Abstract
Background
Angiotensin II may be involved in amyloid metabolism in the brain. Angiotensin receptor blockers (ARB) may also prevent cognitive decline. We evaluated the impact of ARBs on the neuropathology in the National Alzheimer’s Coordinating Center (NACC) database which includes aggregated data and brain autopsies from 29 Alzheimer’s Disease Centers throughout the USA.
Methods
Data from Participants were self- or provider-referred and included those with and without cognitive disorders. Our sample included hypertensive participants and excluded cognitively and neuro-pathologically normal participants (n=890, mean (range) age at death 81 (39–107) years, 43% women, 94% white). Neuropathological data included neuritic plaque and neurofibrillary tangle densities assessed by NIA-Reagan criteria and vascular injury markers. Multiple logistic regression was used to compare the pathological findings in subjects on ARBs to other antihypertensive treatment and to those who did not receive antihypertensive medications.
Results
Participants who were exposed to ARBs, with or without AD, showed less amyloid deposition markers compared to those treated with other antihypertensives (lower CERAD OR=0.47, 95% confidence interval =0.27 to 0.81; ADRDA OR=0.43, CI=0.21 to 0.91; BRAAK & BRAAK OR=0.52, CI= 0.31 to 0.85; neuritic plaques OR=0.59, CI=0.37 to 0.96). They also had less AD-related pathology compared to untreated hypertensives. Participants receiving ARBs were more likely to have had a stroke and hence had more frequent pathological evidence of large vessel infarct and hemorrhage.
Conclusion
Treatment with ARBs is associated with less AD-related pathology on autopsy evaluations. The effect of ARBs on cognitive decline in those with dementia or AD needs further investigation.
Introduction
Observational studies suggest that angiotensin receptor blockers (ARBs) may have superior effects on cognitive function compared to other antihypertensives. In a recent large cohort of American veterans, Li et al reported that ARBs are associated with lower risk of dementia and Alzheimer's Disease (AD) compared to other antihypertensive agents, including angiotensin converting enzyme inhibitors (ACEI).1 The association between concurrent elevation in blood pressure and AD is not consistent.2–4 This may be in part related to the inconsistent processes by which AD was adjudicated and confirmed. A pathological examination is the most accurate process to confirm AD but is not feasible in many observational studies and clinical trials.
AD is related to increased amyloid deposition in the brain.5 The impact of antihypertensive medications on amyloid metabolism is not known. Animal studies suggest that Angiotensin II promotes production of amyloid centrally. We therefore hypothesized that ARBs would be associated with decreased amyloid deposition.6 Animal studies suggest that Angiotensin receptor blockers (ARBs) may decrease A-beta (Aβ) oligomerization.7 Additionally, a recent study in APP-PS1 mice showed that treatment with intranasal losartan decreased Aβ plaques by 3.7-fold compared to saline.8 In humans, it is not known if ARBs are also related to decreased AD pathology and amyloid deposition.
Vascular dementia and related vascular brain injury are also common in hypertension and the impact of antihypertensive medications is unknown.9, 10 Few longitudinal studies include brain autopsy and medication and blood pressure data. The National Alzheimer’s Coordinating Center (NACC) includes brain autopsy series. We therefore aimed to investigate the impact of treatment with ARB on the neuropathological changes related to amyloid and vascular pathology.
Methods
Sample
Data from the National Alzheimer’s Coordinating Center (NACC), which included aggregate data from 29 Alzheimer’s Disease Centers (ADCs) across the US collected between September 2005 and June 2011, was used for this analysis (details described elsewhere).11 Participants were either provider-referred or self-referred and were with or without cognitive disorders. Informed consent was obtained from all subjects including an optional brain autopsy. Data were collected annually using the NACC Uniform Dataset (UDS) data collection protocol and included demographic information, education, marital status, tobacco use, family history of dementia, and body mass index (BMI). Medical information included medical history (Cardiovascular Disease (CVD), stroke/transient ischemic attack (TIA), Parkinson’s disease, brain trauma, hypercholesterolemia, hypertension, diabetes, thyroid disease, and B12 deficiency) and current medications (taken within the last two weeks including antihypertensive, lipid lowering, anticoagulant (such as warfarin or aspirin), antidiabetic, antidepressive, and anti-psychotic medications). Neuropsychiatric data elements included Geriatric Depression Scale (GDS), Mini-Mental State Examination (MMSE), Trail Making Test, logical memory test, digit span test and Clinical Dementia Rating scale (CDR), a global measure of dementia stage).11 If more than one evaluation was done, we used the last one before death in our analyses. Data collection and recording was performed by trained personnel and clinicians. Cognitive disorder diagnoses were made by either a consensus team or a physician who performed a detailed examination (http://www.alz.washington.edu/WEB/study-pop.html). Clinicians reviewed all available information and provided a clinical diagnosis for each participant as cognitively normal, mild cognitive impairment (MCI), AD, vascular dementia, mixed dementia, or other types of dementia. A pathological diagnosis was ascertained by the trained neuropathologist and classified participants as normal pathology, AD, vascular dementia, mixed dementia, or other dementia. We restricted this analysis to hypertensive subjects with available brain autopsy data, medication information and blood pressure measurements from at least one visit. We excluded those with normal cognitive function as assessed by the clinician on the last assessment and those with a pathological diagnosis of normal brain as assessed by the neuropathologist.
Hypertension and antihypertensive medications
Subjects meeting criteria for hypertension included self-reported hypertension, systolic blood pressure ≥140 or diastolic blood pressure ≥90, or receiving antihypertensive medication during at least one visit. Antihypertensive medications reported at each visit were identified and categorized into their broad corresponding classes: ARBs, ACEI, beta blockers (BB), calcium channel blockers (CCB), diuretics, anti-adrenergic agents, and aldosterone antagonists. Participants receiving more than one antihypertensive medication were categorized into more than one class. Participants receiving ARBs at any visit were grouped into the ARBs group, including those on more than one class. Our comparison groups were those treated with antihypertensive medications other than ARBs and untreated hypertensives.
Neuropathological outcomes
Neuropathological data were collected using a standard protocol by trained neuropathologists. AD-related pathology was assessed using several measures. (1) The Consortium to Establish a Registry of Alzheimer’s Disease (CERAD) score measured neuritic plaque counts with adjustments for age and clinical history in the most densely populated 1 sq. mm area of each brain region. The neuritic plaque count was measured in the most severely affected cortical region and was rated as none, sparse, moderate, and frequent.12 Neuritic plaques were identified as plaques with argyrophilic, thioflavin-S-positive, or tau-positive dystrophic neuritis with or without dense amyloid cores. (2) Khachaturian (NIA) criteria measured the density of the neocortical plaques per unit area, corrected for age: age <50 years neurofibrillary tangles and senile or neuritic plaques in the neocortex> 2 to 5 per field; 50–65 years: tangles/ plaques>=8 per field; 66–75 years: tangles/ plaques >10 per field; >?75 years: tangles may or may not be found and senile plaques >15 per field.13 (3) The Braak & Braak neuritic plaque score was classified as: no evidence of neurofibrillary degeneration; stages I–II, the neurofibrillary tangles preferentially involve the entorhinal-perirhinal cortex; stages III–IV, tangles also accumulate in hippocampus and other limbic regions with limited neocortical involvement; stage V, neurofibrillary changes occur in association cortices; stage VI, neurofibrillary changes occur in primary sensory cortex.14, 15
Vascular-related measures included: presence or absence of large artery cerebral infarcts (infarcts larger than 1cm in diameter in the distribution of large and medium-sized meningocerebral vessels); micro infarcts or lacunes (1cm or less detected in the cortical areas microscopically). Large and micro infarcts were included regardless of the histologic age and included acute and chronic cystic lesions. Cerebral hemorrhages were also reported irrespective of size and brain region. Subcortical arteriosclerotic leukoencephalopathy was defined as multifocal or diffuse white matter pathology due to arteriosclerotic small vessel disease.16 Measures of the severity of atherosclerotic disease of the circle of Willis and of arteriosclerosis in the small parenchymal and/or leptomeningeal vessels were scored subjectively as none, mild, moderate, or severe.
Data Analysis
We compared participants exposed to ARBs to two groups: participants treated with other antihypertensive medications and hypertensives who were not treated during follow-up. We used Chi-square or analysis of variance (ANOVA) to compare these three groups on demographic, social, cognitive, medication, and clinical characteristics including clinical diagnosis for the cognitive disorder. Neuropathological outcomes were grouped into dichotomous dependent variables (absent vs present) to simplify the interpretation/presentation of results. We used multiple logistic regression with the primary independent variable being exposure to ARBs compared to other antihypertensives or untreated hypertensives. To address the issue of exposure to multiple antihypertensive medications, we initially restricted our sample to those exposed to only one class of antihypertensive medication; since the results did not significantly change, we present the results for the full sample. All models were adjusted for covariates that may impact cognitive function: age at death, sex, BMI, clinical stroke, APOE-e4 alleles (included as with or without the e4 allele), and systolic blood pressure (mean during the evaluation period). All analyses were conducted using SAS version 9.2 (Cary, NC).
Results
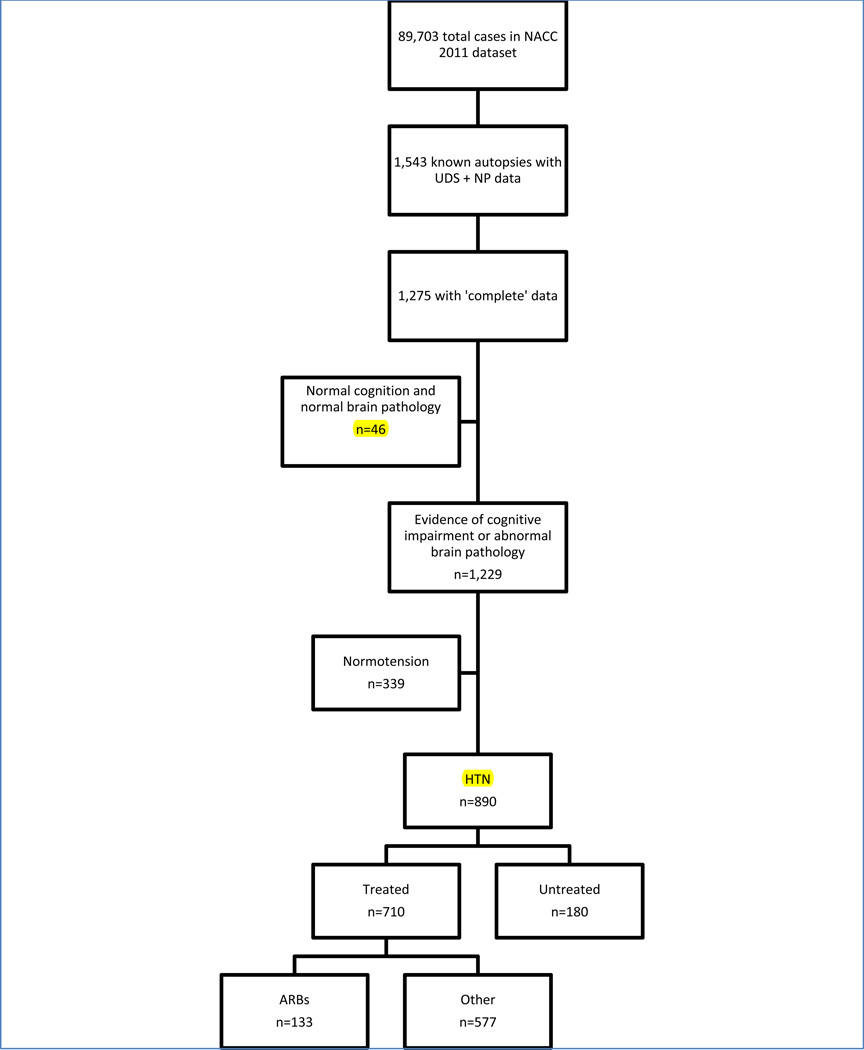
As shown in Figure-1, of the 1,275 subjects who had brain autopsy and available pathological and clinical data, 339 (27%) were not hypertensive and 46 (4%) individuals were clinically and neuropathologically diagnosed as normal and were excluded, leading to a final sample size of 890. Among our analysis sample, 710 (80%) were treated with antihypertensive medications (133 (15%) were treated with ARBs and 577 (64%) were treated with other antihypertensives) and 180 (20%) were untreated. Of the 133 who reported using ARBs, 56% reported using ARBs during only 1 visit, 27% during 2 visits, and 17% during more than 2 visits. The median age at death was 83 years (25th percentile: 75 years and 75th percentile: 90 years), 94% were white, and 43% were women. Table 1 presents the demographic, cognitive, and clinical characteristics of the 3 groups. When comparing all three groups, those who received ARBs were slightly older at death (p<0.001), had significantly higher BMI (p=0.02), and had higher scores on MMSE (p=0.001), logical memory (p=0.013) and CDR (p=0.01) relative to the other two groups. Median time between enrollment and death was 2 years (25th percentile 1 year and 75th percentile: 3 years) and median number of visits was 2 (range 1–6). Participants who were exposed to ARBs were less likely to have a clinical diagnosis of AD compared to those treated with other antihypertensive medications or untreated hypertensive participants (p=0.0001).
Figure 1.
Description of sample sizes from the original NACC 2011 dataset to our final sample of 890 hypertensive participants.
Table 1.
Demographic, social, neuropsychological, and clinical characteristics of the study sample.
| ARBS | Other antihypertensives |
No Treatment |
P-value | |
|---|---|---|---|---|
| n | 133 | 577 | 180 | |
| Age at death, years (SD) | 82.6(9.6) | 82.3(10.7) | 76.7(12.0) | <0.0001 |
| Gender (%female) | 49% | 42% | 42% | 0.35 |
| Race (%white) | 96% | 94% | 96% | 0.42 |
| Education, % ≥12 years | 95% | 90% | 94% | 0.22 |
| Marital Status (%married) | 60% | 58% | 70% | 0.09 |
| Family history of dementia | 60% | 60% | 63% | 0.74 |
| APOE-4, % | 41% | 46% | 50% | 0.38 |
| APOE-2, % | 13% | 12% | 10% | 0.67 |
| Health History | ||||
| Cardiovascular Disease | 46% | 44% | 14% | <0.0001 |
| Stroke | 20% | 17% | 10% | 0.03 |
| Parkinson’s Disease | 6% | 5% | 4% | 0.66 |
| Head Trauma | 11% | 11% | 13% | 0.61 |
| Hypercholesterolemia | 50% | 47% | 37% | 0.02 |
| Diabetes | 24% | 11% | 4% | <0.0001* |
| Never smoked | 52% | 55% | 57% | 0.91 |
| Body Mass Index, kg/m2 (SD) | 27.28 (5.26) | 26.21 (4.71) | 25.79 (4.26) | 0.045 |
| Systolic Blood pressure at enrollment in mm HG, (SD) | 132 (20) | 132 (20) | 143 (18) | <0.0001 |
| Systolic Blood pressure at last visit in mm HG, (SD) | 122 (22) | 129 (22) | 119 (27) | 0.68 |
| Geriatric Depression Scale, (SD) | 3.3(3.1) | 3.2(2.9) | 3.3(2.9) | 0.84 |
| Mini-Mental State-Exam (SD) | 19.7(9.0) | 16.6(9.6) | 15.7(9.2) | 0.001* |
| Logical memory (%<median of whole sample) | 37% | 50% | 57% | 0.013 |
| Trails Making Test, Part B in sec(SD) | 187 (90) | 200(88) | 210 (93) | 0.27 |
| Digit span forward, (SD) | 6 (1) | 6 (1) | 6 (1) | 0.17 |
| Digit span backward (SD) | 4 (1) | 4 (2) | 4(1) | 0.05 |
| Final Clinical Diagnosis | 0.0001 | |||
| AD | 41% | 53% | 62% | |
| VaD | 4% | 1% | 0% | |
| Mixed AD-VaD | 5% | 4% | 2% | |
| Other dementia | 18% | 17% | 22% | |
| Normal** | 14% | 10% | 6% | |
| Mild cognitive impairment | 18% | 16% | 7% | |
| Global Clinical Dementia Rating Scale | 0.01* | |||
| 0** | 14% | 10% | 6% | |
| 0.5 | 22% | 16% | 13% | |
| ≥1 | 64% | 74% | 81% | |
| Antihypertensive Medications | ||||
| Angiotensin Converting enzyme-inhibitors | 11% | 42% | - | <0.0001 |
| Aldosterone antagonists | 4% | 2% | - | 0.39 |
| Alpha-blockers | 8% | 11% | - | 0.20 |
| Beta Blockers | 42% | 40% | - | 0.63 |
| Calcium channel blockers | 29% | 29% | - | 0.96 |
| Diuretics | 56% | 42% | - | 0.003 |
| Mean # antihypertensives (SD) | 2.5(1.2) | 1.7(0.9) | 0 | <0.0001 |
| % on >1 antihypertensive class | 80% | 47% | 0 | <0.0001 |
| Other Medications | ||||
| Nitrates | 6% | 8% | 1% | 0.006 |
| Choline-esterase Inhibitors | 53% | 61% | 69% | 0.01 |
| Antihyperlipidemic | 48% | 40% | 14% | 0.0006 |
| Oral Hypoglycemic | 5% | 2% | 1% | 0.01* |
| Insulin | 2% | 2% | 0% | 0.12 |
| Non-steroidal anti-inflammatory | 35% | 36% | 17% | <0.0001 |
| Anticoagulants | 38% | 40% | 18% | <0.0001 |
| Antipsychotic | 16% | 22% | 22% | 0.24 |
| Antidepressants | 44% | 53% | 51% | 0.15* |
SD: standard deviation. AD: Alzheimer's Disease; VaD: Vascular Dementia;
Except as indicated, p-values are obtained from chi-square or ANOVA tests, comparing differences among the three study groups.
indicates p<0.05 for the 2 groups-comparisons ARB vs other antihypertensive medications
Those who had CDR=0 and were clinically normal had abnormal neuropathology
Alzheimer's disease pathology
As shown in Table 2, participants exposed to ARBs were also less likely to have received a neuropathological diagnosis of AD using the various pathological criteria: CERAD (p=0.005), ADRDA (p=0.04), and the overall neuropathologist diagnosis (p=0.057). After adjusting for covariates, the association between ARBs and lower likelihood of AD diagnosis remained significant as shown in Table 3. Compared to other antihypertensive medications, exposure to ARBs was associated with a 32% to 35% lower likelihood of AD diagnosis, depending on criteria used. This was also true when we compared ARBs to untreated participants as shown in Table 3.
Table 2.
Pathological diagnoses among participants exposed to ARBs, other antihypertensive medications, or no antihypertensive medications.
| Diagnosis | ARBs | Other Antihypertensives |
No Antihypertensives |
P-value |
|---|---|---|---|---|
| N (890) | 133 | 577 | 180 | |
| CERAD | 0.005 | |||
| Normal | 35% | 22% | 21% | |
| Definite AD | 37% | 54% | 61% | |
| Probable AD | 15% | 12% | 9% | |
| Possible AD | 13% | 11% | 9% | |
| ADRDA/Khachaturian | 0.04 | |||
| AD | 58% | 73% | 77% | |
| Criteria not met | 42% | 27% | 23% | |
| Pathological Diagnosis | 0.057 | |||
| AD | 44% | 57% | 59% | |
| VaD | 11% | 7% | 3% | |
| Mixed AD-VaD | 34% | 26% | 30% | |
| Other dementia | 8% | 8% | 6% | |
| Normal* | 2% | 2% | 2% |
CERAD: Consortium to Establish a Registry of Alzheimer’s Disease; ADRDA: Alzheimer's Disease and Related Disorders Association; AD: Alzheimer's Disease; VaD: Vascular Dementia;
Those with normal pathological dx had abnormal cognitive function
Table 3.
Odds ratios for AD neuropathological scores, vascular brain injury measures, and Athero/arteriosclerosis comparing those exposed to ARBs to those exposed to other antihypertensive medications and those who were not exposed to antihypertensive medications
| ARBs vs other Antihypertensive medications |
ARBs vs Untreated | |||||||
|---|---|---|---|---|---|---|---|---|
| Univariate | Multivariate* | Univariate | Multivariate* | |||||
| OR | CI | OR | CI | OR | CI | OR | CI | |
| CERAD Criteria | 0.53 | (0.34–0.82) | 0.47 | (0.27–0.81) | 0.48 | (0.28–0.82) | 0.47 | (0.24–0.93) |
| ADRDA/Khachaturian Criteria | 0.51 | (0.28–0.93) | 0.43 | (0.21–0.91) | 0.40 | (0.19–0.86) | 0.49 | (0.18–1.30) |
| BRAAK and BRAAK | 0.57 | (0.38–0.87) | 0.52 | (0.31–0.85) | 0.62 | (0.38–1.02) | 0.61 | (0.33–1.13) |
| CERAD Neuritic Plaques Score | 0.64 | (0.43–0.94) | 0.59 | (0.37–0.96) | 0.47 | (0.29–0.75) | 0.55 | (0.30–1.01) |
| Large artery infarcts** | 1.78 | (1.02–3.09) | 2.37 | (1.15–4.89) | 3.34 | (1.47–7.61) | 3.94 | (1.20–13.01) |
| Micro infarcts/lacunes** | 1.01 | (0.67–1.52) | 1.03 | (0.63–1.68) | 1.41 | (0.85–2.35) | 1.10 | (0.59–2.04) |
| Hemorrhage** | 2.16 | (1.13–4.11) | 2.3 | (1.11–4.78) | 2.73 | (1.12–6.65) | 2.99 | (0.99–8.96) |
| Atherosclerosis** | 1.08 | (0.66–1.78) | 0.99 | (0.52–1.88) | 2.31 | (1.34–4.0) | 1.18 | (0.54–2.56) |
| Arteriosclerosis** | 1.18 | (0.68–2.03) | 1.52 | (0.76–3.02) | 2.10 | (1.15–3.84) | 2.4 | (1.11–5.18) |
CERAD: Consortium to Establish a Registry of Alzheimer’s Disease; ADRDA: Alzheimer's Disease and Related Disorders Association;
adjusted for age, gender, systolic blood pressure, BMI, stroke, APOE
Also adjusted for anticoagulants/Aspirin
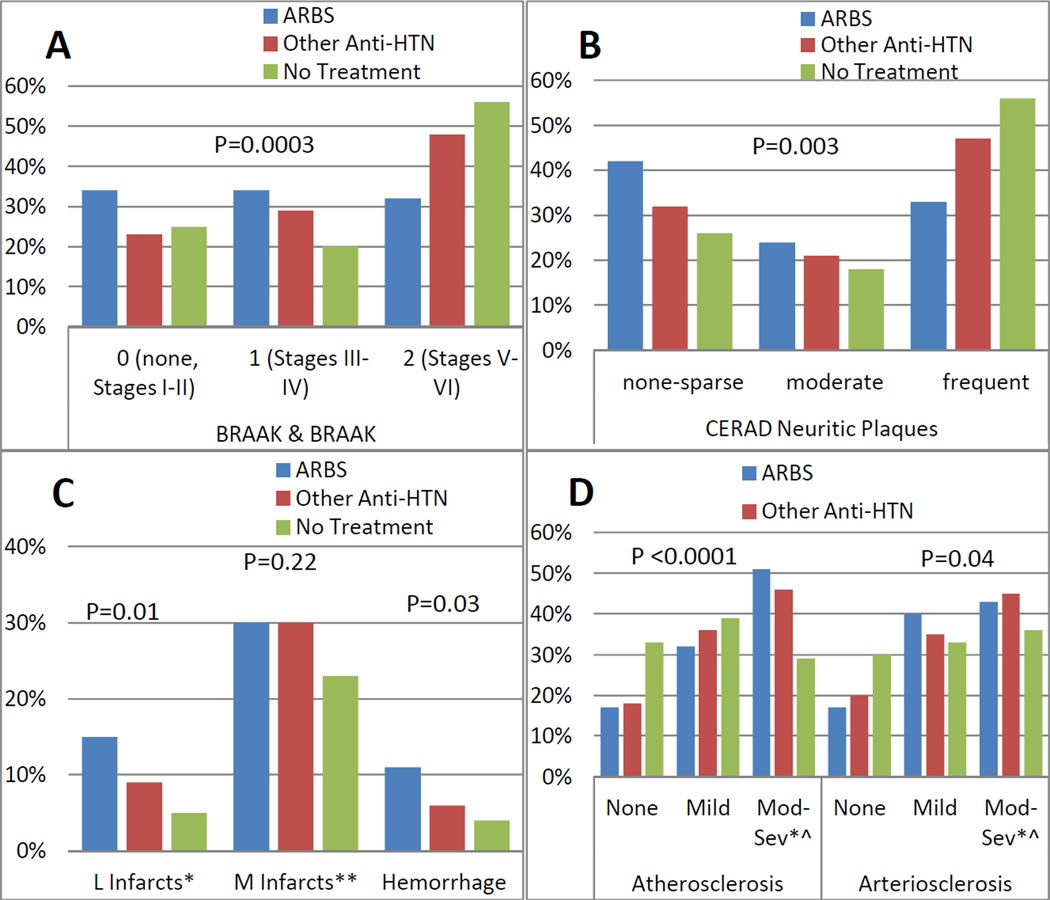
Those exposed to ARBs, with or without a diagnosis of AD, also had less amyloid deposition compared to both untreated participants and those treated with non-ARB antihypertensive medications. As shown in Figure 2A–B, both Braak & Braak (p=0.0003) and the CERAD neuritic plaque count (p=0.003) were lower in the ARB group compared to untreated or treated with other antihypertensives. Odds ratios remained significantly reduced in the multivariate models as shown in Table 3, even after controlling for Apo-E genotype.
Figure 2.
AD neuropathological scores (2A and 2B), vascular brain injury measures (2C), and Athero/arteriosclerosis (2D) in the three groups: those exposed to ARBs, other antihypertensive medications, or no antihypertensive medications.
Vascular-related pathology
Participants who were exposed to ARBs were more likely to have had a stroke (p=0.03). Consistent with this observation, those on ARBs were more likely to show large artery infarcts and hemorrhage on autopsy, as shown in Figure 2C–D. ARB-exposed participants also had greater degrees of atherosclerosis and arteriolosclerosis at autopsy. However, these associations were not significant after adjusting for age, gender, systolic blood pressure, BMI, stroke, APOE-e4 alleles, and prior use of anticoagulants (Table 3), except for higher prevalence of large artery infarct, hemorrhage, and arteriolosclerosis.
When we compared those exposed to ARBs vs those exposed to ACEI (N=362), ARB exposure was associated with lower amyloid deposition (CERAD: adjusted OR=0.43, 95%CI (0.21–0.86), p=0.02) and neuritic plaque count: OR= 0.50, 95%CI (0.28–0.89)p=0.02) compared to ACEI. Finally there was no significant difference between untreated hypertensive participants and those treated with non-ARB antihypertensive medications in either the vascular-related pathology or AD pathology, as shown in Table 4.
Table 4.
Odds ratios for AD neuropathological scores, vascular brain injury measures, and athero/arteriosclerosis comparing those exposed to other antihypertensive medication to those who were not exposed to antihypertensive medications
| Univariate | Multivariate* | |||||
|---|---|---|---|---|---|---|
| OR | Confidence Interval |
P- value |
OR | Confidence Interval |
P- value |
|
| CERAD | 0.81 | (0.51–1.3) | 0.23 | 0.84 | (0.46–1.5) | 0.21 |
| ADRDA/Khachaturian Criteria | 0.74 | (0.39–1.4) | 0.45 | 1.1 | (0.48–2.6) | 0.14 |
| BRAAK Staging | 1.0 | (0.69–1.6) | 0.07 | 1.1 | (0.66–1.9) | 0.06 |
| Neuritic Plaques | 0.62 | (0.41–0.92) | 0.91 | 0.73 | (0.42–1.2) | 0.63 |
| Ischemic, hemorrhagic, or vascular pathology | 1.7 | (1.2–2.7) | 0.12 | 1.5 | (0.88–2.6) | 0.59 |
| Large artery infarcts | 1.8 | (0.85–3.7) | 0.99 | 1.5 | (0.49–4.5) | 0.53 |
| Micro infarcts/lacunes | 1.5 | (0.98–2.2) | 0.24 | 1.0 | (0.62–1.7) | 1.0 |
| Hemorrhage | 1.2 | (0.54–2.6) | 0.3 | 1.3 | (0.46–3.5) | 0.87 |
| Atherosclerosis | 2.0 | (1.3–3.0) | 0.09 | 1.9 | (1.2–3.2) | 0.14 |
| Arteriosclerosis | 1.8 | (1.2–2.7) | 0.29 | 1.5 | (0.86–2.5) | 0.90 |
adjusted for age, gender, systolic blood pressure, BMI, stroke, APOE
Discussion
These autopsy data aggregated across the US Alzheimer Disease Centers demonstrate that hypertensive individuals treated with ARBs are less likely to have a neuropathological diagnosis of AD post-mortem, independent of blood pressure, APOE status, and prior strokes. ARB exposure was also associated with fewer amyloid plaques compared to exposure to other antihypertensive medications or to no antihypertensive medications in those with or without AD diagnosis. Further, ARBs were associated with less AD pathology compared to ACEI.
In a study of 291 post mortem brains, Hoffman et al showed that antihypertensive therapy is associated with lower AD neuropathology, even compared to normotensive individuals.17 In this current study, we provide the first autopsy evidence suggesting that ARBs, rather than other classes of antihypertensive medications, may be associated with reduced amyloid accumulation and AD-related pathological changes. This study provides pathological confirmation to the observation that ARB use is associated with lower AD risk.1
Animal studies have demonstrated that ARBs are associated with decreased amyloid deposition in the brain compared to other antihypertensives.16 Using high throughput drug screening in AD mouse models, Wang et al showed that only valsartan attenuated the oligomerization of Aβ peptides into the high-molecular weight peptides which are known to be associated with cognitive deterioration.7 None of the other antihypertensive classes had this specific positive effect. More recently, Danielyan et al showed that treatment of APP/PS1 mice with intranasal losartan decreased Aβ plaques by 3.7 fold.3 Our study provides the first clinical translational evidence for an association of ARBs with reduced amyloid accumulation in humans.
The association between ARBs and decreased AD-pathology and amyloid accumulation is unique. None of the other antihypertensive medications, including ACE-inhibitors, showed a similar association. Prior evidence suggests that angiotensin converting enzyme (ACE) significantly inhibits fibril formation and aggregation of Aβ in a dose dependent manner suggesting that inhibiting ACE may lead to an increase in Aβ deposition.18 In this analysis, we observed greater amyloid content in those treated with ACEI compared to those treated with ARBs suggesting that ARBs may have a preferential positive effect on AD pathology. These are in agreement with observational data suggesting that ARBs are superior to ACEI in preventing AD and other related dementias. The mechanisms by which ARBs may decrease amyloid accumulation have not been described, but ARB treatment may reduce total Aβ content in the brain in part by facilitating membrane-associated insulin degrading enzyme (IDE)-mediated proteolytic cleavage of Aβ peptides.7
In contrast to the positive effects of ARBS on amyloid deposition and AD-related pathology, we observed greater vascular-related pathology in the ARB group. This is likely related to confounding by indication where ARB users were selected due to their increased vascular risk. This is further confirmed by the greater prevalence of stroke and cardiovascular disease compared to those treated with other antihypertensive medications and those untreated. Adjusting for covariates including anticoagulation accounted for some of the observed associations between ARBs and increased vascular neuropathology. Nevertheless, caution should be used when interpreting our results due to the observational nature of this study. Since fewer individuals on ARBs had a clinical diagnosis of AD, our results should also be interpreted with caution regarding the AD-pathology.
A main limitations to this study is that this a primarily educated Caucasian group who volunteered for this observational study. This limits the generalizability to different populations. Another limitation is the possibility that ARBs were more likely to be prescribed for those with non-AD diagnosis leading to a greater likelihood of diagnosis of vascular dementia and mixed dementia in the ARBs group. Although this is a possible explanation for our findings, the fact that ACEIs which are commonly used in high vascular disease patients did not show this inverse association makes this less likely. Although there may be an intraclass variation in the effects of ARBs on pathology, we were unable to perfrom subgroup analyses due to the relatively small number of participants on ARB. Finally, due to the variable number of followup visits and that the majority reported using ARBs during one or two visits, we were unable to test if the duration of exposure to ARBs have an impact on neuropathology.
The implications of our results are that ARBs may offer an advantage over other antihypertensives in regards to AD risk and pathology. the effect of ARBs in AD under randomized conditions needs further investigation. Further, pre-mortem assessments of amyloid in the brain should be considered in future studies related to antihypertensives and cognitive function as recent evidence suggest an increase in amyloid in healthy older adults measured by Pittsburgh Compound-B (PiB) positron emission tomography.19
Conclusion
This autopsy study suggests that treatment with ARBs is associated with less amyloid accumulation and AD-related pathology independent of other AD risk factors. It is the first human evidence to suggest that ARBs may have a selective beneficial effect on amyloid metabolism. Prospective studies are needed to investigate whether ARBs may have an effect on cognitive decline in those with dementia or AD.
Acknowledgement
The funding supporting the National Alzheimer’s Coordinating Center (NACC) database is grant number U01 AG016976 and enables their ability to deliver data to researchers. Dr. Hajjar is supported by grant 1 K23 AG030057 from the National Institute on Aging (NIA). This work is also supported by grant P50 AG05142 from the NIA to Dr. Mack and Dr. Chui and P01 AG12435 to Dr. Chui.
Contributor Information
Ihab Hajjar, Division of Geriatric, Hospital & General Internal Medicine, Department of Medicine, University of Southern California, Los Angeles, California.
Lauren Brown, Division of Geriatric, Hospital & General Internal Medicine, Department of Medicine, University of Southern California, Los Angeles, California.
Wendy J. Mack, Department of Preventive Medicine, University of Southern California, Los Angeles, California.
Helena Chui, Chair, Department of Neurology, Raymond and Betty McCarron Chair in Neurology, University of Southern California, Los Angeles, California.
References
- 1.Li NC, Lee A, Whitmer RA, et al. Use of angiotensin receptor blockers and risk of dementia in a predominantly male population: prospective cohort analysis. BMJ. 2010;340:b5465. doi: 10.1136/bmj.b5465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guan JW, Huang CQ, Li YH, et al. No Association Between Hypertension and Risk for Alzheimer's Disease: A Meta-Analysis of Longitudinal Studies. Journal of Alzheimer's disease : JAD. 2011 Aug 26; doi: 10.3233/JAD-2011-111160. [DOI] [PubMed] [Google Scholar]
- 3.Israeli-Korn SD, Masarwa M, Schechtman E, et al. Hypertension increases the probability of Alzheimer's disease and of mild cognitive impairment in an Arab community in northern Israel. Neuroepidemiology. 2010;34(2):99–105. doi: 10.1159/000264828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yang YH, Roe CM, Morris JC. Relationship Between Late-Life Hypertension, Blood Pressure, and Alzheimer's Disease. American journal of Alzheimer's disease and other dementias. 2011 Sep 15; doi: 10.1177/1533317511421779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Selkoe DJ. Toward a comprehensive theory for Alzheimer's disease. Hypothesis: Alzheimer's disease is caused by the cerebral accumulation and cytotoxicity of amyloid beta-protein. Annals of the New York Academy of Sciences. 2000;924:17–25. doi: 10.1111/j.1749-6632.2000.tb05554.x. [DOI] [PubMed] [Google Scholar]
- 6.Zhu D, Shi J, Zhang Y, et al. Central angiotensin II stimulation promotes beta amyloid production in Sprague Dawley rats. PloS one. 2011;6(1):e16037. doi: 10.1371/journal.pone.0016037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang J, Ho L, Chen L, et al. Valsartan lowers brain beta-amyloid protein levels and improves spatial learning in a mouse model of Alzheimer disease. The Journal of clinical investigation. 2007 Nov;117(11):3393–3402. doi: 10.1172/JCI31547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Danielyan L, Klein R, Hanson LR, et al. Protective effects of intranasal losartan in the APP/PS1 transgenic mouse model of Alzheimer disease. Rejuvenation research. 2010 Apr-Jun;13(2–3):195–201. doi: 10.1089/rej.2009.0944. [DOI] [PubMed] [Google Scholar]
- 9.Paglieri C, Bisbocci D, Di Tullio MA, Tomassoni D, Amenta F, Veglio F. Arterial hypertension: a cause of cognitive impairment and of vascular dementia. Clinical and experimental hypertension. 2004 May;26(4):277–285. doi: 10.1081/ceh-120034134. [DOI] [PubMed] [Google Scholar]
- 10.Sharp SI, Aarsland D, Day S, Sonnesyn H, Ballard C. Hypertension is a potential risk factor for vascular dementia: systematic review. International journal of geriatric psychiatry. 2011 Jul;26(7):661–669. doi: 10.1002/gps.2572. [DOI] [PubMed] [Google Scholar]
- 11.Beekly DL, Ramos EM, van Belle G, et al. The National Alzheimer's Coordinating Center (NACC) Database: an Alzheimer disease database. Alzheimer disease and associated disorders. 2004 Oct-Dec;18(4):270–277. [PubMed] [Google Scholar]
- 12.Mirra SS, Hart MN, Terry RD. Making the diagnosis of Alzheimer's disease. A primer for practicing pathologists. Archives of pathology & laboratory medicine. 1993 Feb;117(2):132–144. [PubMed] [Google Scholar]
- 13.Khachaturian ZS. Revised criteria for diagnosis of Alzheimer's disease: National Institute on Aging-Alzheimer's Association diagnostic guidelines for Alzheimer's disease. Alzheimer's & dementia : the journal of the Alzheimer's Association. 2011 May;7(3):253–256. doi: 10.1016/j.jalz.2011.04.003. [DOI] [PubMed] [Google Scholar]
- 14.Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta neuropathologica. 1991;82(4):239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- 15.Nagy Z, Yilmazer-Hanke DM, Braak H, Braak E, Schultz C, Hanke J. Assessment of the pathological stages of Alzheimer's disease in thin paraffin sections: a comparative study. Dementia and geriatric cognitive disorders. 1998 May-Jun;9(3):140–144. doi: 10.1159/000017038. [DOI] [PubMed] [Google Scholar]
- 16.Roman GC. Senile dementia of the Binswanger type. A vascular form of dementia in the elderly. JAMA : the journal of the American Medical Association. 1987 Oct 2;258(13):1782–1788. doi: 10.1001/jama.1987.03400130096040. [DOI] [PubMed] [Google Scholar]
- 17.Hoffman LB, Schmeidler J, Lesser GT, et al. Less Alzheimer disease neuropathology in medicated hypertensive than nonhypertensive persons. Neurology. 2009 May 19;72(20):1720–1726. doi: 10.1212/01.wnl.0000345881.82856.d5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hu J, Igarashi A, Kamata M, Nakagawa H. Angiotensin-converting enzyme degrades Alzheimer amyloid beta-peptide (A beta) retards A beta aggregation, deposition, fibril formation inhibits cytotoxicity. The Journal of biological chemistry. 2001 Dec 21;276(51):47863–47868. doi: 10.1074/jbc.M104068200. [DOI] [PubMed] [Google Scholar]
- 19.Langbaum JB, Chen K, Launer LJ, et al. Blood pressure is associated with higher brain amyloid burden and lower glucose metabolism in healthy late middle-age persons. Neurobiology of aging. 2011 Aug 5; doi: 10.1016/j.neurobiolaging.2011.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]