: not mentioned; (2) relapse rate: not mentioned; (3) length of hospital stay: not mentioned; (4) TCM outcomes, such as tongue coat and pulse condition: not mentioned; (5) economic index: not mentioned; (6) withdrawal rates: not specified; (7) source of funding: None; (8) time to measure outcomes: not mentioned; (9) 2. clinical recovery (e.g., cough, fever, rales, and chest films): not mentioned |
|
| |
| Zhang, 2012 [18] | |
|
| |
| RCT: randomization mentioned, but not described in detail | |
| Allocation concealment: not mentioned | |
| Followup: not mentioned | |
| Methods | Study duration: 20 days |
| Parallel/crossover/factorial RCT: parallel | |
| Randomization method: a telephone interview with the author revealed that a random number table was used to generate the random sequence | |
| Blinding: no detailed information on blindness was offered. Telephone interview with author revealed that blinding was not used | |
| ITT: not mentioned | |
| Setting: not mentioned | |
| Country: China | |
| Participants | Number: 200 patients with childhood pneumonia |
| Treatment group: 100 patients with childhood pneumonia: 74 boys (74%) and 26 girls (47%); mean age: 6.28 years; disease duration: not mentioned | |
| Control group: 100 patients with childhood pneumonia: 68 boys (68%) and 32 girls (32%); age and duration of disease not mentioned | |
| Interventions | Treatment group: modified Ma Xing Shi Gan Tang formula plus basic therapy. Mahuang 3 g, Xingren 4 g, Shigao 18 g, Gancao 3 g, Yuxingcao 9 g, and Huangqin 3 g boiled in 3 L water and decocted to 300 mL, taken orally twice daily (bid) |
| Control group: basic therapy included intravenous infusion of azithromycin and azithromycin orally | |
| Outcomes | (1) Total effective rate |
| (2) Clinical recovery (e.g., cough, fever, rales, and chest films) | |
| Notes | (1) Mortality: not mentioned; (2) relapse rate: not mentioned; (3) length of hospital stay: not mentioned; (4) TCM outcomes, such as the tongue coat and pulse condition: not mentioned; (5) economic index: not mentioned; (6) withdrawal rates: not mentioned; (7) source of funding: none; (8) time to measure outcomes: not mentioned; (9) adverse effects: not mentioned |
|
| |
| He, 2011 [19] | |
|
| |
| RCT: randomization mentioned, but not described in detail | |
| Allocation concealment: not mentioned | |
| Followup: not mentioned | |
| Methods | Study duration: 5–7 days |
| Parallel/crossover/factorial RCT: parallel | |
| Randomization method: a telephone interview with the author revealed that a random number table was used to generate the random sequence | |
| Blinding: no detailed information on blindness was offered. A telephone interview with the author revealed that blinding was not used | |
| ITT: not mentioned | |
| Setting: inpatients | |
| Country: China | |
| Participants | Number: 100 patients with childhood pneumonia |
| Treatment group: 50 patients with childhood pneumonia: 29 boys (54%) and 21 girls (42%); age: 9.6 months–12 years; disease duration: 4–8.5 days | |
| Control group: 50 patients with childhood pneumonia: 27 boys (54%) and 23 girls (46%); age: 10.8 months–13 years; disease duration: 5–8 days | |
| Interventions | Treatment group: modified Ma Xing Shi Gan Tang formula plus basic therapy: Mahuang 3 g, Xingren 6 g, Jinhua 6 g, Yinhua 6 g, Shigao 12 g, Yuxingcao 9 g, Banxia 6 g, and Zhigancao 3 g boiled in 2 L water and decocted to 300 mL; taken orally twice daily (bid) |
| Control group: intravenous infusion of azithromycin (10 mg/k · d) | |
| Outcomes | (1) Total effective rate |
| Notes | (1) Mortality: not mentioned; (2) relapse rate: not mentioned; (3) length of hospital stay: not mentioned; (4) TCM outcomes, such as tongue coat and pulse condition: not mentioned; (5) economic index: not mentioned; (6) withdrawal rates: not specified; (7) source of funding: none; (8) time to measure outcomes: not mentioned; (9) adverse effects: not mentioned; (10) clinical recovery (e.g., cough, fever, rales, and chest films): not mentioned |
|
| |
| He et al., 2011[20] | |
|
| |
| RCT: randomization mentioned, but not described in detail | |
| Allocation concealment: not mentioned | |
| Followup: not mentioned | |
| Methods | Study duration: 7 days |
| Parallel/crossover/factorial RCT: parallel | |
| Randomization method: a telephone interview with the author revealed that a random number table was used to generate the random sequence | |
| Blinding: no detailed information on blindness was offered. A telephone interview with the author revealed that blinding was used on outcome assessment | |
| ITT: not mentioned | |
| Setting: inpatients | |
| Country: China | |
| Participants | Number: 80 patients with childhood pneumonia |
| Treatment group: 40 patients with childhood pneumonia: 22 boys (55%) and 18 girls (45%); mean age: 1.8 ± 1.10 years | |
| Control group: 40 patients with childhood pneumonia: 21 boys (52.5%) and 19 girls (47.5%); mean age: 1.75 ± 1.151 years | |
| Disease duration: 7.50 ± 0.50 days | |
| Interventions | Treatment group: modified Ma Xing Shi Gan Tang formula plus basic therapy: Mahuang 3 g, Xingren 3 g, Shigao 9 g, Suzi 3 g, Shangbaipi 6 g, Kuandonghua 6 g, Banxia 6 g, Tinglizi 3 g, Yuxingcao 3 g, and Gancao 3 g boiled in 2 L water and decocted to 300 mL, taken orally twice daily (bid) |
| Control group: basic therapy included supporting treatment and intravenous infusion of ceftazidime (0.1 g/kg · d) | |
| Outcomes | (1) Total effective rate |
| Notes | (1) Mortality: not mentioned; (2) relapse rate: not mentioned; (3) length of hospital stay: not mentioned; (4) TCM outcomes, such as tongue coat and pulse condition: not mentioned; (5) adverse effects: not mentioned; (6) economic index: not mentioned; (7) withdrawal rates: not mentioned; (8) source of funding: none; (9) time to measure outcomes: not mentioned; (10) clinical recovery (e.g., cough, fever, rales, and chest films): not mentioned |
|
| |
| Zhang, 2012 [21] | |
|
| |
| RCT: randomization mentioned, but not described in detail | |
| Allocation concealment: not mentioned | |
| Followup: not mentioned | |
| Methods | Study duration: 5–7 days |
| Parallel/crossover/factorial RCT: parallel | |
| Randomization method: a telephone interview with the author revealed that a computer-generated random-number table was used | |
| Blinding: no detailed information on blindness was offered. A telephone interview with the author revealed that blinding was used on the outcome assessment | |
| ITT: not mentioned | |
| Setting: inpatients | |
| Country: China | |
| Participants | Number: 128 patients with childhood pneumonia |
| Treatment group: 64 patients with childhood pneumonia in the treatment group | |
| Control group: 64 patients with childhood pneumonia in the control group | |
| In two groups, 79 boys (55%) and 49 girls (45%); age 1–14 years old; disease duration: 5–7 days | |
| Interventions | Treatment group: San Ao Tang formula plus basic therapy; Mahuang 3 g, Xingren 12 g, Gancao 3 g boiled in 2 L water and decocted to 250 mL. Taken orally three times daily (tid) |
| Control group: basic therapy included symptomatic therapy and orally azithromycin 10 mg/kg one time per day (qd) | |
| Outcomes | (1) Total effective rate; (2) clinical recovery (e.g., cough, fever, rales, and chest films); (3) adverse effects (e.g., nausea) |
| Notes | (1) Mortality: not mentioned; (2) relapse rate: not mentioned; (3) length of hospital stay: not mentioned; (4) TCM outcomes, such as tongue coat and pulse condition: not mentioned; (5) economic index: not mentioned; (6) withdrawal rates: not mentioned; (7) source of funding: none; (8) time to measure outcomes: not mentioned |
|
| |
| Wang et al., 2009 [22] | |
|
| |
| RCT: randomization mentioned, but not described in detail | |
| Allocation concealment: not mentioned | |
| Followup: not mentioned | |
| Study duration: 7 days | |
| Methods | Parallel/crossover/factorial RCT: parallel |
| Randomization method: a telephone interview with the author revealed that a computer-generated random-number table was used | |
| Blinding: no detailed information on blindness was offered. A telephone interview with the author revealed that blinding was used on outcome assessment | |
| ITT: not mentioned | |
| Setting: patient source not mentioned | |
| Country: China | |
| Number: 200 patients with childhood pneumonia | |
| Participants | Treatment group: 100 patients with childhood pneumonia in the treatment group |
| Control group: 100 patients with childhood pneumonia in the control group | |
| Did not mention the number of boys and girls | |
| Disease duration: 5–7 days | |
| Interventions | Treatment group: Zhi Sou San formula plus basic therapy: Jiegeng 6–9 g, Gancao 3–6 g, Ziwan 3–6 g, Chenpi 3–6 g, Xingren 3–6 g, Baiguo 3–6 g, Huangqi 3–6 g, Chaomaiya 3–6 g, Yunling 3–6 g, and Baiqian 3–6 g boiled in 2 L water. Taken orally three times daily (tid) |
| Control group: basic therapy included symptomatic therapy and intravenous infusion of erythrocin (30 mg/kg · d) | |
| Outcomes | (1) Total effective rate; (2) clinical recovery (e.g., cough, fever, and rales); (3) adverse effects (e.g., nausea, vomiting, and gastrointestinal bleeding) |
| Notes | (1) Mortality: not mentioned; (2) relapse rate: not mentioned; (3) length of hospital stay: not mentioned; (4) TCM outcomes, such as tongue coat and pulse condition: not mentioned; (5) economic index: not mentioned; (6) withdrawal rates: not mentioned; (7) source of funding: none; (8) time to measure outcomes: not mentioned; (9) chest films: not mentioned |
|
| |
| Lv et al., 2009 [23] | |
|
| |
| RCT: randomization mentioned, but not described in detail | |
| Allocation concealment: not mentioned | |
| Followup was not mentioned | |
| Methods | Study duration: 10 days |
| Parallel/crossover/factorial RCT: parallel | |
| Randomization method: a telephone interview with the author revealed that a random number table was used to generate the random sequence | |
| Blinding: no detailed information on blindness was offered. A telephone interview with the author revealed that blinding was used on the outcome assessment | |
| ITT: not mentioned | |
| Setting: inpatients | |
| Country: China | |
| Participants | Number: 60 patients with childhood pneumonia |
| Treatment group: 30 patients with childhood pneumonia: 17 boys (56.7%) and 13 girls (43.3%); age: 8 months–13 years; disease duration: 7–18 days; mean: 14 days | |
| Control group: 30 patients with childhood pneumonia: 16 boys (53.3%) and 14 girls (46.7%); age: 7 months–12 years; disease duration: 7–18 days; mean: 13.5 days | |
| Interventions | Treatment group: self-developed TCM prescription plus basic therapy: Mahuang 3 g, Xingren 3 g, Rengongniuhuang 3 g, Bingpian 2 g, Shengshigao 3 g, Zhusha 2 g, Chuanbeimu 2 g, Huanglian 2 g, Banxia 2 g, Dannanxing 2 g, Shangbaipi 2 g, Huangqin 2 g, and Gancao 2 g boiled in 3 L water and decocted to 300 mL. Taken orally three times daily (tid) |
| Control group: basic therapy included symptomatic therapy and intravenous infusion of erythrocin (20 mg/kg bid) | |
| Outcomes | (1) Total effective rate; (2) clinical recovery (e.g., cough, fever, rales, and chest films) |
| Notes | (1) Mortality: not mentioned; (2) relapse rate: not mentioned; (3) length of hospital stay: not mentioned; (4) TCM outcomes, such as tongue coat and pulse condition: not mentioned; (5) economic index: not mentioned; (6) withdrawal rates: not mentioned; (7) source of funding: none; (8) time to measure outcomes: mentioned; (9) adverse effects: not mentioned |
|
| |
| Lei, 2010 [24] | |
|
| |
| RCT: randomization mentioned, but not described in detail | |
| Allocation concealment: not mentioned | |
| Followup: not mentioned | |
| Methods | Study duration: 7 days |
| Parallel/crossover/factorial RCT: parallel | |
| Randomization method: a telephone interview with the author revealed that a computer-generated random-number table was used | |
| Blinding: no detailed information on blindness was offered. A telephone interview with the author revealed that blinding was used on the outcome assessment | |
| ITT: not mentioned | |
| Setting: inpatients | |
| Country: China | |
| Participants | Number: 160 patients with childhood pneumonia |
| Treatment group: 80 patients with childhood pneumonia: 52 boys (65%) and 28 girls (35%); age: 6 months–12 years; disease duration: 2–7 days | |
| Control group: 80 patients with childhood pneumonia: 54 boys (67.5%) and 26 girls (32.5%); age: 5 months–13 years; disease duration: 1–6 days | |
| Interventions | Treatment group: Tanreqing injection plus basic therapy: 30–50 mL/kg Tanreqing injection + 50–100 mL 10% GS intravenous infusion once daily (qd) |
| Control group: basic therapy included anti-inflammatory, symptomatic therapy. Did not provide any detailed information about the anti-inflammatory, symptomatic therapy | |
| Outcomes | (1) Total effective rate; (2) clinical recovery (e.g., cough, fever, and rales). |
| Notes | (1) Mortality: not mentioned; (2) relapse rate: not mentioned; (3) length of hospital stay: not mentioned; (4) TCM outcomes, such as tongue coat and pulse condition: not mentioned; (5) economic index: not mentioned; (6) withdrawal rates: not mentioned; (7) source of funding: none; (8) time to measure outcomes: mentioned; (9) chest films: not mentioned; (10) adverse effects: not mentioned |
|
| |
| Shi, 2009 [25] | |
|
| |
| RCT: randomization mentioned, but not described in detail | |
| Allocation concealment: not mentioned | |
| Followup: not mentioned | |
| Methods | Study duration: 14 days |
| Parallel/crossover/factorial RCT: parallel | |
| Randomization method: a random number table was used to generate the random sequence | |
| Blinding: no detailed information on blindness was offered. A telephone interview with the author revealed that blinding was not used on study | |
| ITT: not mentioned | |
| Setting: inpatients | |
| Country: China | |
| Participants | Number: 80 patients with childhood pneumonia |
| Treatment group: 40 patients with childhood pneumonia: 21 boys (52.5%) and 19 girls (47.5%) | |
| Control group: 40 patients with childhood pneumonia: 24 boys (60%) and 16 girls (40%); age: 5 months–13 years, in two groups; disease duration: 1–3 days | |
| Interventions | Treatment group: Reduning injection plus basic therapy: 0.5–1.0 mL/kg · d Reduning injection + 250 mL 5% GS intravenous infusion once daily (qd) |
| Control group: basic therapy included symptomatic therapy and intravenous infusion of 10 mg/kg · d azithromycin for 5 days, stop 3 days, then changed to oral 10 mg/kg · d azithromycin for 3 days | |
| Outcomes | (1) Total effective rate; (2) clinical recovery (e.g., cough, fever, rales, and chest films) |
| Notes | (1) Mortality: not mentioned; (2) relapse rate: not mentioned; (3) length of hospital stay: not mentioned; (4) TCM outcomes, such as tongue coat and pulse condition: not mentioned; (5) economic index: not mentioned; (6) withdrawal rates: not mentioned; (7) source of funding: none; (8) time to measure outcomes: mentioned; (9) adverse effects: not mentioned |
|
| |
| Pan, 2011 [26] | |
|
| |
| RCT: randomization mentioned, but not described in detail | |
| Allocation concealment: not mentioned | |
| Followup: not mentioned | |
| Methods | Study duration: 7 days |
| Parallel/crossover/factorial RCT: parallel | |
| Randomization method: a telephone interview with the author revealed that a random number table was used to generate the random sequence | |
| Blinding: no detailed information on blindness was offered. A telephone interview with the author revealed that the outcome assessment was blinding | |
| ITT: not mentioned. | |
| Setting: patient source not mentioned. | |
| Country: China | |
| Participants | Number: 140 patients with childhood pneumonia |
| Treatment group: 70 patients with childhood pneumonia: 42 boys (60%) and 28 girls (40%); age: 1–9 years old (mean: 5.1 ± 1.6 years); disease duration: 2–7 days | |
| Control group: 70 patients with childhood pneumonia: 36 boys (51.4%) and 34 girls (48.6%); age: 1–10 years old (mean: 5.1 ± 1.6 years); disease duration: 1–7 days | |
| Interventions | Treatment group: Reduning injection plus basic therapy: 0.5–0.8 mL/kg Reduning injection + 100 mL 5% GS intravenous infusion once daily (qd) |
| Control group: intravenous infusion of 10 mg/kg · d azithromycin + 5% GS once daily (qd) | |
| Outcomes | (1) Total effective rate; (2) clinical recovery (e.g., cough, fever, and rales) |
| Notes | (1) Mortality: not mentioned; (2) relapse rate: not mentioned; (3) length of hospital stay: not mentioned; (4) TCM outcomes, such as tongue coat and pulse condition: not mentioned; (5) economic index: not mentioned; (6) withdrawal rates: not mentioned; (7) source of funding: mentioned; (8) time to measure outcomes: not mentioned; (9) adverse effects: not mentioned; (10) chest films: not mentioned |
|
| |
| Duan and Feng, 2011 [27] | |
|
| |
| RCT: randomization mentioned, but not described in detail | |
| Allocation concealment: not mentioned | |
| Followup was not mentioned | |
| Methods | Study duration: 14 days |
| Parallel/crossover/factorial RCT: parallel | |
| Randomization method: a telephone interview with the author revealed that a random number table was used to generate the random sequence | |
| Blinding: no detailed information on blindness was offered. A telephone interview with the author revealed that the outcome assessment was blinded | |
| ITT: not mentioned | |
| Setting: outpatients and inpatients | |
| Participants | Country: China |
| Number: 60 patients with childhood pneumonia | |
| In two groups: 35 boys (58.3%) and 25 girls (41.7%); age: 1–13 years; disease duration: 2–5 days | |
| Interventions | Treatment group: Reduning injection plus basic therapy: 10–15 mL Reduning injection + 100 mL 5% GS intravenous infusion once daily (qd) |
| Control group: intravenous infusion of 10 mg/kg · d azithromycin + 5% GS once daily (qd) | |
| Outcomes | (1) Total effective rate; (2) clinical recovery (e.g., fever) |
| Notes | (1) Mortality: not mentioned; (2) relapse rate: not mentioned; (3) length of hospital stay: not mentioned; (4) TCM outcomes, such as tongue coat and pulse condition: not mentioned; (5) economic index: not mentioned; (6) clinical recovery (e.g., cough, rales, and chest films): not mentioned; (7) withdrawal rates: not mentioned; (8) source of funding: none; (9) time to measure outcomes: not mentioned; (10) adverse effects: not mentioned |
|
| |
| Wei and Feng, 2003 [28] | |
|
| |
| RCT: randomization mentioned, but not described in detail | |
| Allocation concealment: not mentioned | |
| Followup: not mentioned | |
| Methods | Study duration: 7–10 days |
| Parallel/crossover/factorial RCT: parallel | |
| Randomization method: a telephone interview with the author revealed that a computer-generated random-number table was used | |
| Blinding: no detailed information on blindness was offered. A telephone interview with the author revealed that blinding was not used | |
| ITT: not mentioned | |
| Setting: inpatients | |
| Country: China | |
| Participants | Number: 180 patients with childhood |
| In two groups: 100 boys (55.6%) and 80 girls (44.4%); age: 2 months–5 years (mean: 2.3 years); disease duration: 1–7 days | |
| Treatment group: 90 patients with childhood pneumonia; 52 boys (57.8%) and 38 girls (42.2%) | |
| Control group: 90 patients with childhood pneumonia; 48 boys (53.3%) and 42 girls (46.7%) | |
| Interventions | Treatment group: Chuanhuning injection plus basic therapy: 10 mg/kg · d + 50–100 mL 10% GS or NS intravenous infusion once daily (qd) |
| Control group: intravenous infusion of 100 mg/kg · d piperacillin twice daily (bid) | |
| Outcomes | (1) Total effective rate; (2) length of hospital stay; (3) clinical recovery (e.g., cough, fever, rales, and chest films) |
| Notes | (1) Mortality: not mentioned; (2) relapse rate: not mentioned; (3) TCM outcomes, such as tongue coat and pulse condition: not mentioned; (4) economic index: not mentioned; (5) withdrawal rates: not mentioned; (6) source of funding: none; (7) time to measure outcomes: not mentioned; (8) adverse effects: not mentioned |
3.3. Risk of Bias in Included Studies
The methodological quality of each study's randomization sequence, allocation concealment, blinding, incomplete outcome data, selective outcome reporting, and potential threats are summarized in Figures 2 and 3.
Figure 2.
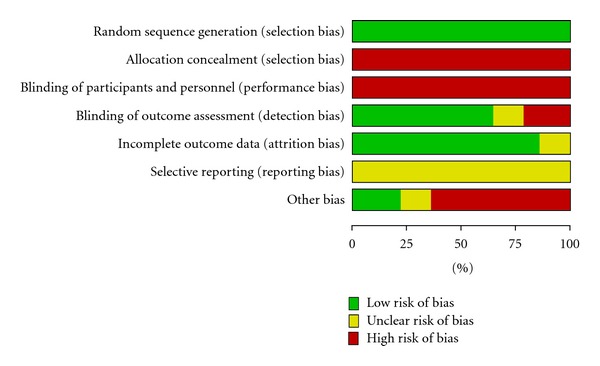
Methodological quality. Judgments about each item are presented as percentages across all included studies.
Figure 3.
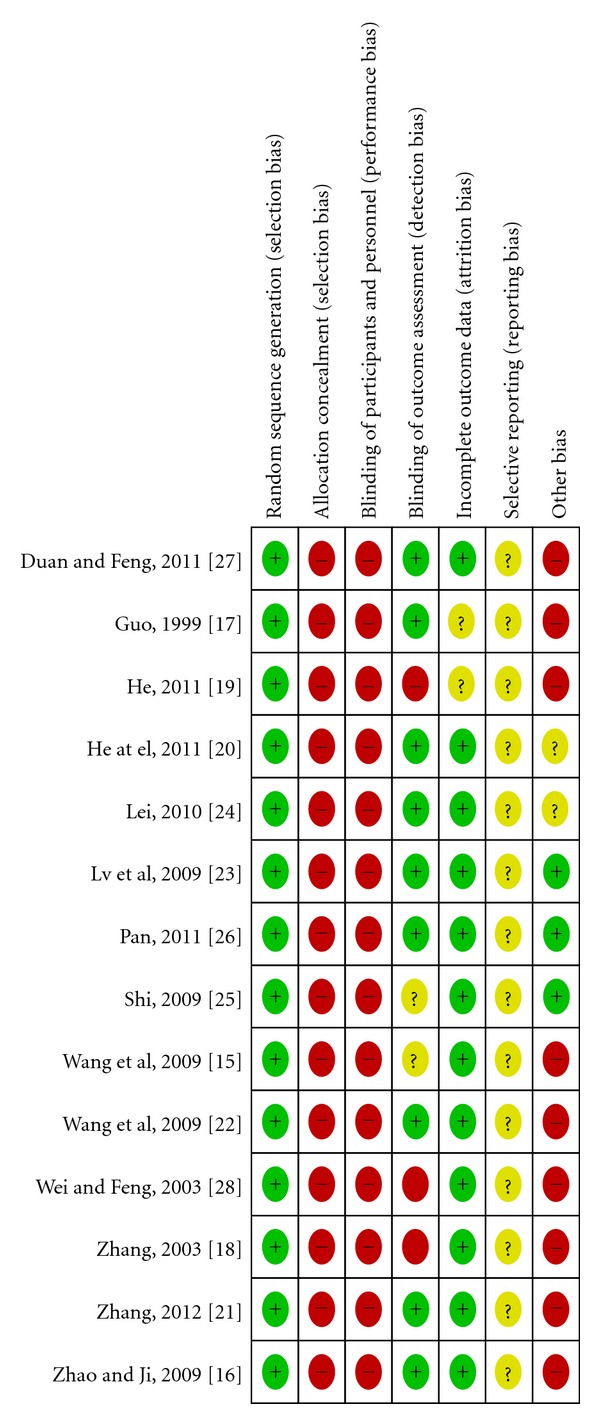
Methodological quality summary.
3.3.1. Randomization and Allocation Concealment
Ten studies [15–20, 23, 25–27] reported using a random-number table, and four [21, 22, 24, 28] reported using a computer-generated random-number table. None of the trials used allocation concealment. Therefore, all studies had a high risk of selection bias.
3.3.2. Blinding
Ten studies [15–17, 20–24, 26, 27] used single blinding (outcome assessment was blinded). We interviewed the original authors by telephone to determine blinding because the blinding methods were not described. Thus, these studies had a low risk of performance bias and low detection bias. The other studies [18, 19, 25, 28] did not use blinding methods and had a high risk of performance bias or a strong detection bias.
3.3.3. Flow of Participants and Intention-to-Treat
None of the studies reported withdrawal, dropout, and/or loss during followup. The method of handling missing data regarding intention-to-treat or per-protocol analysis was not addressed.
3.3.4. Selective Reporting (Reporting Bias)
No detailed evidence of selective reporting was found in any of the 14 studies [15–28]. However, we believed there is a high risk of selective reporting bias because we were unable to compare the protocol with published studies.
3.3.5. Other Potential Sources of Bias
None of the 14 studies [15–28] did not describe patient compliance. The appropriateness of the statistical analyses used was assessed, and the methods of all studies were considered appropriate. Although we conducted comprehensive searches and tried to avoid bias, we could not exclude potential publication bias because all 14 studies were published in China.
4. Effects of Interventions
All studies compared Chinese medicinal herbs plus basic therapy to basic therapy alone. The Chinese medicinal herb treatments included the modified Ma Xing Shi Gan Tang formula, the San Ao Tang formula, the Zhi Sou San formula, a self-developed TCM prescription, Tanreqing injection, Reduning injection, and Chuanhuning injection. Antibiotics were one of main basic therapies for Childhood Pneumonia.
4.1. Total Effective Rate
A significant increase in total effective rate was observed with Chinese medicinal herbs plus basic therapy versus basic therapy (Figure 4, analysis 1.1; RR, 1.18; 95% CI, 1.11–1.26).
Figure 4.
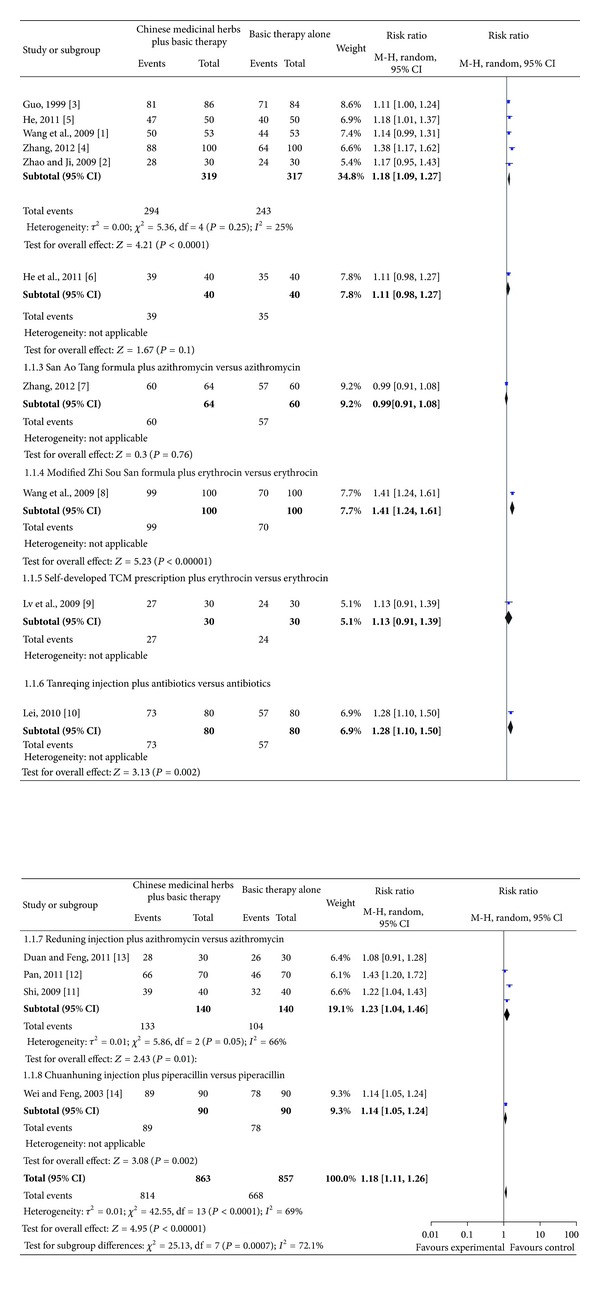
Comparison. Chinese medicinal herbs plus basic therapy versus basic therapy: outcome 1 total effective rate.
Subgroup five studies [15–19] compared the modified Ma Xing Shi Gan Tang formula plus azithromycin versus azithromycin and showed a significant increase in total effective rate (Figure 4; analysis 1.1.1 of analysis 1.1; RR, 1.18; 95% CI, 1.09–1.27).
Subgroup one study [20] compared the modified Ma Xing Shi Gan Tang formula plus ceftazidime versus ceftazidime and showed no difference in total effective rate (Figure 4; analysis 1.1.2 of analysis 1.1; RR, 1.11; 95% CI, 0.98–1.27).
Subgroup one study [21] compared the San Ao Tang formula plus azithromycin versus azithromycin and showed no difference in total effective rate (Figure 4; analysis 1.1.3 of analysis 1.1; RR, 0.99; 95% CI, 0.91–1.08).
Subgroup one study [22] compared the modified Zhi Sou San formula plus erythrocin versus erythrocin and showed a significant increase in total effective rate (Figure 4; analysis 1.1.4 of analysis 1.1; RR, 1.41; 95% CI, 1.24–1.61).
Subgroup one study [23] compared a self-developed TCM prescription plus erythrocin versus erythrocin and showed no difference in total effective rate (Figure 4; analysis 1.1.5 of analysis 1.1; RR, 1.13; 95% CI, 0.91–1.39).
Subgroup one study [24] compared Tanreqing injection plus antibiotics versus antibiotics and showed a significant difference in total effective rate (Figure 4; analysis 1.1.6 of analysis 1.1; RR, 1.28; 95% CI, 1.10–1.50).
Subgroup three studies [25–27] compared Reduning injection plus azithromycin versus azithromycin and showed a significant difference in total effective rate (Figure 4; analysis 1.1.7 of analysis 1.1; RR, 1.23; 95% CI, 1.04–1.46).
Subgroup one study [28] compared Chuanhuning injection plus piperacillin versus piperacillin and showed a significant difference in total effective rate (Figure 4; analysis 1.1.8 of analysis 1.1; RR, 1.14; 95% CI, 1.05–1.24).
4.2. Adverse Effects
Three studies [15, 21, 22] compared Chinese medicinal herbs plus basic therapy versus basic therapy and showed no difference in adverse effects (Figure 5; analysis 1.2; RR, 0.39; 95% Cl, 0.09–1.72).
Figure 5.
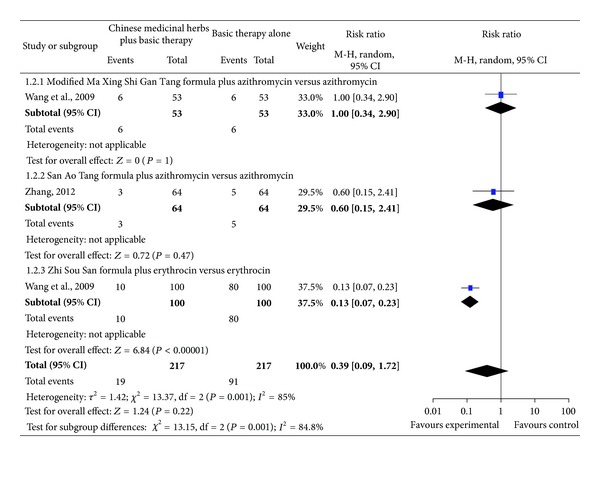
Comparison. Chinese medicinal herbs plus basic therapy versus basic therapy: outcome 2 adverse effects.
Subgroup one study [15] compared the modified Ma Xing Shi Gan Tang formula plus azithromycin versus azithromycin and showed no difference in adverse effects (Figure 5; analysis 1.2.1 of analysis 1.2; RR, 1.00; 95% CI, 0.34–2.90).
Subgroup one study [21] compared the San Ao Tang formula plus azithromycin versus azithromycin and showed no difference in adverse effects (Figure 5; analysis 1.2.2 of analysis 1.2; RR, 0.60; 95% CI, 0.15–2.41).
Subgroup one study [22] compared the modified Zhi Sou San formula plus erythrocin versus erythrocin and showed a significant decrease in adverse effects (Figure 5; analysis 1.2.3 of analysis 1.2; RR, 0.13; 95% CI, 0.07–0.23).
4.3. Time (Day) to Clinical Recovery Including Cough, Fever, Rales, and Chest Films
Studies investigating Chinese medicinal herbs plus basic therapy versus basic therapy showed a significant difference in cough (Figure 6; analysis 1.3; total MD, −2.18; 95% Cl, (−2.66)–(−1.71)), fever (Figure 7; analysis 1.4; total MD, −1.85; 95% Cl, (−2.29)–(−1.40)), rales (Figure 8; analysis 1.5; total MD, −1.53; 95% Cl, (−1.84)–(−1.23)), and chest films (Figure 9; analysis 1.6; total MD, −3.10; 95% Cl, (−4.11)–(−2.08)).
Figure 6.
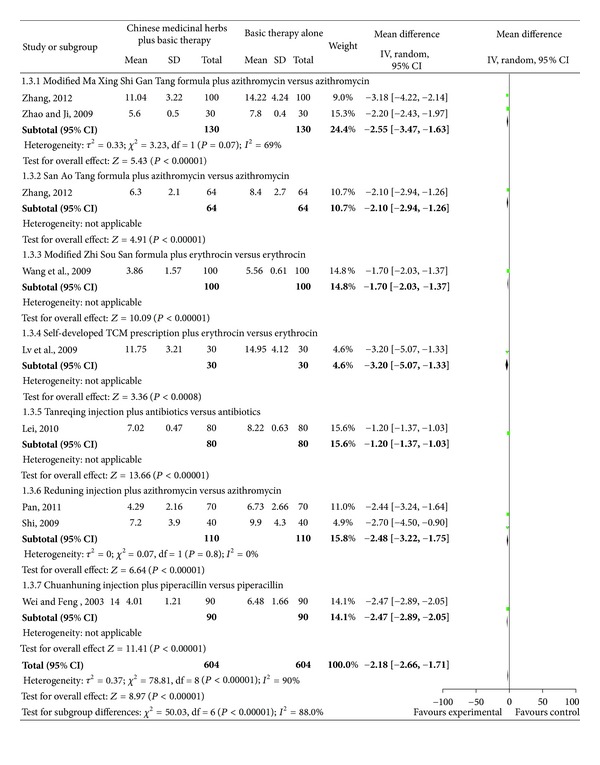
Comparison. Chinese Medicinal herbs plus basic therapy versus basic therapy: outcome 3 cough.
Figure 7.
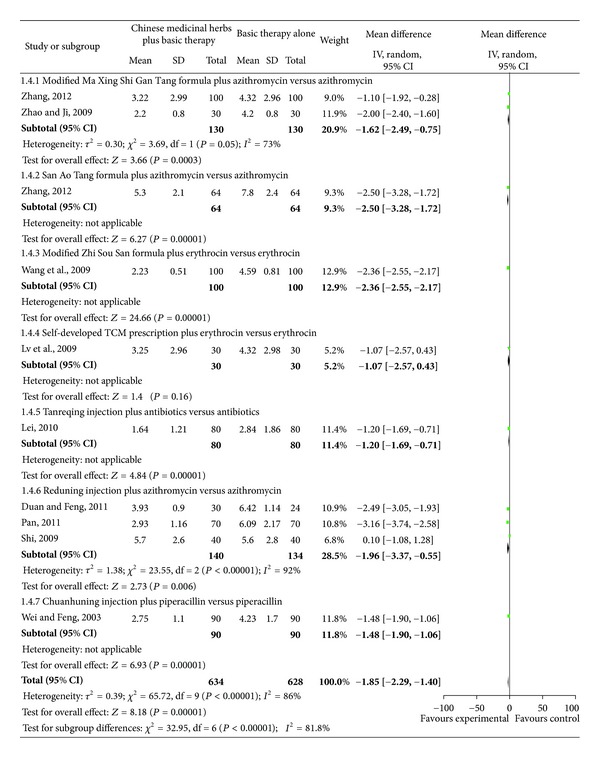
Comparison. Chinese medicinal herbs plus basic therapy versus basic therapy: outcome 4 fever.
Figure 8.
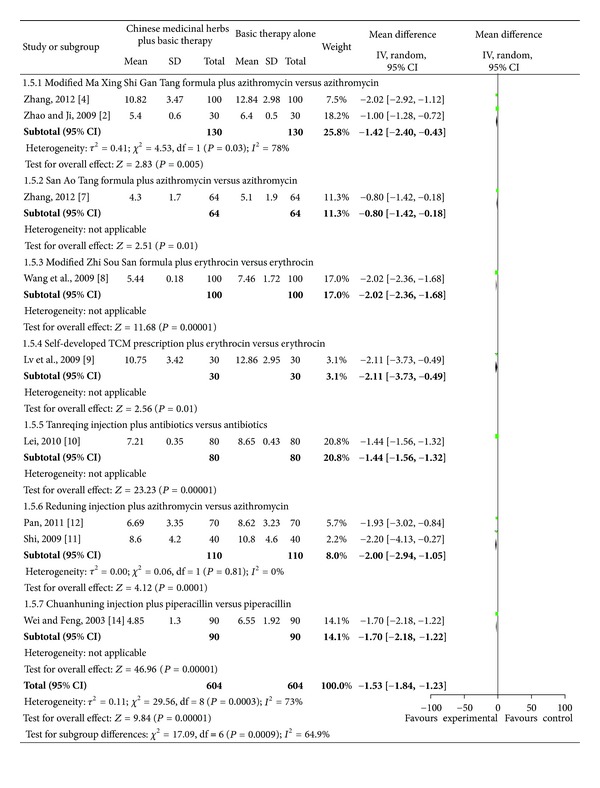
Comparison. Chinese medicinal herbs plus basic therapy versus basic therapy: outcome 5 rales.
Figure 9.
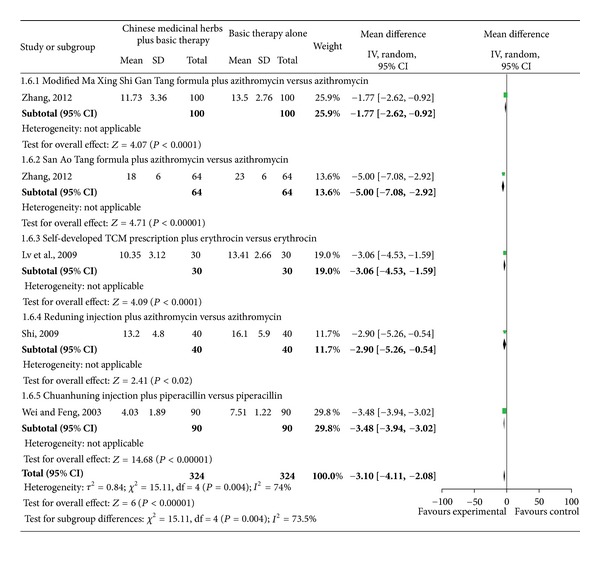
Comparison. Chinese medicinal herbs plus basic therapy versus basic therapy: outcome 6 chest films.
Subgroup two studies [16, 18] compared the modified Ma Xing Shi Gan Tang formula plus azithromycin versus azithromycin and showed a significant difference for cough (Figure 6; analysis 1.3.1 of analysis 1.3; MD, −2.55; 95% Cl, (−3.47)–(−1.63)), fever (Figure 7; analysis 1.4.1 of analysis 1.4; MD, −1.62; 95% Cl, (−2.49)–(−0.75)), and rales (Figure 8; analysis 1.5.1 of analysis 1.5; MD, −1.42; 95% Cl, (−2.40)–(−0.43)). Subgroup one study [18] compared the modified Ma Xing Shi Gan Tang formula plus azithromycin versus azithromycin and showed a significant difference on chest films (Figure 9; analysis 1.6.1 of analysis 1.6; MD, −1.77; 95% Cl, (−2.62)–(−0.92)).
Subgroup one study [21] compared the San Ao Tang formula plus azithromycin versus azithromycin and showed a significant difference in cough (Figure 6; analysis 1.3.2 of analysis 1.3; MD, −2.10; 95% Cl, (−2.94)–(−1.26)), fever (Figure 7; analysis 1.4.2 of analysis 1.4; MD, −2.50; 95% Cl, (−3.28)–(−1.72)), rales (Figure 8; analysis 1.5.2 of analysis 1.5; MD, −0.80; 95% Cl, (−1.42)–(−0.18)), and chest films (Figure 9; analysis 1.6.2 of analysis 1.6; MD, −5.00; 95% Cl, (−7.08)–(−2.92)).
Subgroup one study [22] compared the modified Zhi Sou San formula plus erythrocin versus erythrocin and showed a significant difference in cough (Figure 6; analysis 1.3.3 of analysis 1.3; MD, −1.70; 95% CI, (−2.03)–(−1.37)), fever (Figure 7 analysis 1.4.3 of analysis 1.4; MD, −2.36; 95% Cl, (−2.55)–(−2.17)), and rales (Figure 8 analysis 1.5.3 of analysis 1.5; MD, −2.02; 95% Cl, (−2.36)–(−1.68)).
Subgroup one study [23] compared a self-developed TCM prescription plus erythrocin versus erythrocin and showed a significant difference in cough (Figure 6 analysis 1.3.4 of analysis 1.3; MD, 3.20; 95% CI, (−5.07)–(−1.33)), rales (Figure 8 analysis 1.5.4 of analysis 1.5; MD, −2.11; 95% Cl, (−3.73)–(−0.49)), and chest films (Figure 9 analysis 1.6.3 of analysis 1.6; MD, −3.06; 95% Cl, (−4.53)–(−1.59)), but no difference in fever (Figure 7 analysis 1.4.4 of analysis 1.4; MD, −1.07; 95% Cl, −2.57–0.43).
Subgroup one study [24] compared Tanreqing injection plus antibiotics versus antibiotics and showed a significant difference in cough (Figure 6 analysis 1.3.5 of analysis 1.3; MD, −1.20; 95% CI, (−1.37)–(−1.03)), fever (Figure 7 analysis 1.4.5 of analysis 1.4; MD, −1.20; 95% Cl, (−1.69)–(−0.71)), and rales (Figure 8 analysis 1.5.5 of analysis 1.5; MD, −1.44; 95% Cl, (−1.56)–(−1.32)).
Subgroup two studies [25, 26] compared Reduning injection plus azithromycin versus azithromycin and showed a significant difference in cough (Figure 6 analysis 1.3.6 of analysis 1.3; MD, −2.48; 95% CI, (−3.22)–(−1.75)) and rales (Figure 8 analysis 1.5.6 of analysis 1.5; MD, −2.00; 95% CI, (−2.94)–(−1.05)). Subgroup three studies [25–27] compared Reduning injection plus azithromycin versus azithromycin and showed a significant difference in fever (Figure 7 analysis 1.4.6 of analysis 1.4; MD, −1.96; 95% CI, (−3.37)–(−0.55)). Subgroup one study [25] compared Reduning injection plus azithromycin versus azithromycin and showed a significant difference on chest films (Figure 9 analysis 1.6.4 of analysis 1.6; MD, −2.90; 95% CI, (−5.26)–(−0.54)).
Subgroup one study [28] compared Chuanhuning injection plus piperacillin versus piperacillin and showed a significant difference in cough (Figure 6 analysis 1.3.7 of analysis 1.3; MD, −2.47; 95% CI, (−2.89)–(−2.05)), fever (Figure 7 analysis 1.4.7 of analysis 1.4; MD, −1.48; 95% CI, (−1.90)–(−1.06)), rales (Figure 8 analysis 1.5.7 of analysis 1.5; MD, −1.70; 95% CI, (−2.18)–(−1.22)), and chest films (Figure 9 analysis 1.6.5 of analysis 1.6; MD, −3.48; 95% CI, (−3.94)–(−3.02)).
4.4. Length of Hospital Stay
One study [28] compared Chuanhuning injection plus piperacillin versus piperacillin and showed a significant difference in length of hospital stay (Figure 10 analysis 1.7; total MD, −3.00; 95% CI, (−3.52)–(−2.48)).
Figure 10.
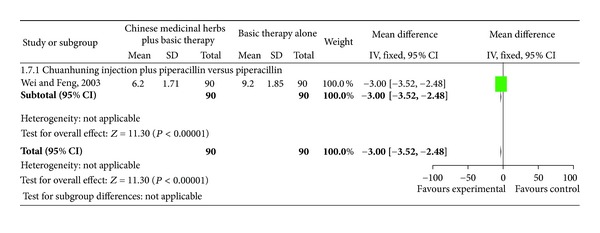
Comparison. Chinese medicinal herbs plus basic therapy versus basic therapy: outcome 7 length of hospital stay.
4.5. Other Outcomes
Mortality, relapse rate, TCM outcomes (e.g., tongue coat and pulse condition), and economic index were not reported in any of the 14 studies.
5. GRADE Quality of Evidence
The “GRADEprofiler” of the Cochrane Collaboration Network was used to classify the systematic review results. The quality of evidence was low to very low (Table 3).
Table 3.
Grade quality of evidence.
| Chinese medicinal herbs plus basic therapy versus basic therapy alone for childhood pneumonia | ||||||
|---|---|---|---|---|---|---|
| Patient or population: patients with childhood pneumonia | ||||||
| Settings: inpatients or outpatients | ||||||
| Intervention: Chinese medicinal herbs plus basic therapy versus basic therapy alone | ||||||
| Illustrative comparative risks (95% CI)* | ||||||
| Outcomes | Assumed risk | Corresponding risk | Relative effect (95% CI) |
Number of Participants (studies) |
Quality of evidence (grade) |
Comments |
| Control | Chinese medicinal herbs plus basic therapy versus basic therapy alone | |||||
|
| ||||||
| Study population | ||||||
| Total effective rate | 779 per 1000 |
920 per 1000
(865–982) |
||||
| Moderate |
RR 1.18
(1.11–1.26) |
1720 (14 studies) |
⊕⊕○○ Low 1,2 |
Important | ||
| 800 per 1000 |
944 per 1000
(888–1000) |
|||||
|
| ||||||
| Study population | ||||||
| Adverse effects | 419 per 1000 |
164 per 1000
(38–721) |
||||
| Moderate |
RR 0.39
(0.09–1.72) |
434 (3 studies) |
⊕○○○ Very low 1,3 |
Important | ||
| 113 per 1000 |
44 per 1000
(10 to 194) |
|||||
|
| ||||||
| Time (day) to improvement of cough | The mean time (days) to improvement in cough in the intervention groups was 2.18 lower (2.66 to 1.71 lower) |
1208 (9 studies) |
⊕⊕○○ Low 1,4 |
Important | ||
|
| ||||||
| Time (day) to improvement of fever | The mean time (days) to improvement in fever in the intervention groups was 2.12 lower (2.25 to 1.98 lower) |
1262 (10 studies) |
⊕⊕○○ Low 1,2 |
Important | ||
|
| ||||||
| Time (day) to improvement of rales | The mean time (days) to improvement in rales in the intervention groups was 1.53 lower (1.84 to 1.23 lower) |
1208 (9 studies) |
⊕⊕○○ Low 1,4 |
Important | ||
|
| ||||||
| Time (day) to improvement in chest films | The mean time (days) to improvement in chest films in the intervention groups was 3.1 lower (4.11 to 2.08 lower) |
648 (5 studies) |
⊕⊕○○ Low 1,4 |
Important | ||
|
| ||||||
| Length of hospital stay | The mean length of hospital stay in the intervention groups was 3 lower (3.52 to 2.48 lower) |
180 (1 study) |
⊕○○○ Very low 1,5 |
Important | ||
*The basis for the assumed risk (e.g., the median control group risk across studies) is provided in the footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio.
Grade: working group grades of evidence.
High quality: further research is very unlikely to change our confidence in the estimate of effect. Handbook description: randomized controlled trial.
Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Cochrane Handbook description: relegation randomized controlled trial.
Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Cochrane Handbook description: two or more degradation factors of randomized controlled trials.
Very low quality: we are very uncertain about the estimate. Cochrane Handbook description: more than three degradation factors of randomized controlled trials.
Reduce the evidence quality factors: methodology defect, included in the research results of the inconsistency, indirect evidence, inexactness, and publication bias.
Increase the level of evidence factor: large effect quantity, confounding factors cannot change effect quantity, or the existing concentration-response relationship.
1There is a high risk of selection bias, performance bias, and detection bias.
2Some studies showed a significant difference, but some studies showed no significant difference.
3Few studies included.
4The protocol of the published studies could not be compared.
5Only one study included.
6. Discussion
Based on the 14 [15–28] RCTs conducted in China, Chinese medicinal herbs increased total effective rates (e.g., ratio of signs and symptoms improvement or recovery) and improved clinical symptoms and signs (e.g., cough, fever, rales, and chest films). However, the evidence that Chinese medicinal herbs decreased adverse effects, mortality, or improved TCM outcomes (e.g., tongue coat and pulse condition) was insufficient. The quality of the evidence was weak due to selective bias, measurement bias, selective reporting bias, and imprecision. Therefore, evidence from the included studies was not enough to make any recommendations.
First, randomization was mentioned in all 14 studies. However, one study [25] described the randomization method in detail, whereas 13 did not [15–24, 26–28]. We interviewed the authors by telephone and determined that a random number table or a computer-generated random-number table was used to generate the allocation sequence. Second, none of the studies mentioned a blinding method, but we interviewed the authors by telephone and found that 10 studies [15–17, 20–24, 26, 27] used single blinding (i.e., the outcome assessment was blinded), and four [18, 19, 25, 28] did not. The lack of blinding participants, health-care providers, and assessors can introduce performance and detection bias. Third, none of the studies addressed the incomplete outcome data, such as missing data due to attrition or exclusion. The inadequate handling of missing data can compromise statistical analyses. Fourth, the majority of experimental Chinese herbal medicine interventions were prepared by the investigators without detailed information describing their underlying rationale for the formulation, dosage, or the manufacturing process, and the quality control processes for their tested interventions are unknown. Thus, independent validation of these findings is necessary.
This paper has several methodological limitations. First, although all data were collected from reports or from direct contact with the authors, many items on the “risk of bias” assessment tool could only be classified as “unclear.” Second, different Chinese herbal medicine interventions were grouped together for analysis in some cases. The results might have been compromised by the heterogeneity within each Chinese herbal medicine intervention and the study design. Third, the concept of TCM syndrome was not considered when analyzing the data, as all studies only considered Childhood Pneumonia, not TCM symptoms. Therefore, the actual therapeutic effect might not have been fully captured.
Based on these reasons, the TCM RCTs should be conducted in accordance with the Consolidated Standards of Reporting Trials for Traditional Chinese Medicine [29] detailed report.
7. Conclusions
Chinese herbal medicines may increase total effective rates, improve clinical symptoms and signs, and shorten the length of hospital stay for children with pneumonia. In a word, Chinese herbal medicines are effective for Childhood Pneumonia. However, there is insufficient evidence to confirm whether Chinese herbal medicines decrease adverse effects, mortality, and TCM outcomes (such as tongue coat and pulse condition). All results were supported by poor methodological quality studies. Thus, larger, multicenter, high methodological quality studies of Chinese herbal medicines for Childhood Pneumonia are needed. These studies should include patients with Childhood Pneumonia and interventions with Chinese herbal medicines. More data, particularly concerning adverse events, are necessary. Meanwhile, future studies should determine the most appropriate drug and dosage for Childhood Pneumonia. So, further trials would help to clarify the validity of the findings of this paper and could determine more clearly the role of Chinese herbal medicines in Childhood Pneumonia in comparison with other therapies. Consequently, the purpose is to guide our application in clinical.
Conflict of Interests
The authors declare no conflict of interests.
Acknowledgments
The authors thank the Department of Nephrology Laboratory, Guangdong Province Hospital of Chinese Medicine. The authors thank the Department of Nephropathy Medicine, Guangdong Province Hospital Of Chinese Medicine. The project was support by the Scientific Research Project of Public Welfare Industry, State Administration of Traditional Chinese Medicine of China (no. 200707004).
References
- 1.Cohen AL, Hyde TB, Verani J, et al. Integrating pneumonia prevention and treatment interventions with immunization services in resource-poor countries. Bulletin of the World Health Organization. 2012;90:289–294. doi: 10.2471/BLT.11.094029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anthony J, Scott G, Wonodi C, Moisi JC, et al. The definition of pneumonia, the assessment of severity, and clinical standardization in the pneumonia etiology research for child health study. Clinical Infectious Diseases. 2012;54(S2):S109–S116. doi: 10.1093/cid/cir1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mathisen M, Strand TA, Sharma BN, et al. RNA viruses in community-acquired childhood pneumonia in semi-urban Nepal; a cross-sectional study. BMC Medicine. 2009;7, article no. 35 doi: 10.1186/1741-7015-7-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Michelow IC, Olsen K, Lozano J, et al. Epidemiology and clinical characteristics of community-acquired pneumonia in hospitalized children. Pediatrics. 2004;113(4 I):701–707.e7. doi: 10.1542/peds.113.4.701. [DOI] [PubMed] [Google Scholar]
- 5.Tafuri S, Martinelli D, Grimaldi A, et al. 23-valent pneumococcal vaccine failure in a patient who developed pneumonia: a case report. The New Microbiologica. 2011;34:417–420. [PubMed] [Google Scholar]
- 6.United Nations Children's Fund. Pneumonia: the Forgotten Killer of Children. New York, NY, USA: United Nations Children's Fund; 2006. [Google Scholar]
- 7.Zhao GM, Black S, Shinefield H, Eskola J. Rates of hospitalization and mortality for pneumonia and respiratory illness in China. Proceedings of the 5th International Symposium on Pneumococci and Pneumococcal Diseases; 2006; PO2.08. [Google Scholar]
- 8.Rudan I, Boschi-Pinto C, Biloglav Z, Mulholland K, Campbell H. Epidemiology and etiology of childhood pneumonia. Bulletin of the World Health Organization. 2008;86(5):408–416. doi: 10.2471/BLT.07.048769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.The WHO Young Infant Study Group. Bacterial etiology of serious infections in young infants in developing countries: results of multicentre study. Pediatric Infectious Diseases Journal. 1999;18:17–22. doi: 10.1097/00006454-199910001-00004. [DOI] [PubMed] [Google Scholar]
- 10.Korppi M, Kiekara O, Heiskanen-Kosma T, Soimakallio S. Comparison of radiological findings and microbial aetiology of childhood pneumonia. Acta Paediatrica, International Journal of Paediatrics. 1993;82(4):360–363. doi: 10.1111/j.1651-2227.1993.tb12697.x. [DOI] [PubMed] [Google Scholar]
- 11.Hersh AL, Shapiro DJ, Newland JG, Polgreen PM, Beekmann SE, Shah SS. Variability in pediatric infectious disease consultants’ recommendations for management of community-acquired pneumonia. PLoS ONE. 2011;6(5):1–6. doi: 10.1371/journal.pone.0020325.e20325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kabra SK, Lodha R, Pandey RM. Antibiotics for community acquired pneumonia in children. doi: 10.1002/14651858.CD004874.pub2. Cochrane Database of Systematic Reviews. In press. [DOI] [PubMed] [Google Scholar]
- 13.Wu T, Zhang J, Qiu Y, Xie L, Liu GJ. Chinese medicinal herbs for the common cold. Cochrane Database of Systematic Reviews. 2007;(1) doi: 10.1002/14651858.CD004782.pub2.CD004782 [DOI] [PubMed] [Google Scholar]
- 14.Li X, Zhang YP, Lou JB. Ma Xing Shi Gan Tang to treat respiratory disease research progress. Journal of Guiyang College of Traditional Chinese Medicine. 2010;32(5):66–68. [Google Scholar]
- 15.Wang HM, Ge JL, Mo D. Azithromycin combined with traditional Chinese Ma Xing Shi Gan Tang for children pneumonia and mycoplasma in 106 cases. Medical Journal of Chinese People's Health. 2009;21(19):2387–2389. [Google Scholar]
- 16.Zhao XC, Ji WY. Maxing Shigan decoction combined with azithromycin for children pneumonia clinical observation. Chinese Journal of Drug Abuse Prevention and Treatment. 2009;15(6):358–360. [Google Scholar]
- 17.Guo XH. Integration of traditional Chinese and Western medicine for Children Pneumonia on 86 cases. Fujian Medical Journal. 1999;21(2, article 90) [Google Scholar]
- 18.Zhang YM. San Ao Tang auxiliary treatment for children mycoplasma pneumonia infection observation of curative effect. Chinese Traditional and Herbal Drugs. 2012;43(2):341–342. [Google Scholar]
- 19.He DJ. Combination of traditional Chinese and Western medicine for Childhood pneumonia in observation of clinical effect. Journal China Health Monthly. 2011;30(7):10–11. [Google Scholar]
- 20.He GP, Lei HX, Du HH. Combination of traditional Chinese and Western medicine for 40 cases of pneumonia cough. Journal of Shaanxi College of Traditional Chinese Medicine. 2011;34(6):36–37. [Google Scholar]
- 21.Zhang YM. San Ao Tang auxiliary treatment for children mycoplasma pneumonia infection observation of curative effect. Chinese Traditional and Herbal Drugs. 2012;43(2):341–342. [Google Scholar]
- 22.Wang SY, Pang WD, Chu QY, et al. Modified Zhi Sou San combined with erythromycin in treatment of Mycoplasma pneumonia curative effect analysis. Modern Journal of Integrated Traditional Chinese and Western Medicine. 2009;18(23):2797–2798. [Google Scholar]
- 23.Lv SQ, Xie D, Xia SF, et al. Combination of traditional Chinese and Western medicine for childhood mycoplasma pneumonia clinical practice. Chinese Journal of Maternal and Child Health Care. 2009;24(22):3093–3094. [Google Scholar]
- 24.Lei Z. Combination of traditional Chinese and Western medicine for 80 cases childhood pneumonia clinical observation. Journal of Traditional Chinese Medicine Information. 2010;2(1, article 91) [Google Scholar]
- 25.Shi YF. Combination of traditional Chinese and Western medicine for childhood mycoplasma pneumonia in 40 cases. Chinese Pediatrics of Integrated Traditional and Western Medicine. 2009;1(4):364–365. [Google Scholar]
- 26.Pan WQ. Combined medication for community acquired pneumonia in children 70 cases. Chinese Medicine Modern Distance Education of China. 2011;9(20):56–57. [Google Scholar]
- 27.Duan XZ, Feng XC. Reduning Injection for childhood Mycoplasma pneumoniae on clinical study. Journal of Changchun University of Traditional Chinese Medicine. 2011;27(2):171–172. [Google Scholar]
- 28.Wei H, Feng SK. Observation on effect of Chuanhuning Injection for childhood pneumonia. Journal of Youjiang Medical College for Nationalities. 2003;25(4, article 516) [Google Scholar]
- 29.Wu TX, Shang HC, Bian ZX. Randomized controlled pragmatic trial: concept, design, practice. Chinese Journal of Evidence-Based Medicine. 2009;9(12):1277–1280. [Google Scholar]


