Abstract
Background
Although opioid substitution therapy is an effective clinical tool used to manage opioid abuse and dependence, concerns regarding the current FDA-approved medications have lead to a search for efficacious, non-opioid medications. Preclinical data indicate that neurokinin 1 (NK1) receptor activity may modulate opioid effects and withdrawal. This investigation sought to examine the ability of the NK1 antagonist aprepitant to alter the effects of methadone as well as withdrawal symptoms induced by brief methadone discontinuation.
Methods
This blinded, placebo-controlled, within-subjects study consisted of placebo and aprepitant conditions. Experimental assessments occurred on the first three days (Days 1–3: placebo or aprepitant + methadone) and again on Days 8–10 (aprepitant or placebo + methadone). Fifteen methadone-maintained patients completed the investigation. Outcome measures were assessments of opioid withdrawal, as well as subjective measures of opioid-like effects.
Results
Statistical trends indicated that aprepitant may reduce opioid withdrawal symptoms. When an active dose of aprepitant was administered an hour before methadone, participants reported less desire to use methadone. However, ratings of methadone “Liking” also appeared to increase.
Conclusions
These data tentatively suggest that aprepitant has some ability to alleviate withdrawal following methadone abstinence, but also appears to increase subjective indicators of methadone’s abuse liability. Since few of the differences between aprepitant and placebo reached statistical significance, these data should only be viewed as preliminary. Findings from other studies indicate that higher doses of aprepitant may be more clinically effective. Further clinical investigations are needed in order to determine whether aprepitant is useful for alleviating opioid withdrawal.
Introduction
It is estimated that 9.2 million people worldwide are regular heroin users, with an estimated 1.2 million active heroin users in the U.S. 1,2,3,4,5 The United States has also observed a substantial increase in the abuse of opioid analgesics such as hydrocodone, hydromorphone and oxycodone.6 In recent years, more people sought treatment for dependence on prescription opioids than for dependence on heroin.7 Although long-term maintenance on medications such as methadone and buprenorphine has significant clinical utility, these treatment strategies are not without shortcomings.8 In the U.S, methadone must be taken daily at specially licensed clinics, and for some opioid abusers, this inconvenience can act as a barrier to treatment access.9
Buprenorphine is another agonist maintenance treatment option, with a superior safety profile and more convenient dispensing when compared to methadone.10, 11 However, because both medications are potent mu agonists, diversion to illicit use is a concern.12,13,14,15 Furthermore, both methadone and buprenorphine maintain physiological dependence on opioids, which is another aspect of their use that does not appeal to some patients and regulatory authorities. As an alternative to agonist maintenance therapy, opioid receptor antagonists have been available in the U.S. since 1984. However, this treatment strategy has been plagued by poor medication adherence by patients. As a result, only 15.8% of substance abuse treatment facilities in the U.S. report using naltrexone.16
Although newer formulations of naltrexone may improve adherence and outcomes, there is clearly a need for the continued development of efficacious treatments for opioid dependence without abuse liability. One such novel and promising pharmacotherapeutic target may be the NK1 receptor (the endogenous receptor for the neuropeptide substance P), which appears to play a key role in modulating the behavioral effects of the prototypical mu opioid agonist morphine. NK1 receptor knockout (KO) mice fail to demonstrate morphine-induced (10 mg/kg) locomotor activity and conditioned place-preference (CPP), they do not self-administer typically reinforcing doses of intravenous (IV) morphine (0.2 mg/kg), and do not exhibit naloxone-precipitated (1 mg/kg) withdrawal symptoms.17,18 Chemical destruction of NK1 receptors using intracerebroventricular (ICV) administration of the neurotoxin substance P-saporin has been shown to reduce the stimulatory and rewarding effects of morphine in mice.19 Other investigations have found that ICV administration of another NK1 antagonist (GR 82334, 10–50 pmol/2 μl) can attenuate morphine-induced (5 mg/kg) locomotor activity but it enhances heroin (0.06 mg/kg) self-administration.20 ICV injections of another NK1 antagonist (RP 67580, 10–90 μg/5 μl) can inhibit naloxone-precipitated withdrawal.21, 22
These data strongly suggest an involvement of NK1 receptors in opioid reward and withdrawal and highlight the need for investigations of this receptor system in clinical populations. In 2003, aprepitant (Emend®) became the first FDA-approved NK-1 receptor antagonist on the market for the treatment of chemotherapy-induced nausea and vomiting.23 The primary aim of this pilot study was to assess interactions between aprepitant and methadone, and between aprepitant and early methadone withdrawal in methadone-maintained patients (MMP).
Materials and Methods
Participants
Male and female methadone-maintained patients were recruited through flyers and newspaper advertisements throughout the New York City metropolitan area. Participants were required to be between the ages of 18 and 65 years, and must have been maintained on the same approximate dose of methadone for at least 4 weeks prior to enrollment. All patients received methadone to treat opioid abuse and dependence and not for the treatment of physical pain. Potential participants were excluded for: any current significant Axis 1 psychiatric diagnoses (other than those related to drug abuse or opioid dependence), organ dysfunction or serious unstable disease state, regular use of any psychoactive medication and use of any investigational medication within the past 30 days. Participants were screened over the course of approximately 1–3 visits, after providing written informed consent. Participants were compensated $60 for completing screening and baseline evaluations, $30 for completing each visit on Days 1, 2, 8, and 9, and $100 for completing each visit on Days 3 and 10. Participants who completed all test procedures received a $60 bonus, making the total possible compensation $440. This study and all of its procedures were approved by the IRB of the VA New York Harbor Healthcare System (VA NYHHS).
Experimental Design
This outpatient, placebo-controlled, double-blind crossover study consisted of placebo and aprepitant conditions separated by a medication washout period of at least 5 days (greater than 5x the half-life of aprepitant). Participants and staff were blinded to the study medication but not methadone. The total duration of the study was approximately 10 days (Figure 1). Experimental assessments occurred on Days 1–3 (placebo or aprepitant) and again, following the washout period, on Days 8–10 (aprepitant or placebo).
Figure 1.
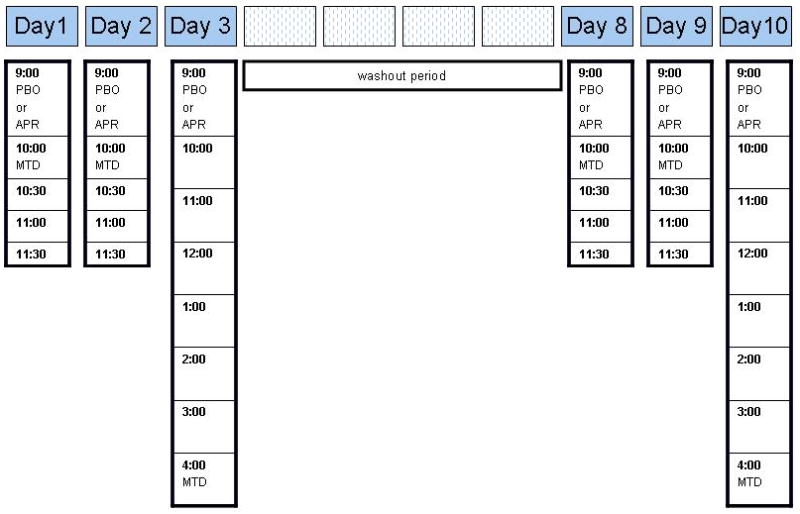
Time points throughout the day at which study drug (aprepitant or placebo) and methadone were administered over the approximate 10-day duration of the study. PBO = placebo, APR = aprepitant, MTD = methadone.
Prior to each experimental session, non-fasting participants provided a urine sample to verify that they had not used illicit drugs. If participants tested positive for illicit substances (illegal drugs or legal medications like benzodiazepines), that were not originally reported as one of their concomitant medications, their session was rescheduled. Exceptions were made for occasional THC positive urine tests, if the participant was not acutely intoxicated, since urine screens for this drug test positive long after use.
On the first two days (Days 1, 2 and 8, 9) of each testing period, participants received the study medication (placebo or aprepitant) at 9 am (after ≈ 24 hrs of methadone abstinence), followed by their usual dose of methadone at 10 am. Study assessments occurred at baseline (just prior to study medication) and every 30 min between 10 am and 11:30 am. On the third day of each testing period (Days 3 and 10), participants received study medication (placebo or aprepitant following ≈ 24 hrs of methadone abstinence) at 9 am, but did not received their methadone dose until 4 pm. Study assessments were performed every 60 minutes between 9 am and 4 pm (Figure 1). Aprepitant and methadone dosing regimens employed for this study were based upon standard clinical practice for their respective medical use.
Questionnaires
A number of questionnaires were used to assess medication effects and opioid withdrawal symptoms. The first questionnaire was a 13-item visual analog scale (VAS) designed to assess subjective mood and physiological effects of aprepitant and methadone. Participants rated each item on the visual analog scale from ‘Not at all’ (0 mm) to ‘Extremely’ (100 mm). Similarly, a shortened form of the Addiction Research Center Inventory (ARCI) allowed participants to rate a broad range of physical, emotive, cognitive, and drug effects.24 Additionally, the Subjective Opioid Withdrawal Scale (SOWS), Clinical Opioid Withdrawal Scale (COWS) and Brief Substance Craving Scale (BSCS) were used to detect opioid withdrawal and assess its severity.25
Drugs
Aprepitant capsules were obtained from commercial vendors via the VA NYHHS Investigational Pharmacy. The dose used in this study (80 mg, p.o.) is the recommended dose for its FDA-approved use as an anti-emetic agent. The investigational pharmacy prepared blinded active aprepitant and matching placebo using over-encapsulation.
Participants’ methadone doses were dispensed in accordance with the standard clinical practices at their respective methadone maintenance programs. During experimental testing days (Days 1–3 and 8–10), methadone was administered in the research clinic at the VA NYHHS. During all other periods, methadone was consumed at the participants’ respective methadone-maintenance program (MMP) clinics.
Statistical Analyses
The effects of aprepitant were analyzed in 2 models. The first model evaluated the interaction between aprepitant and methadone on the second day of each treatment condition (Days 2 and 9). Repeated-measures ANOVAs compared differences between the dosing conditions (aprepitant vs. placebo) over the various post-medication time points. Only 8 of 15 participants were able to complete testing on the first day of both testing periods (Day 1 and 8). Participants missed the first day of each session due to: scheduling conflicts, arriving too late to complete the session, and postponing of the session due to urine drug toxicology results. Therefore, few complete data sets from Days 1 and 8 were available and the data were not analyzed. Regardless of whether a Day 1/8 session was performed, participants were administered a dose of aprepitant or placebo on the days preceding their Day 2/9 and Day 3/10 session, consistent with the dosing procedures mentioned above.
The second model assessed the effects of aprepitant on methadone withdrawal measured on the 3rd day of treatment (Days 3, 10). Outcome measures were analyzed using repeated-measures ANOVA comparing aprepitant and placebo conditions, over the various post-medication time points. When appropriate for both models, planned comparisons were used as follow-up analyses to compare aprepitant and placebo at specific time points. Given the preliminary nature of the study, the significance level of α was set at 0.05 with an α of less than or equal to 0.1 considered as approaching statistical significance. All data analyses were performed using SPSS version fifteen 26 and SuperANOVA.27
Results
Participant demographics
Twenty MMP patients were enrolled into this study, of which 15 completed it. The sample of completers consisted of 11 men and 4 women; 6 African-Americans, 5 Caucasians and 4 Latinos. The participants’ average age was 47.3 ± 9 years, ranging from 31 to 59 years. All participants were daily oral methadone users and had been under methadone therapy for a minimum of 6 months. Participants’ current mean daily methadone dose was 80.9 mg ± 9, ranging from 28–130 mg. For all participants, methadone doses administered during the study matched the dose on which they had been maintained for at least 4 weeks. Nine of the participants entered the aprepitant dosing condition first, and 6 began with placebo. Only 3 mild adverse events (AEs) were judged by a physician to be “possibly” study related (Fatigue, Upset stomach, Insomnia). No adverse events were determined as being “definitely” related to aprepitant.
Interaction of Aprepitant and Methadone
ANOVA revealed that aprepitant slightly decreased participants’ desire to use methadone [F (1, 14) = 3.12, p=0.09]. Follow-up analyses at each of the individual time points revealed that when placebo was given in combination with methadone, participants reported more “desire to use methadone” at 90 min (p=0.06) and 120 min (p=0.05) following drug administration. In support of this finding, a statistical trend was found indicating that aprepitant decreased opioid craving (p=0.10). COWS and SOWS assessments of opioid withdrawal did not show significant differences between aprepitant and placebo pretreatments.
An active dose of aprepitant also appears to increase participants’ ratings of methadone “Liking” [F (1, 14) = 2.14, p=0.08], with a nearly 41% increase being observed at time 120 min [t (14) = 2.45, p=0.028: Figure 3]. Interestingly, a similar finding at the same time point was observed for participants’ rating of “High” (30% increase, [t (14) = 2.09, p=0.05].
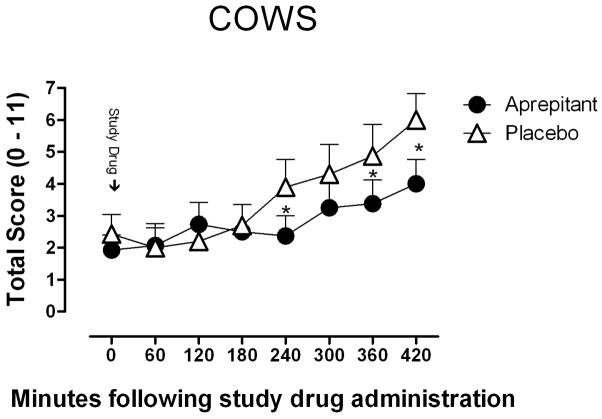
Figure 3.
Mean (± SEM) observer-rated COWS scores as a function of study drug (aprepitant or placebo) and time throughout the session. Following 24 hrs of methadone abstinence, aprepitant or placebo was administered at time 0. * Indicates a significant difference of less than 0.10.
The Effects of Aprepitant on Withdrawal
Aprepitant slightly reduced COWS scores [F (1, 9) = 1.97, p=0.10)] during short-term methadone abstinence, but did not affect SOWS scores or any other subjective indicators of withdrawal severity.
Discussion
In this small cohort of methadone-maintenance patients, the data indicate that aprepitant shows some utility for alleviating opioid withdrawal and craving. Interestingly, despite not having any positive subjective effects (based on subjective assessments at the 60-min time point, immediately prior to methadone administration), aprepitant did increase the positive subjective effects of methadone. This finding suggests that co-administration of aprepitant with opioid drugs may increase their abuse liability. These findings are consistent with another recent clinical study reporting that 200 mg aprepitant significantly increased the amount of money participants would pay for doses of oral and intranasal oxycodone.28 Aprepitant also increased participants’ subjective ratings of oxycodone-induced “High” and drug “Liking”, while increasing oxycodone-induced miosis. In that investigation, the magnitude of the effects of the lower dose of aprepitant (40 mg) on these abuse liability measures were comparable to those observed in the current investigation.
Because of the small sample size and minimal statistical significance, conclusions drawn from the current study should be considered very tentative. One possible explanation for the lack of a robust drug effect may be the design of the study. The observation window selected for assessment of aprepitant effects may have been too narrow and not have overlapped adequately with methadone’s peak effects or when methadone withdrawal reached its peak. Additionally, the study only employed a single, moderate dose of aprepitant. Since other clinical studies have found that higher doses of aprepitant have stronger interactions with opioids, higher doses deserve further systematic study. Because oral doses of aprepitant greater than 300 mg are reportedly safe in patients undergoing chemotherapy, there is ample room for clinical exploration with this drug.23
Aprepitant currently is approved as an anti-emetic medication. Since nausea and vomiting are common clinical manifestations of opioid withdrawal29, aprepitant may be useful in managing opioid withdrawal symptoms. In addition to its utility in treating opioid withdrawal, the management of opioid craving may be important in maintaining opioid abstinence. Although other non-opioid medications like clonidine and lofexidine are available, these medications are limited in their ability to attenuate opioid craving and autonomic signs of withdrawal.30 As such, these findings underscore the importance of continued development of non-opioid medications such as aprepitant.
Figure 2.
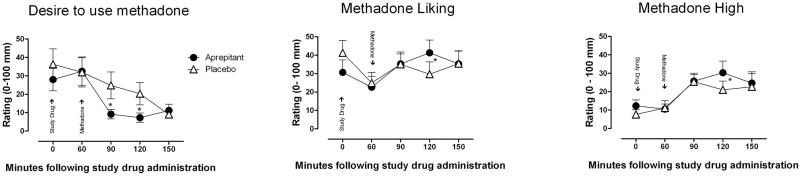
Participant ratings (mean ± SEM) of methadone “Desire,” “Liking,” and “High” as a function of study drug (aprepitant or placebo) and time throughout the session (Day 2 and 9). Following 24 hrs of methadone abstinence, aprepitant or placebo was administered at time 0 and methadone was administered 60 minutes later. * Indicates a significant difference of less than 0.10.
Acknowledgments
Study supported by an NYU Langone Medical Center Seed Grant to MSR through the NYULMC Center of Excellence on Addiction.
References
- 1.Bammer G, Dobler-Mikola A, Philip M, Strang J, Uchtenhagen A. The heroin prescribing debate: integrating science and politics. Science. 1999;284:1277–1278. doi: 10.1126/science.284.5418.1277. [DOI] [PubMed] [Google Scholar]
- 2.Epstein JF, Groerer JC. Heroin Abuse in United States. [Accessed February 20th, 2011];The National Clearing house of Alcohol and Drug Information. 1997 Available at: http://www.oas.samhsa.gov/treatan/treana11.htm.
- 3.United Nations Drug Control Programme (UNDCP) Annual Report Questionnaire. International Narcotics Control Strategy Report(s) Washington: US Department of State; 2001. Retrieved on Jan 26th, 2011 from: http://www.state.gov/p/inl/rls/nrcrpt/2001/c6085.htm. [Google Scholar]
- 4.United Nations Office for Drug Control, Crime Prevention. Global Illicit Drug Trends. New York: United Nations; 2002. Retrieved on Jan 26th, 2011 from: http://www.unodc.org/pdf/report_2002-06-26_1/report_2002-06-26_1.pdf. [Google Scholar]
- 5.United Nations Office on Drugs and Crime (UNODC) Report: Promoting health, security and justice. 2010. New York USA: United Nations; 2010. Retrieved on Jan 26th, 2011 from: http://www.unodc.org/documents/frontpage/UNODC_Annual_Report_2010_LowRes.pdf. [Google Scholar]
- 6.DASIS Report. TEDS reports on treatment admissions and treatment discharges. Office of Applied Studies, Substance Abuse and Mental Health Services Administration; 2007. [Accessed: June 23rd, 2011]. Available online at: http://oas.samhsa.gov/TEDS2k7highlights/toc.cfm. [Google Scholar]
- 7.Office of Applied Studies. The TEDS Report: Substance Abuse Treatment Admissions Involving Abuse of Pain Relievers: 1998 and 2008. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2009. [Accessed March 15th, 2012]. Available at: http://oas.samhsa.gov/2k10/230/230PainRelvr2k10.cfm. [Google Scholar]
- 8.Fareed A, Casarella J, Amar R, Vayalapalli S, Drexler K. Benefits of retention in methadone maintenance and chronic medical conditions as risk factors for premature death among older heroin addicts. J Psychiatric Prac. 2009;15(3):227–234. doi: 10.1097/01.pra.0000351884.83377.e2. [DOI] [PubMed] [Google Scholar]
- 9.Joseph H, Stancliff S, Langrod Methadone Maintenance Treatment (MMT): A review of historical and clinical issues. The Mount Sinai Medical Journal. 2000;(5–6):347–364. [PubMed] [Google Scholar]
- 10.Mattick RP, Kimber J, Breen C, Davoli M. Buprenorphine maintenance versus placebo or methadone maintenance for opioid dependence. Cochrane Database Systematic Reviews. 2008;16:CD002207. doi: 10.1002/14651858.CD002207.pub3. [DOI] [PubMed] [Google Scholar]
- 11.Walsh SL, Preston KL, Stitzer ML, Cone EJ, Bigelow GE. Clinical pharmacology of buprenorphine: ceiling effects at high doses. Clin Pharmacol Therapeu. 1994;55:569–580. doi: 10.1038/clpt.1994.71. [DOI] [PubMed] [Google Scholar]
- 12.Comer SD, Sullivan MA, Walker EA. Comparison of intravenous buprenorphine and methadone self-administration by recently detoxified heroin-dependent individuals. J Pharmacol Exp Ther. 2005;315:1320–1330. doi: 10.1124/jpet.105.090423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Comer SD, Sullivan MA, Whittington RA, Vosburg SK, Kowalczyk WJ. Abuse liability of prescription opioids compared to heroin in morphine-maintained heroin abusers. Neuropsychopharmacol. 2008;33:1179–1191. doi: 10.1038/sj.npp.1301479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Comer SD, Bickel WK, Yi R, de Wit H, Higgins ST, Wenger GR, Johanson CE, Kreek MJ. Human behavioral pharmacology, past, present, and future: symposium presented at the 50th annual meeting of the Behavioral Pharmacology Society. Behav Pharmacol. 2010;21:251–277. doi: 10.1097/FBP.0b013e32833bb9f8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.National Drug Intelligence Center (NDIC) [Accessed June 20th, 2011];Methadone Diversion, Abuse, and Misuse: Deaths Increasing at Alarming Rate. 2007 Available at: http://www.justice.gov/ndic/pubs25/25930/index.htm.
- 16.Substance Abuse and Mental Health Services Administration (SAMHSA) National Survey of Substance Abuse Treatment Services. Data on Substance Abuse Treatment Facilities. 2009. Rockville MD: US Department of Health and Human Services; DHHS publication SMA 05–4112. [Google Scholar]
- 17.Murtra P, Sheasby AM, Hunt SP, De Felipe C. Rewarding effects of opiates are absent in mice lacking the receptor for substance P. Nature. 2000;405:180–183. doi: 10.1038/35012069. [DOI] [PubMed] [Google Scholar]
- 18.Ripley TL, Gadd CA, De Felipe C, Hunt SP, Stephens DN. Lack of self-administration and behavioral sensitization to morphine, but not cocaine, in mice lacking NK1 receptors. Neuropharmacol. 2002;43(8):1258–1268. doi: 10.1016/s0028-3908(02)00295-2. [DOI] [PubMed] [Google Scholar]
- 19.Gadd CA, Murtra P, De Felipe C, Hunt SP. Neurokinin-1 receptor-expressing neurons in the amygdala modulate morphine reward and anxiety behaviors in the mouse. J of Neurosci. 2003;23:8271–8280. doi: 10.1523/JNEUROSCI.23-23-08271.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Placenza FM, Fletcher PJ, Vaccarino FJ, Erb S. Effects of central neurokinin-1 receptor antagonism on cocaine- and opiate-induced locomotor activity and self-administration behaviour in rats. Pharmacol Biochem Behav. 2006;84:94–101. doi: 10.1016/j.pbb.2006.04.011. [DOI] [PubMed] [Google Scholar]
- 21.Maldonado R, Girdlestone D, Roques BP. RP 67580, a selective antagonist of neurokinin-1 receptors, modifies some of the naloxone-precipitated morphine withdrawal signs in rats. Neurosci Letters. 1993;156:135–140. doi: 10.1016/0304-3940(93)90457-v. [DOI] [PubMed] [Google Scholar]
- 22.Chahl LA, Johnston PA. Effect of the nonpeptide NK-1 receptor antagonist CP-96,345 on the morphine withdrawal response of guinea-pigs. Reg Peptides. 1993;46:373–375. doi: 10.1016/0167-0115(93)90090-u. [DOI] [PubMed] [Google Scholar]
- 23.Hargreaves R, Ferreira JC, Hughes D, Brands J, Hale J, Mattson B, Mills S. Development of aprepitant, the first neurokinin-1 receptor antagonist for the prevention of chemotherapy-induced nausea and vomiting. Ann NY Acad Sci. 2011;1222:40–48. doi: 10.1111/j.1749-6632.2011.05961.x. [DOI] [PubMed] [Google Scholar]
- 24.Haertzen CA. An Overview of Addiction Research Center Inventory Scales (ARCI): An Appendix and Manual of Scales, 1974. National Institutes on Drug Abuse; Rockville, MD: [Google Scholar]
- 25.Handelsman L, Cochrane KJ, Aronson MJ, Ness R, Rubinstein KJ, Kanof PD. Two new rating scales for opiate withdrawal. Am J Drug Alc Abuse. 1987;13:293–308. doi: 10.3109/00952998709001515. [DOI] [PubMed] [Google Scholar]
- 26.SPSS I. SPSS 15.0.0 for windows. Chicago, Illinois: 2006. [Google Scholar]
- 27.Gagnon J, Roth JM, Carroll M, Haycock KA, Plamondon J, Feldman DS, Simpson J. Superanova accessible general linear modeling. Yale J Biol Med. 1990;63:191–192. [Google Scholar]
- 28.Walsh SL, Heilig MA, Nuzzo PA, Lofwall MR. Effects of the NK(1) antagonist, aprepitant, on response to oral and intranasal oxycodone in prescription opioid abusers. Addic Biol. 2012 doi: 10.1111/j.1369–1600.2011.00419.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.DSM-IV. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4. Washington DC: American Psychiatric Association; 1994. [Google Scholar]
- 30.Walsh SL, Strain EC, Bigelow GE. Evaluation of the effects of lofexidine and clonidine on naloxone-precipitated withdrawal in opioid-dependent humans. Addiction. 2003;98:427–439. doi: 10.1046/j.1360-0443.2003.00372.x. [DOI] [PubMed] [Google Scholar]