Abstract
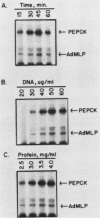
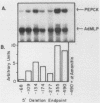
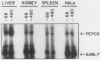
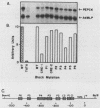
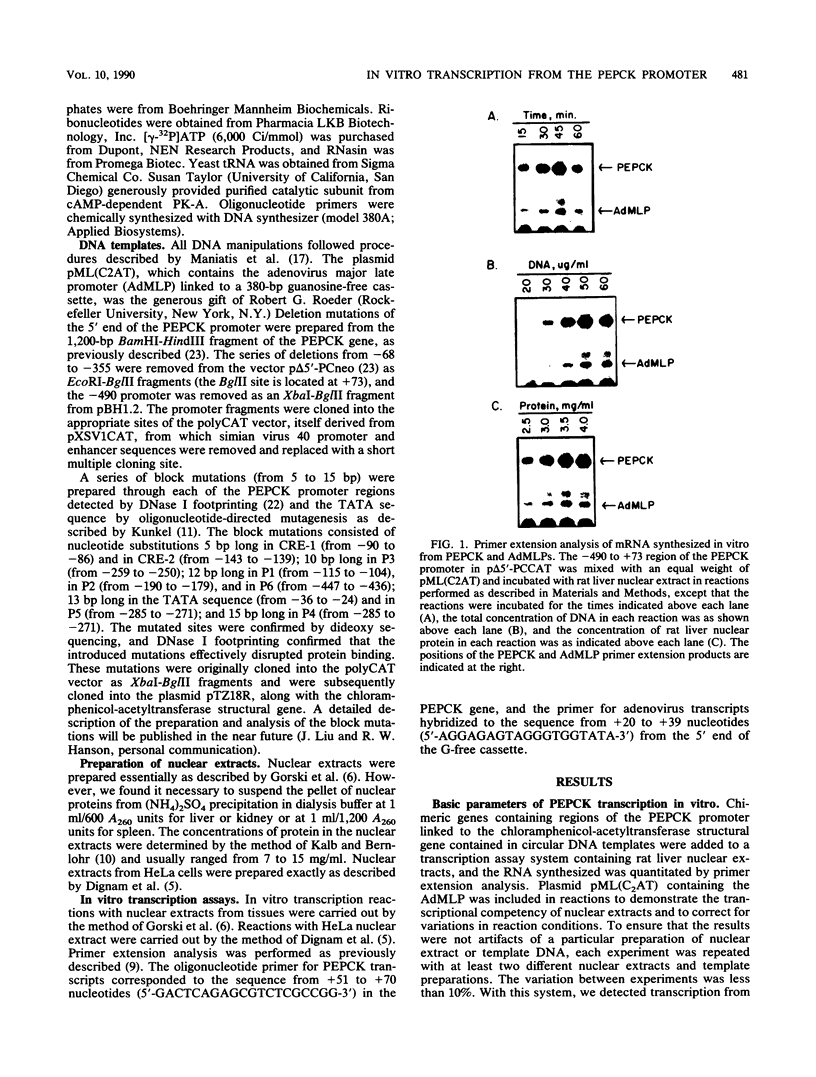
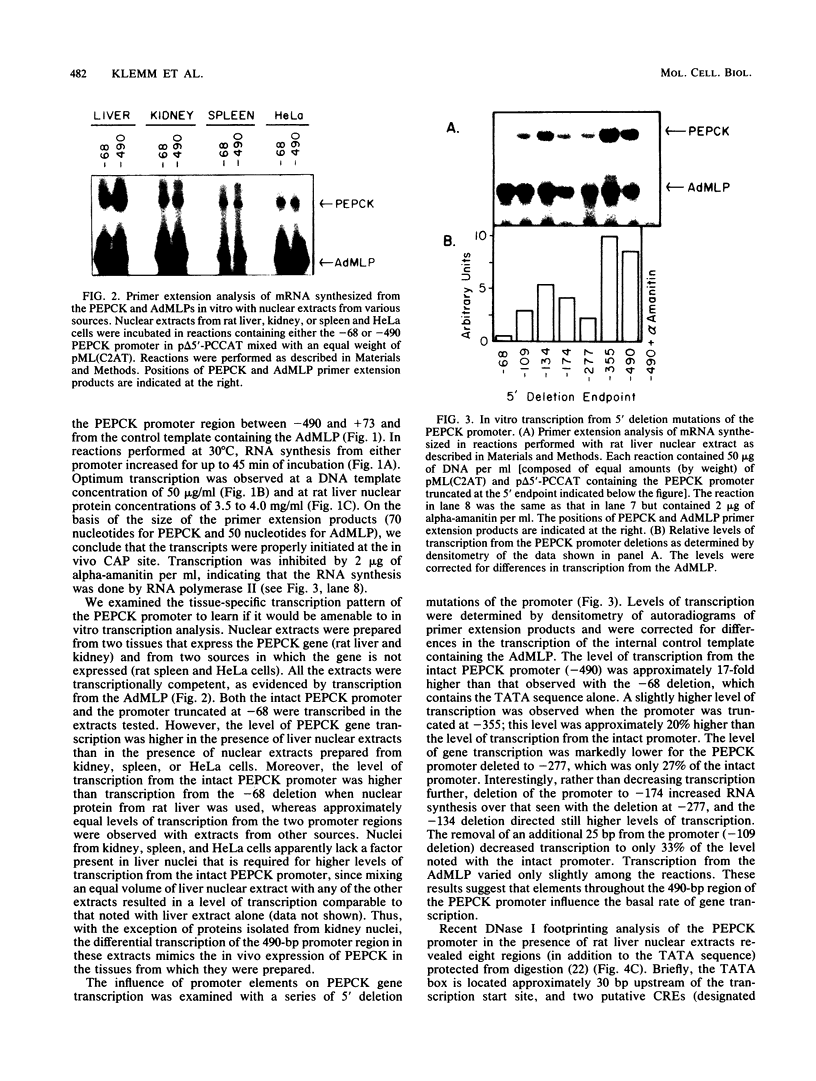
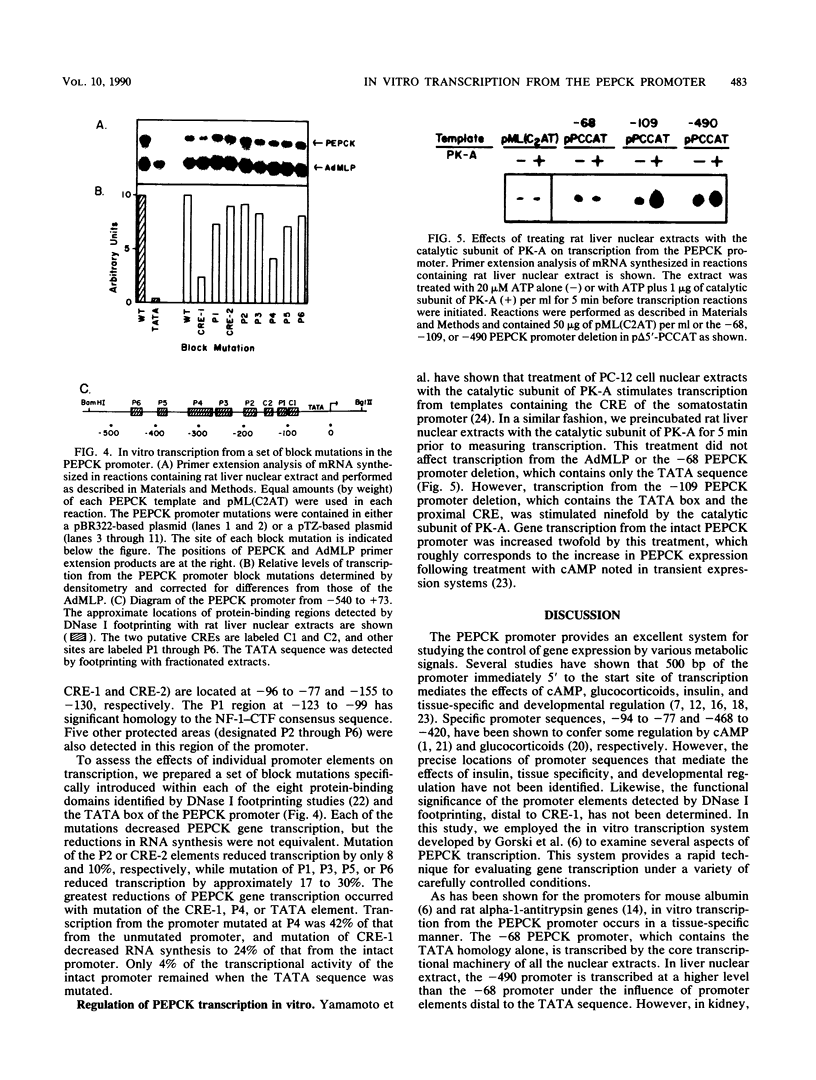
A cell-free system for the study of transcription from the promoter of the phosphoenolpyruvate carboxykinase (GTP) gene by using nuclear extracts from rat tissues was developed. The level of basal transcription from the phosphoenolpyruvate carboxykinase (PEPCK) promoter between -490 and +73 was highest when extracts from liver nuclei, rather than kidney, spleen, and HeLa nuclear extracts, were used. A series of 5' deletions and block mutations were also tested for their effects on basal transcription in vitro. The promoter truncated to -355 had the highest rate of basal transcription, while subsequent deletion to -277 markedly decreased the rate of transcription. Further deletion of the promoter to -134 resulted in a twofold increase in the basal level of transcription compared with that of the promoter deleted to -277. However, subsequent deletion of the NF-1-CCAAT-binding transcription factor binding site or the proximal cyclic AMP (cAMP) regulatory element caused a decrease in basal transcription. Block mutations were inserted into nine specific protein-binding regions of the PEPCK promoter previously shown to be of functional significance or to bind nuclear proteins. Mutation of the TATA box resulted in a 94% decrease in the level of transcription noted with the intact promoter, while sequence substitutions within the proximal cAMP regulatory element decreased the transcription rate to 25%. The addition of the catalytic subunit of cAMP-dependent protein kinase to the in vitro system stimulated transcription from the intact promoter or from a promoter deletion to -109. However, a promoter deletion to -68, which removes the proximal cAMP regulatory element, was unresponsive to added protein kinase catalytic subunit. These findings indicate that the PEPCK promoter between -490 and +73 contains sequences responsive to hormonal and tissue-specific factors in nuclei from rat tissues. The sensitivity of this in vitro transcription system closely mimics the process regulating PEPCK transcription in rat tissues and should make it ideal for testing the function of purified transcription factors.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bokar J. A., Roesler W. J., Vandenbark G. R., Kaetzel D. M., Hanson R. W., Nilson J. H. Characterization of the cAMP responsive elements from the genes for the alpha-subunit of glycoprotein hormones and phosphoenolpyruvate carboxykinase (GTP). Conserved features of nuclear protein binding between tissues and species. J Biol Chem. 1988 Dec 25;263(36):19740–19747. [PubMed] [Google Scholar]
- Corthésy B., Hipskind R., Theulaz I., Wahli W. Estrogen-dependent in vitro transcription from the vitellogenin promoter in liver nuclear extracts. Science. 1988 Mar 4;239(4844):1137–1139. doi: 10.1126/science.2830672. [DOI] [PubMed] [Google Scholar]
- Delegeane A. M., Ferland L. H., Mellon P. L. Tissue-specific enhancer of the human glycoprotein hormone alpha-subunit gene: dependence on cyclic AMP-inducible elements. Mol Cell Biol. 1987 Nov;7(11):3994–4002. doi: 10.1128/mcb.7.11.3994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deutsch P. J., Jameson J. L., Habener J. F. Cyclic AMP responsiveness of human gonadotropin-alpha gene transcription is directed by a repeated 18-base pair enhancer. Alpha-promoter receptivity to the enhancer confers cell-preferential expression. J Biol Chem. 1987 Sep 5;262(25):12169–12174. [PubMed] [Google Scholar]
- Dignam J. D., Lebovitz R. M., Roeder R. G. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 1983 Mar 11;11(5):1475–1489. doi: 10.1093/nar/11.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorski K., Carneiro M., Schibler U. Tissue-specific in vitro transcription from the mouse albumin promoter. Cell. 1986 Dec 5;47(5):767–776. doi: 10.1016/0092-8674(86)90519-2. [DOI] [PubMed] [Google Scholar]
- Granner D., Andreone T., Sasaki K., Beale E. Inhibition of transcription of the phosphoenolpyruvate carboxykinase gene by insulin. Nature. 1983 Oct 6;305(5934):549–551. doi: 10.1038/305549a0. [DOI] [PubMed] [Google Scholar]
- Jameson J. L., Jaffe R. C., Deutsch P. J., Albanese C., Habener J. F. The gonadotropin alpha-gene contains multiple protein binding domains that interact to modulate basal and cAMP-responsive transcription. J Biol Chem. 1988 Jul 15;263(20):9879–9886. [PubMed] [Google Scholar]
- Kalb V. F., Jr, Bernlohr R. W. A new spectrophotometric assay for protein in cell extracts. Anal Biochem. 1977 Oct;82(2):362–371. doi: 10.1016/0003-2697(77)90173-7. [DOI] [PubMed] [Google Scholar]
- Kunkel T. A., Roberts J. D., Zakour R. A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Methods Enzymol. 1987;154:367–382. doi: 10.1016/0076-6879(87)54085-x. [DOI] [PubMed] [Google Scholar]
- Lamers W. H., Hanson R. W., Meisner H. M. cAMP stimulates transcription of the gene for cytosolic phosphoenolpyruvate carboxykinase in rat liver nuclei. Proc Natl Acad Sci U S A. 1982 Sep;79(17):5137–5141. doi: 10.1073/pnas.79.17.5137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langer S. J., Ostrowski M. C. Negative regulation of transcription in vitro by a glucocorticoid response element is mediated by a trans-acting factor. Mol Cell Biol. 1988 Sep;8(9):3872–3881. doi: 10.1128/mcb.8.9.3872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Shen R. F., Tsai S. Y., Woo S. L. Multiple hepatic trans-acting factors are required for in vitro transcription of the human alpha-1-antitrypsin gene. Mol Cell Biol. 1988 Oct;8(10):4362–4369. doi: 10.1128/mcb.8.10.4362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loose D. S., Cameron D. K., Short H. P., Hanson R. W. Thyroid hormone regulates transcription of the gene for cytosolic phosphoenolpyruvate carboxykinase (GTP) in rat liver. Biochemistry. 1985 Aug 13;24(17):4509–4512. doi: 10.1021/bi00338a004. [DOI] [PubMed] [Google Scholar]
- Magnuson M. A., Quinn P. G., Granner D. K. Multihormonal regulation of phosphoenolpyruvate carboxykinase-chloramphenicol acetyltransferase fusion genes. Insulin's effects oppose those of cAMP and dexamethasone. J Biol Chem. 1987 Nov 5;262(31):14917–14920. [PubMed] [Google Scholar]
- McGrane M. M., de Vente J., Yun J., Bloom J., Park E., Wynshaw-Boris A., Wagner T., Rottman F. M., Hanson R. W. Tissue-specific expression and dietary regulation of a chimeric phosphoenolpyruvate carboxykinase/bovine growth hormone gene in transgenic mice. J Biol Chem. 1988 Aug 15;263(23):11443–11451. [PubMed] [Google Scholar]
- Nakagawa J., von der Ahe D., Pearson D., Hemmings B. A., Shibahara S., Nagamine Y. Transcriptional regulation of a plasminogen activator gene by cyclic AMP in a homologous cell-free system. Involvement of cyclic AMP-dependent protein kinase in transcriptional control. J Biol Chem. 1988 Feb 15;263(5):2460–2468. [PubMed] [Google Scholar]
- Petersen D. D., Magnuson M. A., Granner D. K. Location and characterization of two widely separated glucocorticoid response elements in the phosphoenolpyruvate carboxykinase gene. Mol Cell Biol. 1988 Jan;8(1):96–104. doi: 10.1128/mcb.8.1.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn P. G., Wong T. W., Magnuson M. A., Shabb J. B., Granner D. K. Identification of basal and cyclic AMP regulatory elements in the promoter of the phosphoenolpyruvate carboxykinase gene. Mol Cell Biol. 1988 Aug;8(8):3467–3475. doi: 10.1128/mcb.8.8.3467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roesler W. J., Vandenbark G. R., Hanson R. W. Identification of multiple protein binding domains in the promoter-regulatory region of the phosphoenolpyruvate carboxykinase (GTP) gene. J Biol Chem. 1989 Jun 5;264(16):9657–9664. [PubMed] [Google Scholar]
- Short J. M., Wynshaw-Boris A., Short H. P., Hanson R. W. Characterization of the phosphoenolpyruvate carboxykinase (GTP) promoter-regulatory region. II. Identification of cAMP and glucocorticoid regulatory domains. J Biol Chem. 1986 Jul 25;261(21):9721–9726. [PubMed] [Google Scholar]
- Yamamoto K. K., Gonzalez G. A., Biggs W. H., 3rd, Montminy M. R. Phosphorylation-induced binding and transcriptional efficacy of nuclear factor CREB. Nature. 1988 Aug 11;334(6182):494–498. doi: 10.1038/334494a0. [DOI] [PubMed] [Google Scholar]