Abstract
Background
Arrhythmogenic cardiomyopathy (AC) is tightly associated with desmosomal mutations in the majority of patients. Arrhythmogenesis in AC patients is likely related to remodeling of cardiac gap junctions and increased levels of fibrosis. Recently, using experimental models, we also identified sodium channel dysfunction secondary to desmosomal dysfunction. The aim of the present study was to assess the immunoreactive signal levels of the sodium channel protein NaV1.5, as well as Connexin43 and Plakoglobin, in myocardial specimens obtained from AC patients.
Methods
Left and right ventricular free wall (LVFW/RVFW) post-mortem material was obtained from 5 AC patients and 5 age and sex-matched controls. RV septal biopsies (RVSB) were taken from another 15 AC patients. All patients fulfilled the 2010 revised Task Force Criteria for AC diagnosis. Immunohistochemical analyses were performed using antibodies against Connexin43 (Cx43), Plakoglobin, NaV1.5, Plakophilin-2 and N-Cadherin.
Results
N-Cadherin and Desmoplakin immunoreactive signals and distribution were normal in AC patients compared to control. Plakophilin-2 signals were unaffected unless a PKP2 mutation predicting haploinsufficiency was present. Distribution was unchanged compared to control. Immunoreactive signal levels of PKG, Cx43 and NaV1.5 were disturbed in 74%, 70% and 65% of the patients, respectively.
Conclusions
Reduced immunoreactive signal of PKG, Cx43 and NaV1.5 at the intercalated disks can be observed in a large majority of the patients. Decreased levels of Nav1.5 might contribute to arrhythmia vulnerability and, in the future, potentially could serve as a new clinically relevant tool for risk assessment strategies.
Introduction
Arrhythmogenic cardiomyopathy (AC), previously known as arrhythmogenic right ventricular cardiomyopathy/dysplasia (ARVC/D), is a heart muscle disease characterized by replacement of predominantly the right ventricle (RV) with fibro-fatty scar tissue. In later stages of the disease, also the left ventricle (LV) and the interventricular septum (IVS) can be affected. Recently, patients with a predominant LV involvement also have been described.1 Patients generally present with syncope, palpitations and sudden cardiac death.2 Mutations in genes encoding for desmosomal proteins, which are important for intercellular mechanical coupling, are associated with this disease in about 60% of the patients.3–9
The arrhythmogenic phenotype of AC suggests that, although the mutated genes do not code for channel proteins, these gene products associate with molecules that are relevant for electrical function. In particular, several studies have identified a disturbed immuno-localization of Connexin43 (Cx43), the major ventricular gap junction protein, in AC myocardium.10–12 In several other forms of cardiomyopathy, a heterogeneous downregulation and de-phosphorylation of Cx43 are strongly associated with an increased propensity for development of life-threatening ventricular arrhythmias.13–16
AC is clinically diagnosed according to the revised Task Force Criteria (TFC)17 based on global or regional dysfunction and structural alterations, tissue characterization of the ventricular wall, re- and depolarization or conduction abnormalities, arrhythmias, genetics and family history. Still, many cases remain un- or misdiagnosed because of the multiple facets of the clinical manifestation of the disease.
It has been described that immunoreactivity of plakoglobin (PKG) was reduced in a high percentage of AC patients compared either to controls, or to patients with other underlying cardiac disease such as dilated or hypertrophic cardiomyopathy.12 This early reduction in immunoreactivity for PKG appeared not only present in the RV, but also in the macroscopically unaffected LV and IVS. Based on this finding, it was suggested that immunoreactivity of PKG could be a tool to discriminate AC patients from healthy subjects and patients with other forms of heart disease. However, more recent studies have shown that PKG signals also are reduced in sarcoidosis and giant cell myocarditis.18, 19
Recently, we reported that a reduction in Cx43 protein can lead to reduced sodium channel (NaV1.5) expression and function in a mouse model of severely reduced Cx43 and in isolated neonatal rat ventricular cardiomyocytes.20 Furthermore, in vitro silencing of plakophilin-2 (PKP2), one of the desmosomal proteins that is often mutated in AC patients, also leads to a decreased sodium current.21 In addition, PKP2-haploinsufficent mice showed a significant sodium current deficit.22 Whether desmosomal deficiency and the AC phenotype correlate with changes in the distribution of proteins relevant to the sodium channel complex in the human heart remains to be defined.
In this study, we used immunohistochemistry to identify the immunoreactive signal levels and distribution of Cx43, PKG and NaV1.5 in AC patients as compared to controls. Our data show that these levels and distribution of Cx43, PKG and NaV1.5 are affected in the large majority of AC patients.
Methods
Patient samples and tissue processing
Left and right ventricular free wall (LVFW and RVFW) myocardium (on average 2–4 cm3) was obtained from 5 AC patients (AC1–5, post-mortem) and from 5 age and sex matched controls with no underlying heart disease (C1–5). Right ventricular septal biopsies (RVSB, 2–4 mm3) were obtained from another 15 AC patients (AC6–20). All patients consented to clinical evaluation according to the TFC. All material used in this study was flash frozen in liquid nitrogen. Frozen samples were cryo-sectioned at a thickness of 10 µm.
Patient screening
From 18/20 AC patients, DNA was available to screen for mutations in the PKP2, DSP, DSG2, DSC2, PKG, TMEM43 and PLN genes by direct sequencing. In addition, using multiple ligation-dependent probe amplification, the PKP2 gene was screened for large exon deletions. Genetic screening was performed upon the patients’ written consent.
Immunohistochemistry
Frozen material was serially sectioned, generating sections of 10µm thickness that were collected on aminopropyltriethoxysilane (AAS) coated glass slides. Immunohistochemistry was performed as described previously.23 Once the labeling was completed, analysis of the results was perform using a Nikon eclipse 80i microscope equipped for epifluorescence. Independent analysis of the different patients was performed blinded by 3 observers/experiment. First, for each and every patient, an overall conclusion was drawn upon which pictures supportive to the overall conclusion were captured using a Nikon digital sight DS-BMWe camera and NIS Elements BR3.0 software (using equal exposure times). Primary antibodies against N-Cadherin (mouse, Sigma, 1:800), PKP2 (mouse, Progen, undiluted-1:1000), PKG (mouse, Sigma, 1:100,000), NaV1.5 (rabbit, custom-made,23 1:100), and Cx43 (mouse, Transduction Labs, 1:200 and rabbit, Zymed, 1:250) were used. Secondary labeling was performed with appropriate Texas Red (1:100) and FITC (1:250) conjugated whole IgG antibodies (Jackson Laboratories). Blinded cross-evaluation for NaV1.5 labeling of material from 9 patients was performed in Utrecht and in New York.
Results
Patient characteristics
Characteristics of the 5 controls and the 20 AC patients are shown in Table 1. Molecular-genetic analysis of 18 out of 20 AC patients revealed 5 different PKP2 mutations in 11 patients. In addition to a pathogenic PKP2 mutation, one of those patients also carried unclassified variants in TMEM43 (p.Arg240Cys) and DSG2 (p.Ala358Thr). One patient had unclassified variants in DSC2 and DSG2, 2 showed a mutation in PLN (p.Arg14del) and 4 did not present any mutations in the analyzed genes.
Table 1.
Clinical characteristics of Control and AC patients
| Patient | Age | Age 1st symptoms |
Sex | TFC | Mutation |
|---|---|---|---|---|---|
| Control 1 | 36 | NA | M | NA | NA |
| Control 2 | 38 | NA | M | NA | NA |
| Control 3 | 40 | NA | M | NA | NA |
| Control 4 | 35 | NA | F | NA | NA |
| Control 5 | 44 | NA | F | NA | NA |
| AC 1 | 27 | 16 | M | autopsy | None |
| AC 2 | 43 | 24 | M | 8 | None |
| AC 3 | 63 | 55 | F | autopsy | None |
| AC 4 | 63 | 59 | M | HTX | ND |
| AC 5 | 25 | 25 | M | autopsy | ND |
| AC 6 | 16 | 16 | M | 9 | PKP2 deletion exons 1–14, DSG2 uv, TMEM43 p.Arg240Cys uv |
| AC 7 | 39 | 34 | M | 10 | PKP2 deletion exons 1–4 |
| AC 8 | 72 | 69 | M | 8 | PKP2 deletion exons 1–4 |
| AC 9 | 74 | 65 | M | 9 | PKP2 p.Cys796Arg |
| AC 10 | 77 | 76 | M | 7 | None |
| AC 11 | 47 | 43 | F | 5 | PLN c.40_42delAGA p.Arg14del |
| AC 12 | 38 | 37 | M | 7 | UV in DSC2 and UV in DSG2 (not pathogenic) |
| AC 13 | 30 | 22 | F | 5 | PKP2 c.235C>T p.Arg79X |
| AC 14 | 65 | 59 | F | 8 | PKP2 c.235C>T p.Arg79X |
| AC 15 | 39 | 17 | F | 6 | PKP2 c.2146-1G>C p.IVS10-1G>C |
| AC 16 | 49 | 48 | F | 6 | PKP2 c.1211-1212insT p.Leu404fs |
| AC 17 | 58 | 34 | M | 10 | PKP2: c.1211-1212insT p. Leu404fs |
| AC 18 | 48 | 41 | M | 4 | PLN c.40_42delAGA p.Arg14del |
| AC 19 | 41 | 29 | F | 9 | PKP2 c.2146-1G>C p.IVS10-1G>C |
| AC 20 | 23 | 17 | F | 7 | PKP2: c.1211-1212insT p. Leu404fs |
RVFW and LVFW post-mortem material was used from control 1–5 and AC 1–5. Right ventricular septal biopsies were examined from AC 6–20. All AC patients had a TFC score≥4 or were diagnosed after autopsy or heart transplantation.
Abbreviations: NA: not applicable, ND: not determined, HTX: heart transplantation. TFC: Task Force Criteria (TFC value indicates number of criteria; major criterion counts for 2 point, minor criterion for 1 point). UV: unclassified variant
In supplemental Table 1, additional clinical characteristics of the AC patients are provided, including age at which first symptoms occurred, and separate Task Force criteria for AC diagnosis.
Immunoreactive signals and distribution of desmosomal proteins
As depicted in Figure 1, double-labeling with antibodies against N-Cadherin (N-Cad) and PKP2 revealed that the immunoreactive proteins co-localized at the intercalated disks (IDs) of AC patients (exampled by AC8, -9 and -10), identical to the pattern seen in controls (see C1 as an example). N-Cad, an adherens junction protein in which no mutations in humans have been documented, was used as a marker for the ID. Reduced immunoreactive signals of N-cad have never been documented in AC patients, and as such, also serves as a control to verify tissue quality/preservation. Indeed, in all controls and patients we studied the distribution of immunoreactive N-cad was similar.
Figure 1. Undisturbed Plakophilin2 distribution in AC regardless of mutations.
PKP2 is normally present in the intercalated disk in AC patients, regardless of whether a mutation is present in the PKP2 gene. Haploinsuffiency of PKP2 (in AC8) also showed undisturbed PKP2 distribution. However, reduced signal intensity of PKP2 was identified. N-Cad is used as a marker for the intercalated disk. Scale bar equals 100 µm.
Next, we studied the signals and distribution of PKP2 in tissue from AC patients with deletion of exons 1–4 of PKP2, which likely causes PKP2 haplo-insufficiency (AC6, -7, and -8). Results were compared to those obtained from controls, AC patients with no identified mutation (AC10), or AC patients having a missense mutation that thus far has not been associated with trafficking defects (AC9). In controls, as well as in tissue from AC patients without identified PKP2 mutation (AC10) or a p.Cys796Arg mutation (AC9), PKP2 immunoreactive signal levels and distribution were highly comparable. However, in the two AC patients with a deletion of PKP2 exons 1–4 (AC7 and -8) and one with a deletion of exons 1–14 (AC6), the intensity of the PKP2 immunoreactive signal was clearly reduced (see Figure 1 by AC 8). In contrast to this decreased signal intensity, distribution of PKP2 was not affected, since it still showed a complete overlap with N-Cad at the ID.
Distribution and immunoreactive signal levels of PKG, Cx43 and NaV1.5
Previous studies have shown that alterations in PKG expression and distribution can serve as biomarkers to facilitate diagnosis of AC.12 Complementary studies also revealed that tissue preservation and dilution of the anti-PKG antibody critically determined the appropriateness of evaluation.25 Serial antibody dilutions were therefore used to determine the best conditions under which the presence or absence of a PKG signal at the intercalated disc, segregated with the clinical diagnosis of AC. At a dilution of 1:100,000 PKG signals were clearly present in the 5 control patients where they co-localized with N-Cad at the ID (Figure 2A, right panels). In 5/19 AC patients, PKG labeling was comparable to controls but in 14/19 (74%) patients, PKG signals were clearly reduced and sometimes even completely absent whereas double labeling with antibodies raised against N-Cad always revealed a normal pattern of N-Cad at the ID (Figure 2B, right panel).
Figure 2. Expression patterns for Cx43, NaV1.5 and PKG.
A. Double labeling with N-Cadherin. A large subset of the patients (B) showed reduced immunoreactive signal levels for Cx43, NaV1.5 and/or PKG compared to control (A).
Analysis of cryo-material from the RVFW (AC 1–5) and RVSB (ARVC 6–20) revealed that Cx43 immunoreactive signal was disturbed (ranging from mild to severe) in 14/20 (70%) patients when compared to the pattern found in the 5 control individuals. Figure 2A shows that in control material (left panels), Cx43 and N-Cad closely overlap, while this overlay is partially disrupted in a large percentage of AC patients (Figure 2B, left panels). In AC patients, labeling of Cx43 was not only reduced but also clearly heterogeneously distributed. In controls, Cx43 was found almost exclusively at the ID. However, in AC patients, Cx43 signal was not only reduced at the ID, but also sparely present at the lateral sides of the myocytes.
Similarly, double labeling of NaV1.5 and N-Cad revealed a reduced immunoreactive signal of NaV1.5 in 12/17 (65%) patients with labeling in all 5 controls being normal (Figure 2A, mid panels). Again, the alterations ranged from reduced signal intensity of NaV1.5 (with normal N-Cad counterstaining) to complete absence of NaV1.5 (Figure 2B, mid panels). A blinded cross- evaluation on material of 9 AC patients, performed by the group in NYU (New York) confirmed the assessment made in a separate center in 8/9 cases. Three patients classified as unaffected were recognized as such, and only in 1/6 affected patients the evaluation of NaV1.5 downregulation was not independently confirmed (patient AC18).
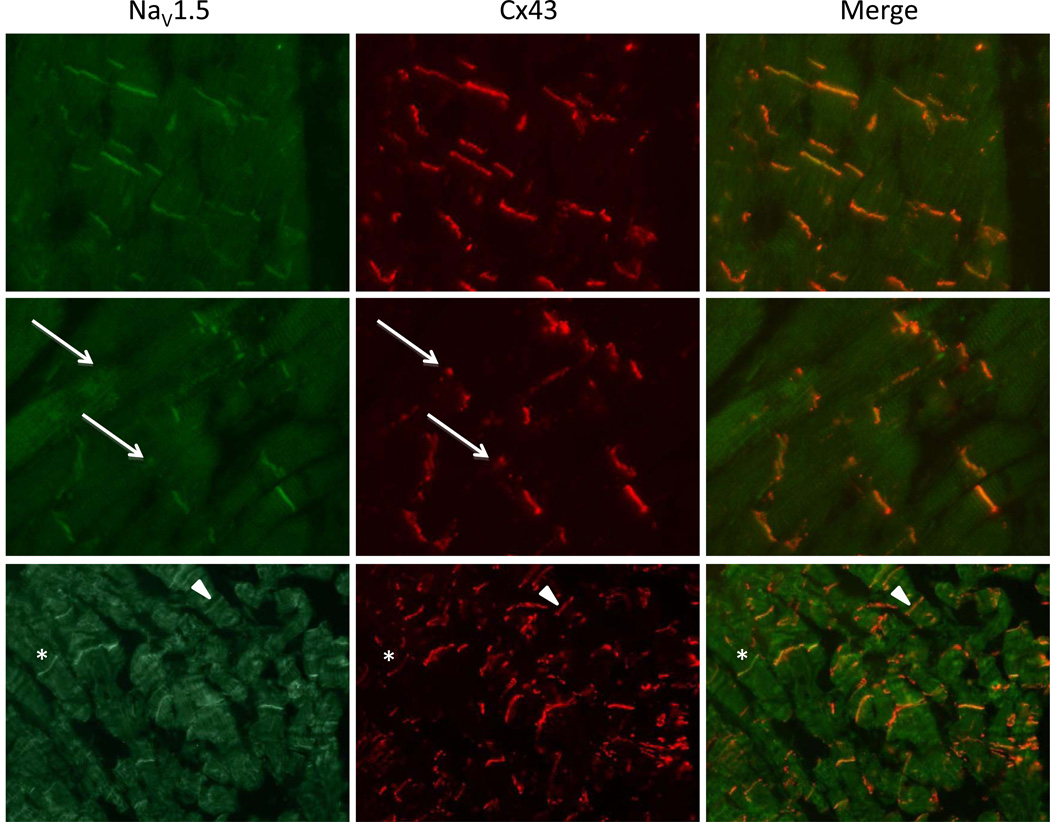
We also performed a double labeling of NaV1.5 and Cx43 (Figure 3). In areas where Cx43 was normally present in the intercalated disk (upper panels) both in controls and in patients, NaV1.5 was also present at the intercalated disk. We expected, based on previous results in mice,20 that in areas with a disturbed Cx43 signal, NaV1.5 would also show a similarly disturbed pattern. In figure 3 (middle panels), an area of disturbed Cx43 expression is shown (arrows) and in this place also NaV1.5 signal is reduced. However, we were unable to confirm that in every patient with a disturbed pattern of Cx43, NaV1.5 was concomitantly reduced. We also identified patients who presented a disturbance in Cx43 pattern without a reduced NaV1.5, and the other way around: patients with reduced NaV1.5 and unchanged Cx43. This is shown by the lower panels in Figure 3 where in one area both phenomena are presented. The asterisk illustrates an ID where Cx43 signal is disturbed while Nav1.5 is normal and the arrow-head illustrates the opposite: normal Cx43 signal with disturbed Nav1.5.
Figure 3. Double labeling of Cx43 and NaV1.5.
Upper panels show a region with unaffected Cx43 and NaV1.5 resulting in a strong overlay. Middle panels show a region of disturbed Cx43 signal, where also immunoreactive signal intensity of NaV1.5 is reduced (respective IDs marked with arrows). The lower panels display examples of IDs where either Nav1.5 is normal with a reduced signal of Cx43 (asterisk) or disturbed Nav1.5 with normal Cx43 (arrowheads).
Supplemental table 2 displays an overview of the results for each and every patient studied (AC1-AC20) regarding the evaluation of immunoreactive signals for Nav1.5, PKG and Cx43. Interestingly, of the 12 patients who showed disturbed signals for Nav1.5, 9 patients also showed disturbed signals for PKG and of those 9 double-affected patients, 6 patients showed in addition disturbed immunoreactive signals for Cx43 too. Another interesting observation is that in all patients in whom PKG was disturbed (14/19), the disturbance in PKG was always accompanied with a disturbance in signals for Nav1.5, Cx43, or both (AC18 excluded being judged inconclusive for Nav1.5). Supplemental Figure 1 summarizes these results as displayed in a Venn-diagram. When we combined these data with the individual TFC scores as listed in table 1, the data might suggest that those patients that based on immunohistochemical analysis were the most affected (meaning disturbed immunoreactive signals for all three proteins tested: Nav1.5, Cx43 and PKG) tended to have slightly higher TFC scores than those that were only affected for 1 or 2 proteins. The average TFC score of those patients was 8.0±0.9, (SEM, n=5 since AC5 did not have a TFC score) while the TFC score in the remaining other 11 with one or two factors affected was 7.1±0.5. Statistical analysis, however, did not result in a statistically significant correlation (r=0.37). In addition, no statistically significant correlation could be detected when we tested the individual TFC scores against the amount of disturbed parameters (being 1, 2 or 3), thereby not selecting the nature of the disturbed parameter. Finally, there was no significant relation between being affected for PKG and the amount of additional affected other parameters (p=0.41). Obviously, this statistical testing is highly limited by the number of included patients and the even smaller number of patients who were present in the different subgroups as illustrated in supplemental Figure 1.
Discussion
We have used an immunohistochemical approach to assess the signal intensity and distribution of immunoreactive proteins in samples of human tissue obtained from patients with the clinical diagnosis of AC. Our results showed that in a large majority of tissue specimens, immunoreactive signals for Cx43, PKG, and/or NaV1.5 were substantially disturbed and heterogeneously distributed. The observations were apparent, regardless of whether mutations in desmosomal genes were found. In contrast, immunoreactive signals for N-Cadherin and PKP2 were normal, except in the case of tissue from patients with a genotype that likely would probably lead to PKP2 haploinsufficiency through deletion of the first 4, or all of the exons.
Several studies have shown a connection between disturbances in mechanical coupling, electrical coupling and excitability of cardiomyocytes.20, 21, 26 These observations were merely derived from artificial systems, where an extreme reduction of one of the components was induced. In the present study, we have used human AC patient material where immunoreactive signals of the mechanical components appeared generally unaffected, despite the fact that mutations in the mechanical components were present in a large proportion of cases. Since the protein immunoreactivity is highly subjected to tissue preservation and experimental conditions, we assessed detection of a given protein with various antibody dilutions. The observed reduction of PKP2 signal-intensity in patients predicted to be haplo-insufficient suggests that the PKP2 antibody dilution was adequate to detect variations in signal intensity (see Figure 1). In addition, control experiments in which the antibody used against PKP2 was further diluted up to 1000 times more than the regular concentration used, still revealed equal but lower intensities of PKP2 in control and patients without a mutation in PKP2 (data not shown).
Mutations in desmosomal proteins are associated with the AC phenotype. A common occurrence is a heterogeneous distribution of cardiac Cx43 at the intercalated disks.10–12 In the present study, we show for the first time that junctional immunoreactive signal of NaV1.5 expression also is decreased in a large subset of our patients. In general, safe and normal conduction depends on appropriate excitability (facilitated through the NaV1.5 channels), cell-to-cell conduction through Cx43 gap junctions, and tissue geometry (preferred absence of massive insulating fibrosis). Pro-arrhythmic alterations in the latter two factors have been recognized in AC patients before. The common disturbance of Cx43 signals but not of the desmosomal proteins that we described is consistent with the results of Fidler et al,27 which showed that RVSB of four patients with a PKP2 mutation all displayed a disturbed Cx43 pattern, with no changes in PKP2 distribution in 3 of the four cases. Of note, the PKP2 mutations included in our study and those described in the study of Fidler et al are different. In the latter case, the mutations were not expected to impair the trafficking of PKP2 to the intercalated disc.
Though multiple components involved in the molecular basis of AC have been identified, the sequence of events that lead to disruption of the macromolecular complex that is situated at the ID still remains fairly unresolved. Whether the molecules of the desmosome, the gap junctions and the sodium channel complex interact directly, or through unknown molecular partners, remains to be defined. In vitro studies in which PKP2 was deleted by interference strategies revealed a concomitant decrease in intercellular communication, excitability and impulse propagation.21, 28 On the other hand, reduced presence of PKG (also known as γ-catenin) at the ID may lead to replacement with β-catenin which in turn reduces intra-nuclear levels of β-catenin and as such transcriptional reduction of Cx43.29, 30 Notably, in all patients within this study, in which PKG signals appeared disturbed, this was always accompanied with a disturbance in immunoreactive signal either of Cx43, or Nav1.5, or both.
Animal studies in genetically engineered mice have shown that a combination of genetically reduced NaV1.5 and Cx43 levels did induce conduction slowing without, however, exhausting conduction reserve and a resulting increased propensity to arrhythmias.31 Genetically reduced Cx43 levels on its own also appeared able to induce a reduction of Nav1.5 dependent sodium current.20, 32 When these conditions were exacerbated in a model of aged mice with reduced levels of Cx43 and Nav1.5, as well as increased amounts of fibrosis, conduction reserve exhausted and a significant incidence of arrhythmias could be recorded.33 The latter situation fits very well with the progressive deterioration as seen in the AC disease model where increasing amounts of fibrosis are found in the later phase of the disease. Additional insight into the inter-relation of Cx43 and the preservation of cardiac structure was provided in a follow up study in which we showed enhanced fibrosis in mice with reduced Cx43 expression; the latter seemed consequent to enhanced fibroblast activity rather than increased proliferation of these cells.34
Though the present study concentrated on a few molecules thought to be relevant to the electrical phenotype, the spectrum of molecular remodeling in AC hearts is likely to be much broader. Within our limitations, our data do suggest that a reduced abundance of Nav1.5 immunoreactive protein at the intercalated disc may be a component of the molecular profile in some AC cases. The latter may, in turn, be a component of the electrophysiological substrate present in AC patients. As in the case of patients suspected of Brugada syndrome, a sodium channel blocker challenge potentially might help for identification and/or stratification of patients at risk of AC, particularly those in the concealed phase of the disease. As a proof of principle, we recently showed that flecainide administration to young heterozygous PKP2 mice with structurally normal hearts (no fibrosis or adiposis detected) led to a high incidence of ventricular arrhythmias and sudden death, whereas the same flecainide challenge did not cause either arrhythmias or death in control littermates.22
Study limitations
The immunohistochemical data presented in this study merely show qualitative differences between controls and patients. Immunoreactive signals of several intercalated disk-associated proteins have been studied in autopsy material from controls and patients and these data have been compared to data obtained with RVSB. These septal biopsies were taken from AC patients who were still under clinical evaluation and were both limited in amount and size, which excludes introduction of additional quantification methodology via Western blotting or by patch clamp. We also did not include a detailed analysis of the degree of fibro-fatty replacement. Though fibrosis was apparent in al biopsies studied, the septal tissue is regarded to be less representative in this aspect.
One of the important limitations of our study is the narrow range of contrast that we have been able to obtain in our microscopic images. It is important to note, though, that working with human material poses particular technical challenges. We have gone to great lengths to protect the quality of the tissue, despite the limitations inherent to its collection. Yet, across the board, issues like background fluorescence, autofluorescence of fibrotic material, or protein immunoreactivity, prevent the signal obtained in human tissue samples from being as sharp and crisp as those that can be obtained from animal tissue, or from cell preparations. Within those limitations, we have placed emphasis on collecting and processing the samples in the most homogeneous, consistent way. This has allowed us to compare the immunoreactive signals obtained from different patients, and this method of analysis is consistent with a number of previous publications on this subject and the best alternative to the realities of studies involving human subjects.
Conclusion
Immunohistochemical analysis in AC reveals reduced signals for Cx43, PKG and/or NaV1.5 in a majority of patients. The newly identified reduction of Nav1.5 sodium channels might importantly contribute to arrhythmia vulnerability in AC patients and could, in the future, be added as an element of evaluation for risk stratification. Our data further support the notion that deficiency in the abundance and/or function of the sodium channel complex may be one of the multiple arrhythmogenic substrates present in the hearts of patients afflicted with mutations in desmosomal proteins and as such, at risk of ventricular fibrillation and sudden cardiac death.
Supplementary Material
Acknowledgments
This work is financially supported by the Netherlands Heart Foundation grant 2007B139 and the Interuniversity Cardiology Institute of the Netherlands (ICIN) project # 06901. Dr. Saffitz was supported by a grant from the US National Institutes of Health (HL102361). Dr. Asimaki was supported by a fellowship grant from the Heart Rhythm Society. Dr. Delmar was supported by grants RO1-HL106632, PO1-HL087226 and RO1-GM67691 from the National Institutes of Health, and a Foundation Leducq Transatlantic Network. Dr. Mohler is supported by the Saving Tiny Hearts Society, the US National Institutes of Health (HL084583, HL083422), Fondation Leducq, and the American Heart Association. Dr. Hund is supported by the US National Institutes of Health (HL096805) and the Gilead Sciences Research Scholars Program.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors maybe discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
No conflict of interest
References
- 1.Sen-Chowdhry S, Syrris P, Prasad SK, et al. Left-dominant arrhythmogenic cardiomyopathy: An under-recognized clinical entity. J Am Coll Cardiol. 2008;52:2175–2187. doi: 10.1016/j.jacc.2008.09.019. [DOI] [PubMed] [Google Scholar]
- 2.Saffitz JE. The pathobiology of arrhythmogenic cardiomyopathy. Annu Rev Pathol. 2011;6:299–321. doi: 10.1146/annurev-pathol-011110-130151. [DOI] [PubMed] [Google Scholar]
- 3.Asimaki A, Syrris P, Wichter T, Matthias P, Saffitz JE, McKenna WJ. A novel dominant mutation in plakoglobin causes arrhythmogenic right ventricular cardiomyopathy. Am J Hum Genet. 2007;81:964–973. doi: 10.1086/521633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Heuser A, Plovie ER, Ellinor PT, et al. Mutant desmocollin-2 causes arrhythmogenic right ventricular cardiomyopathy. Am J Hum Genet. 2006;79:1081–1088. doi: 10.1086/509044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Syrris P, Ward D, Evans A, Asimaki A, et al. Arrhythmogenic right ventricular dysplasia/cardiomyopathy associated with mutations in the desmosomal gene desmocollin-2. Am J Hum Genet. 2006;79:978–984. doi: 10.1086/509122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pilichou K, Nava A, Basso C, et al. Mutations in desmoglein-2 gene are associated with arrhythmogenic right ventricular cardiomyopathy. Circulation. 2006;113:1171–1179. doi: 10.1161/CIRCULATIONAHA.105.583674. [DOI] [PubMed] [Google Scholar]
- 7.Rampazzo A, Nava A, Malacrida S, et al. Mutation in human desmoplakin domain binding to plakoglobin causes a dominant form of arrhythmogenic right ventricular cardiomyopathy. Am J Hum Genet. 2002;71:1200–1206. doi: 10.1086/344208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gerull B, Heuser A, Wichter T, et al. Mutations in the desmosomal protein plakophilin-2 are common in arrhythmogenic right ventricular cardiomyopathy. Nat Genet. 2004;36:1162–1164. doi: 10.1038/ng1461. [DOI] [PubMed] [Google Scholar]
- 9.Cox MG, van der Zwaag PA, van der Werf C, et al. Arrhythmogenic right ventricular dysplasia/cardiomyopathy: Pathogenic desmosome mutations in index-patients predict outcome of family screening: Dutch arrhythmogenic right ventricular dysplasia/cardiomyopathy genotype-phenotype follow-up study. Circulation. 2011;123:2690–2700. doi: 10.1161/CIRCULATIONAHA.110.988287. [DOI] [PubMed] [Google Scholar]
- 10.Kaplan SR, Gard JJ, Carvajal-Huerta L, Ruiz-Cabezas JC, Thiene G, Saffitz JE. Structural and molecular pathology of the heart in carvajal syndrome. Cardiovasc Pathol. 2004;13:26–32. doi: 10.1016/S1054-8807(03)00107-8. [DOI] [PubMed] [Google Scholar]
- 11.Kaplan SR, Gard JJ, Protonotarios N, et al. Remodeling of myocyte gap junctions in arrhythmogenic right ventricular cardiomyopathy due to a deletion in plakoglobin (naxos disease) Heart Rhythm. 2004;1:3–11. doi: 10.1016/j.hrthm.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 12.Asimaki A, Tandri H, Huang H, et al. A new diagnostic test for arrhythmogenic right ventricular cardiomyopathy. N Engl J Med. 2009;360:1075–1084. doi: 10.1056/NEJMoa0808138. [DOI] [PubMed] [Google Scholar]
- 13.van Rijen HV, Eckardt D, Degen J, et al. Slow conduction and enhanced anisotropy increase the propensity for ventricular tachyarrhythmias in adult mice with induced deletion of connexin43. Circulation. 2004;109:1048–1055. doi: 10.1161/01.CIR.0000117402.70689.75. [DOI] [PubMed] [Google Scholar]
- 14.Gutstein DE, Morley GE, Vaidya D, et al. Heterogeneous expression of gap junction channels in the heart leads to conduction defects and ventricular dysfunction. Circulation. 2001;104:1194–1199. doi: 10.1161/hc3601.093990. [DOI] [PubMed] [Google Scholar]
- 15.Wiegerinck RF, van Veen TA, Belterman CN, et al. Transmural dispersion of refractoriness and conduction velocity is associated with heterogeneously reduced connexin43 in a rabbit model of heart failure. Heart Rhythm. 2008;5:1178–1185. doi: 10.1016/j.hrthm.2008.04.026. [DOI] [PubMed] [Google Scholar]
- 16.Boulaksil M, Winckels SK, Engelen MA, et al. Heterogeneous connexin43 distribution in heart failure is associated with dispersed conduction and enhanced susceptibility to ventricular arrhythmias. Eur J Heart Fail. 2010;12:913–921. doi: 10.1093/eurjhf/hfq092. [DOI] [PubMed] [Google Scholar]
- 17.Marcus FI, McKenna WJ, Sherrill D, et al. Diagnosis of arrhythmogenic right ventricular cardiomyopathy/dysplasia: Proposed modification of the task force criteria. Circulation. 2010;121:1533–1541. doi: 10.1161/CIRCULATIONAHA.108.840827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Winterfield JR, Lee J, Asimaki A, et al. Plakoglobin abnormalities and arrhythmogenesis in idiopathic cardiomyopathies. Heart Rhythm. 2010;7:S113. [Google Scholar]
- 19.Asimaki A, Tandri H, Duffy ER, et al. Altered desmosomal proteins in granulomatous myocarditis and potential pathogenic links to arrhythmogenic right ventricular cardiomyopathy. Circ Arrhythm Electrophysiol. 2011;4:743–752. doi: 10.1161/CIRCEP.111.964890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jansen JA, Noorman M, Musa H, et al. Reduced heterogeneous expression of cx43 results in decreased nav1.5 expression and reduced sodium current that accounts for arrhythmia vulnerability in conditional cx43 knockout mice. Heart Rhythm. 2012;9:600–607. doi: 10.1016/j.hrthm.2011.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sato PY, Musa H, Coombs W, et al. Loss of plakophilin-2 expression leads to decreased sodium current and slower conduction velocity in cultured cardiac myocytes. Circ Res. 2009;105:523–526. doi: 10.1161/CIRCRESAHA.109.201418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cerrone M, Noorman M, Lin X, et al. Sodium current deficit and arrhythmogenesis in a murine model of plakophilin-2 haploinsufficiency. Cardiovasc Res. 2012 doi: 10.1093/cvr/cvs218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.van Veen TA, van Rijen HV, Wiegerinck RF, et al. Remodeling of gap junctions in mouse hearts hypertrophied by forced retinoic acid signaling. J Mol Cell Cardiol. 2002;34:1411–1423. doi: 10.1006/jmcc.2002.2102. [DOI] [PubMed] [Google Scholar]
- 24.Hund TJ, Koval OM, Li J, et al. A beta(iv)-spectrin/camkii signaling complex is essential for membrane excitability in mice. J Clin Invest. 2010;120:3508–3519. doi: 10.1172/JCI43621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Munkholm J, Christensen AH, Svendsen JH, Andersen CB. Usefulness of immunostaining for plakoglobin as a diagnostic marker of arrhythmogenic right ventricular cardiomyopathy. Am J Cardiol. 2012;109:272–275. doi: 10.1016/j.amjcard.2011.08.044. [DOI] [PubMed] [Google Scholar]
- 26.Oxford EM, Musa H, Maass K, Coombs W, Taffet SM, Delmar M. Connexin43 remodeling caused by inhibition of plakophilin-2 expression in cardiac cells. Circ Res. 2007;101:703–711. doi: 10.1161/CIRCRESAHA.107.154252. [DOI] [PubMed] [Google Scholar]
- 27.Fidler LM, Wilson GJ, Liu F, et al. Abnormal connexin43 in arrhythmogenic right ventricular cardiomyopathy caused by plakophilin-2 mutations. J Cell Mol Med. 2009;13:4219–4228. doi: 10.1111/j.1582-4934.2008.00438.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sato PY, Coombs W, Lin X, et al. Interactions between ankyrin-g, plakophilin-2, and connexin43 at the cardiac intercalated disc. Circ Res. 2011;109:193–201. doi: 10.1161/CIRCRESAHA.111.247023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.van der Heyden MA, Rook MB, Hermans MM, et al. Identification of connexin43 as a functional target for wnt signalling. J Cell Sci. 1998;111(Pt 12):1741–1749. doi: 10.1242/jcs.111.12.1741. [DOI] [PubMed] [Google Scholar]
- 30.Garcia-Gras E, Lombardi R, Giocondo MJ, et al. Suppression of canonical wnt/beta-catenin signaling by nuclear plakoglobin recapitulates phenotype of arrhythmogenic right ventricular cardiomyopathy. J Clin Invest. 2006;116:2012–2021. doi: 10.1172/JCI27751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stein M, van Veen TA, Remme CA, et al. Combined reduction of intercellular coupling and membrane excitability differentially affects transverse and longitudinal cardiac conduction. Cardiovasc Res. 2009;83:52–60. doi: 10.1093/cvr/cvp124. [DOI] [PubMed] [Google Scholar]
- 32.Desplantez T, McCain ML, Beauchamp P, et al. Connexin43 ablation in foetal atrial myocytes decreases electrical coupling, partner connexins, and sodium current. Cardiovasc Res. 2012;94:58–65. doi: 10.1093/cvr/cvs025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stein M, Boulaksil M, Jansen JA, et al. Reduction of fibrosis-related arrhythmias by chronic renin-angiotensin-aldosterone system inhibitors in an aged mouse model. Am J Physiol Heart Circ Physiol. 2010;299:H310–H321. doi: 10.1152/ajpheart.01137.2009. [DOI] [PubMed] [Google Scholar]
- 34.Jansen JA, van Veen TA, de Jong S, et al. Reduced cx43 expression triggers increased fibrosis due to enhanced fibroblast activity. Circ Arrhythm Electrophysiol. 2012 doi: 10.1161/CIRCEP.111.966580. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.