Abstract
Background
The comparative effects of antihypertensive therapy on cerebral hemodynamics in the contest of cognitive decline related to hypertension are unknown.
Objectives
To compare antihypertensive medicationsthat modulate the renin angiotensin system in cerebral hemodynamic and cognitive effects in hypertensive individuals with executive dysfunction.
Design
double-blind randomized clinical trial.
Setting
Community-dwelling
Participants
Subjects were ≥60 years with hypertension and executive dysfunction.
Intervention
lisinopril, candesartan, or hydrochlorothiazide for 1 year
Measurements
Cerebral blood flow velocity (Transcranial Doppler ultrasonography during rest, sitting, standing, hypercapnia, and hypocapnia), cognition and blood pressure were measured at baseline, 6 and 12 months. Linear mixed models were used to compare the3 groups.
Results
Of the 53 enrolled, 47 had successful insonation (mean age=72 years; 70% white; 57% women). There was a tendency for increased blood flow velocity (BFV) in the candesartan groupcompared to a decline in the lisinopril or HCTZ groups (between group p-value =0.57). This was significant in those with relative low BFV at baseline (<median 27.6 cm/sec, between group p-value =0.03). The candesartan group also had the greatest improvement in executive function (Trail Making Test, part B improved by 17.1 seconds vs HCTZ improved by 4.2 seconds and lisinopril worsened by 14.4 seconds, p=0.008). CO2-vasoreactivity and vasomotor range significantly declined in the lisinopril (within-group p-value=0.001 and 0.02 for vasoreactivity and vasomotor range) and HCTZ groups (within-group p-value=0.10 and 0.009 respectively) but not in the candesartan group (within-group p-value=0.25 and 0.38 respectively; between-group p-values= 0.3 and 0.46 respectively).
Conclusion
This pilot study suggests that angiotensin receptor blockers may preferentially preserve cerebral hemodynamics and improved executive function in those with executive dysfunction. These findings warrant further investigation in a larger trial.
Keywords: angiotensin receptor blocker, cerebrovascular circulation, executive function hemodynamics, hypertension
Introduction
Hypertension is associated with cognitive impairment, especially in the executive domain.1–3 Hypertensive individuals who develop executive dysfunction have similar mortality and institutionalization rates as those with dementia,4 have higher mortality rates and greater disability compared to hypertensive individuals without executive dysfunction.5 Hypertension is also associated with a decline in cerebral blood flow velocity (BFV) and cerebrovascular reserve as assessed by vasoreactivity to CO2.6,7 Impaired cerebral blood flow may further contribute to cognitive decline.8 The comparable impact of antihypertensive medications on cerebral hemodynamics especially in the context of executive dysfunction is not well investigated.
Recent evidence suggests that the renin angiotensin system (RAS) is involved in the regulation and maintenance of cerebral blood flow.9 In hypertension, angiotensin II decreases cerebral blood flow10 and impairs neurovascular coupling.11 Our work suggests that polymorphisms in RAS genes are associated with cerebral vasoreactivity to CO2.12 In the brain, angiotensin II exerts its main effects by activating 2 receptors; type 1 which leads to vasoconstriction, endothelial dysfunction and vascular remodeling and type 2 which leads to vasodilatation, neuronal differentiation, decreased inflammation and axonal regeneration.13 Angiotensin receptor blockers (ARB) block the type 1 but not type 2 whereas angiotensin converting enzyme inhibitors (ACEI) decrease angiotensin II production and hence decrease activation of both receptors. We therefore hypothesized that an ARB-based regimen would be superior to other antihypertensive treatments, including ACEI, on cerebral hemodynamics and executive function.
Our objective was to conduct a double blind randomized clinical trial comparing the effect of ARB (candesartan), ACEI (lisinopril) and an active control (hydrochlorothiazide, HCTZ) on cerebral blood flow, cerebrovascular reserve and hemodynamics, and executive function in hypertensive individuals with executive cognitive impairment without dementia.
Methods
The study design is fully described elsewhere.14 Briefly, this was a 12-month double-blind randomized controlled clinical trial of candesartan, lisinopril, or HCTZ. Inclusion criteria were: 60 years or older; hypertension (systolic blood pressure (SBP) of 140 mm Hg or greater or diastolic blood pressure (DBP) 90 mm Hg or greater or receiving antihypertensive medications); and executive dysfunction based on a score less than 10 on the executive clock draw test (CLOX1).15 To exclude those with possible dementia we did not enroll those with a Mini-Mental-State-Exam (MMSE)<2016 or those with a clinical diagnosis of Alzheimer’s disease or other dementias. Exclusion criteria included: intolerance to the study medications; SBP >200 or DBP >110 mm Hg; elevated serum creatinine (>2.0 mg/dl) or serum potassium (5.3 meq/dl) at baseline; receiving >2 antihypertensive medications; congestive heart failure, diabetes mellitus; stroke; and inability to perform the study procedures or unwilling to stop currently used antihypertensive medications. Antihypertensive medications were tapered using a standard protocol described elsewhere.14
Subjects were recruited from the greater Boston area using newspaper announcements, mail-out fliers, and through blood pressure screening activities in the general community. After approval by their primary care providers, subjects receiving antihypertensive medications were tapered and stopped over three weeks. Baseline measurements (off antihypertensive medications) of blood pressure, cognitive function, physical performance, and cerebral blood flow hemodynamics using Transcranial Doppler (TCD) procedures were then completed. Randomization using a computer generated random allocation sequence occurred after baseline data collection. Participants were seen every 2 weeks until blood pressure control (<140/90 mm Hg) was achieved. The Institutional Review Board of the Hebrew SeniorLife approved the study and all participants provided written informed consent. The study was also registered in ClinicalTrials.gov (NCT00605072).
The intervention
Participants were treated with either lisinopril (10 mg increased to 20 mg then 40 mg if needed), candesartan (8 mg increased to 16 mg then 32 mg if needed), or HCTZ (12.5 mg increased to 25 mg if needed). The goal of the intervention was to achieve SBP<140 mm Hg and DBP<90 mm Hg. If this goal was not achieved after maximum doses of the study drugs, then long acting nifedipine (30 mg increased to 60 mg and 90 mg) was added followed by long-acting metoprolol (12.5 mg increased to 25 mg and 50 mg).
Study Procedures
Baseline, 6 and 12 months assessments included questionnaires regarding social habits, family history, and self-reported medical history, a medication inventory, height, weight, amount of physical activity using the Physical Activity Scale for the Elderly17 and functional status using Instrumental Activities of Daily Living (IADL).18 Blood pressure was measured according to the American Heart Association guidelines.19 Two seated blood pressure readings were performed and averaged at each visit. The cognitive battery was described previously and included Trail Making Test (TMT), Hopkins Verbal Learning Test – Revised (HVLT), and the Digit Span Test. 14
Cerebral blood flow hemodynamics
Cerebral blood flow velocity (BFV) was measured at the middle cerebral artery using TCD ultrasonography (2-MHz probe placed over the temporal bone, MultiDop X4, DWL-Transcranial Doppler Systems Inc., Sterling, VA) . End-tidal CO2 was measured using a CO2 Analyzer (Vacumed, Ventura, CA) attached to a nasal cannula. Mean blood flow velocity (BFV) was measured at rest, during changes in end-tidal CO2(breathing a gas with 8% CO2 for 2 minutes and then mildly hyperventilated to an end-tidal CO2 of approximately 25 mmHg for 2 minutes); and blood pressure changes during a sit-to-stand protocol20. Beat to beat heart rate and blood pressure were simultaneously measured using continuous ECG recording and a FinometerTM (FMS, Finapres Measurement Systems, Arnhem, Netherlands). Obtained data were analysed offline using Matlab (Mathworks, Natik, MA). Cerebrovascular resistance (CVR) was calculated as mean arterial pressure/BFV. The difference between CVR sitting and standing (del CVR=CVRstand − CVRsit) was used as an indicator of autoregulation. Vasoreactivity was calculated as the slope of the regression between mean BFV and end-tidal CO2 at each R-R interval. Vasomotor range (VMR) was computed as the increments between minimum mean BFV during hyperventilation and maximum BFV during CO2 breathing. Both measures were used as indicators of cerebrovascular reserve.
Statistical analysis
Baseline comparisons between the 3 randomized groups were performed to evaluate randomization effectiveness using analyses of variance (ANOVA) or chi-square tests. An intention-to-treat analysis was used. Linear mixed models for repeated measures were used to compare the progression of outcomes in the three groups. Age-adjusted least square means were computed for each visit by treatment group; differences among least square means provided tests of mean differences within-group (change over visits) and between-groups. We performed a predefined subgroup analysis for those with low baseline BFV to test the hypothesis that ARBs would improve perfusion in those with significant baseline hypoperfusion (defined as below the median of the enrolled sample). To explore if the change in executive function is related to the changes in cerebral hemodynamics, we first characterized those with stable executive function over the study period (defined as no change or improved scores on TMT, Part B) and those with stable cerebral hemodynamics (defined as no change or improved BFV, CO2-vasoreactivity, and VMR during the study period) . For those who did not have TCD data at 12 months, we used the measure at 6 months to characterize their change. We calculated a concordance rate within each treatment group as the proportion of subjects with both stable cognitive function and stable hemodynamics divided by the number of individuals treated within that group. A higher concordance rate may suggest a greater contribution of hemodynamics in the executive cognitive change. We used the Cochran–Mantel–Haenszel statistics to test the hypotheses that the concordance rates between the three groups differed.21
Results
Of the 63 eligible participants, 53 were successful in tapering their antihypertensive medications and were randomized. Of those 47(89%) had successful insonation of the middle cerebral artery. Of the 53 randomized, 47 completed 6 months (40 had successful TCD insonation) and 31 completed 12 months evaluations (29 had successful TCD insonation). This analysis was restricted to those with successful insonation at baseline(N=47). As shown in Table 1, the three groups were similar in all baseline clinical characteristics, blood pressure and cerebral hemodynamics. They also had similar reported adverse events as shown in Table 2.
Table 1.
Baseline characteristics, sitting and orthostatic blood pressure, and cerebral hemodynamics of those randomized and had successful TCD insonation by study group
| Measure | Lisinopril | Candesartan | HCTZ | p-value |
|---|---|---|---|---|
| N | 17 | 17 | 13 | |
| Age, years | 72 (6) | 72 (9) | 71 (7) | 0.91 |
| Women, % | 59% | 47% | 69% | 0.47 |
| African American, % | 29% | 12 | 31% | 0.71 |
| White, % | 65% | 82% | 62% | |
| High school education or lower,% | 18% | 24% | 15% | 0.84 |
| College education or higher, % | 82% | 76% | 84% | |
| Body Mass Index, Kg/m2 | 29.09 (5.90) | 28.09 (4.12) | 28.96 (7.94) | 0.87 |
| Baseline cognitive function | ||||
| Mini Mental State Examination | 26 (2) | 26 (2) | 25 (2) | 0.15 |
| Executive Clock Draw test | 9 (2) | 9 (2) | 9 (2) | 0.80 |
| Baseline Functional and mood measures | ||||
| Gait speed, m/sec | 1.17 (0.21) | 1.12 (0.38) | 1.03 (0.21) | 0.44 |
| Instrumental Activities of Daily Living | 8 (0) | 8 (0) | 8 (0) | 0.60 |
| Physical Activity Scale in the Elderly | 179 (59) | 150 (61) | 175 (52) | 0.33 |
| Center for Epidemiologic Studies Depression Scale | 8 (7) | 8 (7) | 6 (6) | 0.81 |
| Baseline Biochemical profile | ||||
| Serum Creatinine, mg/dl | 0.90 (0.25) | 1.00 (0.24) | 0.88 (0.30) | 0.37 |
| Serum Potassium, meq/dl | 4.46 (0.37) | 4.47 (0.32) | 4.41 (0.45) | 0.89 |
| Medications | ||||
| Aspirin use, % | 35% | 29% | 30% | 0.93 |
| Statin use, % | 24% | 41% | 31% | 0.54 |
| PrestudyAntihypertensives | ||||
| Diuretics, % | 41% | 24% | 31% | 0.54 |
| ACEI, % | 29% | 29% | 31% | 0.99 |
| ARB, % | 29% | 0% | 23% | 0.06 |
| Calcium channel blockers. % | 0% | 18% | 8% | 0.18 |
| Beta Blockers, % | 24% | 12% | 38% | 0.23 |
| Relevant Medical HX | ||||
| Coronary artery disease, % | 35% | 56% | 46% | 0.48 |
| Hyperlipidemia | 35% | 56% | 38% | 0.44 |
| Blood pressure and Heart Rate | ||||
| Sitting | ||||
| Systolic Blood Pressure, mm Hg | 153 (18) | 149 (13) | 155 (15) | 0.60 |
| Diastolic Blood pressure, mm Hg | 85 (10) | 81 (8) | 83 (8) | 0.41 |
| Heart rate, beats per min | 64 (11) | 65 (8) | 66 (9) | 0.82 |
| Sit-to-stand after 1 minute* | ||||
| Systolic Blood Pressure, mm Hg | −4 (7) | −10 (10) | −10 (6) | 0.10 |
| Diastolic Blood pressure, mm Hg | 1 (5) | −2 (7) | −3 (4) | 0.19 |
| Heart rate, beats per min | 2 (4) | 2 (6) | 1 (5) | 0.91 |
| Sit-to-stand after 3 minute* | ||||
| Systolic Blood Pressure, mm Hg | 1 (6) | −1 (10) | −1 (5) | 0.78 |
| Diastolic Blood pressure, mm Hg | 3 (5) | −2 (6) | −0.1 (4) | 0.02 |
| Heart rate, beats per min | 2 (4) | 2 (5) | 2 (3) | 0.96 |
| Cerebral hemodynamics | ||||
| Sitting BFV, cm/sec | 28.09 (6.16) | 29.14 (5.75) | 29.85 (10.56) | 0.81 |
| Orthostatic change*in BFV, cm/sec | −3.13 (2.51) | −4.01 (3.53) | −3.57 (2.67) | 0.69 |
| Sitting CVR, mm Hg/cm/sec | 3.46 (0.82) | 3.45 (1.14) | 3.45 (1.20) | 0.99 |
| Orthostatic change*in CVR, mm Hg/cm/sec | −0.50 (0.60) | −0.26 (0.74) | −0.35 (0.39) | 0.54 |
| CO2 Vasoreactivity, slope | 0.56 (0.20) | 0.51 (0.16) | 0.59 (0.41) | 0.71 |
| CO2Vasomotor range | 0.61 (0.22) | 0.60 (0.22) | 0.72 (0.41) | 0.50 |
BFV: blood flow velocity. CVR: cerebrovascular resistance.
Numbers are means (standard deviation) or % for categorical variables
P-values from ANOVA for continuous variables and Chi-square test for categorical variables.
Orthostatic =Standing measure-sitting measure
ACEI: Angiotensin Converting Enzyme Inhibitor; ARB: Angiotensin Receptor Blocker
Table 2.
Most common adverse and serious adverse events reported during the study period in all participants (with and without successful TCD insonation)
| Lisinopril | Candesartan | HCTZ | p-value | |
|---|---|---|---|---|
| N | 18 | 20 | 15 | |
| Dizziness | 28% | 30% | 40% | 0.73 |
| Weakness or fatigue | 17% | 5% | 7% | 0.43 |
| Fall, non-injurious | 22% | 5% | 13% | 0.29 |
| Cough | 28% | 20% | 20% | 0.81 |
| Hospitalization (non-elective) during study period* | 22% | 15% | 20% | 0.84 |
Reasons for hospitalization included pneumonia, chest pain and leg pain from a traumatic muscle injury. HCTZ: Hydrochlorothiazide
P-values from Chi-square test for categorical variables.
Blood pressure control
Systolic blood pressure reductions were equivalent in all three groups (lisinopril group (mean reduction ± standard error)=27±5 mm Hg; candesartan=26±5 mm Hg, and HCTZ=25±6 mm Hg; p=0.93). Blood pressure control levels were also equivalent (lisinopril 91%, candesartan 100% and HCTZ 100%, p=0.40). The average number of visits to achieve control was lowest for candesartan (1.3 visits vs 2.5 visits for lisinopril and 2 visits for HCTZ; p<0.001).
Resting cerebral blood flow velocity
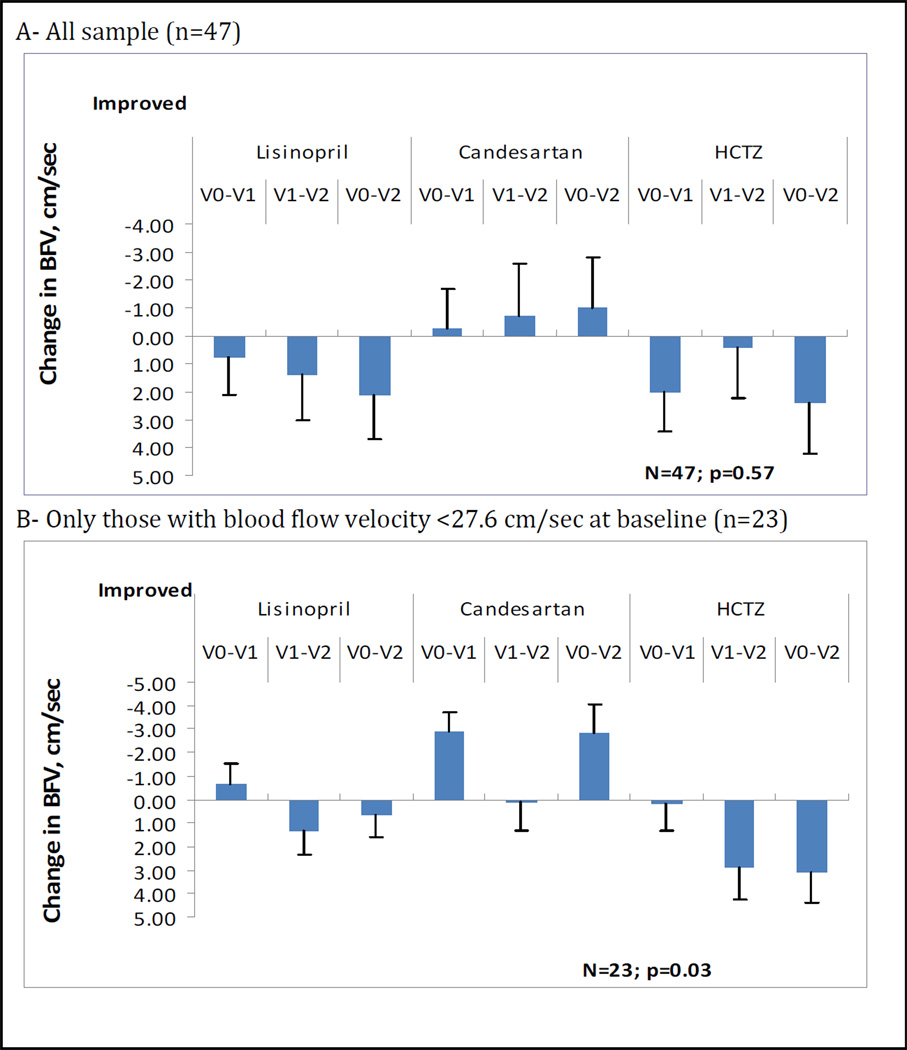
The three groups did not differ in baseline cerebral hemodynamic measures. There was a trend for an increase in BFV (increase by 1.03 cm/sec over 12 months) in the candesartan group whereas there was a decline in the lisinopril group of −2.12 cm/sec and in the HCTZ group of −2.40 cm/sec. The between-group p-value was 0.57. However, in those with relatively low BFV (below the median of 27.6 cm/sec), at baseline (n=23) the candesartan effectwas more pronounced (BFV increased by 2.79 cm/sec in the candesartan group vs decline in the lisinopril and HCTZ groups) and the between-group p-value was 0.03. [Figure 1]
Figure 1.
Changes over study period in the three groups in cerebral blood flow velocity in the overall sample (A) and in those with baseline blood flow velocity below the median.
Footnote (figure 1): Least square means were adjusted for age. P-values were obtained from the linear mixed model for the visit by group interaction parameter. V0-V1: change from baseline to 6 months; V0–V2: change from baseline to 12 months; V1–V2: change from 6 months to 12 months. BFV: cerebral blood flow velocity. HCTZ: hydrochlorothiazide.
Orthostatic hemodynamics and autoregulation
Despite the significant decreases in sitting blood pressure after treatment, there were no increases in the 1-minute and 3-minute orthostatic blood pressure declines in the three groups, as shown in Table 3. Further, the BFV declines during active standing did not worsen in all three groups. However, there was a group difference in orthostatic changes in CVR: those treated with candesartan or lisinopril showed less changes in CVR upon standing, whereas those treated with HCTZ showed greater changes in CVR upon standing (p-value between-groups=0.05). These data are shown in Table 4.
Table 3.
Sitting and orthostatic changes in blood pressure and heart rate in the three groups during the study period.
| Lisinopril | Candesartan | HCTZ |
Between group P-value |
||
|---|---|---|---|---|---|
| N | 17 | 17 | 13 | ||
| Sitting | |||||
| Systolic Blood Pressure, mm Hg |
Baseline | 153±3 | 150±3 | 156±3 | 0.93 |
| 6 months | 129±4 | 130±4 | 132±4 | ||
| 12 month | 126±4 | 124±5 | 131±5 | ||
|
Within-group p-value |
<0.0001 | 0.0001 | <0.0001 | ||
| Diastolic Blood Pressure, mm Hg |
Baseline | 85±2 | 81±2 | 83±2 | 0.63 |
| 6 months | 72±2 | 71±2 | 76±2 | ||
| 12 month | 70±3 | 69±3 | 74±3 | ||
|
Within-group p-value |
<0.0001 | 0.001 | 0.03 | ||
| Heart Rate, BPM | Baseline | 64±2 | 65±2 | 66±3 | 0.28 |
| 6 months | 63±3 | 67±3 | 64±3 | ||
| 12 month | 65±3 | 63±4 | 73±4 | ||
|
Within-group p-value |
0.74 | 0.58 | 0.09 | ||
| 1-minute orthostatic change* | |||||
| Systolic Blood Pressure, mm Hg |
Baseline | −5±2 | −10±2 | −10±2 | 0.77 |
| 6 months | −3±2 | −7±3 | −4±3 | ||
| 12 month | −3±3 | −9±3 | −4±3 | ||
|
Within-group p-value |
0.71 | 0.59 | 0.08 | ||
| Diastolic Blood Pressure, mm Hg |
Baseline | 1±2 | −2±2 | −3±2 | 0.24 |
| 6 months | −2±2 | −2±2 | −1±2 | ||
| 12 month | −1±2 | −5±3 | 3±3 | ||
|
Within-group p-value |
0.36 | 0.73 | 0.20 | ||
| Heart Rate, BPM | Baseline | 2±1 | 2±1 | 1±1 | 0.66 |
| 6 months | 5±1 | 3±1 | 5±1 | ||
| 12 month | 5±2 | 4±2 | 6±2 | ||
|
Within-group p-value |
0.06 | 0.67 | 0.04 | ||
| 3-minutes orthostatic change | |||||
| Systolic Blood Pressure, mm Hg |
Baseline | 1±2 | −1±2 | −1±2 | 0.78 |
| 6 months | 4±2 | −2±3 | 1±2 | ||
| 12 month | 2±2 | −2±4 | −2±3 | ||
|
Within-group p-value |
0.43 | 0.84 | 0.65 | ||
| Diastolic Blood Pressure, mm Hg |
Baseline | 3±1 | −2±1 | 0±1 | 0.16 |
| 6 months | 1±1 | 1±2 | 2±2 | ||
| 12 month | 2±2 | 3±2 | 4±2 | ||
|
Within-group p-value |
0.65 | 0.04 | 0.17 | ||
| Heart Rate, BPM | Baseline | 2±1 | 2±1 | 2±1 | 0.27 |
| 6 months | 3±1 | 3±1 | 3±1 | ||
| 12 month | 3±1 | 2±2 | 8±2 | ||
|
Within-group p-value |
0.48 | 0.89 | 0.03 | ||
Numbers are age-adjusted least square means± standard error.
Orthostatic =Standing measure-sitting measure
Table 4.
Age-adjusted least square mean of the hemodynamic and cerebrovascular reactivity measures in the three groups over the study period
| Lisinopril | Candesartan | HCTZ |
Between group P-value |
||
|---|---|---|---|---|---|
| N | 17 | 17 | 13 | ||
| Sitting Blood Flow velocity, cm/sec |
Baseline | 28.00±1.49 | 29.12±1.57 | 29.52±1.79 | 0.57 |
| 6 months | 27.27±1.58 | 29.40±1.68 | 27.52±1.78 | ||
| 12 month | 25.89±1.80 | 30.13±2.02 | 27.12±2.10 | ||
|
Within-group p-value |
0.43 | 0.85 | 0.27 | ||
| Standing change in Blood Flow Velocity, cm/sec |
Baseline | −3.10±0.55 | −4.00±0.74 | −3.46±0.72 | 0.81 |
| 6 months | −3.26±0.61 | −3.61±0.80 | −2.92±0.72 | ||
| 12 month | −2.84±0.74 | −2.28±0.99 | −2.38±0.92 | ||
|
Within-group p-value |
0.88 | 0.20 | 0.51 | ||
| Sitting Cerebrovascular Resistance, mm Hg*s/cm |
Baseline | 3.48±0.20 | 3.46±0.22 | 3.52±0.24 | 0.70 |
| 6 months | 2.97±0.22 | 2.81±0.24 | 3.26±0.24 | ||
| 12 month | 3.02±0.26 | 3.04±0.30 | 3.55±0.31 | ||
|
Within-group p-value |
0.07 | 0.03 | 0.51 | ||
| Standing change in Cerebrovascular Resistance, mm Hg*s/cm |
Baseline | −0.50±0.17 | −0.26±0.17 | −0.35±0.19 | 0.05 |
| 6 months | −0.85±0.19 | −0.24±0.19 | −0.44±0.19 | ||
| 12 month | −0.14±0.23 | −0.23±0.25 | −0.89±0.25 | ||
|
Within-group p-value |
0.03 | 0.99 | 0.17 | ||
| CO2 Vasoreactivity, slope |
Baseline | 0.55±0.05 | 0.51±0.06 | 0.59±0.06 | 0.30 |
| 6 months | 0.42±0.05 | 0.48±0.06 | 0.47±0.06 | ||
| 12 month | 0.32±0.06 | 0.39±0.08 | 0.50±0.07 | ||
|
Within-group p-value |
0.001 | 0.25 | 0.10 | ||
| CO2Vasomotor range |
Baseline | 0.61±0.06 | 0.61±0.06 | 0.72±0.07 | 0.46 |
| 6 months | 0.51±0.06 | 0.53±0.07 | 0.51±0.07 | ||
| 12 month | 0.39±0.07 | 0.50±0.09 | 0.56±0.08 | ||
|
Within-group p-value |
0.02 | 0.39 | 0.009 | ||
Values are obtained from the Mixed Model adjusted for age at baseline. Between group p-value is obtained from the Mixed Model output of the Group*visit term, and within-group p-values is obtained from the Mixed Model.
Cerebrovascular reserve
As shown in Table 4, subjects treated with candesartan had no significant decline in vasoreactivity (within-group p for trend = 0.25) or vasomotor range (p=0.38) over the 12 months period; in contrast, subjects randomized to lisinopril and HCTZ had declines in both measures over the study period (vasoreactivity: p=0.001 for lisinopril and 0.1 for HCTZ; VMR: p=0.02 for lisinopril and 0.009 for HCTZ). However, the between group comparisons did not reach statistical significance (p=0.30 for vasoreactivity and 0.46 for VMR).
Executive function and cerebral hemodynamics
After adjusting for age and baseline MMSE, those randomized to candesartan demonstrated the greatest improvement in TMT, part B (12-month least square mean decrease of 17.1 seconds; vs 4.2 seconds in the HCTZ group and an increase of 14.4 seconds in the lisinopril group, between-group p-value=0.008). Those in the candesartan group also showed improved performance on the recognition portion of the HVLT which assesses in part aspects of executive function (between-group p-value=0.034). There were no group differences in the change in the immediate and delayed recall of HVLT or in the Digit Span test. In the candesartan group, 8 (47%) had stable or improved executive function and BFV vs 3 (18%)in the lisinopril and 1 (13%)in the HCTZ groups. These group differences did not reach statistical significance (p=0.71). The concordance rate tended to be highest in the candesartan group for VMR (Candesartan: 3 (18%), lisinopril 1 (6%) and HCTZ: 2 (15%); p=0.39) but notCO2-vasoreactivity (4(31%)for the HCTZ vs 3(18%) for candesartan and 1(6%) for lisinopril, p=0.78). Due to the small number of individuals within each group, these results should be interpreted with great caution.
Discussion
In this pilot study we found that an ARB-based regimen in older adults with hypertension and mild executive dysfunction may be associated with preservedexecutive function and blood flow velocity, especially in those with relatively lower pretreatment BFV. These effects may contribute to the positive effects of candesartan on executive function. ARB treatment wasalso associated with preservation of cerebrovascular reserve, measured by CO2-vasoreactivity and VMR, whereas an ACEI or diuretic based regimens may not provide this protection. Finally, improved blood pressure control was not associated with increased orthostatic hypotension or orthostatic declines in BFV.
To our knowledge, this is the first head-to-head comparison of the effects of 3 commonly used antihypertensive medications on cerebral hemodynamics in hypertensive older adults. Prior animal studies suggested that ARBs improved cerebral blood flow, increase cerebrovascular reserve, and ameliorate ischemic changes from atherosclerosis and hypoperfusion.22–26 In humans, 2 studies have shown that ARB treatment preserves or improves cerebral hemodynamics in post stroke patients and those with cerebral small-vessel disease.27,28 Our findings further extend these possible positive effects of ARBs to non-stroke individuals.
Recent evidence suggests that there is an alternative pathway in the brain renin angiotensin system that may counterbalance the negative effects of AT1 through the activation of AT2. 29–31 We previously hypothesized that ARBs may have a superior effect to ACEI since ARBs but not ACEI is associated with AT2 activation. Our study provides preliminary human support that AT2 activation in the brain may be beneficial on both executive function and cerebral hemodynamics.
Our study suggests that candesartan may have a positive effect on executive function in those with existing limitations in this cognitive domain.32 Decline in perfusion is associated with executive dysfunction33–35 and a decrease in CO2 vasoreactivity has been observed in patients with dementia.36,37 There was a trend for a higher degree of concordance between improved or unchanged scores on the TMT and BFV and VMR in those treated with candesartan. Hence, the differential effect of ARBs on BFV and cerebrovascular reserve may have a role in the differential effects of ARBs relative to other antihypertensives on executive function. These results however, need to be interpreted with caution due to the sample size within each group.
Antihypertensive therapy was not associated with greater orthostatic blood pressure or BFV reductions despite a decrease of 21–28 mm Hg in sitting systolic blood pressure after treatment. In fact, there was a trend of less orthostatic declines in blood pressure and BFV. Clinically this study suggests that achieving blood pressure control to below 140/90 mm Hg is unlikely to lead to decline in cerebral blood flow or orthostatic hypotension. However, due to our small sample size these findings should be interpreted cautiously.
The mechanisms of these potential superior cerebrovascular effects of ARB may be related to restoring proper central endothelial function, decreasing inflammation, and preventing neuronal degeneration, partially via an activated AT2-receptor pathway.25,38–40. This unique effect of ARBs on AT2 needs further investigation and may offer new therapeutic paradigm for vascular brain disease and cognitive dysfunction.
The main limitation of this study is the small sample size as this is a pilot study and a larger clinical trial is needed to further confirm our findings. The validity of TCD measurements as an index of cerebral blood flow is based on the assumption that the cerebral vessel diameters are constant.41 Since we did not have brain imaging, our ability to validate this assumption over the study period is limited.
Conclusion
In this pilot study of older adults with hypertension and evidence of executive dysfunction,an ARB-based regimen may be associated with improved cerebral blood flow and maintain cerebrovascular reserve compared to ACEI- or HCTZ- based regimens. These positive effects on cerebral hemodynamics may be partially contributing to the improved executive function observed with Candesartan. However these findings should be considered cautiously due to the small sample size of this pilot study. Since no treatment is available for executive dysfunction, future studies exploring the effects of ARB on executive cognitive impairment is a critical priority.
Acknowledgment
Funding sources: Dr. Hajjar and the AVEC trial are supported by grant 1 K23 AG030057 from the National Institute on Aging. This work is also supported by P01-AG004390 and R37-AG025037 from the NIA to Dr. Lipsitz, the Irving and Edyth S. Usen and Family Chair in Geriatric Medicine at Hebrew SeniorLife, NIH/NCRR grant UL1 RR031986 for the USC CTSI and Dr. Mack, and the generous donation Hinda Marcus to the Cardiovascular Research Laboratory at Hebrew SeniorLife and Harvard Medical School. The study was also registered in ClinicalTrials.gov (NCT00605072).
Footnotes
Conflicts of Interest: none
Author Contributions: Ihab Hajjar, MD, MS- study concept and design, data collection and analysis, manuscript preparation. Meaghan Hart- data collection. Wendy Mack, PhD- data analysis. Yu-Ling Chen, MS- data analysis. Vera Novak, MD, PhD- study concept, manuscript editing. Helena Chui, MD- result interpretation, manuscript preparation. Lewis Lipsitz, MD- study concept and design, result interpretation and manuscript preparation
Sponsor’s Role: Funding
Contributor Information
Ihab Hajjar, Division of Geriatric, Hospital & General Internal Medicine, Department of Medicine, University of Southern California, Los Angeles, California.
Meaghan Hart, Institute for Aging Research, Hebrew Senior Life, Boston, Massachusetts.
Yu-Ling Chen, Alzheimer Disease Research Center and Department of Preventive Medicine, University of Southern California, Los Angeles, California.
Wendy Mack, Department of Preventive Medicine, University of Southern California, Los Angeles, California.
Vera Novak, Beth Israel Deaconess Medical Center, Harvard Medical School.
Helena Chui, Department of Neurology, Raymond and Betty McCarron Chair in Neurology, Professor, University of Southern California, Los Angeles, California.
Lewis Lipsitz, Harvard School of Medicine, Co-Director, Institute for Aging Research, Hebrew SeniorLife, Chief of division of Gerontology, Beth Israel Deaconess Medical Centre, Boston, Massachusetts.
References
- 1.Vicario A, Martinez CD, Baretto D, Diaz Casale A, Nicolosi L. Hypertension and cognitive decline: impact on executive function. J Clin Hypertens (Greenwich) 2005 Oct;7(10):598–604. doi: 10.1111/j.1524-6175.2005.04498.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grigsby J, Kaye K, Shetterly SM, Baxter J, Morgenstern NE, Hamman RF. Prevalence of disorders of executive cognitive functioning among the elderly: findings from the San Luis Valley Health and Aging Study. Neuroepidemiology. 2002 Sep-Oct;21(5):213–220. doi: 10.1159/000065638. [DOI] [PubMed] [Google Scholar]
- 3.Royall DR, Espino DV, Polk MJ, Palmer RF, Markides KS. Prevalence and patterns of executive impairment in community dwelling Mexican Americans: results from the Hispanic EPESE Study. International journal of geriatric psychiatry. 2004 Oct;19(10):926–934. doi: 10.1002/gps.1185. [DOI] [PubMed] [Google Scholar]
- 4.Rockwood K, Wentzel C, Hachinski V, Hogan DB, MacKnight C, McDowell I. Prevalence and outcomes of vascular cognitive impairment. Vascular Cognitive Impairment Investigators of the Canadian Study of Health and Aging. Neurology. 2000 Jan 25;54(2):447–451. doi: 10.1212/wnl.54.2.447. [DOI] [PubMed] [Google Scholar]
- 5.Hajjar I, Quach L, Yang F, et al. Hypertension, white matter hyperintensities, and concurrent impairments in mobility, cognition, and mood: the cardiovascular health study. Circulation. 2011 Mar 1;123(8):858–865. doi: 10.1161/CIRCULATIONAHA.110.978114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zazulia AR. Regulation of cerebral blood flow in untreated mild-to-moderate hypertension. Am J Hypertens. 2009 Apr;22(4):344. doi: 10.1038/ajh.2009.20. [DOI] [PubMed] [Google Scholar]
- 7.Ficzere A, Valikovics A, Fulesdi B, Juhasz A, Czuriga I, Csiba L. Cerebrovascular reactivity in hypertensive patients: a transcranial Doppler study. J Clin Ultrasound. 1997 Sep;25(7):383–389. doi: 10.1002/(sici)1097-0096(199709)25:7<383::aid-jcu6>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 8.Poels MM, Ikram MA, Vernooij MW, et al. Total cerebral blood flow in relation to cognitive function: the Rotterdam Scan Study. J Cereb Blood Flow Metab. 2008 Oct;28(10):1652–1655. doi: 10.1038/jcbfm.2008.62. [DOI] [PubMed] [Google Scholar]
- 9.Kazama K, Anrather J, Zhou P, et al. Angiotensin II impairs neurovascular coupling in neocortex through NADPH oxidase-derived radicals. Circ Res. 2004 Nov 12;95(10):1019–1026. doi: 10.1161/01.RES.0000148637.85595.c5. [DOI] [PubMed] [Google Scholar]
- 10.Saavedra JM, Nishimura Y. Angiotensin and cerebral blood flow. Cell Mol Neurobiol. 1999 Oct;19(5):553–573. doi: 10.1023/A:1006995016403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kazama K, Anrather J, Zhou P, et al. Angiotensin II impairs neurovascular coupling in neocortex through NADPH oxidase-derived radicals. Circ Res. 2004 Nov 12;95(10):1019–1026. doi: 10.1161/01.RES.0000148637.85595.c5. [DOI] [PubMed] [Google Scholar]
- 12.Hajjar I, Sorond F, Hsu YH, Galica A, Cupples LA, Lipsitz LA. Renin angiotensin system gene polymorphisms and cerebral blood flow regulation: the MOBILIZE Boston study. Stroke. 2010 Apr;41(4):635–640. doi: 10.1161/STROKEAHA.109.572669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Horiuchi M, Mogi M, Iwai M. The angiotensin II type 2 receptor in the brain. J Renin Angiotensin Aldosterone Syst. 2010 Mar;11(1):1–6. doi: 10.1177/1470320309347793. [DOI] [PubMed] [Google Scholar]
- 14.Hajjar I, Hart M, Milberg W, Novak V, Lipsitz L. The rationale and design of the antihypertensives and vascular, endothelial, and cognitive function (AVEC) trial in elderly hypertensives with early cognitive impairment: role of the renin angiotensin system inhibition. BMC Geriatr. 2009;9:48. doi: 10.1186/1471-2318-9-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Royall DR, Cordes JA, Polk M. CLOX: an executive clock drawing task. J Neurol Neurosurg Psychiatry. 1998 May;64(5):588–594. doi: 10.1136/jnnp.64.5.588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Crum RM, Anthony JC, Bassett SS, Folstein MF. Population-based norms for the Mini-Mental State Examination by age and educational level. Jama. 1993 May 12;269(18):2386–2391. [PubMed] [Google Scholar]
- 17.Washburn RA, Ficker JL. Physical Activity Scale for the Elderly (PASE): the relationship with activity measured by a portable accelerometer. J Sports Med Phys Fitness. 1999 Dec;39(4):336–340. [PubMed] [Google Scholar]
- 18.Lawton MP, Brody EM. Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist. 1969;9(3):179–186. Autumn. [PubMed] [Google Scholar]
- 19.Pickering TG, Hall JE, Appel LJ, et al. Recommendations for blood pressure measurement in humans and experimental animals: Part 1: blood pressure measurement in humans: a statement for professionals from the Subcommittee of Professional and Public Education of the American Heart Association Council on High Blood Pressure Research. Hypertension. 2005 Jan;45(1):142–161. doi: 10.1161/01.HYP.0000150859.47929.8e. [DOI] [PubMed] [Google Scholar]
- 20.Sorond FA, Serrador JM, Jones RN, Shaffer ML, Lipsitz LA. The sit-to-stand technique for the measurement of dynamic cerebral autoregulation. Ultrasound Med Biol. 2009 Jan;35(1):21–29. doi: 10.1016/j.ultrasmedbio.2008.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wallenstein S, Wittes J. The power of the Mantel-Haenszel test for grouped failure time data. Biometrics. 1993 Dec;49(4):1077–1087. [PubMed] [Google Scholar]
- 22.Iwai M, Inaba S, Tomono Y, et al. Attenuation of focal brain ischemia by telmisartan, an angiotensin II type 1 receptor blocker, in atherosclerotic apolipoprotein E-deficient mice. Hypertens Res. 2008 Jan;31(1):161–168. doi: 10.1291/hypres.31.161. [DOI] [PubMed] [Google Scholar]
- 23.Kobayashi T, Kawamata T, Shibata N, Okada Y, Kobayashi M, Hori T. Angiotensin II type 1 receptor blocker telmisartan reduces cerebral infarct volume and peri-infarct cytosolic phospholipase A(2) level in experimental stroke. J Neurotrauma. 2009 Dec;26(12):2355–2364. doi: 10.1089/neu.2009.0965. [DOI] [PubMed] [Google Scholar]
- 24.Omura-Matsuoka E, Yagita Y, Sasaki T, et al. Postischemic administration of angiotensin II type 1 receptor blocker reduces cerebral infarction size in hypertensive rats. Hypertens Res. 2009 Jul;32(7):548–553. doi: 10.1038/hr.2009.69. [DOI] [PubMed] [Google Scholar]
- 25.Oyama N, Yagita Y, Sasaki T, et al. An angiotensin II type 1 receptor blocker can preserve endothelial function and attenuate brain ischemic damage in spontaneously hypertensive rats. J Neurosci Res. 2010 Oct;88(13):2889–2898. doi: 10.1002/jnr.22441. [DOI] [PubMed] [Google Scholar]
- 26.Takeda S, Sato N, Takeuchi D, et al. Angiotensin receptor blocker prevented beta-amyloid-induced cognitive impairment associated with recovery of neurovascular coupling. Hypertension. 2009 Dec;54(6):1345–1352. doi: 10.1161/HYPERTENSIONAHA.109.138586. [DOI] [PubMed] [Google Scholar]
- 27.Kimura Y, Kitagawa K, Oku N, et al. Blood pressure lowering with valsartan is associated with maintenance of cerebral blood flow and cerebral perfusion reserve in hypertensive patients with cerebral small vessel disease. J Stroke Cerebrovasc Dis. 2010 Mar;19(2):85–91. doi: 10.1016/j.jstrokecerebrovasdis.2009.03.010. [DOI] [PubMed] [Google Scholar]
- 28.Matsumoto S, Shimodozono M, Miyata R, Kawahira K. Effect of the angiotensin II type 1 receptor antagonist olmesartan on cerebral hemodynamics and rehabilitation outcomes in hypertensive post-stroke patients. Brain Inj. 2009 Dec;23(13–14):1065–1072. doi: 10.3109/02699050903379404. [DOI] [PubMed] [Google Scholar]
- 29.Gelosa P, Pignieri A, Fandriks L, et al. Stimulation of AT2 receptor exerts beneficial effects in stroke-prone rats: focus on renal damage. J Hypertens. 2009 Dec;27(12):2444–2451. doi: 10.1097/HJH.0b013e3283311ba1. [DOI] [PubMed] [Google Scholar]
- 30.Kaschina E, Unger T. Angiotensin AT1/AT2 receptors: regulation, signalling and function. Blood Press. 2003;12(2):70–88. doi: 10.1080/08037050310001057. [DOI] [PubMed] [Google Scholar]
- 31.Li J, Culman J, Hortnagl H, et al. Angiotensin AT2 receptor protects against cerebral ischemia-induced neuronal injury. Faseb J. 2005 Apr;19(6):617–619. doi: 10.1096/fj.04-2960fje. [DOI] [PubMed] [Google Scholar]
- 32.Hajjar I, Hart M, Chen YL, et al. Effect of antihypertensive therapy on cognitive function in early executive cognitive impairment: a double-blind randomized clinical trial. Arch Intern Med. 2012 Mar 12;172(5):442–444. doi: 10.1001/archinternmed.2011.1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cohen RA. Hypertension and cerebral blood flow: implications for the development of vascular cognitive impairment in the elderly. Stroke. 2007 Jun;38(6):1715–1717. doi: 10.1161/STROKEAHA.107.487165. [DOI] [PubMed] [Google Scholar]
- 34.Girouard H, Iadecola C. Neurovascular coupling in the normal brain and in hypertension, stroke, and Alzheimer disease. J Appl Physiol. 2006 Jan;100(1):328–335. doi: 10.1152/japplphysiol.00966.2005. [DOI] [PubMed] [Google Scholar]
- 35.Chao LL, Pa J, Duarte A, et al. Patterns of cerebral hypoperfusion in amnestic and dysexecutive MCI. Alzheimer Dis Assoc Disord. 2009 Jul-Sep;23(3):245–252. doi: 10.1097/WAD.0b013e318199ff46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Silvestrini M, Pasqualetti P, Baruffaldi R, et al. Cerebrovascular reactivity and cognitive decline in patients with Alzheimer disease. Stroke. 2006 Apr;37(4):1010–1015. doi: 10.1161/01.STR.0000206439.62025.97. [DOI] [PubMed] [Google Scholar]
- 37.Vicenzini E, Ricciardi MC, Altieri M, et al. Cerebrovascular reactivity in degenerative and vascular dementia: a transcranial Doppler study. European neurology. 2007;58(2):84–89. doi: 10.1159/000103642. [DOI] [PubMed] [Google Scholar]
- 38.Rompe F, Artuc M, Hallberg A, et al. Direct angiotensin II type 2 receptor stimulation acts anti-inflammatory through epoxyeicosatrienoic acid and inhibition of nuclear factor kappaB. Hypertension. 2010 Apr;55(4):924–931. doi: 10.1161/HYPERTENSIONAHA.109.147843. [DOI] [PubMed] [Google Scholar]
- 39.Wilms H, Rosenstiel P, Unger T, Deuschl G, Lucius R. Neuroprotection with angiotensin receptor antagonists: a review of the evidence and potential mechanisms. American journal of cardiovascular drugs : drugs, devices, and other interventions. 2005;5(4):245–253. doi: 10.2165/00129784-200505040-00004. [DOI] [PubMed] [Google Scholar]
- 40.Ghiadoni L, Virdis A, Magagna A, Taddei S, Salvetti A. Effect of the angiotensin II type 1 receptor blocker candesartan on endothelial function in patients with essential hypertension. Hypertension. 2000 Jan;35(1 Pt 2):501–506. doi: 10.1161/01.hyp.35.1.501. [DOI] [PubMed] [Google Scholar]
- 41.Kontos HA. Validity of cerebral arterial blood flow calculations from velocity measurements. Stroke. 1989 Jan;20(1):1–3. doi: 10.1161/01.str.20.1.1. [DOI] [PubMed] [Google Scholar]